Abstract
In this chapter, an overview of current medical implant devices and infection problems associated with implantation is provided, bridging the gap between material engineering and clinical practice. The pathogenesis, common pathogens, and infection sites are listed, alongside the details of up-to-date strategies and guidelines for diagnosis and treatment of biomaterials-associated infections. Through the combined understanding of microbial pathogenicity, drug resistance, patients’ immune response processes, and current clinical practices, we can tackle the problem of biomaterials-associated infection via multidisciplinary approaches. To meet the clinical demands and challenges in future, strategic design of intelligent biomaterials is in need to reduce implantation device-caused infections, improving the patient’s quality of life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biomaterial-associated infection
- Implant-related infection
- Nosocomial infection
- Drug resistance
- Intelligent biomaterials
Introduction
Biomaterial-associated infection is one of the major complications in the clinical use of implanted materials, occurring in both permanent implants and temporary devices. Since the first permanent pacemaker was successfully implanted into the human body 60 years ago, the number of surgical cases using implants has increased significantly in the past decades, such as arthroplasty in joint surgery, intervertebral disc implants in spinal surgery, fracture internal fixation in traumatology, prosthetic valves in cardiac surgery, pacemakers, and various implants and filling materials in orthopedics, improving the quality of life of many patients. In recent 30 years of biomaterial evolution, biomaterials have been used in many implantation occasions such as fiber membranes for dialysis, artificial lung, auxiliary heart (segmented polyurethane), intraocular lens, dental adhesive, artificial bone, guide wire, and drug delivery system (e.g., microcapsule). In China, the output capacity of biomaterials such as bio-polyamide (bio-PA) and bio-polytrimethylene terephthalate (bio-PTT) has been put into large-scale industrial production which reached about 678,710 tonnes in total and 170,960 tonnes in the year of 2015 alone [1,2,3,4]. The compound annual growth rate (CAGR) of biomaterials was predicted to be over 10–20% till the year of 2020 [1, 4]; the Asia pacific orthopedic biomaterial market is predicted to grow with a CAGR of 12.6% during 2017–2023 [3] (Fig. 1).
While the industry of biomaterials has been thriving recently, the annual overall incidence of implantation device-caused infection is about 2–3% [2], and relatively few biomaterials have been designed with effective infection prevention property. Apart from the surgical operation and perioperative preventive measures, development of intelligent biomaterials is the key factor for implant design. Microbial proliferation can cause physical damage to the implant, such as loosening, dislocation, and structural instability, apart from causing systemic infection symptoms such as fever or embolism. Alongside bioactivity and biocompatibility, the chemical composition and physical properties are crucial for biomaterial design.
Infection around prosthesis implantation is a serious complication. Infections around implant and/or implant device often greatly reduce the patient’s quality of life, by subjecting them to chronic pain and inconvenience. According to recent studies, biomaterial-associated infections are the most common cause of revision in the first 5 years after the initial replacement of the implantation [5]. In many cases, infection around the prosthesis also means prolonged hospital stays, from weeks to months. For implantation-related nosocomial infections, long-term hospitalization, multiple surgeries, and anesthesia will increase the risk of patients’ exposure to multidrug-resistant pathogens, resulting in secondary complications (pulmonary embolism, intubation-related sepsis, antibiotic-associated diarrhea, hemorrhoids, etc.), even the risk of death. Replacement surgery often requires more than one additional surgery to treat these infections with treatment of peripheral bone, muscle, and soft tissues. Consequences of biomaterial-associated infection have become a socioeconomic problem for the medical resource distribution and public health care system. Although progress has been made in preoperative, intraoperative, and postoperative management alongside the greatly improved surgical techniques, the infection rate has not decreased significantly over the past two decades. In the case of implantation infection, the only solution is systemic management of infection prevention before its occurrence. Treatment involving complete removal of all infected soft tissue and bone around the prosthesis has devastating consequences for patients. Therefore, no effort should be spared in reducing the risk of biomaterial-associated infections and effectively diagnosing and treating existing infections (Scheme 1).
