Abstract
Graphene is currently one of the most promising carbon-based materials being studied in the world due to its novel electronic, thermal and optical properties. Nowadays, graphene can be considered the basis for the whole family of carbon nanomaterials, except for diamond structure. Because of that, several methods of graphene production have been studied; however, such methods need to be improved and the scaling is still a bottleneck for the productive sector. This article presents the main way to produce graphene and some techniques to modify its surface. In addition, here we present a review about graphene and its derivatives in the adsorption from the aqueous medium of the most diverse types of organic contaminants in the environment, such as pharmaceuticals, dyes, surfactants, pesticides, etc. We have shown that graphene and its derivatives are efficient adsorbents for the removal of emerging organic pollutants from the environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The rapid growth of industrial and agricultural activities has led to a growing increase in the release of pollutants into the environment, such as persistent organic pollutants, for example, the industrial dyes [27, 122]. This has led to several environmental concerns, because the accumulations of toxic waste are potentially harmful to human health and the environment [17, 84, 119].
Therefore, efficient solutions are needed to lessen the effects caused by these pollutants in the environment. Thus, several methods have been used to remove these pollutants from the wastewater, where it is possible to highlight the adsorption, degradation, coagulation, flocculation, ion exchange, membrane filtration, electrochemical and catalytic oxidation, catalytic ozonization, all these technologies have limitations and restrictions [29, 75]. Among the remediation options, adsorption is one of the most used techniques due to its simplicity, ease of operation, low cost and efficient removal in low concentrations [121].
Among the materials used as adsorbents in the removal of organic compounds in wastewater, the carbon nanomaterials, such as graphene [49, 79, 99], which have recently been received special attention because of their specific surface area associated to relatively high adsorption capacity [21, 79, 107].
Therefore, graphene has been widely studied as an adsorbent for water decontamination because of its physico-chemical properties, which include, besides the high specific surface area, its mechanical, thermal and chemical stabilities [16, 36, 68, 120]. Therefore, these characteristics make graphene a potential material to be used in the treatment of effluents [35, 93].
Although recent studies have shown that graphene is being used as an adsorbent for emerging organic pollutants from the aquatic environment, there are few studies with this approach and there is a need for further studies and applicability on an industrial scale [33].
2 Graphene
The term “graphene” was first used in 1947, but the official definition was given by the International Union of Pure and Applied Chemistry (IUPAC) in 1994. In 1947, Walace in 1947 first studied to understand the structure of graphite, which consists of superimposed and weakly bonded graphene layers to each other, contrasting with the interatomic bonds in each plane, which are the strongest known bonds in a crystal [112].
In 2004, researchers were able to isolate small fragments exfoliated from graphite and after characterization verified that it was a single-layer structure of carbon atoms [77]. This material has attracted much attention from the scientific community because of its specific physical and chemical properties, large outer surface area, and ease of modification [110]. Some of the main properties of graphene are shown in Table 1.
These properties provide graphene with properties that translate into greater mechanical strength than steel, higher electron mobility than silicon, higher thermal conductivity than copper, higher surface area than graphite and lighter material than many others [98].
2.1 Structure
Graphene is a two-dimensional (2D) network, composed of a hexagonal structure, which looks like a honeycomb [34]. Graphene has a sp2 hybridization, where the carbon atoms form covalent bonds with each other (δ bonds), where the distances of the C–C bonds are approximately 1.42 Å, with a thickness of one carbon atom (approximately 1 Angstrom = 10−8 cm) (Fig. 1) [88].
Structure of a Graphene Blade. Reproduced with permission from Ref. [80]. Copyright: 2017 Elsevier
In the graphene structure, the sp2 hybrid orbital is a result of the bonding in the plane of the pure orbitals s, px and py, while the pure orbital pz is free and perpendicular to the plane. This produces a sharing of sp2 orbital hybridization of a carbon with three neighboring carbon atoms forming the hexagonal and 2D structure of graphene, as shown in Fig. 2 [105].
a Energy levels of outer electrons in carbon atoms. b The formation of sp2 hybrids. c The crystal lattice of graphene, where A and B are carbon atoms belonging to different sub-lattices, a1 and a2 are unit-cell vectors. d Sigma bond and pi bond formed by sp2 hybridization. Reproduced with permission from Ref. [105]. Copyright: 2019 Elsevier
2.2 Synthesis of Graphene
Graphene production can be performed through two different strategies, which are designated as Top-Down and Bottom-Up [30].
2.2.1 Top Down Approach
The Top Dow strategy (top to bottom) is characterized by the attack of graphite powder, which will eventually separate its layer to generate graphene sheets [11]. Among Top Dow methods, we can highlight mechanical and chemical exfoliation and chemical reduction.
2.2.1.1 Mechanical Exfoliation
Graphite forms when the layers of mono-atomic graphene are stacked together by weak forces of van der Waals, the method of exfoliating the inverse of the stacking [87, 125]. Hummers and Offman were the first to synthesize graphene in 1958, they obtained graphene through graphite exfoliation, although they did not obtain only carbon graphite as it is now known as graphene [41].
Andre Geim and Konstantin Novoselov reported in 2004, the synthesis of graphene nanosheets through the mechanical exfoliation. It was observed for the first time that it was possible to isolate a sheet of carbon atoms at room conditions [73]. Mechanical exfoliation is an ancient method and the most popular, since it was the process that led to the discovery of graphene [74].
This method involves exfoliating the graphite sheets until a single sheet is obtained. Exfoliation has the purpose of removing the graphite layers, this can be done using a variety of agents, such as adhesive tape [73], ultrasound [25] and electric field [58], and this technique does not require special equipment, but has a low performance disadvantage [100].
2.2.1.2 Chemical Exfoliation
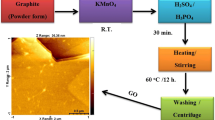
Chemical exfoliation is the best route to be used to obtain graphene. This method consists in using the graphite to produce the graphene through the continuous reduction of the forces of Van der Waals that occurs through the insertion of special chemicals in the atomic planes of the graphite [113].
It is important to stress that chemical exfoliation is performed in two steps. Initially, occurs the reduction of the interlayer van der waals forces to produce compounds interlaced with graphenes [113]. Then, graphene is exfoliated with one or a few layers by rapid heating or sonication [6, 64].
2.2.1.3 Chemical Reduction of Graphite Oxide
In 1859, graphite oxide was first reported by Brodie, this one also presented the possibility of exfoliation, through the use of functional groups introduced in the oxidative graphite [14]. Graphite oxidation can be produced by the oxidation of graphite using oxidants such as concentrated sulfuric acid, nitric acid and potassium permanganate based on the method of Brodie [14], Staudenmaier method [102], Hummers method [41].
The oxidized graphite disperses easily in polar solvents, and the sonication process separates the leaves to obtain a smaller number of leaves than in graphite [7]. The number of leaves varies according to the experimental conditions of oxidation and sonication [24].
2.2.2 Bottom-Up Approach
The Bottom-Up strategy explore carbon dioxide to generate graphene [11]. Among the methods used in this strategy are: pyrolysis, epitaxial growth and CVD.
2.2.2.1 Pyrolysis
The graphene can be obtained through the pyrolysis of sodium ethoxide via sonication. This process improves the performance of graphene sheet detachment. Thus, the generated graphene sheets are measured up to 10 μm [22].
