Abstract
Purpose of Review
Obstructive sleep apnea syndrome (OSAS) has a high prevalence in western countries. Many papers have been published with the purpose of demonstrating that OSAS acts as an arrhythmia trigger and is responsible for an increase in cardiovascular morbidity and mortality. The aim of this study was to review our knowledge on this topic.
Recent Findings
There is a lot of evidence demonstrating the relationship between OSAS and arrhythmias, but there remains a lack of an interventional randomized trial to demonstrate that by treating OSAS we can reduce arrhythmia burden.
Summary
OSAS is a highly prevalent illness in western countries and is clearly related to an increase in cardiovascular mortality and morbidity. Cardiac arrhythmias are triggered by a repetitive hypoxemia, hypercapnia, acidosis, intrathoracic pressure fluctuations, reoxygenation, and arousals during apnea and hypopnea episodes. Early diagnosis and treatment of these patients can reduce further cardiovascular morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) consists of repetitive episodes of complete or partial cessation of breathing conducting to apneas (>10 s without breathing) or hypopneas (30% reduction of the breathing flux during 10 s with oxygen desaturation >4% and or an arousal), caused by a collapse of the upper airway path. Polysomnography is the gold standard diagnostic method that uses the number of apneas and hypopneas per hour of sleep, and the apnea-hypopnea index (AHI) is the way to measure it. OSA syndrome (OSAS) means an AHI > 5 with evidence of daytime sleepiness and is considered severe OSAS when AHI ≥ 30. Continuous positive airway pressure (CPAP) is the treatment of choice in severe OSAS [1]. Table 1 shows the related terms and definitions.
OSAS is quite prevalent in the general population (2–3% in women and up to 6–9 % in men), increasing with age; in men over 50, its prevalence is approximately 17%, and 9% in women [2]. It seems logical that the prevalence is rising due to the increasingly aged population and the ongoing obesity epidemic [3]. OSAS increases the risk of traffic accidents by sevenfold [4] and is associated with an increase in morning irritability.
The relationship between OSAS and cardiovascular disease has long time been established and is clearly demonstrated with hypertension [5], as well as with heart failure, atrial fibrillation (AF), coronary disease, and stroke [6•].
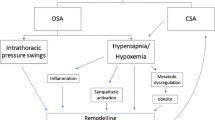
The OSAS pathophysiology can explain this relationship. Indeed, repetitive airway cessation induces hypoxemia, hypercapnia, intrathoracic pressure fluctuations, reoxygenation, and arousals [6•,7•,8]. All these repetitive insults can activate several cardiovascular mechanisms such as sympathetic activation inducing vasoconstriction, tachycardia, acute blood pressure elevations, and a decrease of cardiac variability, also in addition to an increase in left ventricular wall stress, increase in afterload, acute diastolic dysfunction, left atrial stress, left atrial enlargement, hypercoagulability, oxidative stress, and endothelial dysfunction. Finally, all of these mechanisms are related to hypertension, systolic and diastolic dysfunction, coronary artery disease, sinus node dysfunction (sick sinus syndrome), atrioventricular block, AF, ventricular ectopy or even ventricular tachycardia and sudden cardiac death. Figure 1 shows the pathophysiological scenario of OSA.
OSAS and Atrial Fibrillation
Although causality between OSAS and AF has yet to be established, there is a large number of mechanistic pathways that may increase the propensity to develop AF. These pathways are complex, multidirectional, and potentially synergistic. In addition, OSA and AF share a lot of risk factors such as increasing age, obesity, heart failure, and hypertension [6•].
Epidemiologic studies have shown that OSAS doubles the risk of having AF, providing evidence of the relationship between these two illnesses. In 1993, Flemons et al. [9] identified a potential relationship between sleep apnea and a greater incidence of arrhythmias, but failed to produce statistically significant variations among a small sample study with 263 participants. However, the 1998 study by Javaheri et al. [10] found a statistically significant association between sleep apnea and AF. Although the results were significant, the sample includes only males, thereby posing a potential gender bias within the small 81-participant study. In 2004, Gami et al. [11] showed a significant difference of OSA between patients with AF (49%) compared with patients without AF (32%) in a general cardiology practice population. In another study [12] of the same group, they showed that in patients under 65 years, in the multivariate analysis, age, male gender, history of coronary artery disease, and obesity, increased the risk of incident atrial fibrillation, but the factor that increased the risk most was a decrease of nocturnal oxygen saturation with a HR of 3.29 (1.35-8.04) 95%CI. Mehra et al. [13] in the Sleep Heart Health study showed that in 566 patients with severe OSA, up to 5% had AF in a 24 h Holter monitoring compared with 1% in patients without OSA.
