Abstract
Obstructive sleep apnoea syndrome (OSAS) is a sleep-related breathing (SBD) disorder characterized by intermittent episodes of partial or complete obstruction of the upper airway, leading to cessation of breathing while asleep. OSAS elicits a series of mechanical, hemodynamic, chemical, neural, and inflammatory responses with adverse consequences on the cardiovascular system. Among these, cardiac arrhythmias are a common problem in affected patients, including both brady and tachy-arrhythmic manifestations. The strong association between OSAS and arrhythmia has prompted in last decades significant research aimed to understanding the mechanism connecting the two conditions, to define prevalence, to prospect new therapies and to explain the synergic negative effects in terms of morbidity and mortality in affected patients. In our center experience since 2009, we have focused our interest on atrial fibrillation (AF) and sleep apnea with the aim to define prevalence of SBD in a real-life population affected by AF and to assess the role of SBD on atrial remodelling, AF recurrence and therapies. Moreover, we have studied the complex relation between sleep apnoea and heart failure in patients treated with cardiac resynchronization therapy. We will report a revision of literature about sleep apnoea, AF and furthermore the main findings of our research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
1.1 OSAS and Arrhythmias
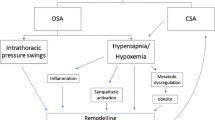
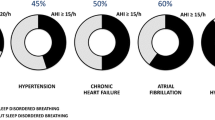
Obstructive sleep apnoea syndrome (OSAS) is a sleep-disordered breathing (SDB) characterized by upper airway occlusion in the face of continued activity of inspiratory thoracic pump muscle. OSAS affects 3–7% of the adult population [1], has been shown to be independently associated with increased cardiovascular morbidity, including hypertension, congestive heart failure, ischemic heart disease, and stroke [2]. Moreover, OSAS has been consistently associated with increased risk of cardiac arrhythmias, in particular atrial fibrillation and ventricular arrhythmias [3,4,5].
Proposed mechanisms to explain these relationships include and intermittent hypoxia, oxidative stress, sympathetic activation, and endothelial dysfunction, all of which are critical mediators of cardiovascular disease [6,7,8]. In particular, the pro-arrhythmic contributions are the apnoea-induced hypoxia and the increased sympathetic nervous system activity.
Patients with OSA have a higher prevalence of atrial fibrillation (AF) than patients without OSA, and are more likely to develop new-onset AF, independent of obesity [3]. Obstructive sleep apnea is also associated with an increased risk of AF after coronary bypass surgery, higher recurrence rates of AF after cardioversion [9], and higher relapse rates of AF after catheter ablation [10]. Data from observational studies show that CPAP treatment is associated with a significantly decreased recurrence rate of AF, even after electrical cardioversion or ablative therapies, and that patients are less likely to progress to more permanent forms of AF and have significantly reduced occurrence of paroxysmal AF compared with untreated patients [11,12,13,14]. Moreover, OSAS is associated with increased risk of complex ventricular ectopy, ventricular tachycardia [15] and life-threatening arrhythmias [16]. In addition to well known risk factors, a greater QT dispersion might contribute to ventricular arrhythmias in patients with OSAS [17]. In heart failure patients with impaired left-ventricular ejection fraction (LVEF) and implantable cardioverter defibrillator (ICD), severity of OSAS correlates with the risk for complex ventricular arrhythmias and appropriate implantable defibrillator therapy [18]. Treatment with continuous positive airways pressure (CPAP) reduces ventricular ectopy in patients with SDB, but there are no evidences against the occurrence of malignant arrhythmias in OSAS patients [19]. OSAS is also associated with greater incidence of bradyarrhythmia in small-sample studies, and the severity of SDB correlates with duration and severity of bradycardia, whereas large-cohort studies generally do not report such association [20].
2 Research Topics Analysed by Our Group
2.1 Sleep Disordered Breathing and Atrial Fibrillation
Considering the great prevalence of both atrial fibrillation and SDB, the mutual relationship among the two diseases and impact on prognosis of affected patients, in 2009 we decided to focus our research on this topic. The aim of our research was to characterize the prevalence of SDB in a cohort of AF real-life population. Moreover, we aimed to assess the role of SDB on atrial remodelling, AF recurrence and therapies together with the evaluation of quality of life of patients presenting both AF and SDB.