2.2.2.2 Epitaxial Growth
The term “epitaxy” derives from the Greek, the prefix epi means “on” and taxis means “order” or “agreement”. When deposition of a single crystalline film on a single crystalline substrate produces epitaxial film and the process is known as epitaxial growth [9]. In this process, graphene is produced in the substrate (semiconductor) and the growth process is followed by lithography to make electronic devices based on graphene [9, 114]. The graphene produced by this process is called epitaxial graphene that can be grown on various substrates, such as silicon carbide [31], iron (Fe) [71] and copper (Cu) [32].
A promising substrate used in the growth of epitaxial graphene is silicon carbide. The preparation of graphene can be accomplished by applying heat and cooling of a silicon crystal (SiC). Usually, on the Si face of the crystal, there will be single layer or double layer graphene, however, on face C, graphene of few layers is cultivated [18].
2.2.2.3 Chemical Vapor Deposition—CVD
Chemical vapor deposition (CVD) is defined as the placement of a solid on a heated surface from a chemical reaction in the vapor phase where the deposition species are atoms or molecules. The chemical energy required to initiate a specific chemical reaction is provided by heat, light or electric charge [56].
High quality graphene deposition from the CVD process is usually done on various transition metal substrates such as Ni [45], Cu [90] and Pd [22], where graphene growth by CVD has been produced mainly on copper and nickel substrates [108].
3 Modifications
One of the great limitations in the use of graphene is its low reactivity, which hinders its interaction with different chemical species. Thus, there is a constant search for techniques that can be used to increase the reactivity of graphene with the introduction of different functional groups, these groups can be added by functionalization, doping and defects [72].
Among these techniques, chemical functionalization has attracted attention due to the possibility of increasing the solubility, processability and interactions of graphene with organic polymers [48]. An example is the introduction of oxygen-containing groups (−COOH, −CO and −OH) on the surface of the graphene. These groups occupy up to 60% of the surface area of graphene oxide (GO) [92].
Graphene oxide (GO), an oxidized version of graphene, can be synthesized by the oxidation of natural graphite [38, 41, 96]. This reaction occurs through the oxidation and exfoliation of graphite, usually in aqueous solution. This produces carbon-based hydrophilic plates decorated with various oxygenated functional groups (hydroxyl, epoxide and carboxyl), as shown in Fig. 3 [66].
Idealised structures of graphene, graphene oxide and reduced graphene oxide. Reproduced with permission from Ref. [66]. Copyright: 2019 Elsevier
The continuous, atomically thin, two-dimensional (2D) arrangement of carbon atoms that is functionalized with epoxy and hydroxy groups in the basal carbon plane and carboxy groups around the edges is the most accepted structure of GO leaves [66]. The GO may be reduced, partially, to graphene-like sheets by withdrawing the oxygen-containing groups and causing the recovery of a conjugated structure. Reduced GO (rGO) sheets are generally considered as a chemically derived graphene type (Fig. 3) [76]. The reduction of the GO does not completely remove oxygenated functional groups, only promotes a significant reduction of them. One way to accomplish GO reduction is to use high temperature treatment or use chemical agents such as hydrazine to form rGO [66, 76].
When performing the oxidative removal of the functional groups of oxygen from the GO there is the formation of another derivative of the graphene, named of graphene acid. The experimental procedure is based on the multiple oxidation of graphite with potassium permanganate in acidic medium. This procedure leads to the formation of a graphene derivative with an approximate composition of carboxyl groups, [C1(COOH)1]n, of reaches 30 wt%, and simultaneously causing the oxidative elimination of other functional oxygen groups [42].
GO has a negative surface when in aqueous medium due to the formation of COO−. Thus, when there is functionalization on the GO surface with a functional group which in aqueous medium produces a positive charge occurs the formation of the zwitterionic graphene oxide (ZGO). Zwitterionic compound containing both anionic and cationic groups would convert charge with the pH values. One way of producing ZGO is through the introduction of silane groups. For example, the preparation of the ZGO nanosheets with two silanes ((3-glycidyloxypropyl)trimethoxysilane (GPTMS) and (3-aminopropyl)triethoxysilane (APTES)), as shown in Fig. 4. Covalent bonds are responsible for grafting the silane molecules onto the GO surface and when the pH of the aqueous solution is changed from 12 to 2, there is also the variation of the potential (ζ) of ZGO of −29.0 mV to +28.5 mV [26].
Reaction scheme for the two steps silanization by GPTMS and APTES. Reproduced with permission from Ref. [26]. Copyright: 2018 Elsevier
Another important functionalization of graphene is the incorporation of thiol groups on its surface. An example of this reaction is shown in Fig. 5. From the Figure, it can be observed that, initially, the graphene is oxidized and forms graphene oxide (GO), which is then heated to above 400 °C in order to exfoliate the GO structure in layers in thermally reduced graphite oxide (TRGO) sheets. TRGO contains different oxygen groups directly bound to the carbon skeleton of a two-dimensional graphene-derived backbone (Fig. 5a). Soon after, The TRGO is placed to react with propylene sulfide forming the TRGO-SH, as shown in Fig. 5b [65].
a Schematic presentation of a model structure for thermally reduced graphite oxide (TRGO) with its functional carboxyl, aldehyde and especially hydroxyl groups according to recent studies. Reproduced with permission from Ref. [65]. Copyright: 2014 Elsevier. b Schematic presentation of the ring opening reaction of propylene sulfide (2-methylthiirane) with deprotonated hydroxyl groups of TRGO to yield the material TRGO-SH. Reproduced with permission from Ref. [65]. Copyright: 2014 Elsevier
Pristine graphene can be functionalized by organic functionalities such as free radicals and dienophiles. For example, sp2 carbon atoms of graphene are easily attacked by a free radical generated by heating a diazonium salt, causing the formation of a covalent bond. The sulfonic groups (−SO3H) can be incorporated into the graphene by this reaction. Figure 6 shows the sulfonation reaction of graphene using 4-sulfobenzenediazonium with added of the sodium dodecylbenzensulfonate (SDBS) to the graphene dispersion to perform surfactant wrapped graphene [85].
The reaction of sulfonation of the graphene using 4-sulfobenzenediazonium. Reproduced with permission from Ref. [85]. Copyright: 2018 Elsevier
In addition, on the graphene surface can also be incorporated phosphate groups. One way to accomplish this incorporation is the phosphating of GO from the covalent attachment of triethylphosphite on the GO surface via the Arbuzov reaction (Fig. 7). This reaction occurs at about 150 °C and is characterized by the reaction of triethylphosphite with electrophilic site of graphene oxide like epoxide or near to carbonyl group. After the non-localized attack of the GO surface by the triethylphosphite is formed the diethyl phosphonate. Then the ethyl group reacts with the bromide obtained from the LiBr to form the ethyl bromide. This process is carried out until the formation of the phosphene oxide graphene. Finally, hydrolyzing the ethyl groups present in the phosphonated GO is carried out to give hydroxyl groups and to form the phosphated graphene oxide (PGO) [2].
Schematic diagram for the synthesis of PGO. Reproduced with permission from Ref. [2]. Copyright: 2018 Elsevier
4 Graphene Nanocomposites
The development of nanotechnology has attracted the interest of researchers to improve the performance of composites by introducing nanomaterials to them [54]. Graphene and its derivatives are considered promising candidates as nanomaterials that can be used to enhance composites, due to its excellent properties such as high mechanical and chemical stability [54, 55, 117].