Guidelines for prevention of implantation infection and antibiotic resistance. (US Center for Disease Control and Prevention [6])
For implantation such as artificial joint devices, infection after long-term implantation is a severe problem. The presence of foreign biomaterials in the human body for a long time may cause the patient’s innate immune function to decline. When the surface of implant biomaterials becomes colonized with an infectious flora, the risk of developing infectious diseases cannot be avoided. The difficulty in controlling biomaterial-associated infectious diseases is that it is necessary not only to evaluate the antimicrobial properties at the time of manufacture but also to confirm the effectiveness after long-term placement [7]. However, methods for evaluating the long-term usage of biomaterials in human body environments and their associated material properties still have not been fully investigated. Although evaluation methods for cultured cells and tissues can be studied in various ways, there are fewer studies to investigate changes that occur within human physiological conditions [7]. To confirm whether a newly developed implant meets the required criteria, it is essential to evaluate the long-term characteristics of the biomaterial. Meanwhile, in order to facilitate biomaterial development, it is important to set long-term performance evaluation methods. In the case of medical surgery biomaterials, it is necessary to evaluate what may eventually occur 10 or 20 years after implantation in the human body. Now it is difficult to carry out long-term monitoring even in animal model experiments, which is challenging for biomaterial characterization and evaluation.
Strategies for the prevention, diagnosis, and treatment of biomaterial-associated infections have evolved over the past few years. Most hospitals comply with strategies agreed on by major professional societies. Since infections around the prosthesis have been recognized as the most serious complication of artificial implantation, more attention has been paid to the development of intelligent biomaterials with infection resilience [8,9,10]. In order to effectively prevent and treat infections in the future while maintaining the function of implants, multidisciplinary collaboration between medical specialists, material science researchers, and the industry needs to be established. This chapter focuses on the pathogenesis of biomaterial-associated infections, current clinical demands of infection-reducing biomaterials, and recent research of infection-reducing strategies, intended to further facilitate research in this area.
Pathogenesis of Biomaterial-Associated Infection
As the phrase “the race for the surface” suggests [11, 12], the fate of biomaterial implants is influenced by a competition between host tissue cell integration and bacterial colonization at their surfaces. Microorganisms may enter the patient’s body during the surgery. Recent studies also suggested that biomaterial-associated infections might be lifestyle related. Physical conditions including past surgical history, diabetes (blood glucose >200 mg/L or HbA1C >7%), nutrition deficiency, obesity (BMI > 40 kg/m2), chronic liver disease or kidney diseases, excessive smoking (>1 pack/day), excessive drinking, and drug abuse would put the patient at higher risk of biomaterial-associated infections [13].
In due course of implantation, if biomaterials cause damage to the epithelium and the mucosal barrier, the implant or implant device may weaken the host’s defense system and provide a growing niche for microorganisms, allowing pathogens to access blood circulation and deep tissues. Meanwhile, biomaterials may release soluble components and form high-density fibrous tissue membranes around the implant or implant device, which would act as a mechanical barrier preventing immune responsive macrophages from migrating to the interface and allowing pathogens to survive near the implant. Implanted biomaterials may also interfere with the physiological process of anti-infection through surface–media interactions; tissues around the implant site may be prone to infection diffusion [14]. The choice of implantation biomaterials is crucial because the physical and chemical properties of the biomaterial you choose determine their capacity for preventing or inducing adsorption, infection, and inflammation under healthy physiological conditions when it interacts with different microorganisms.
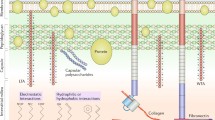
Mechanistic studies of bacterial and fungal biofilm formation on implantation biomaterials has not received sufficient attention yet. Microorganisms can form biofilms that protect microbes against antibiotics and from the body’s own immune system. Biofilm formation helps pathogens adapt to chemical and physical conditions of microenvironment, the biochemical interactions of the host defense, and also antibiotic regimes, assisting in intercellular communication and nutrition for pathogen proliferation [15,16,17,18,19].
As shown in Fig. 2, once attached to the surfaces, bacteria or fungi adhere firmly. The pathogens rapidly grow into microcolonies and secrete extracellular polymeric substances (EPS) to form a three-dimensional matrix cell structure termed biofilm. EPS consists of polysaccharides, proteins, and sometimes extracellular DNA (eDNA). Polysaccharide intercellular adhesin (PIA) process also happens which involves staphylococcal surface protein (60 kDa) [20, 21]. After maturation, the biofilm can disperse causing the bacteria to diffuse and spread [22], seeding acute infections [23]. It is difficult to eradicate biofilms due to their characteristics; the host cells around the biofilm are in a dormant state. The only effective solution is to prevent the formation of bacterial biofilms via strategic design of biomaterials.
