Abstract
Hydrogen sulfide (H2S) gas can be traced once the groundwater is pumped out from a deep well which is located <10 km from the costal line. The groundwater contains 5.1 ± 0.1 ppt of salinity which is classified as saline groundwater. The initial color of the groundwater is green yellowish. After 40 s exposed to the oxygen, its colour suddenly turned to black and become sludgy. Afterwards, the black colour turns to partially cloudy after 8 h being exposed to the oxygen, subsequently, the H2S gas vanishes along with the disappearance of the black colour. Hence, from this reaction, this study aims to investigate the cause of the black precipitate formation which comes from the oxidation of the deep well saline groundwater. Based on the XRD and DSC results, the black precipitate is a troilite mineral (FeS). The elements that contained in the groundwater mostly originated from the seawater. The fast precipitation is caused by the Cl− content which is increasing the oxidation rate. The increase of Fe2+ is caused by the weathering process during the travel of the groundwater through the aquifer. Meanwhile, \( {\text{SO}}_{4}^{2 - } \) is decomposed by microorganism to produce S2− and this causes the reaction of Fe2+ and S2− to form FeS despite in saline condition.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Deep well is defined as a depth of well greater than 30 m from the water table. Pollution of deep well usually occurred from a long-term impact of local activities. In general, the water quality of the deep well changes much slower than the shallow well. This happens because the pollution of deep well is affected by the land use for more than 10 years and influenced by weathering of rocks through the aquifer [23]. In this case, the water will slowly move in the aquifer which can take many years before it can reach above the 30 m depth; in between a foot to a mile per day based on the type of soil and rock [3].
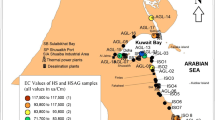
In the Engineering Campus of Universiti Sains Malaysia, a tube well with a diameter of 6 in. and 60 m body length was dug for geomorphology and groundwater study. According to the water table in this study area at the coordinate of 5°08′50.25″ N, 100°29′36.23″ E, it is ±1.76 m from the surface area and the depth of the tube well is 58.24 m [14]. Based on the bore hole data of the tube well and the self-potential (SP) prospecting data before pumping at the study area taken by Nik Adik [1], the final water table was around 1.62–1.86 m. Therefore, in this case, this study will be focusing on the deep well where the pollutants may consist of various minerals that dissolve in water and anaerobic living organisms. Based on Fig. 1, coastline distance is about 8 km away from the site area. According to Tawnie et al. [20] report, the saline intrusion can extend over 10 km from the coastal line even in groundwater. This will affect the intrusion of salt into the river and the groundwater [14]. Table 1 shows the classification of salinity status.
Due to proximity to the sea, the occurrence of salinity is unavoidable in groundwater or surface water due to the intrusion from the seawater. Salinity also can be composed from the agriculture activities such as the usage of fertilizer for plantation. Fertilizer that flows beneath the underground reacts with the free ion in groundwater such as Mg2+ and hence, forms Epsom salt or in its chemical name known as magnesium sulfate (MgSO4). Based on this research area, salinity is formed from the natural process such as weathering of rocks and salt inflation in the rainfall for thousands of years. Seawater moves into the aquifer and mixes with the fresh groundwater [12].
H2S is exists more in groundwater compared to surface water due to sulfate reducing bacteria (SRB) which is one of the biotic factors. With the presence of SRB, it decomposes sulfate (\( {\text{SO}}_{4}^{2 - } \)) to S2− and arouses the nucleation of Fe2+ and S2− to form FeS despite unexposed to the oxygen [17]. FeS is also named as troilite mineral. It has been proved by Picard et al. [17] that the FeS formation was accelerated by SRB whereby the experiment was conducted in an anaerobic vinyl chamber and nanocrystalline. Furthermore, their data also unveiled about the nucleation of FeS which occurred on the cell surface of the living SRB and dead SRB and also on extracellular materials. Besides that, the presence of oxidants in the groundwater such as chloride and fluoride also tend to oxidize \( {\text{SO}}_{4}^{2 - } \) to S2− and subsequently reacts with Fe2+ to form FeS. This factor is categorized as an abiotic factor [8]. To consummate their studies, both biotic and abiotic factors play the role to form FeS. However, with the cooperation of living microorganisms, the nucleation can be accelerated and it could take one week to form FeS in anaerobic condition [17].
