Abstract
Male infertility is a widespread problem; approximately in one in five infertile couples, the problem lies solely in the male partner. The pathogenesis of male infertility can be reflected by defective spermatogenesis due to pituitary disorders, testicular cancer, germ cell aplasia, varicocele, and environmental factors or due to defective sperm transport resulting from congenital abnormalities or immunological or neurological factors. Recent findings have shown that male infertility increases due to exposure to environmental toxicants that leads to abnormal apoptosis of either germ cells or Sertoli cells. Some of the environmental contaminants have the ability to interfere with natural hormones and have been shown to induce programmed cell death. Apoptosis, also known as programmed cell death (PCD), is an important phenomenon required for normal spermatogenesis in mammals and is believed to ensure cellular homeostasis. Under normal conditions, an adequate number of germ cells are eliminated via the process of apoptosis in order to maintain a precise germ cell population is compliance with the supportive capacity of the Sertoli cells. This chapter briefs both physiological and pathological events that can trigger apoptosis and their effects on the male reproductive system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Apoptosis
- Male infertility
- Programmed cell death
- Sertoli cells
- Tumor necrosis factor
- Extrinsic pathway
- Intrinsic pathway
- Steroidogenesis
- Environmental contaminants
-
Increasing evidence from epidemiological and clinical studies suggests that male reproductive health has been deteriorating due to several factors including exposure to environmental contaminants and changing lifestyle, but the mechanistic pathway has not yet been fully elucidated.
-
Recent findings have shown that the incidence of male infertility has increased due to genetic disorders and pathological apoptosis.
-
Apoptosis is a highly regulated process characterized by distinct changes in the cell morphology including membrane blebbing, shrinkage of cell volume, cytoplasmic vacuolization, nuclear condensation, and DNA fragmentation, followed by the disassembly of the cell into membrane-bound apoptotic bodies. The biochemical features of apoptosis include phosphatidylserine exposure to the external leaflet of the plasma membrane, activation of caspase cascades, DNA cleavage, and DNA laddering.
-
Environmental contaminants possessing endocrine-disrupting properties are known to induce oxidative stress and cause pathological apoptosis, thereby affecting the male reproductive health.
1 Introduction
Infertility is a global health issue and is usually defined as the failure to achieve clinical pregnancy after regular and unprotected sex for at least a year or more. Even though infertility affects 15–20% of couples worldwide [1], male factor infertility is contributing approximately 50% of cases, with sole responsibility in 30% and co-contributing with the female in 20% of cases. According to WHO, the overall prevalence of primary infertility in India is between 3.9% and 16.8% [2]. In some African countries, the problem even exceeds where one-third of the couples are infertile [3, 4]. Increasing evidence from epidemiological and clinical studies suggests that male reproductive health has been deteriorating due to several factors including exposure to environmental contaminants and changing lifestyle. The pathogenesis of male infertility can be reflected by defective spermatogenesis due to pituitary disorders , testicular cancer , germ cell aplasia , varicocele , and environmental factors or due to defective sperm transport resulting from congenital abnormalities or immunological or neurological factors. In 30–40% of male infertility cases, no cause is identified (idiopathic male infertility). Recent findings have shown that the incidence of male infertility has increased due to genetic disorders and pathological apoptosis. Of these, apoptosis has been identified as a major factor contributing to male infertility and has been studied extensively in recent years.
Apoptosis, also known as programmed cell death (PCD) , is required for normal spermatogenesis in mammals and is believed to ensure cellular homeostasis, and an adequate amount of germ cells is eliminated via the process of apoptosis in order to maintain a precise number of germ cell population in compliance with the supportive capacity of the Sertoli cells . Apoptosis is a highly regulated process characterized by distinct changes in the cell morphology including membrane blebbing, shrinkage of cell volume, cytoplasmic vacuolization, nuclear condensation, and DNA fragmentation, followed by the disassembly of the cell into membrane-bound apoptotic bodies. The biochemical features of apoptosis include phosphatidylserine exposure to the external leaflet of the plasma membrane, activation of caspase cascades, DNA cleavage, and DNA laddering. This chapter briefs both physiological and pathological events that trigger apoptosis and their effects on the male reproductive system.