It is also worth noting that when treating AF, the presence of OSA has recurrence consequences. Kanagala et al. [14], in 118 patients undergoing electrical cardioversion with 39 with documented OSA, showed that after a 12-month follow-up period, recurrences were significantly higher in non-treated OSA patients compared with well treated OSA patients with CPAP and a control group with no respiratory disturbances. Matiello et al. [15], in 174 patients undergoing pulmonary veins isolation, found an increase in AF recurrences in patients with severe OSA compared with patients without. Other studies have shown an increase in recurrences due to reconduction, after isolation of pulmonary veins, in patients with OSAS compared with patients without. Indeed, Sauer et al. [16] published some years ago that the five factors associated with the acute reconduction in pulmonary veins, were age, hypertension, non-paroxysmal AF, large left atrium, and sleep breathing disorders.
Several authors have also shown that CPAP treatment can reduce recurrences of AF after pulmonary veins isolation in patients with OSAS [17]. Table 2 shows the most significant studies on this issue.
Recent studies [21,22,23] have examined atrial remodeling of structural and electrical components in patients with OSA to account for predisposition to AF. Sudden negative intrathoracic pressures may lead to repetitive atrial stretch and gradually lead to left atrial enlargement [24]. As a result of left atrial enlargement, there may be remodeling at the pulmonary vein ostia, a site known to initiate and propagate AF [25]. Patients with moderate–severe OSA (AHI > 15) undergoing catheter ablation had significantly larger left atria indexed to body surface area compared to those patients with an AHI < 15 (p= 0.009) [21]. Because an enlarged left atrium is a risk factor for AF, a plausible mechanism for OSA leading to a predisposition to AF is structural remodeling.
OSAS and Other Arrhythmias
OSAS and Sick Sinus Syndrome (SSS)
SSS consisting of bradycardia with chronotropic incompetence, different degrees of sino-atrial block, and the well-known tachycardia–bradycardia syndrome are quite common in OSAS patients [6, 7]. Simantirakis et al. [26] included 23 patients with moderate to severe OSAS with an implantation of a loop recorder, and followed them for 16 months. The study conclude that the prevalence of long pauses and bradycardia was 22%. Martí-Almor et al. [27] performed a PSG and the Epworth sleepiness scale (ESS) in 38 patients with SSS, showing that up to 87% of patients had OSAS and 32% were considered severe OSAS compared with 3% in the general population, supporting the association between OSAS and SSS. Garrigue et al. [28] studied 98 patients, with an implanted pacemaker, with PSG and ESS for identification of OSAS patients, identifying 59% of pacemaker patients with undiagnosed OSAS. In 2014, Velasco et al. [29] performed a cross-sectional survey study to evaluate symptomatic bradycardia with OSAS. They included 190 patients and categorized them according to their risk for OSA, with a high-risk and low-risk group based on the Berlin questionnaire [30]. Multivariate regression was used to control for the effect of confounding influences. No statistically significant variations between OSA risk and the presence of brady-arrhythmias were identified. Nevertheless the study had several limitations especially related to the diagnosis of brady-arrhythmias based upon medical chart reviews instead on ECG recordings.
OSAS and Atrial Flutter
Increased pressure in the pulmonary artery—one of the consequences of severe OSAS—has been associated with atrial arrhythmias, such as AF and typical atrial flutter. Although there is a paucity of literature regarding the relationship of typical atrial flutter and OSAS, Bazan et al. [31] in a prospective series of 56 patients undergoing typical atrial flutter ablation, screened for OSA, found that up to 82% of patients had OSAS and 45% of them were considered as severe OSAS. In this study, 38% of patients had AF during follow-up after flutter ablation. Both freedom of AF prior to flutter ablation and CPAP initiation were associated with a significant reduction of AF during follow-up; inversely, CPAP was not protective from AF recurrences when AF was documented prior to flutter ablation. This finding can be explained by the fact that the left atrium may already be damaged and atrial fibrosis cannot be reversed by CPAP treatment.