Diagnosis and Treatment of Biomaterial-Associated Infections
Early diagnosis of biomaterial-associated infections and the severity of the infections are still quite challenging. At present, there is a lack of consensus of treatment procedure among the specialists, the clinical features of infection around the prosthesis are still not clear, especially how to distinguish biomaterial-associated infections from the failure of the implant to remain sterile; the diagnostic criteria are still controversial, and the choice of suitable antibiotics or surgical methods for treatment is still inconclusive. There is an unmet need of a global-scale survey-based statistics to develop guidelines for handling biomaterial-associated infections and general clinically supported guidelines for the use of various treatments. Patients with persistent or recurrent infections often require multiple surgeries, which can lead to anatomical damage (muscle contractures, bone defects, loss of soft tissue coverage, etc.), which may require additional operation for joint fixation, Girdlestone procedure, or even amputation. Patients with persistent infections are often under great stress due to chronic pain (Fig. 3).
(a) Infection after knee arthroplasty with visible sinus; (b) tissue infection around the prosthesis after total hip arthroplasty with visible osteonecrosis after removal of the prosthesis. (Photographs courtesy of Prof. Fanpu Ji at Department of Infectious Diseases, 2nd Hospital of Xi’an Jiaotong University, with consent of patients)
There are many classifications of infections around prosthesis based on different stages, each with its own criteria. As commonly agreed by many specialists, the simplest classification is to divide biomaterial-associated infections into early infections and late infections. Early infection refers to an infection that occurs within 3–4 weeks after the implantation of the prosthesis or the onset of symptoms [24]. Early acute infection symptoms are usually caused by intraoperative misconducts; biofilm production is less or immature in this stage [25]. Infections that occur after 3–4 weeks are classified as late infections, meaning that these infections are caused by blood sources, even several years or decades after surgery. Infections that occur after more than 4 weeks after surgery often accompanied with persistent pain at infection site, and low-virulence pathogens such as coagulase-negative staphylococci, enterococci, or Propionibacterium acnes (P. acnes) are more common source. Acute homogenous infection may happen more than 2 years after surgery, due to bloodborne dissemination; the clinical symptoms are typical redness, heat, and pain, the pathogen source often includes streptococcus and gram-negative bacilli with culture-positive rate <50% [24]. Infection stage classification has certain significance for the treatment plan, but it must be emphasized that infection is a continuous and coherent process. Follow-up treatment must not be based solely on the stage classification; other factors such as the stability of the prosthesis, the presence of sinus, pathogenic virulence, and patients’ relevant medical history should also be taken into consideration.
For early infection cases, it may be reasonable to retain the prosthesis. For advanced infections in late stages, the prosthesis, all foreign bodies, and infected bone and soft tissue should be removed [26]. If the prosthesis is implanted close to the surface of the skin, infections are usually discovered in early stages with redness, swelling, heat, and pain around the implant device or implant. Pain is the most important clinical symbol of infection; if pain suddenly occurs after an asymptomatic period, then clinical examination must be performed. Formation of fistula and/or exudation around the implantation part of the body is also considered as a sign of local infection; serum examination of biomarkers should be carried out [25, 27]. Systemic immune and neural symptoms such as fever and muscle dysfunction may occur later as implants and devices gradually become impaired. Infected artificial joints such as hip or knee implants can cause walking pain and walking instability. Infected prosthetic heart valve may cause fatigue as the patient has less cardiac output, eventually leading to severe heart failure. An effective surgery with antibiotic treatment plan is needed to alleviate the infection and pain, and restore function, yet there are still no clear treatment guidelines to ensure more than 90% success rate of long-term treatment.