2 Physiological Role of Apoptosis in Male Reproduction
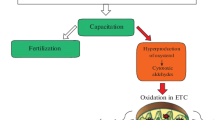
Testes accomplish one of the complex events called spermatogenesis, which is necessary for the propagation of germplasm. Spermatogenesis is a highly dynamic and synchronized process of germ cell maturation from diploid spermatogonia to mature haploid spermatozoa that takes place in the seminiferous epithelium of the testis. This highly intricate cellular development is fostered by the somatic cells, the Sertoli cells , which envelope the germ cells [5]. During testicular development, the Sertoli cell number increases gradually and thereafter their proliferative capacity declines to produce a stable population of nondividing Sertoli cells [6]. On the other hand, the germ cells continuously proliferate and differentiate to become mature spermatozoa. Under normal condition, overproliferation of germ cells is tempered by selective apoptosis of their progeny in order to maintain a precise germ cell population in compliance with the supportive capacity of the Sertoli cells [6]. Apoptosis also occurs as a defense mechanism, such as in immune reactions or when cells are damaged by disease or environmental agents. Necrosis and apoptosis are the two major mechanisms of cell death. Necrosis occurs in cells that are damaged by external injury, whereas apoptosis occurs in cells that are induced to commit programmed death from internal or external stimuli. Apoptosis is broadly divided into an initiation phase followed by a signaling phase and an execution phase in which cells rapidly execute a death program. Apoptosis consists of highly intricate, sophisticated, and energy-dependent cascade mechanisms and occurs through two main pathways (Fig. 37.1). The first, referred to as the extrinsic or cytoplasmic pathway, is triggered through the Fas death receptor, a member of the tumor necrosis factor (TNF) receptor superfamily [7]. The second pathway is the intrinsic or mitochondrial pathway that when stimulated leads to the release of cytochrome c from the mitochondria and activation of downstream death signal [8]. Both the pathways converge into a final common pathway involving the activation of a cascade of proteases called caspases that cleave regulatory and structural molecules, culminating in the death of the cell. The pathways are linked; thus, the distinction between the two pathways is simplistic. Overexpression of antiapoptotic protein Bcl-2 in the intrinsic pathway may lead to the inhibition of extrinsic-mediated apoptosis [9]; conversely, TNFα may increase the expression of NFκB and stimulates antiapoptotic members of the Bcl-2 family proteins. Lindane, an organochlorine pesticide, is known to impair rat testicular functions and fertility through modulation of NFκB and FasL [10].
3 Extrinsic Pathway
Extrinsic pathway comprises several protein members including the death receptors, the membrane-bound Fas ligand, the Fas complexes, the Fas-associated death domain, and caspases 8 and 10, which ultimately activate the rest of the downstream caspases leading to apoptosis. Activation of the extrinsic pathway is initiated with the ligation of cell surface receptors called death receptors (DRs). Fas is a member of the TNF receptor superfamily and is also called Apo-1. Fas signaling plays an important role in apoptosis. The Fas ligand (FasL)-Fas system is mainly recognized for its death-related functions. When a death stimulus triggers the pathway, the membrane-bound FasL interacts with the inactive Fas complexes and forms the death-inducing signaling complex. The Fas death-inducing signaling complex contains the adaptor protein Fas-associated death domain protein and caspases 8 and 10 and leads to activation of caspase 8, which, in turn, can activate the rest of the downstream caspases. Caspase 8 interacts with the intrinsic apoptotic pathway by cleaving Bid (a proapoptotic member of the Bcl-2 family), leading to the subsequent release of cytochrome c [11].
4 Intrinsic Pathway
One of the most important regulators of this pathway is the Bcl-2 family of proteins. The Bcl-2 family includes proapoptotic members such as Bax, Bak, Bad, Bcl-Xs, Bid, Bik, Bim, and Hrk and antiapoptotic members. Bax is a multidomain proapoptotic member of the Bcl-2 family and its deficiency results in a large accumulation of premeiotic germ cells in mature animals and a near complete absence of spermatocytes and mature sperm [12]. Antiapoptotic Bcl-2 members act as repressors of apoptosis by blocking the release of cytochrome c, whereas proapoptotic members act as promoters. Following a death signal, proapoptotic proteins undergo posttranslational modifications that include dephosphorylation and cleavage resulting in their activation and translocation to the mitochondria leading to apoptosis [8]. In response to apoptotic stimuli, the outer mitochondrial membrane becomes permeable, leading to the release of cytochrome c. Once cytochrome c is released into the cytosol, it interacts with Apaf-1, leading to the activation of caspase-9 proenzymes. Active caspase 9 then activates caspase 3, which subsequently activates the rest of the caspase cascade and leads to apoptosis [9].