OSAS and Ventricular Tachycardia/Sudden Cardiac Death
Ventricular arrhythmias have also been associated with OSAS, probably related to several factors such as ventricular hypertrophy, QT interval prolongation, and triggered and abnormal automaticity due to the autonomic imbalance in OSAS patients [32]. In a study by Koshino et al. [33], 35 patients with ventricular arrhythmias were screened for OSAS. They showed that up to 60% had some kind of sleep breathing disorder and 22% of them showed an IAH > 30/h (severe OSAS). Gami et al. [34], in a revision of PSG in 112 patients with sudden cardiac death, showed that people with OSAS have a peak in sudden death from cardiac causes during sleeping hours, which contrasts strikingly with the nadir of sudden death from cardiac causes during this period in people without obstructive sleep apnea and in the general population. There was a clear relationship between AHI and sudden death, with a clear increase in sudden death when AHI is over 40.
The earlier research of Gami et al. was reaffirmed in a 15-year, longitudinal follow-up study: SCD was the significant and elevated risk among individuals suffering from sleep apnea [35]. Strengthening their prior findings, the researchers revealed predictors of SCD through regression analysis, indicating that along with age (>60 years), a mean nocturnal oxygen saturation <93%, and specifically a low nocturnal oxygen saturation of <78%, as well as an AHI > 20, all significantly predict risk for sudden cardiac arrest. In fact, the researchers revealed that when oxygen saturation reached 78% or less, the inversely related risk for SCD was approximately 80% in OSAS patients.
OSAS in Pacemaker Population
There was an increase in OSAS prevalence in pacemaker patients. In addition, in the past, pacemaker implementation was offered as a therapeutic tool for treating OSAS patients.
A study from Garrige et al. [36] concluded that atrial overdrive pacing (AOP) was an effective treatment to decrease AHI in OSA patients. No other study has reproduced these findings, even the same group published years later that AOP was not useful for OSA patients [37]. Finally, Simantirakis et al. [38] in 16 patients with OSAS compared CPAP vs AOP switching therapy after one month, and described that only CPAP is able to ameliorate OSAS, with any effect on AOP. Only resynchronization therapy is able to ameliorate sleep breathing disorders related to central sleep apnea, due to the fact of improving heart failure [39].
Interestingly pacemaker manufacturers have been very interested in the role of cardiac pacing as a treatment of breathing disorders. This means that nowadays we have the possibility to use the ventilation minute rate responsive sensor (transthoracic impedance sensor) as a surrogate of PSG to early detect apneas; this system is called Sleep Apnea Monitoring (SAM). In the DREAM study [40], SAM was tested with PSG and the authors found that a respiratory disturbance index (RDI) > 20 was equivalent to an AHI > 30 in a PSG with a very high sensitivity and specificity.
Nevertheless, as pointed out before the prevalence of OSA in pacemaker patients is extremely high compared with the general population. In the European Multicenter polysomnographic study [28], the prevalence of OSA was 68% in patients with a long term pacing for atrio-ventricular block (AVB) and 58% in patients with SSS. Using the RDI as a diagnostic tool in RESPIRE registry Marti-Almor et al. [41•] described a high proportion of unselected pacemaker patients with OSAS (31.1%), interestingly the study showed a higher incidence of significant AF, defined as cumulative AF episodes lasting more than 24 hours over two consecutive days on the basis of the duration of the fallback mode switch, in patients with severe OSAS than in patients with non-severe OSAS (25% vs 13.9%, p = 0.002)
The relationship of SSS and OSAS has already been described but there is some discrepancy regarding the relationship of atrioventricular block (AVB) and OSAS. Tilkian and co-workers [42] reported that more than 50% of patients with sleep apnea develop episodes of heart block during sleep. In 400 patients studied by Guilleminault et al. [43], bradycardic arrhythmias were reported in almost 18% of the patients and later studies found heart block in 9–13% of patients with sleep apnea. Due to ventricular asystoles of up to 13 s in duration, it was hypothesized that heart block might lead to an increased mortality risk in these patients. Becker et al. [44] performed Holter monitoring in 239 consecutive patients diagnosed as having sleep apnea (AHI >10/h). Episodes of second and third degree AVB and/or sinus arrest > 2 s in duration occurred in 17 of the 239 patients. There was no significant difference in age between patients with and without heart block (mean (SD) 50.7 (12.8) versus 52.1 (9.8) years), but those with heart block were significantly more obese (body mass index 38.7 (7.3) versus 30.7 (4.6). Bradycardic arrhythmias exclusively occurred during apneas and hypopneas and were not found during hyperventilation. The occurrence of AVB was related to the severity of apneas, showing that patients with AHI < 60/h did not have any heart block while 17/97 patients with AHI > 60 did.