Thus, various materials are used together with graphene and its derivatives for the production of composites for the most diverse applications. For example, one study showed a production of GO/bentonite (BG) compounds, prepared from GO interspersed with an intermediate layer of bentonite, as shown in Fig. 8. In these composites, it can be observed that after the addition of the GO there was an increase of the BET surface area, because the presence of GO between the BG layers formed an exfoliated structure. In addition, BG composites exhibited a greater adsorption capacity of toluidine blue dye (TB) from aqueous solutions than normal bentonite due to the synergistic effect between bentonite and GO. Finally, it was concluded that the adsorption of TB in BG composites occurs mainly through ion exchange, electrostatic interaction and intermolecular interactions (Fig. 8) [116].
Illustration of the synthesis of BG composites and TB adsorption using BG composites. Reproduced with permission from Ref. [116]. Copyright: 2019 Elsevier
The GO may also be introduced onto the surface of silica gel to decorate/functionalize GO through specific functional groups. To prepare this composite (SiO2@GO–PO3H2) four steps are required which are schematically presented in Fig. 9. (i) Silica gel (SG) is grafted using 3-aminopropyltriethoxysilane (APTS) producing the compound SG-APTS. (ii) The GO is added to the surface of the SG-APTS forming SiO2@GO. (iii) An aminosilane group is added to the surface of the SiO2@GO generating the SiO2@GO-NH2. (iv) The vinylphosphonic acid is added on the SiO2@GO-NH2 forming the SiO2@GO–PO3H2- composite and the incorporation of the phosphate group is confirmed by the appearance of the peaks at 2325 and 1695 cm−1, which were assigned to P(O)-OH stretching vibration. The adsorption of ln (III) ions on the surface of SiO2@GO–PO3H2 occurs by a chemical adsorption process, due to the coordination of the ln (III) ions to the element P, present on the surface of the adsorbent, generating a lnP bond [53].
The scheme for preparation of SiO2@GO–PO3H2. Reproduced with permission from Ref. [53]. Copyright: 2019 Elsevier
In addition, some researchers have prepared composite materials from of graphene and polymeric materials. For example, a recent study prepared a nanocomposite (CNF-GNS/NR) using cellulose nanofibrils (CNF), graphene (GNS) and natural rubber (NR). The CNF-GNS/NR nanocomposite was produced through the formation of a three-dimensional multilayer network structure generated by the dispersion and overlap of CNF and GNS in NR (Fig. 10). The results showed that the incorporation of CNF increased the electrical conductivity of the composite GNS/NR, in addition, the addition of CNF reduced the percolation threshold of the composites. This confirms that using the CNF to construct a multi-layer multi-layer conductive network in NR is effective to promote the increase of the electrical conductivity of these composites [115].
Schematic of the CNF-GNS/NR fabrication process. Reproduced with permission from Ref. [115]. Copyright: 2019 Elsevier
Another polymer widely used in the production of composites with graphene is chitosan (CS). As an example, it may be mentioned a research recent which produced a composite using CS and graphene. For the production of this composite SiO2 was used to improve the dispersibility of the grafene in chitosan suspensions. The CS/SiO2-loaded graphene composite beads were cross-linked with ethylene glycol diglycidyl ether (EGDE) and prepared by the phase inversion method (Fig. 11). The results showed that the spheres Cs and Cs/graphene-SiO2 presented spherical shapes and the incorporation of graphene improved the mechanical strength of the composites compared to the CS spheres [19].
Schematic illustration of the preparation of CS/graphene-SiO2 composite beads. Reproduced with permission from Ref. [19]. Copyright: 2019 Elsevier
In addition to studies related to graphene/polymer composites, research related to metal matrix graphene composites (CMMs) has also gained prominence. An example is the graphene/Cu composites, these composites attracted attention because of their electrical, thermal and mechanical properties, which made them promising materials for a wide range of applications [23].
The two main difficulties in the production of reinforced copper based graphene composites are the non-uniform dispersion of the graphene flakes inside the copper matrix and the low bond strength of the graphene/Cu matrix interface. An effective preparing approach of graphene-strengthening copper-based composites is an easy electrochemical deposition and atmosphere sintering. Figure 12 shows the schematic of preparation of graphene-nanoplatelets/copper (GNP/Cu) composite by electrochemical deposition and atmosphere sintering. The results showed that when the graphene content is 1.8 wt%, it is dispersed homogeneously in the matrix and forms a coherent interface in the GNP/Cu composites. In addition, it is observed that Cu-O-C bond formation is promoted by nickel (Ni) in the formation of graphene nanoplatelets (Ni-GNPs) during electrochemical deposition. Finally, the results showed that oxygen mediates the formation of the Cu-O-C bond during the electrochemical deposition process and that this bond modifies the interstitial oxygen atoms by combining them with ionic or covalent bonding in the crystal lattice of copper [126].
Schematic of preparation of GNP/Cu composite. Reproduced with permission from Ref. [126]. Copyright: 2018 Elsevier
Another interesting example of graphene/metal composites are graphene composites reinforced with aluminum (graphene/Al). These composites when dispersed uniformly in graphene show an improvement in their properties related to strength and stiffness [43]. A way to prepare the graphene/Al composite is to use the pressure infiltration method. The schematic preparation process of the GNPs/Al composites is given in Fig. 13. Initially, graphene nanoplates (GNPs) are mixed with pure aluminum powder (Al) using a planetary mill at a speed of rotation. Soon after, the mixture is placed in a steel mold up to a defined height to prepare the preforms. This step is performed with addition of pressure to the defined height. The infiltration of molten Al is carried out at low temperature and with higher pressure (15 MPa) to inhibit the reaction and overcome the poor wettability between the graphene and the Al matrix. No Al4C3 phase was detected in the composites, while GNPs have been well bonded with the Al matrix. It is also suggested that the use of graphene with less defect may inhibit the reaction between the pure Al matrix and graphene. In addition, it has been found that the addition of graphene significantly improves the mechanical properties of the composites [118].
The schematic preparation process of the GNPs/Al composites. Reproduced with permission from Ref. [118]. Copyright: 2018 Elsevier
5 Adsorption of Organic Compounds
In recent years, graphene-based nanomaterials have sparked great interest into potential applications in a wide range of industrial or agricultural fields. Among these applications, it is possible to mention the adsorption of polluting organic compounds from aqueous medium. These materials can interact with organic pollutants and alter the transport, fate and bioavailability of these contaminants in the environment [91].
5.1 Pharmaceutical Drugs
Pharmaceutical drugs are organic pollutants that have attracted increasing attention from recent research. These pollutants are resistant and have become a hazard to the aquatic ecosystem. An example of this type of contaminant is the antibiotic ciprofloxacin (CIP), a type of quinolone. Recent research has produced a hydrogen and graphene adsorbent (GH) from a hydrothermal reduction for the removal of CIP from the aqueous medium. In the adsorption process of CIP on GH, The interactions responsible for the adsorption process were based on the π–π interaction of electron donor-receptor (EDA), which are proven interactions as responsible for the adsorption of organic pollutants with benzene rings on carbon materials [103].
Another example of organic pollutants of pharmaceutical origin are the substances that can affect the endocrine system, called endocrine disrupting chemicals (EDCs). These contaminants can generate serious environmental and health problems. In recent years, EDCs have been found in effluents from different sources, which makes their removal an important issue. Thus, the process of removal of this contaminant type has been well reported in the literature. For example, researchers performed the adsorption of endocrine disrupting chemicals (EDCs), 17α-ethynyl estradiol and β-estradiol, sp2 hybridized GO sheets. The study showed that 17α-ethynylestradiol and β-estradiol molecules interacted with GO through hydrogen bonds and electrostatic interactions. These interactions originate through the GO-containing oxygen groups and the OH groups of the 17α-ethynyloestradiol molecules as β-estradiol. In addition, there are also the π–π interactions between the π electrons of the GO and the aromatic rings of the molecules of the pollutants. Finally, it was verified that the adsorption process was more efficient in acid medium, with a maximum adsorption of 98.46% and 97.19% for 17α-ethinyl estradiol and β-estradiol, respectively [12].