5 Fas/FasL
Fas is a type I transmembrane receptor protein that belongs to the TNF/nerve growth factor family [13, 14], and Fas ligand (FasL) has been identified as a TNF-related type II transmembrane protein [15]. The binding of the surface protein FasL to the Fas receptor triggers apoptosis in Fas-bearing cells by the activation of various caspases. It is widely accepted that FasL is normally expressed in Sertoli cells, whereas Fas antigen is expressed in the germ cells of rodents and humans. Caspase 8 is involved in the upstream of the apoptosis process [16, 17]. Activation of caspase 8 is followed by activation of caspase 3, which is known as executioner protease in this process [18]. In the rodent testis, apoptosis, induced by FasL, has been suggested to be one of the mechanisms that limits the number of germ cells during normal spermatogenesis or after testicular injuries. Recent studies have also shown that, in the human testis, apoptosis is a conspicuous event during spermatogenesis; Fas–FasL interaction is reportedly involved in the regulation of this event [19, 20].
6 Caspase and Calpain Families
Caspases (cysteinyl aspartate-specific proteases) are aspartic acid-directed cysteine proteases. These proteases are synthesized as precursors that have insignificant catalytic activity. The precursor caspase is converted to the active enzyme by proteolytic processing either by another protease or by autocatalysis and triggered by the binding of cofactors or removal of inhibitors . Caspases share similarities in amino acid sequence, structure, and substrate specificity. They are all expressed as proenzymes (30–50 kDa). They contain three domains and they are the amino terminal domain, a large subunit (~20 kDa), and a small subunit (~10 kDa). During the caspase activation, proteolytic processing occurs between domains, followed by association of the large and small subunits to form a heterodimer [21]. Although the majority of caspases are situated within the cytoplasm, some of the members can be found in the Golgi apparatus (caspase 12) or in association with the mitochondria (caspases 2, 3, and 9) [22, 23]. Caspase 3 is the most important effector caspase. Its activation is important in PCD signaling [24]. Calpains are a superfamily of related proteins, some of which have been shown to function as calcium-dependent cysteine proteases. Calpain also plays an important role in apoptosis and necrosis, and Rojas et al. [25] demonstrated the presence of a calpain–calpastatin system in human spermatozoa.
7 Cytochrome c
Different proapoptotic proteins, such as cytochrome c and Smac/Diablo, that are normally present in the intermembrane space of mitochondria, are released during the early stages of apoptosis. Suppression of the antiapoptotic members or activation of the proapoptotic members of the Bcl-2 family leads to altered mitochondrial membrane permeability resulting in release of cytochrome c into the cytosol. Binding of cytochrome c to Apaf-1 triggers the activation of caspase 9, which then accelerates apoptosis by activating other caspases. In the cytosol, cytochrome c participates in the formation of the apoptosome complex together with its adaptor molecule, Apaf-1, resulting in the recruitment, processing, and activation of procaspase 9 in the presence of adenosine triphosphate [26]. Subsequently, caspase 9 cleaves and activates procaspases 3 and 7; these effector caspases are responsible for the cleavage of various proteins leading to the biochemical and morphological features characteristic of apoptosis [27]. The release of cytochrome c is, therefore, considered a key initiative step in the apoptotic process.
8 Nuclear Factor Kappa B
The classical NFκB transcriptional factors are composed of homodimers or heterodimers of Rel protein, of which p65/p50 heterodimer is the predominant complex, in testicular germ cells [28]. In unstimulated cells, NFκB dimers are sequestered in the cytoplasm by inhibitory kappa B (IκB) protein. Upon exposure to various extracellular signals that leads to phosphorylation and degradation of IκB, free NFκB dimers rapidly translocate to the nucleus, wherein they activate transcription of target genes [29].