Sleep Disorders Breathing and Cardiac Electrophysiology
A variety of electrophysiological parameters including abnormal automaticity, triggered activity, shortening of the atrial effective period, QT interval prolongation or even re-entrant mechanism due to the presence of slow conduction areas in a fibrosis border zone after structural atrial or ventricular damage, may be included in OSA pathophysiology. Enhanced arrhythmogenesis occurs when automaticity is either increased or reduced by sympathetic or parasympathetic activation due to repetitive hypoxemia and reoxygenation episodes occurring during sleep in OSAS patients. Abnormal automaticity is generated in response to potassium dysregulation that is observed during the hyperpneic phase of periodic sleep apnea [45, 46].
Triggered activity occurs when early or delayed depolarizations reach a membrane potential threshold resulting in a spontaneous action potential. This triggered response can result in extrasystoles that can precipitate tachyarrhythmia. Established triggered activity precipitants via early after depolarizations include hypoxia, acidosis, and ventricular hypertrophy [45, 47], all intrinsic characteristics of OSAS pathophysiology. Alternatively, delayed after depolarizations resulting in triggered activity often occurs in response to increased catecholamine levels, which are also inherent to OSAS.
The interval between the T wave peak and T wave end in the ECG has been studied as a measure of cardiac dispersion and repolarization. An increase in this interval is associated with ventricular arrhythmias and sudden cardiac death [48,49,50]. In patients with OSAS, an increase in these intervals and a correction of them after CPAP treatment has been described [51, 52].
A decrease in the atrial effective refractory period (ERP) [53] has also been described in OSA patients that refers to the time when the cardiomyocyte is not excitable because it is recovering from the last action potential. This shortening in ERP may predispose to AF generation.
In a recent study, Schlatzer et al. [54] in a simulated obstructive sleep apnea model, observed that intrathoracic pressure swings induce atrial premature beats and alter measures of ventricular repolarization in 44 patients with previous paroxysmal atrial fibrillation. They concluded that atrial premature beats may induce paroxysmal atrial fibrillation. Figure 2 summarizes the arrhythmogenic mechanisms present in OSAS patients.
Conclusion
OSAS is a highly prevalent illness in western countries and is clearly related to an increase in cardiovascular mortality and morbidity. Cardiac arrhythmias are triggered by a repetitive hypoxemia, hypercapnia, acidosis, intrathoracic pressure fluctuations, reoxygenation, and arousals during apnea-hypopnoea episodes. An early diagnosis and treatment of these patients can reduce further cardiovascular morbidity and mortality.
Nevertheless, some problems remain to be resolved regarding OSAS and arrhythmias.
-
1)
OSAS has been related to multiple arrhythmias, with a huge amount of observational evidence, but there remains a necessity for an interventional randomized trial to demonstrate a causative relationship between OSAS and cardiac arrhythmias, demonstrating a reduction of arrhythmic burden with effective OSAS treatment.
-
2)
Most of the patients with OSAS are mildly symptomatic, and the diagnosis is done late, after much of the damage to the cardiac chamber has already occurred. When there is a structural remodeling rather than electrical remodeling, CPAP is not always able to reverse the situation. PSG is the gold standard diagnosis tool, but the waiting list in western countries is long. Using SAM in pacemaker patients is an option to detect OSAS in this setting, but it is not an early diagnosis in patients already implanted with a pacemaker. It is possible that in the future, smartphones will be able to detect snoring or may have some breathing sensor that can diagnose the patients early to avoid cardiac arrhythmias.
-
3)
It is imperative to know if we have to act earlier, specifically in moderate OSA (AHI 15–30), to avoid structural remodeling.