In aerobic condition, a low temperature and low concentration of H2S also can form FeS as shown in Eqs. (1) and (2) [18, 19]. According to Eq. (3), if the reaction time is prolonged, the FeS may transform to greigite, Fe3S4 or also known as ferrimagnetic where it is identical to magnetite, Fe3O4 [6, 17]. A further reaction under hotter conditions and a higher concentration of H2S gas may trigger the transformation of pyrite, FeS2 as shown in Eq. (4) [22].
The alteration of the composition above depends on the environmental condition such as temperature, pH, pressure etc. An example of pyrite in Eq. (1), in weakly acidic condition (pH = 4), FeS and FeS2 are formed [13, 16]. Meanwhile, in neutral and alkaline mixtures (pH 6–9), only FeS and S are formed as shown in Eq. (2) [22].
A redox process at the aqueous-solid interface from the reaction of iron and sulfur has been detailed out by Fan et al. [8] as shown in Fig. 2. The mechanism of iron and sulfur reaction based on pH and Eh variable has been illustrated in graphs to see the changes of iron and sulfur species. The fixed-concentration species is (A: [ST] = 10−6 M, B: [FeT] = 10−6 M) and the contours are total ions (A: [FeT], B: [ST]) displayed in different colors which are yellow: 10−6 M, red: 10−4 M, and blue: 10−5 M.
This is the Eh-pH diagram for the F–S–H2O system at room temperature, 25 °C drawn by Fan et al. [8]
Based on Fig. 2 which sulfur is a source of an oxidizing agent for ferric, Fe3+ to Fe2+ by electron transfer at pH of 5–6 and approximately around −0.6 to 0.1 volts. Reaction of Fe2+ can form a goethite (FeO(OH)); non-magnetite, troilite (FeS); low magnetite, pyrrhotite (Fe(1 − x)S) where x = 0–0.2, mackinawite (Fe(x + 1)S) where x = 0–0.1; high magnetite, greigite (Fe3S4) or pyrite (FeS2) depending on the environmental condition. There are three stable states that describe the transition phase of the mineral which include the excited state (unstable), meta state (metastable/partial stable) and ground state (stable). However, the graph only illustrates the excited state and meta state.
In the graph, the interval between the Fe2+ and the area of blue color represents the transition of aqueous-solid phase in which the minerals are in the excited state. Excited phase is a phase with a high solubility and it often favors to form before the meta state phase with lower solubility which is also known as mackinawite. According to Bleam [4], FeS is highly soluble in acidic and less soluble in alkaline condition as shown in Eq. (5). Based on the graph at Fig. 2a, it was found that when S increased in FeS, it tends to rise its magnetic force such as greigite. Mackinawite is divided into two magnetic properties which are magnetic and non-magnetic, and caused by the amount of sulfur [4, 10, 15].
The other graph at Fig. 2b describes the same concept as the graph in Fig. 2a with the same reaction of Fe and S but different amount of Fe. As stated in Taylor et al.’s [21] article, by applying the temperature 160 °C to the aqueous hydrogen sulfide that mixed with iron monosulfide powder, the reaction sequence of Fe2+ and H2S is (troilite → pyrrhotite → pyrite). According to the properties and amount of Fe and S, the blue colour could be an amorphous troilite which requires higher temperature to stable and the red colour is pyrrhotite [4, 7, 13, 21].