9 Spermatogenesis
Spermatogenesis is a dynamic and well-regulated process that involves multiplication, maturation, and differentiation of germ cells resulting in the formation of mature spermatozoa. The process is subdivided into spermatogoniogenesis known as mitotic multiplication of spermatogonia, maturation of spermatocytes, spermiogenesis , and spermiation. Germ cells undergo mitosis to produce primary spermatocytes. The primary spermatocytes enter meiosis to form secondary spermatocytes and proceed through meiosis to produce haploid spermatids. These in turn undergo a complex process of morphological and functional differentiation resulting in the production of mature spermatozoa, which is known as spermiogenesis . In mammalian species, to maintain proper germ cell numbers, apoptosis takes place in the testis [17]. Loss of spermatogenic cells is incurred mostly during maturity of spermatogonia and to a lesser extent during maturation of spermatocytes and spermatid in adult rat testis. The sign of spermatogenesis is started when germ cells differentiate into spermatogonia. While some spermatogonia become self-renewing spermatogonial stem cells, most differentiate into spermatocytes and, at ~10 days after birth in mice and at puberty in man, initiate meiosis and are accompanied by extensive germ cell apoptosis [17].
10 Steroidogenesis
Leydig cells are the principal cells involved in the process of steroidogenesis. Leydig cells secrete androgens, particularly testosterone, which is extremely essential for the initiation and maintenance of spermatogenesis [30]. Any factor affecting the Leydig cell viability, in turn, can interrupt the endocrine regulation of spermatogenesis and consequently affect the reproductive performance. Aroclor 1254, a commercial mixture of polychlorinated biphenyls, brought about a state of oxidative stress in cultured Leydig cells characterized by decline in the levels of enzymatic and nonenzymatic antioxidants accompanied by an elevation in the levels of lipid peroxidation and reactive oxygen species (ROS) . In addition, the activities of steroidogenic enzymes were inhibited at the level of gene expression causing diminished testosterone production [31]. Exposure of rats to a single dose of cadmium (0.20 mg/100 g body weight) inhibited the activities of testicular 3β and 17β-hydroxysteroid dehydrogenase along with reduced expression of StAR protein resulting in lowered serum testosterone levels. The observed effects have been attributed to the excess generation of ROS in the testis resulting from depletion of antioxidant enzymes like superoxide dismutase (SOD) and glutathione peroxidase. Exposure of primary cultured Leydig cells to cadmium at the concentrations of 10 mm caused increased oxidative DNA damage resulting in decreased viability of cells and testosterone secretion [32]. The high levels of corticosterone associated with stress are known to induce apoptosis in Leydig cells . The activation of Fas system, cleavage of procaspase 3, loss of mitochondrial membrane potential, and increased ROS generation are reported to be the possible mechanisms involved in corticosterone-induced Leydig cell death [33]. The decline in testosterone production following toxicant exposure may be in part due to apoptosis of Leydig cells caused by induction of stress by corticosterone.
11 Effect of Environmental Contaminants
Apoptosis of spermatogenic cells is essential for the maintenance of testicular homeostasis, although increased cell death can result in defective spermatogenesis leading to infertility [34]. In the testis, apoptotic death is a common programmed event that reduces 75% of germ cells [35]. However, excessive or inadequate apoptosis of testicular cells results in abnormal spermatogenesis or testicular tumors [36]. Various testicular toxicants have been reported to induce massive germ cell apoptosis indicating that the seminiferous epithelium responds to most of the adverse stimuli by eliminating germ cells through PCD [37]. Recent studies showed that dichlorodiphenyltrichloroethane (DDT) and its metabolite induced apoptosis through either in vitro or in vivo experiments [38, 39]. Song et al. demonstrated that exposure to p, p’-dichlorodiphenyldichloroethylene (DDE), a metabolite of DDT, at over 30 µM dose level showed induction of apoptotic cell death in cultured rat Sertoli cells by inducing mitochondria-mediated apoptotic changes including elevation in reactive oxygen species (ROS) generation, decrease in mitochondrial membrane potential, and release of cytochrome c into the cytosol which could be blocked by N-acetylcysteine, an antioxidant with an elevated ratios of Bax/Bcl-w and Bak/Bcl-w, and cleavages of procaspases 3 and 9 were induced by p, p’-DDE [32]. Metabolite of DDT (p, p’-DDE) at a dose of 30 µM for 24-h exposure could induce apoptosis of Sertoli cells through a FasL-dependent pathway including nuclear translocation of NFκB, increase of the FasL mRNA, and