Alongside echocardiography and scintigraphic imaging (X-ray, CT, fMRI) methods, laboratory-based biochemistry and immunoassay play an important role in the development in the diagnosis of biomaterial-associated infections. In serum testing, elevated levels of indicators such as procalcitonin (PCT), erythrocyte sedimentation rate (ESR), sedimentation rate (sed rate), C-reactive protein (CRP) may be associated with infections. In urine testing, a positive leukocyte esterase test indicates infection. If noninvasive tests fail to diagnose infections, puncturing to collect cerebrospinal fluid and/or synovial fluid from the implantation area that is suspected of being infected must be performed in the operating room with strict aseptic procedures. Patients should cease their antibiotic doses 10–14 days prior to puncture. Specimens obtained by puncture should be sent to the nearest qualified laboratory as soon as possible for further tests and must be cultured for at least 14 days to ensure that slow-growing pathogens can be detected. Increased white blood cell (WBC) count or increased percentage of neutrophils (PMN%) should be considered a red flag. If clinical manifestations and serological tests are highly suspected of infection around the prosthesis, but bacterial culture test is negative, an open surgical biopsy should be performed. Biopsy specimens collected from around the prosthesis area are more accurate for examination of bacterial culture or histological analysis [5]. If at least two tissue culture tests around the prosthesis have found the same pathogen, an infection could be concluded. Special attention is due if the patient is seriously suspected to have a periprosthetic infection, even if the above diagnostic criteria are not met; infection should be considered with the help of further examination and treatment. Formation of biofilm may significantly reduce the sensitivity of traditional microbial culture techniques, making pathogenic examinations difficult. Currently, there is limited consensus in the diagnosis gold standards and treatment methods; thus, different guidelines should be considered to understand the limitations of each type of detection method. The application and analysis combined with the examination of actual patients’ condition need multidisciplinary cooperation. In addition, the sensitivity of qualitative and quantitative examination via biochemical and histological analyses could be further improved with techniques such as sonication, real-time quantitative PCR, metagenomic next-generation sequencing (mNGS), and Ibis T5000 universal biosensor system.
Causative Pathogens of Biomaterial-Associated Infections
Upon usage, biomaterials directly or indirectly contact or interact with the human body components (e.g., organs, tissues, cells, and proteins). Most prostheses such as vascular and blood vessel stains are embodied under the skin within the body and do not have an opening surface for infection. However, implants used in dental treatments usually have extended structure from within the tissue to outside the tissue implant contact point. Biomaterials placed in such a fashion with exposed parts which create the niche of polysaccharide and hemidesmosome secretion are susceptible to infection. Although adhesion, repair, and immune function are retained in the surrounding tissues of implants, the binding part between implanted biomaterials and the tissue mucosa has a much weaker protective mechanism. If inflammation reaches the bones along the tissue surface, especially if an implant has uneven structure, it is difficult to remove the infected surrounding tissue, since at present there is no effective early diagnostic techniques against peri-implant inflammation.
Biomaterial-Associated Infection-Related Drug Resistance
Drug resistance of pathogens is the main enemy we face in the first line of designing anti-infection biomaterials. Just as penicillin-resistant bacteria have already existed before the appearance of penicillin, most of the drug-resistant pathogens have existed in nature long before drug discovery. If antibiotic drugs are continually applied, the susceptible strains of pathogens may be destroyed, and the resistant strains may survive and eventually proliferate through mutation and evolution. The use of antibiotics means the selection of more resistant pathogens. The prevalence of drug-resistant pathogens may increase when pathogenic microorganisms are frequently exposed to antibiotics. As shown in Table 1, the pathogens in biomaterial-associated infections often include gram-negative bacteria, aerobic gram-positive bacteria, fungi, and even mixed strain of pathogens. The American College of Orthopaedic Surgeons (AAOS) clinical guidelines for the diagnosis of prosthetic infections strongly recommend against the use of antibiotics prior to infection diagnosis. If antibiotics are applied before sample collection for diagnostic tests, the influence of biofilm formation often leads to negative culture results of pathogen culture tests. At present, about 15–20% of implantation infection patients have negative clinical bacterial culture, and the negative results may make diagnosis by doctors perplexing.