-
4)
We also have to know what to do with older patients with OSAS, specifically those diagnosed with SAM in some pacemakers. Is it worth to treat them, or are we too late to avoid further morbidity and mortality?
-
5)
OSA has been seen by the medical community as a pneumologist problem, but with increasing age, the obesity pandemic, and lifestyles in western countries, it is important to recognize that others specialists may help to decrease the major consequences of OSAS. Cardiologists, neurologists, and internists must look for OSA in patients with arrhythmias or stroke and treat them as soon as possible with CPAP in order to reduce cardiovascular morbidity.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Berry RB, Budhiraja R, Gottlieb DJ, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597–619.
Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–39.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14.
Ellen RL, Marshall SC, Palayew M, et al. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2009;5:573–81.
Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and the risk of hypertension. JAMA. 2012;307(20):2169–76.
• May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis. Mechanistic Insights. Contemporary reviews in sleep medicine. Chest. 2017;151(1):225–41. Excellent review of OSA-related electrophysiological mechanisms leading to arrhythmia generation in humans and in animal models.
• Latina JM, Mark Estes NA III, Garlitski A. The Relationship between Obstructive Sleep Apnea and Atrial Fibrillation: A Complex Interplay. Pulmon Med. 2013;(Article ID 621736):11. https://doi.org/10.1155/2013/621736. This paper explores the pathophysiology of OSA which may predispose to AF, clinical implications of stroke risk in this cohort who display overlapping disease processes, and targeted treatment strategies.
Patel N, Donahue C, Shenoy A, et al. Obstructive sleep apnea and arrhythmia: a systemic review. Int J Cardiol. 2017;228:967–70.
Flemons WW, Remmers JE, Gillis AM. Sleep apnea and cardiac arrhythmias: is there a relationship? Am Rev Respir Dis. 1993;148:618–21.
Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences, and presentations. Circulation. 1997;21:2154–9.
Gami AS, Pressman G, Caples SM, Kanagala R, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7.
Gami AS Hodge DO, Herges RM, et al. Obstructive sleep apnea and the risk of incident atrial fibrillation. JACC. 2007;49(5):565–71.
Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–6.
Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–94.
Matiello M, Nadal M, Tamborero D, et al. Low efficacy of atrial fibrillation ablation in severe obstructive sleep Apnoea patients. Europace. 2010;12:1084–9.
Sauer WH, Mckernan ML, Lin D, et al. Clinical predictors and outcomes associated with acute return of pulmonary vein conduction during pulmonary vein isolation for treatment of atrial fibrillation. Heart Rhythm. 2006;3(9):1024–8.
Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment. A review. JAMA Cardiol. 2018;3(6):532–40.
•Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. JACC. 2013;62(4):300–5. Findings from this study suggest that treating with CPAP can reduce atrial fibrillation recurrences after atrial fibrillation ablation.
Abe H, Takahashi M, Yaegashi H, et al. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apnea patients. Heart Vessels. 2010;25:63–9.
Patel D, Mohanty P, Di Biase L, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. 2010;3:445–51.
Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9(3):321–7.
Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–51.
Lau DH, MacKenzie L, Kelly DJ, et al. Hypertension andatrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumentedovine model. Heart Rhythm. 2010;7(9):1282–90.
Otto ME, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99(9):1298–302.
Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–66.
Simantirakis E, Schiza S, Marketou E, Chrysostomakis S, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment—a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070–6.
Martí-Almor J, Felez-Flor M, Balcells E, et al. Prevalence of obstructive sleep apnea syndrome in patients with sick sinus syndrome. Rev Esp Cardiol. 2006;59(1):28–32.
Garrigue S, Pépin JL, Defaye P, Murgatroyd F, Poezevara Y, Clémenty J, et al. High prevalence of sleep apnea syndrome in patients with long-term pacing: the European multicenter polysomnographic study. Circulation. 2007;115(13):1703–9.
Velasco A, Hall C, Perez-Verdia A, Nugent K. Association of high-risk scores for obstructive sleep apnea with symptomatic bradyarrhythmias. J Cardiovasc Med. 2014;15(5):407–10.
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91.
Bazan V, Grau N, Valles E, et al. Obstructive sleep apnea in patients with typical atrial flutter. Prevalence and impact on arrhythmia control outcome. Chest. 2013;143(5):1277–83.