protein expression, which could be blocked by an antioxidant agent N-acetylcysteine. In addition, caspases 3 and 8 were activated by p, p’-DDE treatment in these cells [40]. Ichimura et al. demonstrated the expression and localization of FasL, Fas, and caspase 3 proteins in mouse testis 12 h after the exposure to 4-0.004 mg/g of di(2ethylhexyl) phthalate (DEHP) and correlated the expression of these proteins with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) of the DNA-fragmented nucleus [41]. Immunocytochemical examination of the DEHP-exposed (4 mg/g) mouse revealed a distribution of FasL in Sertoli cell and Fas in nearby spermatocyte and Fas and caspase 3 in the same spermatocyte. Exposure to mono(2-ethylhexyl) phthalate (MEHP), a Sertoli cell-specific toxicant, induced massive germ cell apoptosis associated with increased expression of both the Fas and FasL genes in rat testis. Mono(2-ethylhexyl) phthalate (MEHP), a well-known Sertoli cell toxicant, could decrease the levels of procaspase 8 and increase the levels of procaspase-8 cleavage products in mice testis [42]. β-Benzene hexachloride (BHC), a major metabolite of benzene hexachloride, induced apoptosis by activation of c-Jun N-terminal kinases (JNKs), translocation of NF-κB, expression of FasL, and further activation of caspase cascade [43]. Spontaneous germ cell apoptosis has been observed in several species of mammalian testis. Vaithinathan et al. demonstrated a significant increase in the levels of cytosolic cytochrome c and procaspase 9 as early as 6 h following exposure to a single dose of methoxychlor at 50 mg/kg bodyweight. Time-dependent elevations in the levels of Fas, FasL, and pro- and cleaved caspase 3 demonstrate induction of testicular apoptosis in adult rats following a single dose of methoxychlor [44]. Recent findings reveal that methoxychlor exposure to pregnant female rats from embryonic days 8–15 at the dosage of 100 and 200 mg/kg/day showed an increase in spermatogenic cell apoptosis and decreased sperm number and motility in adult animals of F1 and F2 generation [45, 46]. In another study, oral administration of bisphenol A 480 and 960 mg/kg/day induces apoptosis of Leydig and germ cells in the mouse testis through the Fas signaling pathway [47]. Lindane, a well-known endocrine disruptor, could induce apoptosis of testicular cells by stimulating the mitochondrion-dependent pathway by elevating the levels of cytochrome c with a parallel increase in procaspase 9. A time-dependent elevation of Fas, FasL, and caspase 3 in peritubular germ cells illustrates induction of testicular apoptosis in adult rats following exposure to a single dose of lindane [10].
12 Oxidative Stress
Control of apoptosis may involve various pathways such as the mitochondria-mediated Bcl-2 family, Fas/FasL system that can be engaged by oxidative stress. Reactive oxygen species (ROS) is considered a potential signal for apoptosis. Elevated levels of ROS can cause oxidation of the mitochondrial pores, thereby disrupting the mitochondrial membrane potential and releasing cytochrome c and activating the mitochondria-mediated pathway of apoptosis. In addition, ROS have been shown to induce the expression of Fas receptor and ligand stimulating the Fas/FasL-mediated apoptotic signal transduction pathway. Several environmental disruptors are known to inappropriately activate apoptosis in testicular locale by increasing the levels of ROS [10, 48]. During the transit from undifferentiated germ cells to mature spermatozoa, the sperms are vulnerable to multitudinous threats which are counteracted by the powerful antioxidant defense system of the testis [49]. Many toxicants have been shown to damage this protective shield, thus increasing the susceptibility of this organ to oxidative stress [50, 51]. Experimental studies have demonstrated that exposure to hexachlorocyclohexane (i.p., 20 mg/kg/day) during the critical stages of testicular development induces elevation in the levels of lipid peroxidation and hydrogen peroxide (H2O2), along with reduction in the levels of superoxide dismutase (SOD) , catalase, and ascorbic acid [52]. Doreswamy et al., have demonstrated the induction of oxidative stress, DNA damage, and apoptosis in testis following exposure to multiple doses of nickel chloride [53]. Our earlier studies on various toxicants in rodent models have exemplified the role of oxidative stress in mediating its effects on testis. Oral exposure to lindane (5 mg/kg bodyweight/day) for 30 days resulted in elevated levels of hydrogen peroxide and lipid peroxidation with concomitant decline in the activities of antioxidant and steroidogenic enzymes in testis [54]. Similar impairment of antioxidant system and mitochondria-dependent apoptosis of rat testis has been observed with lindane following single dose of it [10, 55]. Methoxychlor, at dose levels of 50 mg/kg body weight, caused significant diminution in testicular antioxidant enzymes along with Fas–FasL and