Since May 2009 to March 2017 we prospectively enrolled patients admitted to Arrhythmology and Cardiology Clinic of Ancona for elective electrical cardioversion of AF.
At baseline patients, who accepted to participate in this observational trial, underwent to ECG, complete echocardiographic examination, nocturnal cardiorespiratory monitoring, and complete blood sample analysis. Quality of life and diurnal somnolence were respectively assessed trough Short Form [21] Health Survey (SF-36) and the Epworth Sleepiness Scale (ESS). All patients with paroxysmal and persistent atrial fibrillation older than 18 years were candidate for the study. The exclusion criteria included: severe chronic obstructive pulmonary disease, known SDB or/and patients treated with nocturnal respiratory therapy, inability to give informed consent, disagreement to participate to the study.
One hundred and twenty-one patients were enrolled and considered for the final analysis.
Characteristics of patients’ population are reported in Table 1.
Our population presented a male prevalence (n = 88, 73%) and a mean age of 68.7 ± 11.4 years. Patients enrolled have already had a mean of 1.0 ± 3.1 previous AF Episode. The population presented arterial hypertension in 78%, hyperlipidaemia in 52%, and diabetes in 20%, and chronic obstructive pulmonary disease in 17% and lone AF. Mean CHA2DS2-VASC score was 3.0 ± 1.8. Mean left ventricular ejection ischemic cardiomyopathy in 18% of patients. A minority of 7.4% of patients presented DS fraction was 51.0 ± 11.6%.
At blood analysis, we found the following mean value: creatinine 1.1 ± 0.7 mg/dl, haemoglobin 14.1 ± 1.6 g/dl and BNP 327.1 ± 293.9 pg/ml.
At baseline population received RAAS blockers in 63% of cases, betablockers in 61%, calcium channel blockers in 42%, amiodarone in 34%, flecainide in 15%, and propafenone in 1% and digoxin in 6.5%. Almost three quarter of patients were anticoagulated with warfarin and 27% received new oral anticoagulation drugs.
Among 121 patients, 90 (74.4%) finally underwent to electrical cardioversion and 1 patient (0.8%) to pharmacological cardioversion. In 4 patients (4.4%) the cardioversion was unsuccessful and in 10 patients there was an early AF recurrence (within 5 days). In 84.6% of cases electrical cardioversion was effective. In a mean follow-up of 903 ± 796 days, 53% of patients presented AF recurrence.
Patients with AF recurrences presented higher left atrial volumes (84.7 ml3 vs. 70.35 ml3 p = 0.042) when compared with patients with sinus rhythm, while the other echocardiographic variables were comparable.
Then we assessed SDB prevalence in our population: using a diagnostic AHI equal or greater than 5, 90% of patients (n = 109) were affected by SDB with OSAS in 57%, CSA-CSR in 32% ad hypopneas in 1% of cases.
Among patients with sleep apnoea, 36% of subjects presented a mild form (AHI < 15), 27% a moderate (15 ≤ AHI < 30) and 37% a severe one (AHI ≥ 30).
Mean value of the severity apnoea indices were the following: apnoea/hypopnea index (AHI) 24.1 ± 19.4 events/h, hypopnea index (HI) 7.6 ± 6.6 events/h, central apnoea index (CAI) 6.7 ± 11.2 events/h, obstructive apnoea index (OAI) 6.4 ± 6.9 events/h, Cheyne-Stokes respiration index (CSR) 4.1 ± 8.4 events/h, oxygen desaturation index (ODI) 26.9 ± 17.9 events/h, lowest oxygen saturation 71.2 ± 12.7%, mean oxygen saturation 92.3 ± 2.7% and SaO2 < 90% of time in bed (TIB) 13.97 ± 29.7%.