H2S gas which is well known for its rotten egg smell can be traced once the groundwater was pumped out of the tube well. H2S gas smell can as well become stronger when it turns black. Based on Fig. 3, initial color of the groundwater in Universiti Sains Malaysia was green yellowish. After 40 s exposed to the oxygen, the color of the groundwater suddenly turned to black and this attends to the sludgy appearance. Afterwards, the black colour was turned to red after 8 h being exposed to the oxygen, subsequently the H2S gas disappeared along with the disappearance of the black colour. Figure 4 shows the reaction of the groundwater after exposed to the oxygen for 24 h.
Since this groundwater shown to have relation to the FeS contamination which affected by the surrounding condition such the seawater intrusion, it is beneficial to do a deeper study of groundwater based on the condition of the surrounding that affecting the source.
2 Methodology
2.1 Sampling
The groundwater was pumped for 3 h to surpass or equal to the volume of three wells as shown in Eq. (6). The volume of the pumped groundwater was calculated based on the flowrate of the pump as shown in Eq. (7). The flowrate was manually measured via a measuring cylinder. Meanwhile, the well’s depth was measured from the water table until the final depth of the well as displayed in Eq. (8). The distance of the well’s depth will be justifying to the classification of the well either it is a deep well or a shallow well. Besides, the volume of the well was calculated using a formula of cylinder as exhibited in Eq. (9).
where, \( V_{pg } \) = Volume of the pumped groundwater (l)
\( V_{well} \) = Volume of the well (l)
\( f_{p} \) = Flowrate of the pump (l/s)
\( t \) = Time taken of 1 L (s)
\( D_{well} \) = Depth of the well (m)
\( L_{well} \) = Length of the well (m)
\( d_{wt} \) = Distance of the water table from the surface (m).
2.2 Preservation of Sample
Sample was put into 50 ml centrifuge tube and 3% of nitric acid (NHO3) was added to assure that the pH to be less than 2 and to avoid any changes occurred to the sample. pH was measured using a pH meter. This sample was kept in the fridge at a temperature of 4 °C. This preservation sample was prepared for NH3N, COD and ICP-OES test. The time taken between the sampling and the transfer of the sample to the fridge must be less than 2 h. These samples are then kept in the fridge not over 28 days.
2.3 XRD
The solid sample was grinded using a mortar and a pestle. Then, the powder sample was loaded into milled well on a sample holder as shown in Fig. 5. A glass slide was used to compact the sample and slid on the surface of the compaction sample area in order to remove the uneven surface. Then, the sample holder was cleaned using a brush before placing it into a stage.
The XRD machine software was enabled in the “tool”. It was set to be in a condition of 45 kV and 10 mA. The water chiller was checked and it set to be at a temperature of <72 °C with a flow 4–6 m/h. After the sample preparation was ready for scanning, the aluminum sample holder was placed onto a stage in the XRD machine. The sample was scanned from 2 to 35° 2θ.
2.4 SEM-EDX
The size of the crushed marble must be less than 4 cm before being tested via SEM test. The sample was coating for 20 min using the sputter coating to make the sample visible in the SEM-EDX machine.
2.5 ICP-OES
The preserved groundwater samples were filtered using 0.45 μm nylon syringe filter to remove suspended solid. A sample probe rinse solution of 1% ultrapure HNO3 was prepared to rinse the probe during reading the samples. 10 ppm of Scandium (Sc) was put into the 1% ultrapure HNO3 for an internal standard solution. 23 multi-elements, chlorine and sulfur single-element standard were used for identifying inorganic element in the groundwater samples. 1, 2, 3, 10, 25 and 50 ppm were chosen as a standard range. A good wavelength depends on the calibration graph of each element. An accurate concentration was read according to the calibration graph below 5% of (relative standard deviation) RSD and above the detention limit value.
2.6 TGA-DSC
The nitrogen gas and the house air-line were turned on to trigger an oxidizing environment. The water chiller is set to 20 °C. In the “Sample Info”, sample information such as sample name and weight were written. At the zero box, it was written 0.000 in order to continue the program. The sample pan was removed from the crucible and added with 10–30 mg of sample. The sample weight was then recorded and the pan was placed back into the crucible.