mitochondria-mediated apoptosis in a time-dependent manner [44]. Compilation of these studies indicates the generation of free radicals and associated oxidative stress as the pathological mechanism underpinning the adverse effects of testicular toxicants. Innumerable studies have disclosed the involvement of oxidative stress in carrying out the malicious role of apoptosis in testis. Most of the toxicants have been reported to perturb the testicular locale either directly or indirectly targeting pivotal constituents of the testis—the germ cells, Sertoli cells, and Leydig cells. A study on the exposure of testis to a single dose (2 g/kg body weight) of di(2-ethylhexyl) phthalate revealed an augmented generation of ROS with simultaneous decrement in the concentrations of glutathione and ascorbic acid leading to selective apoptosis of spermatocytes [56]. Further exploration revealed the accrual of mono(2-ethylhexyl) phthalate, a toxic metabolite of DEHP, in testis causing mitochondrial respiratory damage and release of cytochrome c inciting apoptosis [56]. Exposure of spermatogenic cells to synthetic organic chemical, methyl tert-butylether (MTBE), enervated cell viability and induced generation of ROS and enhanced lipid peroxidation [57]. Similar damaging effects of oxidative stress followed by apoptosis in maturing germ cells have been observed with multifarious array of toxicants including metals. The uninterrupted close association of germ cells with Sertoli cells is yet another obligatory factor in spermatogenesis. Apart from its fostering role, Sertoli cells play a highly remarkable phagocytic role in eliminating spermatogenic cells undergoing apoptosis in response to chemical insult [58]. Consequently, any agent that confronts Sertoli cells may have a profound effect on spermatogenesis. In vitro exposure to β-BHC can enhance ROS and oxidative stress and then induce activation of JNKs and NF-kB, expression of FasL in rat Sertoli cells. Upon ligation of FasL to Fas, an FasL-mediated apoptotic death is stimulated in a target cell leading to the activation of caspase 8. Finally, apoptosis of Sertoli cells is mediated by executioner caspase 3, thereby disturbing the spermatogenic process [43]. Sertoli cells on exposure to an environmental contaminant, nonylphenol (10–40 mm), caused accumulation of ROS within 2 h of exposure which subsequently resulted in the loss of mitochondrial membrane potential and enhanced lipid peroxidation at 12 h posttreatment [59]. In vitro studies on the effects of 4-tert-octylphenol, a degradation product of alkylphenolpolyethoxylate, at a concentration of 30–60 mm for 6–24 h showed a decrease in the viability of Sertoli cells and increased apoptosis via caspase-3 pathway in a concentration- and time-dependent manner [60]. Diverse studies have cumulated over time, which accentuates the feasible role of oxidative stress in Sertoli cell apoptosis in response to toxicants.
13 Mechanisms Involved in Inducing Apoptosis
The possible mechanisms involved in the action of various factors in mediating testicular apoptosis are summed up. Most of the studies involving toxicants illustrate the undoubted role of ROS in executing its detrimental effects [31, 54, 59, 61]. These elevated levels of ROS can cause oxidation of the mitochondrial pores, thereby disrupting the mitochondrial membrane potential and releasing cytochrome c [56, 62]. Once free of the mitochondrial membrane, cytochrome c rapidly assembles a multi-protein complex involving Apaf-1 and procaspase 9 leading to the activation of the caspase 9, which subsequently triggers the effector caspase 3, 6, and/or 7 [26, 27]. These caspases, in turn, activate endonucleases and proteases resulting in DNA fragmentation and degradation of nuclear and cytoskeletal proteins [63, 64]. Apart from ROS, Bax, a member of proapoptotic Bcl-2 family, can directly influence the release of cytochrome c from mitochondria [65]. It is interesting to note that the Bcl-2 protein family is itself regulated by ROS [66]; however, whether this regulation has any role in toxicant-mediated apoptosis is not known. ROS have been shown to induce the expression of Fas receptor and ligand stimulating the Fas/FasL-mediated apoptotic signal transduction pathway [67]. Interaction of Fas with FasL leads to a cascade of events which begins with the proteolytic cleavage of procaspase 8 to its active form, which consequently activates downstream effector caspase 3, 6, or 7 [68,69,70]. These caspases execute the cells by degrading the constituent proteins [71]. Elimination of apoptotic action of Fas by antioxidants further emphasizes the role of ROS in Fas-mediated death process [72, 73]. Therefore, deprivation of antioxidants and/or generation of free radicals by toxicants is capable of reducing the Fas pathway. In addition, they impair steroidogenesis and may deprive germ cell of the essential growth factor, testosterone, and increase their susceptibility to ROS attack [74].