Patients with SDB presented higher AF recurrence when compared with patients without SDB (p = 0.014). When considering the typology of SDB, patients with AF recurrence showed more frequently CSA-CSR while we did not find any correlation between OSAS and AF relapse. Studying patients’ characteristics, those with CSA-CRS presented higher BNP value e lower LVEF when compared to OSAS ones (respectively 294.5 vs. 168.5 pg/ml, p = 0.015; 45.4 vs. 54.4%, p < 0.0001).
We wanted to define a correlation between SDB severity index and atrial remodelling and we found a significant relation between mean arterial saturation and SaO2 < 90% of Time in bed (TIB) with atrial diameter (respectively p = 0.009 and 0.001).
Moreover, we reported a significant correlation between SDB severity index and the Physical Component Score and the Mental Component Score of the SF-36.
The results of our observational single-centre study let us to focus on some interesting findings.
First, as already reported in literature, we confirmed the high prevalence of SDB in AF population [3, 22, 23]. OSAS prevalence is in line with previous findings and minimal variations of numbers among studies might be related to AHI values criteria used for SDB diagnosis and to patient’s characteristics, with a special regard to obesity prevalence in studied populations.
When considering the prevalence of CSA in AF population, few evidences are available in literature: CSA-CSR is usually related to heart failure and studied in this context. Therefore, our experience is important because it shows a remarkable prevalence of 32% of CSA-CSR in AF patients, independently from HF considerations.
The second important result is that SDB is associated with a higher arrhythmic recurrence after cardioversion. This data has been already described: [24, 25] however, the novel concept of our study is that, when considering the typology of SDB, only CSA and not OSAS maintains a significant correlation with AF recurrence. Our data is in contrast with Kanagala et al’ paper: they reported that patients with untreated OSA had a higher recurrence of AF after cardioversion than patients without a polysomnographic diagnosis of sleep apnoea and appropriate treatment with CPAP in OSA patients was associated with lower recurrence of AF. This difference in results may be due to the small sample size of the studied population and deserves further investigations [9]. The third aspect deduced by our experience is that patients with CSA and AF recurrences present a worst clinical profile with higher BNP e lower LVEF values. It indicates a more compromised patient in whom HF or asymptomatic systolic dysfunction might coexist at the time of electrical cardioversion, therefore impacting the success of CV in the long term.
2.2 Effects of Cardiac Resynchronization Therapy on Both Central and Obstructive Sleep Apnoea Severity
SDB is a common comorbidity diagnosed in about 50% of HF patients, both in the form of central sleep apnoea (CSA) with Cheyne Stokes Respiration (CSR), or in the form of OSA [26, 27].
In the last decade, cardiac resynchronization therapy (CRT) has become an important therapeutic step in the management of HF patients demonstrating a positive effect on cardiac function and hemodynamic profile and an improvement of functional capacity, quality of life and survival [28]. Therefore, different studies dealing with few patients have been focused on the role of CRT on SDB, showing reduction in episodes of central sleep apnoea but no significant effects on OSA [29,30,31,32,33]. Only one study suggested SDB being even a prognostic negative factor with potential impact on response to CRT [34].
Therefore, we elaborate a study to assess the clinical impact of SDB on CRT response, by evaluating clinical and echocardiographic parameters.
We conducted this single-centre, prospective, observational study at the Cardiology and Arrhythmology Clinic, “Ospedali Riuniti” Hospital, Ancona, Italy. Patient enrolment started in June 2010 and ended in July 2014. Patients with a class I or IIa indication for a biventricular pacing (CRT-P) implantation, with or without a cardioverter-defibrillator (CRT-D), according to current recommendations of the European Society of Cardiology [21] were considered for study participation.
Exclusion criteria were the following: life expectancy less than 1 year, acute coronary syndrome or coronary revascularization in the last 40 days before device implant, acute HF, severe aortic stenosis, severe chronic obstructive pulmonary disease, prior stroke in the last 12 weeks.
The primary endpoint of the study was to evaluate the possible relationship between the presence and severity of SDB (AHI > 15) and the clinical and echocardiographic response to CRT during a 12-months follow up. A positive clinical response was defined as an improvement of ≥1 NYHA class from baseline. The echocardiographic response was defined as a reduction of left ventricular end-systolic volume by 15% or more from baseline [35, 36]. Secondary outcomes were SDB interference with plasmatic level of brain natriuretic peptide (BNP), quality of life score (Minnesota Living with Heart Failure Questionnaire, MLHFQ), distance covered during six minutes walking test (6MWT), incidence of AF episodes, ventricular sustained arrhythmias, hospitalization for HF.