Another pollutant drug detected in water due to incomplete treatment is diclofenac. This is a pharmaceutical compound with analgesic and antipyretic properties, categorised under the therapeutic class of non-steroidal anti-inflammatory drug (NSAID). The toxicity of diclofenac has been documented in the literature, indicating the adverse effects of this compound on both the environment and terrestrial organisms. A recent research has developed a three-dimensional rGO aerogel (rGOA) for the removal of diclofenac in aqueous solution. The production of rGOA occurred by reducing the GO using a reducing environment (L-ascorbic acid). The highest adsorption capacity of diclofenac on this adsorbent was 596.71 mg under the conditions of: dosage of 0.25 g/L, initial concentration of 325 mg/L, adsorption velocity of 200 rpm and temperature of 30 °C. The pH strongly influenced the adsorption process of diclofenac on rGOA. This study showed that the highest amount of diclofenac adsorbed on rGOA occurred in acid medium, with an amount of 96% of adsorbed drug at pH < pKa and adsorption mechanisms based on hydrophobic attraction, π–π EDA and precipitation. Under basic conditions, it was observed that the amount of drug adsorbed decreased to 81% when the pH reached the value of 9. In this medium, hydrogen bonds and hydrophobic interactions are responsible for the removal of diclofenac using rGOA [37]. Table 2 shows other pharmaceutical pollutants that are removed through graphene and its derivatives.
5.2 Organic Dyes
In addition of drugs, dyes are also found in the wastewater. The dyes present high biological toxicity, decrease the transparency of the water and consumption of high quantity of oxygen. This causes a reduction in the capacity of water bodies to perform self-purification, in addition to affecting the growth of water organisms and microorganisms. Like this, the removal of the dyes from the aqueous medium is necessary to reduce water pollution. This can be done using the adsorption process which is one of the most effective ways to turn polluted water into clean water. The use of graphene and its derivatives as adsorbents for dyes has been reported in recent research. For example, researchers prepared graphene-tannic acid hydrogel using GO and tannic acid (TA) through one-step hydrothermal method, as shown in Fig. 14 [106].
Structure of TA molecule and formation of the hydrogels. Reproduced with permission from Ref. Tang et al. [106]. Copyright: 2018 Elsevier
The hydrogels produced from graphene were used in adsorption of the organic dye methylene blue (MB). The adsorption process was improved by the presence of tanic acid on the surface of the graphene sheets and microstructures of the hydrogels. In addition, the results showed that the adsorption of the dye on the geis occurs more effectively at alkaline pH. The main mechanism of adsorption of the dye on the surface of the hydrogels is the electrostatic attraction, which increases with increasing pH, reaching a maximum value in pH 10 (the maximum adsorption capacity = 563 mg g−1), due to the presence of tanic acid on the surface of the hydrogels [106]. Thus, graphene and its derivatives can be used as adsorbents for removal of dyes from the aqueous medium. Table 3 shows other examples of dyes removed by graphens and their derivatives.
5.3 Surfactants
Surfactants play a key role in many industrial processes and are chemical substances with The presence of surfactants in aquatic environment is also a major public health concern. the most varied applications. These compounds being biodegraded generate by-products that have been found in aquatic sediments, groundwater, surface water and drinking water. Thus, the adsorption process is also used in the removal of these contaminants from the aqueous medium. Among the adsorbents that can be used in these adsorption processes are compounds derived from graphene, such as GO and rGO. Recently, the GO and rGO were used in the adsorption of the non-ionic surfactant Triton X-100 (TX-100) from water. The adsorption process of TX-100 on rGO was based on hydrophobic interactions and π-stacking. In addition, GO and rGO revealed superior removal capabilities when compared to other adsorbents [82]
5.4 Pesticides
In addition, another serious environmental problem is the contamination of water with pesticides. Pesticides are classified according to their biological function and target organism (herbicides, fungicides, rodenticides, insecticides, etc.). The presence of pesticides in the environment can cause serious damage to human health, such as headaches and nausea to chronic impacts such as cancer, reproductive damage and endocrine disruption. The pesticides are very soluble and cause adverse effects even at levels of μg/L and are therefore considered primary pollutants for aquatic ecosystems. An efficient process for the removal of pesticides from the aqueous medium is to use graphene based nanocomposites as adsorbent through the adsorption process. For example, a study used Fe3O4/rGO nanocomposite for the adsorption of the pesticide ametryn in an aqueous medium. The adsorption process of this pesticide on the Fe3O4/rGO nanocomposite showed was efficient of the 93.61% due to electrostatic, hydrophobic and π–π interactions of composite towards the heterocyclic conjugation of pesticide molecule [13].
Another organic pollutant in the waters is bisphenol A (BPA). BPA is an example of biphenol compounds (BCs) being one of the most produced in the world. BCs exhibit various adverse effects, such as endocrine disruption, cytotoxicity, genotoxicity, reproductive toxicity, dioxin-like effects, and neurotoxicity. These effects caused several materials to be used for the removal of BPA from the aqueous medium, among them we can mention the adsorption process using nanomaterials derived from graphene. For example, a study showed which the graphite (GP), GO and rGO can be used as adsorbents for BPA. The rGO has the highest adsorption capacity for BPA than GO and GP. The study showed that the interactions reponsible for the adsorption of BPA on the GP are the hydrophobic interactions and on the rGO were the π–π interactions [109].
In addition to these pollutants, there is also natural organic matter (NOM) which is a complex of organic substances that are dangerous to the environment and have been found in surface water [3]. One way to remove this type of pollutant is to use the adsorption process with adsorbents derived from graphene. For example, recently, a research prepared an iron-functionalized rGO (frGO)-coated and was that used for the adsorption of natural organic matter, such as fulvic acid (FA), from synthetic water. The production process of the iron-functionalized frGO is shown in Fig. 15.
Flow diagram of fulvic acid removal by iron-functionalized reduced graphene oxide (fRGO). Reproduced with permission from Ref. [89]. Copyright: 2017 Royal Society of Chemistry
The study indicated that iron-functionalized frGO can be used as an effective adsorbent material for the removal of contaminants such as fulvic acid. The pH was a variable that influenced the adsorption process of the FA on the fRGO, being that the greater adsorption occurred in low pH. The adsorption mechanism was based both by electrostatic interactions and π–π interactions (Fig. 16) [89].
a RGO to fRGO conversion by adding FeSO4 b Proposed FA-fRGO interactions complex. Reproduced with permission from Ref. [89]. Copyright: 2017 Royal Society of Chemistry
5.5 Other Organic Pollutants
Other organic molecules that are not include in the different categories defined before, are also considered extremely dangerous for aquatic ecosystems. Table 4 presents other examples of organic pollutants removed from aqueous medium using graphene and its derivatives.