As shown in Table 2, multidrug-resistant pathogens such as methicillin-resistant Staphylococcus aureus (S. aureus) (MRSA) and Staphylococcus epidermidis are common sources of infection. Small colony variants (SCVs) including S. aureus, Pseudomonas aeruginosa (P. aeruginosa), and several other bacteria can even grow within the temporary spacer containing gentamicin [46]. Life-threatening pathogens such as enterobacteria, non-fermenting bacteria (e.g., Acinetobacter spp., Pseudomonas spp.) are resistant to penicillin, cephalosporin, quinolone, and carbapenem, which are classified as 3MRGN (multidrug-resistant gram-negative) or 4MRGN according to Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) [47]. These multidrug-resistant pathogens are resistant to all known Class 3 or Class 4 antibiotic drugs. When nosocomial outbreak occurs or when there are infections caused by drug-resistant pathogens, there is little to do clinically.
With the drug resistance problem in mind, two aspects must be considered while deciding on the treatment of biomaterial-associated infections: the annihilation of the pathogen by effective drug dosing regimens and the suppression of emergence of resistant pathogen strains. In order to suppress the emergence of resistant pathogen strains, firstly, antibiotics should not be prescribed when the patient is only a carrier without symptoms or when test results of infections are inconclusive; secondly, the antibiotic regimens with sufficient dosage should be stopped immediately after the infection symptoms cease to exist; thirdly, the use of a single antibiotic drug should be avoided in order to decrease the selective pressure of drug resistance; last but not least, nosocomial infections should be prevented with strict regulations, and human-to-human transmission routes must also be prohibited effectively.
Clinical Demands: Desirable Properties of Infection-Reducing Biomaterials
The US Public Law 105-230: Biomaterials Access Assurance Act of 1998 and the FDA guidance of the International Standard ISO 10993-1 [48] insist that biomedical evaluations of implantation biomaterials be required carried out before implantation. Low toxicity, nonallergenic, and low inflammatory reaction should be tested as a biocompatibility indicator. Biomaterials with sufficient in vivo stability (corrosion resistance and abrasion resistance) are required. Biomaterials used for implants, implant device, or catheters that penetrate the skin that is in contact with tissue or bone area must have interface compatibility and firm connectivity. Adhesion property is also required to be considered in order to avoid the invasion space of bacteria. In contact with tissue, biomaterials may trigger the surrounding tissue cells to generate extracellular matrix (ECM) components contained in serum. Adsorption of biomolecules onto the surface of implanted biomaterials is followed by cell adhesion behavior as well as immune responses (cell migration, proliferation, differentiation). For instance, if cell adhesion molecules such as fibronectin are adsorbed on the surface of the biomaterial before bacteria colonization, the adhesion between the implanted biomaterial and surrounding tissue cells increases.
The process of biomaterial–cell adhesion within the implant’s surrounding tissue takes place through a series of events as follows: (1) physical adsorption of ECM to the surface of the material; (2) binding between the ECM and the cell membrane protein (integrin) and the adhesion spot associated protein; (3) binding between the adhesion complex protein and the cytoskeleton, that is, the binding proteins penetrate cell membrane in form of chains. In this state, when a shearing force (a force parallel to the adhesion interface) is applied to the cells, the material–cell adhesion breaks at the weakest part, and the cells are detached. It has been reported that the weakest binding point is actually inside the cells rather than between the surface of the substrate and the ECM. As for the improvement of biomaterial design strategies, it is important to facilitate the adsorption step of cell adhesion with the surface of biomaterials, while ensuring that minimum shearing force is applied, to break the material–cell adhesion interface binding. The strength of deformation force is generated at the interface. In each biomaterial–cell/tissue interface, the binding breaking force is different. In addition to controlling the biomaterial adsorption behavior on the material surface, it is important to match the mechanical properties between the material and the biological tissue, in order to maintain the intermolecular binding properties. Ideal biomaterials with intelligence should be able to generate self-organizing and self-governing functionality at their interface with surrounding host tissues. Activation of host tissue–biomaterial interaction and long-term functional retention are also key performance indicators.