Verrier RL, Josephson ME. Impact of sleep on arrhythmogenesis. Circ Arrhythm Electrophysiol. 2009;2:450–9.
Koshino Y, Satoh M, Katayose Y, et al. Association of sleep-disordered breathing and ventricular arrhytmias in patients without heart failure. Am J Cardiol. 2008;101(6):882–6.
Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–79.
Gami A, Olson E, Shen W, RWright RS, Ballman K, Hodge D, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–6.
Garrigue S, Bordier P, Jaïs P, et al. Benefit of atrial pacing in sleep apnea síndrome. N Engl J Med. 2002;346(6):404–12.
JL JP, Defaye P, Garrigue S, Poezevara Y, Levy P. Overdrive atrial pacing does not improve obstructive sleep apnoea syndrome. Eur Respir J. 2005;25(2)):343–7.
Simantirakis E, Shciza S, Chrysostomakis S, et al. Atrial overdrive pacing for the obstructive sleep apnea–hypopnea syndrome. N Engl J Med. 2005;353:2568–77.
Lamba J, Simpson CS, Redfearn DP, et al. Cardiac Resynchronization therapy for the treatment of sleep apnoea: a meta-analysis. Europace. 2011;13(8):1174–9.
Defaye P, de la Cruz I, Marti-Almor J, et al. A pacemaker transthoracic impedance sensor with an advanced algorithm to identify severe sleep apnea: The DREAM European study. Heart Rhythm. 2014;11(5):842–8.
• Marti-Almor J, Marques P, Jesel L, et al. Incidence of sleep apnea and association with atrial fibrillation in an unselected pacemaker population: results of the observational RESPIRE study. Heart Rhythm. 2020;17(2):195–202. Findings from this study showed the usefulness of sleep apnea monitoring sensor in the pacemaker to detect OSA, and the increase in the incidence of atrial fibrillation in the severe OSA group compared with the non severe OSA group.
Tilkian AG, Guilleminault C, Schroeder JS, et al. Sleep inducedapnea syndrome. Prevalence of cardiac arrhythmiasand their reversal after tracheostomy. Am J Med. 1977;63:348–58.
Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4.
HF Becker UK, Stammnitz A, Peter JH. Heart block in patients with sleep apnoea. Thorax. 1998;53(suppl 3):S29–32.
Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52.
Roden DM, Iansmith DH. Effects of low potassium or magnesium concentrations on isolated cardiac tissue. Am J Med. 1987;82(3A):18–23.
Antzelevitch C, Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin. 2011;3(1):23–45.
Gupta P, Patel C, Patel H, et al. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41(6):567–74.
Panikkath R, Reinier K, Uy-Evanado A, et al. Prolonged Tpeak to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4(4):441–7.
Yamaguchi M, Shimizu M, Ino H, et al. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci. 2003;105(6):671–6.
Roche F, Barthélémy JC, Garet M, Duverney D, Pichot V, Sforza E. Continuous positive airway pressure treatment improves the QT rate dependence adaptation of obstructive sleep apnea patients. Pacing Clin Electrophysiol. 2005;28(8):819–25.
Gillis AM, Stoohs R, Guilleminault C. Changes in the QT interval during obstructive sleep apnea. Sleep. 1991;14(4):346–50.
Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Combined blockade of early and late activated atrial potassium currents suppresses atrial fibrillation in a pig model of obstructive apnea. Heart Rhythm Off J Heart Rhythm Soc. 2011;8(12):1933–9.
Schlatzer C, Schwarz EI, Sievi NA. Intrathoracic pressure swings induced by simulated obstructive sleep apnoea promote arrhythmias in paroxysmal atrial fibrillation. Europace. 2016;18:64–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors. All the studies on humans beings performed by the authors follow the Helsinki criteria and all of them were done after signing informed consent approved by the local ethical committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Invasive Electrophysiology and Pacing
Rights and permissions
About this article
Cite this article
Martí-Almor, J., Jiménez-López, J., Casteigt, B. et al. Obstructive Sleep Apnea Syndrome as a Trigger of Cardiac Arrhythmias. Curr Cardiol Rep 23, 20 (2021). https://doi.org/10.1007/s11886-021-01445-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01445-y