From a total of 196 eligible patients admitted to our Clinic for CRT implantation, only 65 patients with available baseline nocturnal cardiorespiratory monitoring and agreeing to participate were considered for primary endpoint analysis. The study followed the principles outlined in the Declaration of Helsinki. The protocol was approved by local ethics committee and all patients gave their written informed consent.
All patients underwent CRT implantation electively in stable conditions. Demographic and clinical data were collected at baseline, at 6-month and at 12-month after device implantation. At each visit, a 12-lead electrocardiogram (ECG), an echocardiographic examination, cardiorespiratory monitoring, device interrogation and 6MWT were performed. Moreover, during each visit, all patients completed the Epworth Sleepiness Scale (ESS) questionnaire for the evaluation of the daytime sleepiness [37] and the MLHFQ [38].
All patients underwent a complete transthoracic echocardiographic examination at baseline and during later visits. Studies were performed with commercially available ultrasound equipment (M4S probe, Vivid 7 PRO, GE-Vingmed Ultrasound). Left ventricular end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were calculated according to the criteria proposed by the American Society of Echocardiography [39]. Left ventricular function was assessed by modified biplane Simpson’s method. A single operator performed all the echocardiographic evaluations and was blinded to results of cardiorespiratory monitoring.
Patients enrolled underwent implantation of a CRT-P or CRT-D device (Cognis P106 or 107, Boston Scientific; Consulta CRT-D or Syncra CRT-P, Medtronic Inc; Promote accel RF, St. Jude Medical; Lumax HF-T, Biotronik). Several types of LV leads were used (Easytrack 3 IS, Easytrack 2, Acuity, Boston Scientific; 4196, Attain ability, Medtronic; Corox OTW, Biotronik; Quickflex 1258, St. Jude Medical). Device implantation was successful in all cases without any major complications.
At each follow-up visit, device interrogation was performed including evaluation of the integrity of the system, leads information, and concomitant arrhythmias with particular interest to AF episodes, sustained ventricular tachycardia and ventricular fibrillation. We also collected data about device interventions (appropriate or inappropriate therapies as shock or ATP).
After enrolment, all participants underwent overnight cardiorespiratory monitoring using Vital Night Plus (VitalAire, Milan, Italy) and data were scored manually by three trained operators according to the recommendations of the American Academy of Sleep Medicine [40]. Continuous nocturnal recordings included thoracic and abdominal respiratory effort, airflow by a nasal-oral thermocouple and nasal pressure recording, finger pulse oximetry, and body position. Apnoea were defined by the absence of airflow for 10 s or more. An episode of obstructive apnoea was defined as the absence of airflow in the presence of ribcage and abdominal excursions while CSA was defined as the absence of airflow without any abdominal or thoracic breathing efforts. Hypopneas were defined by a decrease in breathing amplitude of ≥30% for at least 10 s with an oxygen desaturation of 3% or more. In case of mixed apnoea (absence of airflow with the characteristics of CSA in the first part followed by obstructive breaths at the end) patients were classified to have either predominately CSA or OSA according to the main disorder (>50% of events). The apnoea hypopnea index (AHI) is the severity marker of SDB and is represented by the number of apnoea and hypopnea per hour of sleeping time. An AHI of ≥15/h was chosen as a pathological cut-off because an at least moderate form of SDB were considered as clinically relevant in term of effects on study endpoints.
General characteristics of the population, divided by presence or absence of SBD, are shown in Table 2. According to the cardiorespiratory monitoring testing performed at baseline, 36 patients (55.4%) had significant SDB, 18 patients (27.7%) had OSA, and the remaining 18 patients (27.7%) had CSA. The mean AHI value was 32 ± 11 for OSA patients, 45 ± 24 for CSA ones and 10 ± 3 for those without SDB.