Besides mechanical properties, examination of the intracellular interactions between the biomaterial implants and the surrounding tissue cells also requires biochemical analysis. Infection-reducing components must not interfere with the physicochemical properties of the biomaterial. On the other hand, the biomaterial activities should not be inactivated by the patient’s innate immune response. Therefore, elucidation of various biomarkers for performance evaluation is needed. The recent development of nucleic acid-based microarray analysis has made it possible to examine in a timely manner the gene expression level of surrounding cells interacting with the biomaterial. However, with the emerging research in regenerative medicine and tissue engineering, at present, the correlation between the gene expression profile of cultured cells in vitro and the gene expression of implantation surrounding tissues in vivo of the patient’s body has not been confirmed. At the same time, many studies have reported the optimal culture conditions for inducing functional expression of cells on scaffold biomaterials. In order to resolve the occurrence of infections after long-term implantation, recent reports have shown that it is possible to examine the effectiveness of infection-reducing agents in biomaterials by various tests. The problem that remains to be addressed is the need for conducting an evaluation of the infection-reducing properties not only at the time of manufacture but also after long-term implantation. Current methods for evaluating long-term exposure to the in vivo environment and the long-lasting infection-reducing activity of the biomaterial after implantation have still not been fully studied. As the nature of the interface determines the function of the biomaterial to a large extent, strategic designing of interface with more advanced functions such as sensing or exerting bioactivity and stimuli responsiveness is needed. The strategic design of interface properties and functionalities between biomaterials and surrounding tissue cells is considered to be a major development, namely, the intelligentization of the interface.
Approach from various disciplines could be employed for the design of infection-reducing biomaterials, including chemical and physical methods for alteration of material composition, surface treatment; biomedical methods such as construction of drug releasing materials; molecular biology approach such as using functional proteins. Recent reports have shown that biomaterials releasing drugs such as bisphosphonates, statins, and parathyroid hormone could facilitate bone metabolism.
Summary and Outlook
The reliability of retrospective studies on the rate of infection after prosthesis implantations might be compromised because of individual variability among patients and differences in other aspects (operative time, surgical techniques, blood transfusions, operating room, etc.), which are factors that have a major impact on the infection process. For the same reason, the analysis of implantation registration center data may also lead to biased conclusions given the lack of information about biomaterial-associated infections. It is clear that we need to find a more scientific method to assess the capacity of biomaterials’ resilience to infection. In the future, for long-term implantation with intelligent biomaterials, multidisciplinary collaborations of epidemiology, etiology, surgery, microbiology, infectious disease, and pharmacology should be promoted to conduct in-depth research on the diagnosis and treatment of biomaterial-associated infections and to fully combine the expertise of materials chemistry and physics research with that of industry.
References
Biomaterial industry in China. CCM Data & Business Intelligence 2016
Nana A, Nelson SB, McLaren A, Chen AF (2016) What’s new in musculoskeletal infection: update on biofilms. JBJS 98(14):1226–1234
Europe, Health & Medicine, Pharmaceuticals & Biotech (2019) Asia pacific orthopedic biomaterial market 2019, industry analysis, size, share, trends, product price, profit, forecast to 2023
Patent application trend survey report summary (artificial organs). https://www.jpo.go.jp/shiryou/pdf/gidou-houkoku/h28/28_06.pdf. Accessed 8 May 2019
Kheir MM, Tan TL, Shohat N, Foltz C, Parvizi J (2018) Routine diagnostic tests for periprosthetic joint infection demonstrate a high false-negative rate and are influenced by the infecting organism. JBJS 100(23):2057–2065