Before running the test, the temperature and rate were set up to 1300 °C as the highest temperature and 5 °C/min for the temperature rate. The derivative of heat flow was used to determine the accurate transition temperature data. Last but not least, the line graph was thickened to display an unambiguous data.
3 Results and Discussion
3.1 Salinity
Based on ICP-OES result in Table 2, Na+ and Cl− have concentration in part per thousand (ppt) value compared to the other elements which have concentration in part per million (ppm). Based on the data taken in dry and wet season as shown in Table 3, the result shows 5.1 ± 0.1 ppt and it has been categorized as saline groundwater. In view of the salinity of the groundwater, this has risen questions on the elements that exist in the groundwater.
By looking at the SEM-EDX result in Table 4, the presence of chloride in the groundwater has shown a higher weight percentage as compared to Na. Based on the SEM scanned picture in Fig. 6, the sample shows a cubic shape that represents the NaCl crystal salt shape. According to the XRD analysis in Fig. 8, the dried groundwater sample has been found to have two salt compounds which are magnesium dichloride (MgCl2) and sodium chloride (NaCl).
This means that the melting point value is referring to NaCl’s melting point. Thus, it can be concluded that the salinity of the groundwater is due to the presence of NaCl which comes from the seawater. As illustrated in the Google map in Fig. 1 where the location is 8 km far from the coastline, it has been reported by Tawnie et al. [20] that salinity intrusion can extend over 10 km distance from the coastal line even in the groundwater.
According to the salinity results that were taken along the river to the estuary as shown in Fig. 7, the salinity of the river that located close to the study area is not in a high concentration compared to the salinity level of the groundwater as referred to Table 5. However, the amount of salinity at the estuary at CP10 is almost near to the amount of salinity in the groundwater located at the study area. Thus, it can be concluded that the salinity intrusion has occurred from the estuary and it travels through the aquifer starting from the estuary to the study area. Moreover, it also could justify that the nearby river from the study area does not influenced by the increase of the salinity in the groundwater.
Based on the comparison between the concentration of the groundwater sample to the standard concentration of seawater which the sources referred from Keener-Chavis and Sautter [11], Anthoni [2] and Campbell [5] as shown in Table 6, it was found that the elements that naturally exist in a high concentration in the groundwater are quite similar to the elements that has a high concentration in the seawater. Some of elements in the groundwater such as Ba, Fe and Mn have higher concentration compared to the standard seawater concentration. These elements naturally occurred due to the weathering of rock or through the decomposition process during the transportation in the aquifer. Sulfate (\( {\text{SO}}_{4}^{2 - } \)) in groundwater can be reduced by sulfate reducing bacteria in an anaerobic environment and subsequently produces sulfide (S2−).
3.2 FeS
According to the odor and the color changes of the groundwater sample, this reaction was quite similar to the previous study of FeS formation. The presence of hydrogen sulfide gas and ferrous sulfide was formed due to high sulfur ion content. The characteristic of FeS was reported by Liu et al. [13] that appear to be black or brown just like the color of sludge.
Based on XRD data from a dried groundwater sample in Fig. 8, it has shown that the groundwater contains FeS (Troilite). FeS (Troilite) which is also called as iron rich endmember is an amorphous FeS whereby it is not a stable compound and required other elements to stabilize it. It changes its colour from black to red in 8 h, which is when the H2S will be produced. Then, after the 8 h, the smell finally disappears along with the changes of the black colour to red. This means that S2− ion in FeS (troilite) has detached from the Fe2+ ion and attaches with H+ that exists in the groundwater to produces H2S gas. Apart from that, H+ ion can be increased during evaporation of water.