Heart failure medication prior to enrolment is shown in Table 2. No significant differences were seen among the three groups regarding major therapeutic drug classes along the follow-up.
Biventricular stimulation percentage was comparable between groups at 6 months (96 ± 3% in OSA group, 98 ± 2% in CSA group and 97 ± 4% in patients without sleep apnoea, p = 0.567) and 12 months (96 ± 4% in OSA group, 95 ± 3% in CSA group and 96 ± 3% in patients without SDB, p = 0. 344).
Repeated measurements showed a statistically progressive significant reduction of LVEDV in general population, which decreased from 225.2 ± 57.7 ml at baseline to 191.9 ± 58.2 ml at 6-month to 186.6 ± 68.4 ml at 1-year (p within groups <0.001). Systolic volumes showed a similar trend, decreasing from 145.4 ± 45.9 ml at baseline to 119.48 ± 48.4 ml at 6-month to 114.7 ± 57.3 ml at 1-year (p within groups <0.001). Presence and type of SDB did not meaningfully interfere with volumes reduction (p between groups = 0.638 for diastolic volume and 0.680 for systolic volume). Temporal LV volumes variations in study groups are reported in Table 3.
Left ventricular ejection fraction (LVEF) significantly improved in all the implanted patients independently of SDB (p within groups <0.001; Table 3). Ejection fraction improvement was still significant after dividing the population according to presence or absence of SDB, without any significant difference between no SDB, OSA, and CSA/CSR groups (p between groups = 0.584; Table 3).
NYHA class showed an overall positive improvement over 1 year in the whole population (p within groups = 0.044) and in the SDB groups (p between groups = 0.872) (Table 3).
6MWT distance improved significantly over 1-year, with the best improvement already present at 6 months (p within groups <0.001; Table 3). Moreover, OSA and CSA/CSR did not interfere with any functional improvement, as the prolongation in walking distance was similar between the three groups (p between groups = 0.692; Table 3) and irrespective of NYHA class at enrolment. At 1-year follow-up there was a significant decrease of MLHFQ (p within groups <0.001), which was consistent in each of the three groups, with no major differences between no SDB, OSA and CSA/CSR patients (p between groups = 0.453; Table 3).
BNP levels were significantly reduced over 1 year in all the patients independently of SDB presence (p within groups = 0.032, p between groups = 0.346; Table 3).
We did not find any difference in atrial AF burden, nor in ventricular arrhythmias, hospital admissions over 1 year between no SDB, OSA and CSA/CSR patients.
Seven patients did not complete the follow-up evaluations: 3 patients with no SDB and 1 with CSA died and 3 patients refused to participate to further follow-up visits (Fig. 1).
SDB overall prevalence did not change significantly during follow-up (Fig. 2). However, CSA/CSR episodes number dropped significantly from 27.7% before implant down to 10.0% at 6-months and 3.2% at 1-year (p < 0.001); differently OSA prevalence increased during the first 6 months from 27.7 to 53.3% and then stabilised at 1-year (48.4%).
Also, SDB severity was favourably affected by CRT stimulation both in CSA and OSA patients at 6- and 12-months follow-up (p within groups = 0.031, p between groups = 0.124). The AHI value decreased in CSA patients from baseline 45 to 27 at 12-months follow-up, meanwhile it went from 32 to 21 in OSA patients (Fig. 3). The reduction in CSA/CSR episodes and the improvement of CSA severity were consistent even after sub-dividing the population according to NYHA class.
In the last years, the relation between HF and SDB has been a big focus of interest, showing complex and mutual interactions with secondary effect on patients’ prognosis and treatment. CRT is a main stone in advanced chronic HF therapy and its beneficial effects on morbidity and mortality are well documented [35, 41, 42]. Recently, its role in treatment of sleep apnoea has also been investigated.
Our study shows that more than half of chronic HF patients present sleep apnoea with an equal distribution of central and obstructive sleep apnoea. Moreover, SDB presence, either in the type of OSA or CSA, does not affect CRT clinical effectiveness.
Positive CRT effects on SDB are not limited to the central form, as previously reported in literature [26, 31,32,33], but our study highlights that CRT may reduce also OSA severity, despite its relative increased prevalence: this finding was never reported so far.