US Disease Control and Prevention (CDC). Appropriate antibiotic use in hospitals and long-term care. https://www.cdc.gov/antibiotic-use/healthcare/index.html. Accessed 26 April 2019
Use of International Standard ISO 10993-1. Biological evaluation of medical devices—part 1: evaluation and testing within a risk management process. https://www.iso.org/standard/44908.html. Accessed 26 April 2019
Holzapfel BM, Reichert JC, Schantz JT, Gbureck U, Rackwitz L, Noth U, Jakob F, Rudert M, Groll J, Hutmacher DW (2013) How smart do biomaterials need to be? A translational science and clinical point of view. Adv Drug Deliv Rev 65(4):581–603
Singh B (2018) NanoBioMaterials: nanobiomaterials. CRC Press
Yamamoto R (2017) Changes and prospects of implant materials from the viewpoint of biocompatibility. Materia 56(3):225–228. (in Japanese)
Gristina AG, Naylor P, Myrvik Q (1989) Infections from biomaterials and implants-a race for the surface. Med Prog Technol 14(3–4):205–224
Busscher HJ, van der Mei HC, Subbiahdoss G, Jutte PC, van den Dungen JJ, Zaat SA, Schultz MJ, Grainger DW (2012) Biomaterial-associated infection: locating the finish line in the race for the surface. Sci Transl Med 4(153):153rv10
Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA (2016) Periprosthetic joint infection. Lancet 387(10016):386–394
Kannan MB, Walter R, Yamamoto A (2015) Biocompatibility and in vitro degradation behavior of magnesium–calcium alloy coated with calcium phosphate using an unconventional electrolyte. ACS Biomater Sci Eng 2(1):56–64
Baidya AK, Bhattacharya S, Dubey GP, Mamou G, Ben-Yehuda S (2018) Bacterial nanotubes: a conduit for intercellular molecular trade. Curr Opin Microbiol 42:1–6
Bryers JD (2018) Modeling biofilm accumulation. Physiological models in microbiology volume II, Chapter 11
de la Fuente-Nunez C, Reffuveille F, Fernandez L, Hancock REW (2013) Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16(5):580–589
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633
Romling U, Balsalobre C (2012) Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 272(6):541–561
Sasaki K (2016) Polysaccharide intercellular adhesion (PIA) process also happens which involves staphylococcal surface protein. J Jpn Soc Poweder Powder Metallurgy 63(8). (in Japanese)
Guidelines for Biomaterial Research That Supports Medical Care (2017) Annual meeting of Japan biomaterial engineering committee. (in Japanese)
Wolfmeier H, Pletzer D, Mansour SC, Hancock REW (2018) New perspectives in biofilm eradication. ACS Infect Dis 4(2):93–106
Guilhen C, Forestier C, Balestrino D (2017) Biofilm dispersal: multiple elaborate strategies for dissemination of bacteria with unique properties. Mol Microbiol 105(2):188–210
Guo Y, Shao HY. Characteristics and challenges of human implant infection (in Chinese). Beijing-Hong Kong infection Forum 2018
Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR (2013) Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56(1):e1–e25
Elkins JM, Kates S, Lange J, Lange J, Lichstein P, Otero J, Soriano A, Wagner C, Wouthuyzen-Bakker M (2019) General assembly, diagnosis, definitions: proceedings of international consensus on orthopedic infections. J Arthroplast 34(2):S181–S185
Parvizi J, Della Valle CJ (2010) AAOS Clinical Practice guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg 18(12):771–772
Drago L, Lidgren L, Bottinelli E, Villafañe JH, Berjano P, Banfi G, Romanò CL, Sculco TP (2016) Mapping of microbiological procedures by the members of the International Society of Orthopaedic Centers (ISOC) for diagnosis of periprosthetic infections. J Clin Microbiol 54(5):1402–1403
Higuera CA, Zmistowski B, Malcom T, Barsoum WK, Sporer SM, Mommsen P, Kendoff D, Della Valle CJ, Parvizi J (2017) Synovial fluid cell count for diagnosis of chronic periprosthetic hip infection. JBJS 99(9):753–759
Johns Hopkins ABX Guide. 2018
Guidelines for clinical application of antimicrobial agents (2015 edition, in Chinese). National Health and Family Planning Commission
Thoendel M, Jeraldo P, Greenwood-Quaintance KE, Chia N, Abdel MP, Steckelberg JM, Osmon DR, Patel R (2017) A novel prosthetic joint infection pathogen, Mycoplasma salivarium, identified by metagenomic shotgun sequencing. Clin Infect Dis 65(2):332–335
Cabrita HB, Croci AT, de Camargo OP, de Lima A (2007) Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded cement spacer. Clinics 62(2):99–108