5.6 Conclusions
Thus, it can be observed that there are several ways of producing graphene and an extremely versatile nanomaterial. This versatility causes graphene to undergo various chemical modifications on its surface to improve its physical, chemical and mechanical properties. In addition, graphene can form several composites with a myriad of materials (clays, polymers, metals, etc.). Finally, it can be concluded that graphene and its derivatives have been used as efficient adsorbents in the removal of emerging organic contaminants (pharmaceuticals, dyes, surfactants, pestiscides, etc.) from the aqueous medium, and nanomaterials have become promising for the decontamination of this pollutants of the environment.
References
Abdi, G., Alizadeh, A., Amirian, J., Rezaei, S., Sharma, G.: Polyamine-modified magnetic graphene oxide surface: feasible adsorbent for removal of dyes. J. Mol. Liq. 289, 111118 (2019)
Achary, L.S.K., Kumar, A., Rout, L., Kunapuli, S.V.S., Dhaka, R.S., Dash, P.: Phosphate functionalized graphene oxide with enhanced catalytic activity for Biginelli type reaction under condition. Chem. Eng. J. 331, 300–310 (2018)
Algamdi, M.S., Alsohaimi, I.H., Lawler, J., Ali, H.M., Aldawsari, A.M., Hassan, H.M.A.: Fabrication of graphene oxide incorporated polyethersulfone hydrid ultrafiltration membranes for humic acid removal. Sep. Purif. Technol. 223, 17–23 (2019)
Al-Khateeb, L.A., Almotiry, S., Salam, M.A.: Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 248, 191–199 (2014)
Ali, M.E.A.: Preparation of graphene nanosheets by electrochemical exfotiation of a grafite-nanoclay composite electrode: application for the adsorption of organic dyes. Colloids Surf., A 570, 107–116 (2019)
Allen, M.J., Tung, V.C., Kaner, R.B.: Honeycomb carbon: a review of graphene. Chem. Rev. 110, 132–145 (2009)
Ayan-Varela, M., Paredes, J.I., Rodil, S.V., Rozada, R., Martinez Alonso, A., Tascon, J.M.D.: A quantitative analysis of the dispersion behavior of reduced graphene oxide in solvents. Carbon 75, 390–400 (2014)
Azizi, A., Moniri, E., Hassani, A.H., Panahi, H.A., Miralinaghi, M.: Polymerization of graphene oxide with polystyrene: non-linear isotherms and kinetics studies anionic dyes. Microchem. J. 145, 559–565 (2019)
Berger, C., Song, Z., Li, T., Li, X., Ogbazghi, A.Y., Feng, R., First, P.N.: Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J. Phys. Chem. B 108, 19912–19916 (2004)
Bhattacharyya, A., Mondal, D., Roy, I., Sarkar, G., Saha, N.R., Rana, D., Ghosh, T.K., Mandal, D., Chakraborty, M., Chattopadhyay, C.: Studies of the kinetics and mechanism of the removal process of proflavine dye through adsorption by graphene oxide. J. Mol. Liq. 230, 696–704 (2017)
Bhuyan, M.S.A., Uddin, M.N., Islam, M.M., Bipasha, F.A., Hossain, S.S.: Synthesis of graphene. Int. Nano Lett. 6, 65–83 (2016)
Borthakur, P., Boruah, P.K., Das, M.R., Kulik, N., Minofar, B.: adsorption of 17α-ethynyl estradiol and β-estradiol on graphene oxide surface: An experimental and computational study. J. Mol. Liq. 269, 160–168 (2018)
Boruah, P.K., Sharma, B., Hussain, N., Das, M.R.: Magnetically recoverable Fe3O4/graphene nanocoposite towards efficient removal of triazine pesticides from aqueous solution: investigation of the adsorption phenomenon and specific ion effect. Chemosphere 168, 1058–1067 (2017)
Brodie, B.C.: On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 149, 249–259 (1859)
Burakov, A., Neskoromnaya, E., Babkin, A.: Removal of the Alizarin Red S anionic dye using graphene nanocomposites: a study on kinetic under dynamic conditions. Mater. Today: Proc. 11, 392–397 (2019)
Burakov, A.E., Galunin, E.V., Burakova, I.V., Kucherova, A.E., Agarwal, S., Tkachev, A.G., Gupta, V.K.: Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol. Environ. Saf. 148, 702–712 (2018)
Chappell, G.A., Rager, J.E.: Epigenetics in chemical-induced genotoxic carcinogenesis. Curr. Opin. Toxicol. 6, 10–17 (2017)
Chaste, J., Saadani, A., Jaffre, A., Madouri, A., Alvarez, J., Pierucci, D., Aziza, Z.B., Ouerghi, A.: Nanostructures in suspended mono- and bilayer epitaxial graphene. Carbon 125, 162–167 (2017)
Chen, J., Ma, Y., Wang, L., Han, W., Chai, Y., Wang, T., Li, J., Ou, L.: Preparation of chitosan/SiO2-loaded graphene composite beads for efficient removal of bilirubin. Carbon 143, 352–361 (2019)
Chen, P., Li, H., Song, S., Weng, X., He, D., Zhao, Y.: Adsorption of dodecylamine hydrochloride on graphene oxide in water. Result Phys. 7, 2281–2288 (2017)
Chen, Y., Chen, L., Bai, H., Li, L.: Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 1, 1992–2001 (2013)
Choucair, M., Thordarson, P., Stride, J.A.: Gram-scale production of graphene based on solvothermal synthesis and sonication. J. Mater. Chem. A 4, 30–33 (2009)
Chu, K., Wang, F., Wang, X., Huang, D.: Anisotropic mechanical properties of graphene/copper composites with aligned graphene. Mater. Sci. Eng., A 713, 269–277 (2018)
Chua, C., Pumera, M.: Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem. Soc. Rev. 43, 291–312 (2014)
Ci, L.J., Song, L., Jariwala, D., Elias, A.L., Gao, W., Terrones, M., Ajayan, P.M.: Graphene shape control by multistage cutting and transfer. Adv. Mater. 21, 4487–4491 (2009)
Cui, J., Li, J., Qiu, H., Yang, G., Zheng, S., Yang, J.: Zwitterionic graphene oxide modified with silane molecules for multiple applications. Chem. Phys. Lett. 706, 543–547 (2018)
Deblonde, T., Cossu-Leguille, C., Hartemann, P.: Emerging pollutants in wastewater: a review of the literature. Int. J. Hyg. Environ. Health 214(6), 442–448 (2011)
Delhiraja, K., Vellingiri, K., Boukhvalov, D.W., Philip, L.: Development of highly water stable graphene oxide based composites for the removal of pharmaceuticals and personal care products. Ind. Eng. Chem. Res. 58, 2899–2913 (2019)
Du, Q., Sun, J., Li, Y., Yang, X., Wang, X., Wang, Z., Xia, L.: Highly enhanced adsorption of congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles. Chem. Eng. J. 245, 99–106 (2014)
Edwards, R.S., Coleman, K.S.: Graphene synthesis: relationship to applications. Nanoscale 5, 38–51 (2013)