CSA episodes dropped drastically over the follow-up, from 27.7% at baseline to 3.2% at 12 months. Meanwhile, there was a substantial increase in OSA prevalence (from 27.7% at baseline to 48.8%). CRT improves the hemodynamic, therefore preventing hyperventilation and following apnoea with two mechanisms: reduction of venous pulmonary pressures and reduction of lung-chemoreceptor circulation time. By acting on the pathophysiology of HF, CRT directly should affect CSA, as a direct consequence of HF, and not the total prevalence of OSA, whose development is related to different mechanisms. In our cases, the OSA prevalence increase could be explained with the previously described concept of “unmasked OSA” as CSA disappears [32]. A patient with chronic HF may present a spectrum of SDB, passing from CSA to OSA because of the severity of cardiac dysfunction and its hemodynamic profile. An example of this concept is reported in Fig. 4 that represents the cardiorespiratory monitoring at baseline and at 6 months of one of our study’s patient.
The reduction of the severity of SDB over the 12-months follow-up, with the same effects on central and obstructive type, is one of the more important results of our study. Lamba’s metanalysis reported a reduction of severity of sleep apnoea, with major effect specifically in patients with CSA [43]; similar results on CSA are reported in a more recent systematic review while the CRT effects on OSA appear controversial [44]. In contrast, we found a significant reduction of AHI value also in OSA patients, as previously shown by Stanchina et al. [45] in a small population. The degree of AHI reduction, at least 10 points in our population, is of great importance because the magnitude of the improvement of the respiratory profile has been previously related to prognosis [46].
This positive effect even on OSA patients is not unexpected: in OSA, upper airway occlusion depends not only on anatomical features but also on neck oedema and fluids displacement during night [43, 47]. CRT, improving patient hemodynamic profile, reduces also local oedema, and therefore the apnoea severity. Moreover, CRT increase in cardiac output may reduce OSA events, as it does in CSA, by improving respiratory system control loop gain that acts also on the activity of upper airway dilator muscles [45].
The second main message of our study is that SDB does not impact CRT clinical and echocardiographic improvements. Our results are consistent with previous indirect findings: in a recent meta-analysis a substantial improvement in LVEF by CRT was present in both SDB subgroups [43]. In the present study, however, we did not focus only on LVEF but we systematically assessed other clinical and echocardiographic variables, such as MLHFQ, 6MWT distance, NYHA class and LV volumes. CRT resulted in an overall improvement of all the above-mentioned parameters, independently of the presence or absence of SDB. Similar but partial results were reported in a smaller population of 30 patients with CSA by Luthje et al. [30] and a not significant improvement of MLHFQ score was previously described in OSA patients by Stanchina et al. [45].
In our population, SDB did not influence prognosis at 12 months follow up. These data diverge from Sredniawa’s ones [34] who showed that patients with high baseline AHI value were at greater risk of major cardiovascular events during the first 6 months of CRT.
According to our data, SDB and HF coexistence at baseline did not affect a positive clinical response to CRT; therefore, sleep apnoea should not be considered a negative factor to deny resynchronization therapy to patients with indication to CRT.
2.3 Dasap-Hf
Diagnosing apnoea is a relevant issue in heart failure patients. The automated detection of SBD has been only performed in a limited cohort of patients with preserved LVEF requiring pacemaker (PM) implantation.
We are participating in a multicentre clinical trial called DASAP-HF to evaluate the performance of an implantable ventilation sensor, which detects SBD in patients implanted with an ICD or CRT-D.
3 Conclusions
The prevalence of SDB in AF population appears high. SDB is associated with a higher arrhythmic recurrence after cardioversion, especially in case of CSA.
Patients with CSA and AF recurrences present a worst clinical profile with higher BNP e lower LVEF values. This finding suggests a more compromised patient in whom HF or asymptomatic systolic dysfunction might coexist at the time of electrical cardioversion, therefore impacting the maintenance of sinus rhythm in the long term.
If further investigated, this might have important clinical repercussion, suggesting a relation between the sleep profile and cardioversion success. In case of CSA the optimization of therapy and the achievement of a better hemodynamic profile might contribute to reduce arrhythmic recurrences.