Del Pozo JL, Patel R (2009) Infection associated with prosthetic joints. N Engl J Med 361(8):787–794
Wang Y, Cheng LI, Helfer DR, Ashbaugh AG, Miller RJ, Tzomides AJ, Thompson JM, Ortines RV, Tsai AS, Liu H, Dillen CA, Archer NK, Cohen TS, Tkaczyk C, Stover CK, Sellman BR, Miller LS (2017) Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc Natl Acad Sci U S A 114(26):E5094–E5102
Wang Z, de la Fuente-Nunez C, Shen Y, Haapasalo M, Hancock REW (2015) Treatment of oral multispecies biofilms by an anti-biofilm peptide. PLoS One 10(7):e0132512
Gao Q, Yu M, Su Y, Xie M, Zhao X, Li P, Ma PX (2017) Rationally designed dual functional block copolymers for bottlebrush-like coatings: in vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater 51:112–124
Gross MS, Phillips EA, Carrasquillo RJ, Thornton A, Greenfield JM, Levine LA, Alukal JP, Conners WP, Glina S, Tanrikut C, Honig SC, Becher EF, Bennett NE, Wang R, Perito PE, Stahl PJ, Gaya MR, Barbara MR, Cedeno JD, Gheiler EL, Kalejaiye O, Ralph DJ, Kohler TS, Stember DS, Carrion RE, Maria PP, Brant WO, Bickell MW, Garber BB, Pineda M, Burnett AL, Eid JF, Henry GD, Munarriz RM (2017) Multicenter investigation of the micro-organisms involved in penile prosthesis infection: an analysis of the efficacy of the AUA and EAU guidelines for penile prosthesis prophylaxis. J Sex Med 14(3):455–463
Habous M, Tal R, Tealab A, Soliman T, Nassar M, Mekawi Z, Mahmoud S, Abdelwahab O, Elkhouly M, Kamr H, Remeah A, Binsaleh S, Ralph D, Mulhall J (2018) Defining a glycated haemoglobin (HbA1c) level that predicts increased risk of penile implant infection. BJU Int 121(2):293–300
Scholz HC, Nockler K, Gollner C, Bahn P, Vergnaud G, Tomaso H, Al Dahouk S, Kampfer A, Cloeckaert M, Maquart MS, Zygmunt AM, Whatmore M, Pfeffer B, Huber P, Busse HJ, De BK (2010) Brucella inopinata sp nov., isolated from a breast implant infection. Int J Syst Evol Microbiol 60:801–808
Hoque J, Haldar J (2017) Direct synthesis of dextran-based antibacterial hydrogels for extended release of biocides and eradication of topical biofilms. ACS Appl Mater Interfaces 9(19):15975–15985
Mao X, Cheng R, Zhang H, Bae J, Cheng L, Zhang L, Deng L, Cui W, Zhang Y, Santos HA, Sun X (2019) Self-healing and injectable hydrogel for matching skin flap regeneration. Adv Sci (Weinh) 6(3):1801555
Hoque J, Adhikary U, Yadav V, Samaddar S, Konai MM, Prakash RG, Pararnanandham K, Shome BR, Sanyal K, Haldar J (2016) Chitosan derivatives active against multidrug-resistant bacteria and pathogenic fungi: in vivo evaluation as topical antimicrobials. Mol Pharm 13(10):3578–3589
Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, Grp EMINS (2006) Methicillin-resistant S-aureus infections among patients in the emergency department. N Engl J Med 355(7):666–674
Scott VCS, Haake DA, Churchill BM, Justice SS, Kim JH (2015) Intracellular bacterial communities: a potential etiology for chronic lower urinary tract symptoms. Urology 86(3):425–431
Minassian AM, Newnham R, Kalimeris E, Bejon P, Atkins BL, Bowler IC (2014) Use of an automated blood culture system (BD BACTEC™) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis 14(1):233
Heudorf U, Albert-Braun S, Hunfeld KP, Birne FU, Schulze J, Strobel K, Petscheleit K, Kempf VA, Brandt C (2016) Multidrug-resistant organisms in refugees: prevalences and impact on infection control in hospitals. GMS Hyg Infect Control 11
US Public Law 105-230 105th Congress, Biomaterials Access Assurance Act of 1998. https://www.biomaterials.org/sites/default/files/docs/2014/biomaterials_access_assurance_act_of_1998.pdf. Accessed 26 April 2019
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2018YFC1105402 and 2017YFA0207202), the National Natural Science Foundation of China (21875189 and 81601553), Key R&D Program of Jiangsu Province (BE2017740), the Natural Science Foundation of Zhejiang Province (LGF19H200005), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bai, D., Chen, J., Li, P., Huang, W. (2020). Perspectives on Biomaterial-Associated Infection: Pathogenesis and Current Clinical Demands. In: Li, B., Moriarty, T., Webster, T., Xing, M. (eds) Racing for the Surface. Springer, Cham. https://doi.org/10.1007/978-3-030-34475-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-34475-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34474-0
Online ISBN: 978-3-030-34475-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)