Emtsev, K., Speck, F., Seyller, T., Ley, L., Riley, J.D.: Interaction, growth, and ordering of epitaxial graphene on SiC 0001 surfaces: a comparative photoelectron spectroscopy study. Physcal Rev. B 77, 155303 (2008)
Gao, L., Guest, J.R., Guisinger, N.P.: Epitaxial graphene on Cu (111). Nano Lett. 10, 3512–3516 (2010)
Gautam, R.K., & Chattopadhyaya, M.C.: Graphene-based nanocomposites as nanosorbents. Nanomater. Wastewater Remediat. 49–78 (2016)
Geim, A.K., Novoselov, K.S.: The rise of graphene. Nat. Mater. 6, 183–191 (2007)
González, J.A., Villanueva, M.E., Piehl, L.L., Copello, G.J.: Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: adsorption and desorption study. Chem. Eng. J. 280, 41–48 (2015)
Goodwin Jr., D.G., Adeleye, A.S., Sung, L., Ho, K.T., Burgess, R.M., Petersen, E.J.: Detection and quantification of graphene-family nanomaterials in the environment. Environ. Sci. Technol. 52, 4491–4513 (2018)
Hiew, B.Y.Z., Lee, L.Y., Lai, K.C., Gan, S., Thangalazhy-Gopakumar, S., Pan, G., Yang, T.C.: Adsorptive decontamination of diclofenac by three-dimensional graphene-based adsorbent: Response surface methodology, adsorption equilibrium, kinetic and thermodynamic studies. Environ. Res. 168, 241–253 (2019)
Hong, Y.L., Ryu, S., Jeong, H.S., Kim, Y.: Surface functionalization effect of graphene oxide on its liquid crystalline and assembly behaviors. Appl. Surf. Sci. 480, 514–522 (2019)
Huang, D., Xu, B., Wu, J., Brookes, P.C., Xu, J.: Adsorption and desorption of phenanthrene by magnetic graphene nanomaterials from water: roles of pH, heavy metal ions and natural organic metter. Chem. Eng. J. 368, 390–399 (2019)
Huang, X., Yin, Z., Wu, S., Qi, X., He, Q., Zhang, Q., Zhang, H.: Graphene-based materials: synthesis, characterization, properties, and applications. Small 7(14), 1876–1902 (2011)
Hummers, W., Offeman, R.: Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339–1339 (1958)
Jankovský, O., Novácek, M., Luxa, J., Sedmidubský, D., Fila, V., Pumera, M., Sofer, Z.: A new member of the graphene family: graphene acid. Chem. Eur. J. 22, 17416–17424 (2016)
Jiang, Y., Xu, R., Tan, Z., Ji, G., Fan, G., Li, Z., Xiong, D., Guo, Q., Li, Z., Zhang, D.: Interface-induced strain hardening of graphene nanosheet/aluminum composites. Carbon 146, 17–27 (2019)
Kerkez-Kuyumcu, O., Bayazit, S.S., Salam, M.A.: Antibiotic amoxicillin removal from aqueous solution using magnetically modified graphene nanoplatelets. J. Ind. Eng. Chem. 36, 198–205 (2016)
Kim, K.S., Zhao, Y., Jang, H., Lee, S.Y., Kim, J.M., Kim, K.S., Ahn, J.-H., Kim, P., Choi, J.-Y., Hong, B.H.: Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457, 706–710 (2009)
Konicki, W., Aleksandrzak, M., Mijowska, E.: Equilibrium, kinetic and thermodynamic studies on adsorption of cationic dyes from aqueous solutions using graphene oxide. Chem. Eng. Res. Des. 123, 35–49 (2017)
Konicki, W., Aleksandrzak, M., Moszynski, D., Mijowska, E.: Adsorption of anionic azo-dyes from aqueous solutions onto graphene oxide: equilibrium, kinetic and thermodynamic studies. J. Colloid Interdace Sci. 496, 188–200 (2017)
Kuilla, T., Bhadra, S., Yao, D., Kim, N.H., Bose, S., Lee, J.H.: Recent advances in grapheme based polymer composites. Prog. Polym. Sci. 35, 1350–1375 (2010)
Kyzas, G.Z., Deliyanni, E.A., Bikiaris, D.N.: Mitropoulos, A.C. Graphene composites as dye adsorbents Review. Chem. Eng. Res. Des. 129, 75–88 (2018)
Kyzas, G.Z., Koltsakidou, A., Nanaki, S.G., Bikiaris, D.N., Lambroupoulou, D.A.: Removal of beta-blockers from aqueous media by adsorption onto graphene oxide. Sci. Total Environ. 537, 411–420 (2015)
Lai, K.C., Hiew, B.Y.Z., Lee, L.Y., Gan, S., Thangalazhy-Gopakumar, S., Chiu, W.S., Khiew, P.S.: Ice-templated graphene oxide/chitosan aerogel as an effective adsorbent for sequestration of metanil yellow dye. Biores. Technol. 274, 134–144 (2019)
Lee, C., Wei, X., Kysar, J.W., Hone, J.: Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887), 385–388 (2008)
Li, M., Meng, X., Huang, K., Feng, J., Jiang, S.: A Novel composite adsorbent for the separation and recovery of indium from aqueous solutions. Hydrometallurgy 186, 73–82 (2019)
Li, G., Yuan, J.B., Zhang, Y.H., Zhang, N., Liew, K.M.: Microstructure and mechanical performance of graphene reinforced cementitious composites. Compos. A 114, 188–195 (2018)
Li, Z., Fu, X., Guo, Q., Zhao, L., Fan, G., Li, Z., Xionf, D., Su, Y., Zhang, D.: Graphene quality dominated interface deformation behavior of graphene-metal composite: the defective is better. Int. J. Plast 111, 253–265 (2018)
Li, X., Magnuson, C.W., Venugopal, A., Na, J., Suk, J.W., Han, B., Zhu, Y., Fu, L., Vogel, E.M., Voelkl, E., Colombo, L., Ruoff, R.S.: Graphene films with large domain size by a two-step chemical vapor deposition process. Nano Lett. 10, 4328–4334 (2010)
Li, X., Zhu, Y., Cai, W., Borysiak, M., Han, B., Chen, D., Piner, R.D., Colomb, L., Ruoff, R.S.: Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 9(12), 4359–4363 (2009)
Liang, X., Chang, A.S.P., Zhang, Y., Harteneck, B.D., Choo, H., Olynick, D.L., Cabrini, S.: Electrostatic force assisted exfoliation of prepatterned few-layer graphenes into device sites. Nano Lett. 9, 467–472 (2008)
Liu, C., Liu, H., Zhang, K., Dou, M., Pan, B., He, X., Lu, C.: Partly reduced graphene oxide aerogels induced by proanthocyanidins for efficient dye removal. Biores. Technol. 282, 148–155 (2019)
Liu, S.-H., Tang, W.-T., Yang, Y.-H.: Adsorption of nicotine in aqueous solution by a defective graohene oxide. Sci. Total Environ. 643, 507–515 (2018)
Liu, G., Li, L., Xu, D., Huang, X., Xu, X., Zheng, S., Zhang, Y., Lin, H.: Metal-organic framework preparation using magnetic graphene oxide-β-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohyd. Polym. 175, 584–591 (2017)
Liu, F.-F., Zhao, J., Wang, S., Xing, B.: Adsorption of sulfonamides on reduced graphene oxides as effected by pH and dissolved organic matter. Environ. Pollut. 210, 85–93 (2016)
Mahmoodi, N.M., Ghezelbash, M., Shabanian, M., Aryanasab, F., Saeb, M.R.: Efficient removal of cationic dyes from colored wastewaters by dithiocarbamate-functionalized graphene oxide nanosheets: from synthesis to detailed kinetics studies. J. Taiwan Inst. Chem. Eng. 81, 239–246 (2017)