3.3 Precipitation of Groundwater Using Strong Reducing Agent
As discussed before, the color of the groundwater has rapidly turned to black in 40 s due to the Cl content which is one of the strong reducing agents where it can be a catalyst to accelerate the formation of FeS and destabilized in maintaining the FeS form. Thus, to enable the strong reducing agent to be a great catalyst to precipitate the groundwater sample, NH4OH was used to investigate the reaction of the groundwater via titration as shown in Fig. 9. It was found that the ratio of the titrant with the sample was 1:1 to start a clear precipitate and 2:1 for a clear water as shown in Fig. 10. It is important to note that Cl− is one of the major elements in the groundwater, hence the greater the concentration of the Cl− the faster the precipitation of the groundwater.
By addition of NH4OH solution into the sample, the pH of the sample was increased from 6.78 to 12.01, subsequently it changes its colour to become clear water. According to the XRD result of the residual in Fig. 11, it was found that the compound was mostly made of pyrrhotite. By comparing the DSC result in Fig. 11, the top line of the graph has shown a transition temperature at 795 °C. According to Frost [9], the melting point of pyrrhotite at composition of FeS0.96 is 795 °C. As have been discussed before in introduction (Fig. 2), the increase of pH during titration and the application of heat during evaporation process of the sample at temperature of 100 °C had caused the FeS (troilite) to transformed into unstoichiometric FeS (pyrrhotite). Due to this fact, the black colour precipitate actually represents the FeS reaction.
4 Conclusions
As conclusion, the presence of FeS after the deep well groundwater exposed to the oxygen is caused by the intrusion of seawater which increase the chlorine content, the decomposition of sulfate in the seawater to sulfur by microorganism and the high concentration of iron due to weathering of rock in the aquifer. The oxygen and chlorine accelerate the reaction of Fe2+ and S2− to form FeS in 40 s.
References
Adik NNAN (2010) Use of hydro-geophysics streaming potential (SP) signals in evaluating environmental friendly bio-ecological drainage system (BIOECODS) effectiveness (Master dissertation, Universiti Sains Malaysia, Penang, Malaysia). Retrieved from http://eprints.usm.my/40957/1/Use_of_hydro-geophysics_streaming_potential_%28SP%29_Signals_in_evaluating_environmental_friendly_Bio-ecological_drainage_system_%28BIOECODSTM%29_Effectiveness.pdf
Anthoni JF (2006) The chemical composition of seawater, seafriends. Retrieved 18 Apr 2019, from http://www.seafriends.org.nz/oceano/seawater.htm
Athirah AA, Saad NA, Mohd Akhir MF, Zakaria NA (2019) Manganese removal in groundwater treatment using marble. Int J Integr Eng 11(1):53–60
Bleam WF (2016) Water chemistry. In: Soil and environmental chemistry, 2nd edn. Academic Press, Wisconsin, USA. https://doi.org/10.1016/b978-0-12-415797-2.00005-4
Campbell K (2016) Over 40 minerals and metals contained in seawater, their extraction likely to increase in the future. Mining Weekly. Retrieved 18 Apr 2019, from https://www.miningweekly.com/article/over-40-minerals-and-metals-contained-in-seawater-their-extraction-likely-to-increase-in-the-future-2016-04-01
Csákberényi-Malasics D, Rodriguez-Blanco JD, Kis VK, Rečnik A, Benning LG, Pósfai M (2012) Structural properties and transformations of precipitated FeS. Chem Geol 294:249–258
Esmaeely SN, Bota G, Brown B, Nešić S (2017) Influence of pyrrhotite on the corrosion of mild steel. Corrosion 74(1):37–49
Fan D, Lan Y, Tratnyek PG, Johnson RL, Filip J, O’Carroll DM, Nunez GA, Agrawal A (2017) Sulfidation of iron-based materials: a review of processes and implications for water treatment and remediation. Environ Sci Technol 51(22):13070–13085