SDB does not limit the positive response to CRT in a chronic HF population. Differently from previous studies, CRT favoured not only a statistically significant reduction of CSA prevalence and severity, but improved also OSA severity, despite its increased prevalence. This last finding may be related to night neck oedema and fluid displacement reduction, direct consequence of cardiac output improvement, related to CRT effects.
The algorithm of implantable ventilation sensor in ICD will provide us further insights into the pathophysiological mechanisms linking OSAS to heart failure.
References
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea. Am J Respir Crit Care Med 165(9):1217–1239
Bradley TD, Floras JS (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373:82–93
Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T et al (2007) Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 49(5):565–571
Shepard JW, Garrison MW, Grither DA, Dolan GF (1985) Relationship of ventricular ectopy to oxyhemoglobin desaturation in patients with obstructive sleep apnea. Chest 88(3):335–340
Fichter J, Bauer D, Arampatzis S, Fries R, Heisel A, Sybrecht GW (2002) Sleep-related breathing disorders are associated with ventricular arrhythmias in patients with an implantable cardioverter-defibrillator. Chest 122:558–561
Leuenberger URS, Sweer L, Waravdekar N, Jacob E, Sweer L, Waravdekar N et al (1995) Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol 79(2):581–588
Somers VK, Dyken ME, Clary MP, Abboud FM (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96(4):1897–1904
Xie A, Skatrud JB, Puleo DS, Morgan BJ (2001) Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol 91(4):1555–1562
Kanagala R (2003) Obstructive Sleep apnea and the recurrence of atrial fibrillation. Circulation 107(2):2589–2594
Ng CY (2011) Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 108(1):47–51
Pearse SG, Cowie MR, Nalliah CJ, Sanders P, Kalman JM, Chang C-C et al (2014) Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 62(4):300–305
Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP et al (2013) Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2(6):e000421
Naruse Y, Tada H, Satoh M, Yanagihara M, Tsuneoka H, Hirata Y et al (2013) Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Hear Rhythm 10(3):331–337
Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC et al (2015) Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J 169(5):647–654
Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL et al (2006) Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 173(8):910–916. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16424443
Serizawa N, Yumino D, Kajimoto K, Tagawa Y, Takagi A, Shoda M et al (2008) Impact of sleep-disordered breathing on life-threatening ventricular arrhythmia in heart failure patients with implantable cardioverter- defibrillator. Am J Cardiol 102(8):1064–1068
Voigt L, Haq SA, Mitre CA, Lombardo G, Kassotis J (2011) Effect of obstructive sleep apnea on QT dispersion: a potential mechanism of sudden cardiac death. Cardiology 118(1):68–73
Bitter T, Westerheide N, Prinz C, Hossain MS, Vogt J, Langer C et al (2011) CheyneStokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J 32(1):61–74
Ryan CM, Usui K, Floras JS, Bradley TD (2005) Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 60(9):781–785
Mehra R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T et al (2009) Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 169(12):1147–1155
Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J et al (2010) 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy-Devel. Europace
Bitter T, Langer C, Vogt J, Lange M, Horstkotte D, Oldenburg O (2009) Sleep disordered breathing in patients with atrial fibrillation and normal systolic left ventricular function. Dtsch Aerztebl Int 106(10):164–170
Braga B, Poyares D, Cintra F, Guilleminault C, Cirenza C, Horbach S et al (2009) Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med 10(2):212–216
Mazza A, Bendini MG, Cristofori M, Nardi S, Leggio M, De Cristofaro R et al (2009) Baseline apnoea/hypopnoea index and high-sensitivity C-reactive protein for the risk of recurrence of atrial fibrillation after successful electrical cardioversion: a predictive model based upon the multiple effects of significant variables. Europace 11(7):902–909. Available from: http://dx.doi.org/10.1093/europace/eup107
Monahan K, Brewster J, Wang L, Parvez B, Goyal S, Roden DM et al (2012) Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol 110(3):369–372
Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V (2007) Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 9(3):251–257
Schulz R, Blau A, Börgel J, Duchna HW, Fietze I, Koper I et al (2007) Sleep apnoea in heart failure. Eur Respir J 29(6):1201–1205. Available from: http://erj.ersjournals.com/content/29/6/1201.abstract
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt O-A et al (2013) 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 15(8):1070–1118. Available from: http://dx.doi.org/10.1093/europace/eut206
Shalaby A, Atwood CW, Selzer F, Suffoletto M, Gorcsan J, Strollo P (2011) Cardiac resynchronization therapy and obstructive sleep-related breathing disorder in patients with congestive heart failure. Pacing Clin Electrophysiol 34(5):593–603
Lüthje L, Renner B, Kessels R, Vollmann D, Raupach T, Gerritse B et al (2009) Cardiac resynchronization therapy and atrial overdrive pacing for the treatment of central sleep apnoea. Eur J Heart Fail 11(3):273–280
Simantirakis EN, Schiza SE, Siafakas NS, Vardas PE (2008) Sleep-disordered breathing in heart failure and the effect of cardiac resynchronization therapy. Europace 10(9):1029–1033
Gabor JY, Newman DA, Barnard-Roberts V, Korley V, Mangat I, Dorian P et al (2005) Improvement in Cheyne-Stokes respiration following cardiac resynchronisation therapy. Eur Respir J 26(1):95–100
Oldenburg O, Faber L, Vogt J, Dorszewski A, Szabados F, Horstkotte D et al (2007) Influence of cardiac resynchronisation therapy on different types of sleep disordered breathing. Eur J Heart Fail 9(8):820–826
Sredniawa B, Lenarczyk R, Kowalski O, Pruszkowska-Skrzep P, Kowalczyk J, Musialik-Lydka A et al (2009) Sleep apnoea as a predictor of mid- and long-term outcome in patients undergoing cardiac resynchronization therapy. Europace 11(1):106–114
Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T et al (2004) Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350(21):2140–2150. Available from: https://doi.org/10.1056/NEJMoa032423
Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A et al (2012) A randomized trial of long- term remote monitoring of pacemaker recipients (The COMPAS trial). Eur Heart J 33(9):1105–1111. Available from: http://dx.doi.org/10.1093/eurheartj/ehr419
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14(6):540–545
Rector TS, Kubo SH, Cohn JN (1993) Validity of the minnesota living with heart failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol 71(12):1106–1107
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocard. J Am Soc Echocardiogr 18(12):1440–1463. Available from: https://doi.org/10.1016/j.echo.2005.10.005
Iber C, Ancoli-Israel S, Chesson AL Jr., Quan SF for the American Academy of Sleep Medicine (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st edn, Westchester, IL: American Academy of Sleep Medicine
Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C et al (2001) Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 344(12):873–880
Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, Kappenberger L et al (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352(15):1539–1549. Available from: https://doi.org/10.1056/NEJMoa050496
Lamba J, Simpson CS, Redfearn DP, Michael KA, Fitzpatrick M, Baranchuk A (2011) Cardiac resynchronization therapy for the treatment of sleep apnoea: a meta-analysis. Europace 13(8):1174–1179. Available from: http://dx.doi.org/10.1093/europace/eur128
Anastasopoulos DL, Chalkias A, Iakovidou N, Xanthos T (2016) Effect of cardiac pacing on sleep-related breathing disorders: a systematic review. Heart Fail Rev 21(5):579–590
Stanchina ML, Ellison K, Malhotra A, Anderson M, Kirk M, Benser ME et al (2007) The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest 132(3):433–439
Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D et al (2007) Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial. Circulation 115(25):3173–3180
Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE et al (2010) Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 121(14):1598–1605
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cipolletta, L., Urbinati, A., Matassini, M.V., Francesca, M., Gasparini, S., Capucci, A. (2020). Obstructive Sleep Apnoea Syndrome and Arrhythmia: Results of 10 Years’ Experience. In: Longhi, S., et al. The First Outstanding 50 Years of “Università Politecnica delle Marche”. Springer, Cham. https://doi.org/10.1007/978-3-030-33832-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-33832-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33831-2
Online ISBN: 978-3-030-33832-9
eBook Packages: EducationEducation (R0)