Marcano, D.C., Kosynkin, D.V., Berlin, J.M., Sinitskii, A., Sun, Z., Slesarev, A., Alemany, L.B., Lu, W., Tour, J.M.: Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010)
Marquardt, D., Beckert, F., Pennetreau, F., Tolle, F., Mulhaupt, R., Riant, O., Hermans, S., Barthel, J., Janiak, C.: Hybrid materials of platinum nanoparticles and thiol-functionalized graphene derivatives. Carbon 66, 285–294 (2014)
McCoy, T.M., Turpin, G., Teo, B.M., Tabor, R.F.: Graphene oxide: a surfactant or particle? Curr. Opin. Colloid Interface Sci. 39, 98–109 (2019)
Molla, A., Li, Y., Mandal, B., Kang, S.G., Hur, S.H., Chung, J.S.: Selective adsorption of organic dyes on graphene oxide: theorical and experimental analysis. Appl. Surf. Sci. 464, 170–177 (2019)
Moradi, O., Gupta, V.K., Agarwal, S., Tyagi, I., Asif, M., Makhlouf, A.S.H., Sadegh, H., Shahryari-ghoshekandi, R.: Characteristics and electrical conductivity of graphene and graphene oxide for adsorption cationic dyes from liquids: Kinetic and thermodynamic study. J. Ind. Eng. Chem. 28, 294–301 (2015)
Moussavi, G., Hossaini, Z., Pourakbar, M.: High-rate adsorption of acentaminophen from the contaminated water onto double-oxidized graphene oxide. Chem. Eng. J. 287, 665–673 (2016)
Naeem, H., Ajmal, M., Qureshi, R.B., Muntha, S.T., Farooq, M., Siddiq, M.: Facile synthesis of graphene oxide silver nanocomposite for decontamination of water from multiple pollutants by adsorption, catalysis and antibacterial activity. J. Environ. Manage. 230, 199–211 (2019)
N’Diaye, A.T., Coraux, J., Plasa, T.N., Busse, C., Michely, T.: Structure of epitaxial graphene on Ir(111). New J. Phys. 10(4), 043033 (2008)
Nigar, S., Wang, H., Imtiaz, M., Yu, J., Zhou, Z.: Adsorption mechanism of ferrocene molecule on pristine and functionalized graphene. Appl. Surf. Sci. 481, 1466–1473 (2019)
Novoselov, K.S., Geim, A.K., Morozov, S.V., Jiang, D., Zhang, Y., Dubonos, S.V., Grigorieva, I.V., Firsov, A.A.: Electric field effect in atomically thin carbon films. Science 306, 666–669 (2014)
Novoselov, K.S.: Nobel lecture: graphene: materials in Flatland. Rev. Mod. Phys. 83, 837–849 (2011)
Oliveira, E.H.C., Mendonça, É.T., Barauna, O.S., Ferreira, J.M., Da Motta Sobrinho, M.A.: Study of variables for optimization of the dye indosol adsorption process using red mud and clay as adsorbents. Adsorption 22, 59–69 (2016)
Pei, S., Cheng, H.: The reduction of graphene oxide. Carbon 50, 3210–3228 (2010)
Pei, Q.X., Zhang, Y.W., Shenoy, V.B.: A molecular dynamics study of the mechanical properties of hydrogen functionalized graphene. Carbon 48, 898–904 (2010)
Peng, G., Zhang, M., Deng, S., Shan, D., He, Q., Yu, G.: Adsorption and catalytic oxidation of pharmaceuticals by nitrogen-doped reduced graphene oxide/Fe3O4 nanocoposite. Chem. Eng. J. 341, 361–370 (2018)
Perreault, F., Fonseca De Faria, A., Elimelech, M.: Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 44, 5861–5896 (2015)
Phiri, J., Gane, P., Maloney, T.C.: General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. 215, 9–28 (2017)
Prabhu, S.M., Khan, A., Farzana, M.H., Hwang, G.C., Lee, W., Lee, G.: synthesis and characterization of graphene oxide-doped nano-hydroxyapatite and its adsorption performance of toxic diazo dyes from aqueous solution. J. Mol. Liq. 269, 746–754 (2018)
Prediger, P., Cheminski, T., Neves, T.F., Nunes, W.B., Sabino, L., Picone, C.S.F., Oliveira, R.L., Correia, C.R.D.: Graphene oxide nanomaterials for the removal of non-ionic surfactant from water. J. Environ. Chem. Eng. 6(1), 1536–1545 (2018)
Puri, C., Sumana, G.: Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmotillonite nanocomposite. Appl. Clay Sci. 166, 102–112 (2018)
PuvaneswarI, N., Muthukrishnan, J., Gunasekaran, P.: Toxicity assessment and microbial degradation of azo dyes. Indian J. Exp. Biol. 44, 618–626 (2006)
Radnia, H., Rashidi, A., Nazar, A.R.S., Eskandari, M.M., Jalilian, M.: A novel nanofluid based on sulfonated graphene for enhanced oil recovery. J. Mol. Liq. 271, 795–806 (2018)
Rajabi, M., Mahanpoor, K., Moradi, O.: Preparation of PMMA/GO and PMMA/GO-Fe3O4 nanocomposites for malachite green dye adsorption: kinetic and thermodynamic studies. Compos. B Eng. 167, 544–555 (2019)
Rao, C.N.R., Maitra, U., Matte, H.S.S.R. Synthesis.: Characterization, and selected properties of graphene. In: Rao, C.N.R., Sood, A. K. (Eds.) Graphene: Synthesis, Properties, and Phenomena, 1st ed., pp. 1–47, (2012)
Rao, C.N.R., Sood, A.K.: Graphene: Synthesis, Properties, and Phenomena, p. 436. Wiley-VCH, Verlag GmbH, Weinheim (2012)
Ray, S.K., Majumder, C., Saha, P.: Functionalized reduced graphene oxide (FRGO) for removal of fulvic acid contaminant. RSC Advances 7, 21768–21779 (2017)
Reina, A., Jia, X.T., Ho, J., Nezich, D., Son, H., Bulovic, V., Mildred Dresselhaus, S., Kong, J.: Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 9, 30–35 (2009)
Ren, W., Chang, H., Mao, T., Teng, Y.: Planarity effect of polychlorinated biphenyls adsorption by graphene nanomaterials: the influence of graphene characteristics, solution pH and temperature. Chem. Eng. J. 263, 160–168 (2019)
Ren, H., Kulkarni, D.D., Kodiyath, R., Xu, W., Choi, I., Tsukruk, V.V.: Competitive adsorption of dopamine and rhodamine 6G on the surface of graphene oxide. Appl. Mater. Interfaces 6(4), 2459–2470 (2014)
Robati, D., Rajabi, M., Moradi, O., Najafi, F., Tyagi, I., AgarwaL, S., Gupta, V.K.: Kinetics and thermodynamics of malachite green dye adsorption from aqueous solutions on graphene oxide and reduced graphene oxide. J. Mol. Liq. 214, 259–263 (2016)
Rostamian, R., Behnejad, H.: A comprehensive adsorption study and modeling of antibiotics as a pharmaceutical waste by graphene oxide nanosheets. Ecotoxicol. Environ. Saf. 147, 117–123 (2018)
Rostamian, R., Behnejad, H.: A comparative adsorption study of sulfamethoxazole onto graphene and graphe oxide nanosheets through equilibrium, kinetic and thermodynamic modeling. Process Saf. Environ. Prot. 102, 20–29 (2016)
Sahu, M., Raichur, A.M.: Toughening of high performance tetrafunctional epoxy with poly(allyl amine) grafted graphene oxide. Compos. B Eng. 168, 15–24 (2019)