Frost BRO (2002) Partial melting of sulfide ore deposits during medium and high-grade metamorphism. J Mineral Assoc Can 40(1):1–18
Jeong HY, Jun HL, Hayes KF (2008) Characterization of synthetic nanocrystalline mackinawite: crystal structure, particle size, and specific surface area. Geochim Cosmochim Acta 72(2):493–505
Keener-Chavis P, Sautter LR (2000) Physical and chemical properties of the ocean. In: Of sand and sea: teachings from the southeastern shoreline. South Carolina Sea Grant Consortium, pp 19–31
Li Q, Lian B, Wang Y, Taylor RA, Dong M, Lloyd T, Liu X, Tan J, Ashraf MM, Waghela D, Leslie G (2018) Development of a mobile groundwater desalination system for communities in rural India. Water Res 144:642–655
Liu Y, Zhang Z, Bhandari N, Dai Z, Yan F, Ruan G, Lu AY, Deng G, Zhang F, Al-Saiari H, Kan AT, Tomson MB (2017) New approach to study iron sulfide precipitation kinetics, solubility, and phase transformation. Ind Eng Chem Res 56(31):9016–9027
Mohd Akhir MF, Saad NA, Zakaria NA, Xin KL, Athirah AA (2019) Desalination of groundwater using marble filter. Int J Integr Eng 11(1):92–100
Morse JW, Millero FJ, Cornwell JC, Rickard D (1987) The chemistry of the hydrogen sulfide and iron sulfide systems in natural waters. Earth Sci Rev 24:1–42
O’Day PA, Vlassopoulos D, Root R, Rivera N (2004) The influence of sulfur and iron on dissolved arsenic concentrations in the shallow subsurface under changing redox conditions. Proc Natl Acad Sci 101(38):13703–13708
Picard A, Gartman A, Clarke DR, Girguis PR (2018) Sulfate-reducing bacteria influence the nucleation and growth of mackinawite and greigite. Geochim Cosmochim Acta 220(10):367–384
Rickard D (1995) Kinetics of FeS precipitation: part 1. Competing reaction mechanisms. Geochim Cosmochim Acta 59(21):4367–4379
Rickard D (2006) The solubility of FeS. Geochim Cosmochim Acta 70(23):5779–5789
Tawnie I, Sefie A, Normi IA, Shamsuddin MKN, Mohamed A (2016) Overview of groundwater contamination in Malaysia. In: WEPA Secretariat of Institute for Global Environmental Strategies (IGES) (eds) The 12th international symposium on Southeast Asian water environment (SEAWE12). Water Environment Partnership in Asia (WEPA), Hanoi, Vietnam, pp 76–83. Retrieved from http://wepa-db.net/activities/2016/20161129/PDF/06%20Malaysia_GWater%20Malaysia_WEPA291116.pdf
Taylor P, Rummery TE, Owen DG (1979) Reactions of iron monosulfide solids with aqueous hydrogen sulfide up to 160°C. J Inorg Nucl Chem 41(12):1683–1687
Walker R (2001) Instability of iron sulfides on recently excavated artifacts. Stud Conserv 46(2):141–152
Xin KL, Saad NA, Mohd Akhir MF, Zakaria NA (2019) Removal of iron in groundwater using marble column filter. Int J Integr Eng 11(1):112–118
Acknowledgements
This research was fully funded by HICOE and short-term grant. My deepest appreciation to Director of River Engineering and Urban Drainage Research Center (REDAC), Civil Engineering School and Material and Mineral Resources Engineering School who were giving opportunities to use their equipment without any objection.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Mohd Akhir, M.F., Saad, N.A., Zakaria, N.A. (2020). Oxidation of Deep Well Saline Groundwater Generates the Precipitation of Ferrous Sulfide (FeS). In: Mohamed Nazri, F. (eds) Proceedings of AICCE'19. AICCE 2019. Lecture Notes in Civil Engineering, vol 53. Springer, Cham. https://doi.org/10.1007/978-3-030-32816-0_76
Download citation
DOI: https://doi.org/10.1007/978-3-030-32816-0_76
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32815-3
Online ISBN: 978-3-030-32816-0
eBook Packages: EngineeringEngineering (R0)