Sekulic, M.T., Boskovic, N., Slavkovic, A., Garunovic, J., Kolakovic, S., Pap, S.: Surface functionalized adsorbent for emerging pharmaceutical removal: adsorption performance and mechanisms. Process Saf. Environ. Prot. 215, 50–63 (2019)
Segundo, J.E.D.V., Vilar, E.O.: Grafeno: Uma revisão sobre as propriedades, mecanismos de produção e potenciais aplicações em sistemas energéticos. Rev. Eletrônica Mater. E Process. 11, 54–57 (2016)
Sham, A.Y.W., Notley, S.M.: Adsorption of organic dyes from aqueous solutions using surfactant exfoliated graphene. J. Environ. Chem. Eng. 6, 495–504 (2018)
Singh, V., Joung, D., Zhai, L., Das, S., Khondaker, S.I., Seal, S.: Graphene based materials: past, present and future. Prog. Mater Sci. 56, 1178–1271 (2011)
Song, Z., Ma, Y.-L., Li, C.-E.: The residual tetracycline in pharmaceutical wastewater was effectively removed by using MnO2/graphene nanocomposite. Sci. Total. Environ. 168, 580–590 (2019)
Staudenmaier, L.: Verfahren zur Darstellung der Graphitsaure. Eur. J. Inorg. Chem. 31, 1481–1487 (1898)
Sun, Y., Yang, Y., Yang, M., Yu, F., Ma, J.: Response surface methodological evaluation and optimization for adsorption removal of ciprofloxacin onto graphene hydrogel. J. Mol. Liq. 284, 124–130 (2019)
Szczesniak, B., Choma, J., Jaroniec, M.: Ultrahigh benzene adsorption capacity of graphene-MOF composite fabricated via MOF crystallization in 3D mesoporous graphene. Microporous Mesoporous Mater. 279, 387–394 (2019)
Tahriri, M., Del Monico, M., Moghanian, A., Yaraki, M.T., Torres, R., Yadegari, A., Tayebi, L.: Graphene and its derivatives: opportunities and challenges in destistry. Mater. Sci. Eng., C 102, 171–185 (2019)
Tang, C., Yu, P., Tang, L., Wang, Q., Bao, R., Liu, Z., Yang, M., Yang, W.: Tannic acid functionalized graphene hydrogel for organic dye adsorption. Ecotoxicol. Environ. Saf. 165, 299–306 (2018)
Terracciano, A., Zhang, J., Christodoulatos, C., Wu, F., Meng, X.: Adsorption of Ca2+ on single layer graphene oxide. J. Environ. Sci. 57, 8–14 (2017)
Viculis, L.M., Mack, J.J., Kaner, R.B.: A chemical route to carbon nanoscrolls. Science 299, 1361 (2003)
Wang, P., Zhang, D., Tang, H., Li, H., Pan, B.: New insights on the understanding of the high adsorption of bisphenol compounds on reduced graphene oxide at high pH values via charge assisted hydrogen bond. J. Hazard. Mater. 371, 513–520 (2019)
Wang, J., Chen, B., Xing, B.: Wrinkles and folds of activated graphene nanosheets as fast and efficient adsorptive sites for hydrophobic organic contaminants. Environ. Sci. Technol. 50, 3798–3808 (2016)
Wang, J., Chen, B.: Adsorption and coadsorption of organic pollutanrs and a heavy metal by graphene oxide and rediced graphene materials. Chem. Eng. J. 281, 379–388 (2015)
Wallace, P.R.: The Band Theory of Graphite. Physical Review Journal 71, 622–634 (1947)
Wu, Y.H., Yu, T., Shen, Z.X.: Two-dimensional carbon nanostructures: Fundamental properties, synthesis, characterization, and potential applications. J. Appl. Phys. 108(7), 071301 (2010)
Wu, X., Li, X., Song, Z., Berger, C., de Heer, W.A.: Weak antilocalization in epitaxial graphene: evidence for chiral electrons. Phys. Rev. Lett. 98, 136801 (2007)
Xiong, X.-Q., Bao, Y.-L., Liu, H., Zhu, Q., Lu, R., Miyakoshi, T.: Study on mechanical and electrical properties of cellulose nanofibrils/graphene-modified natural rubber. Mater. Chem. Phys. 223, 535–541 (2019)
Xu, W., Chen, Y., Zhang, W., Li, B.: Fabrication of graphene oxide/bentonite composites with excellent adsorption performances for toluidine blue removal from aqueous solution. Adv. Powder Technol. 30, 493–501 (2019)
Yadav, A., Upadhyaya, A., Gupta, S.K., Verma, A.S., Negi, C.M.S.: Poly-(3-hexylthiophene)/graphene composite based organic photodetectors: the influence of graphene insertion. Thin Solid Films 675, 128–135 (2019)
Yang, W., Zhao, Q., Xin, L., Qiao, J., Zou, J., Shao, P., Yu, Z., Zhang, Q., Wu, G.: Microstructure and mechanical properties of graphene nanoplates reinforced pure Al matrix composites prepared by infiltration method. J. Alloy. Compd. 732, 748–758 (2018)
Yamjala, K., Nainar, M.S., Ramisetti, N.R.: Methods for the analysis of azo dyes employed in food industry–a review. Food Chem. 192, 813–824 (2016)
Yi, H., Huang, D., Zeng, G., Lai, C., Qin, L., Cheng, M., Ye, S., Song, B., Ren, X., Guo, X.: Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B: Environ. B 239, 408–424 (2018)
Yu, L., Wang, L., Xu, W., Chen, L., Fu, M., Wu, J., Ye, D.: Adsorption of VOCs on reduced graphene oxide. J. Environ. Sci. 67, 171–178 (2018)
Zare, E.N., Motahari, A., Sillanpää, M.: Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: a review. Environ. Res. 162, 173–195 (2018)
Zhang, C., Luan, J., Yu, X., Chen, W.: characterization and adsorption performance of graphene oxide-montmorillonite nanocomposite for the simultaneous removal of Pb2+ and p-nitrophenol. J. Hazard. Mater. 378, 120739 (2019)
Zhang, W., Chen, J., Hu, Y., Fang, Z., Cheng, J., Chen, Y.: Adsorption characteristics of tetrabromobisphenol A onto sodium bisulfite reduced graphene oxide aerogels. Colloids Surf. A 538, 781–788 (2018)
Zhang, Y., Small, J.P., Pontius, W.V., Kim, P.: Fabrication and electric-field-dependent transport measurements of mesoscopic graphite devices. Appl. Phys. Lett. 86(7), 073104 (2005)
Zhao, X., Tang, J., Yu, F., Ye, N.: Preparation of graphene nanoplatelets reinforcing copper matrix composites by electrochemical deposition. J. Alloy. Compd. 766, 266–273 (2018)
Zhu, S., Liu, Y.-G., Liu, S.-B., Zeng, G.-M., Jiang, L.-H., Tan, X.-F., Zhou, L., Zeng, W., Li, T.-T., Yang, C.-P.: Adsorption of emerging contaminant metformin using graphene oxide. Chemosphere 179, 20–28 (2017)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bezerra, R.D.S., Teixeira, P.R.S., da Silva-Filho, E.C., Lobo, A.O., Viana, B.C. (2019). Nanostructured Carbon-Based Materials for Adsorption of Organic Contaminants from Water. In: Gonçalves, G., Marques, P. (eds) Nanostructured Materials for Treating Aquatic Pollution. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-33745-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-33745-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33744-5
Online ISBN: 978-3-030-33745-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)