14 Conclusion
The purpose of this chapter was to evaluate the role of various factors influencing apoptosis in male infertility. Mounting evidence suggests that apoptosis occurs as the predominant cell death mechanism in testis in response to several diseases and toxic injuries. Research implies that reactive oxygen species and other secondary free radicals such as nitric oxide and hydroperoxides could be inducers or mediators of apoptosis in testis through downregulation of antioxidant defense system or increased expression of apoptosis-related proteins. However, the exact mechanism of action of apoptosis in inducing male infertility remains a mystery. Further studies are warranted to evaluate the adverse effects of apoptosis on testis.
15 Review Criteria
An extensive literature search probing “the apoptosis and male infertility” was performed using search engines such as ScienceDirect, OVID, Google Scholar, PubMed, and MEDLINE. The start and end dates for these searches were January 2014 to December 2018. The following keywords were used to retrieve the information and the data extraction: “male reproductive health,” “apoptosis,” “extrinsic apoptotic pathway in testis,” “intrinsic apoptotic pathway in testis,” “oxidative stress,” “environmental contaminants,” and “endocrine disruptors.” Articles published in English language were considered and the data that were solely published in conference or meeting proceedings, websites, or books were excluded in writing this review. Websites and book-chapter citations provide conceptual content only.
References
Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am. 2014;41(1):195–204.
Nene UA, Coyaji K, Apte H. Infertility: a label of choice in the case of sexually dysfunctional couples. Patient Educ Couns. 2005;59(3):234–8.
Larsen U. Infertility in Central Africa. Tropical Med Int Health. 2003;8(4):354–67.
Larsen U, Menken J. Individual-level sterility: a new method of estimation with application to sub-Saharan Africa. Demography. 1991;28(2):229–47.
Saez JM, et al. Cell-cell communication in the testis. Horm Res. 1991;36(3-4):104–15.
McLachlan RI, et al. Effects of testosterone on spermatogenic cell populations in the adult rat. Biol Reprod. 1994;51(5):945–55.
Zapata JM, et al. A diverse family of proteins containing tumor necrosis factor receptor-associated factor domains. J Biol Chem. 2001;276(26):24242–52.
Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304(3):437–44.
Reed JC. Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin Hematol. 1997;34(4 Suppl 5):9–19.
Saradha B, Vaithinathan S, Mathur PP. Lindane induces testicular apoptosis in adult Wistar rats through the involvement of Fas-FasL and mitochondria-dependent pathways. Toxicology. 2009;255(3):131–9.
Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296(5573):1635–6.
Knudson CM, et al. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270(5233):96–9.
Watanabe-Fukunaga R, et al. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–7.
Nagata S, Golstein P. The Fas death factor. Science. 1995;267(5203):1449–56.
Suda T, et al. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75(6):1169–78.
Tanaka M, et al. Downregulation of Fas ligand by shedding. Nat Med. 1998;4(1):31–6.
Sinha Hikim AP, et al. Deciphering the pathways of germ cell apoptosis in the testis. J Steroid Biochem Mol Biol. 2003;85(2-5):175–82.
Philchenkov AA. Caspases as regulators of apoptosis and other cell functions. Biochemistry (Mosc). 2003;68(4):365–76.
Pentikainen V, Erkkila K, Dunkel L. Fas regulates germ cell apoptosis in the human testis in vitro. Am J Phys. 1999;276(2 Pt 1):E310–6.
Francavilla S, et al. Fas and Fas ligand expression in fetal and adult human testis with normal or deranged spermatogenesis. J Clin Endocrinol Metab. 2000;85(8):2692–700.
Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–6.
Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6(11):1028–42.
Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16.
Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424.
Rojas FJ, Brush M, Moretti-Rojas I. Calpain-calpastatin: a novel, complete calcium-dependent protease system in human spermatozoa. Mol Hum Reprod. 1999;5(6):520–6.
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6.
Budihardjo I, et al. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90.
Delfino F, Walker WH. Stage-specific nuclear expression of NF-kappaB in mammalian testis. Mol Endocrinol. 1998;12(11):1696–707.
Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18(49):6867–74.
Huleihel M, Lunenfeld E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J Androl. 2004;6(3):259–68.
Murugesan P, et al. Effects of polychlorinated biphenyl (Aroclor 1254) on steroidogenesis and antioxidant system in cultured adult rat Leydig cells. J Endocrinol. 2007;192(2):325–38.
Yang JM, et al. Cadmium-induced damage to primary cultures of rat Leydig cells. Reprod Toxicol. 2003;17(5):553–60.
Gao HB, et al. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Mol Cell Endocrinol. 2003;199(1-2):153–63.
Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. BioEssays. 2000;22(5):423–30.
Allan DJ, Harmon BV, Roberts SA. Spermatogonial apoptosis has three morphologically recognizable phases and shows no circadian rhythm during normal spermatogenesis in the rat. Cell Prolif. 1992;25(3):241–50.
Lin WW, et al. In situ end-labeling of human testicular tissue demonstrates increased apoptosis in conditions of abnormal spermatogenesis. Fertil Steril. 1997;68(6):1065–9.
Blanchard TL, Johnson L. Increased germ cell degeneration and reduced germ cell:Sertoli cell ratio in stallions with low sperm production. Theriogenology. 1997;47(3):665–77.
Tebourbi O, Rhouma KB, Sakly M. DDT induces apoptosis in rat thymocytes. Bull Environ Contam Toxicol. 1998;61(2):216–23.
Perez-Maldonado IN, et al. DDT induces apoptosis in human mononuclear cells in vitro and is associated with increased apoptosis in exposed children. Environ Res. 2004;94(1):38–46.
Shi Y, et al. p, p’-DDE induces apoptosis of rat Sertoli cells via a FasL-dependent pathway. J Biomed Biotechnol. 2009;2009:181282.
Ichimura T, Kawamura M, Mitani A. Co-localized expression of FasL, Fas, Caspase-3 and apoptotic DNA fragmentation in mouse testis after oral exposure to di(2-ethylhexyl)phthalate. Toxicology. 2003;194(1-2):35–42.
Giammona CJ, et al. Death receptor response in rodent testis after mono-(2-ethylhexyl) phthalate exposure. Toxicol Appl Pharmacol. 2002;185(2):119–27.
Shi Y, et al. beta-Benzene hexachloride induces apoptosis of rat Sertoli cells through generation of reactive oxygen species and activation of JNKs and FasL. Environ Toxicol. 2011;26(2):124–35.
Vaithinathan S, Saradha B, Mathur PP. Methoxychlor induces apoptosis via mitochondria- and FasL-mediated pathways in adult rat testis. Chem Biol Interact. 2010;185(2):110–8.
Anway MD, et al. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–9.
Cupp AS, et al. Effect of transient embryonic in vivo exposure to the endocrine disruptor methoxychlor on embryonic and postnatal testis development. J Androl. 2003;24(5):736–45.
Li YJ, et al. Bisphenol a exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci. 2009;108(2):427–36.
Lee J, et al. The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology. 1999;140(2):852–8.
Peltola V, Huhtaniemi I, Ahotupa M. Antioxidant enzyme activity in the maturing rat testis. J Androl. 1992;13(5):450–5.
Abdollahi M, et al. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10(6):RA141–7.
Saradha B, Mathur PP. Effect of environmental contaminants on male reproduction. Environ Toxicol Pharmacol. 2006;21(1):34–41.
Samanta L, Roy A, Chainy GB. Changes in rat testicular antioxidant defence profile as a function of age and its impairment by hexachlorocyclohexane during critical stages of maturation. Andrologia. 1999;31(2):83–90.
Doreswamy K, et al. Nickel-induced oxidative stress in testis of mice: evidence of DNA damage and genotoxic effects. J Androl. 2004;25(6):996–1003.
Sujatha R, et al. Effect of lindane on testicular antioxidant system and steroidogenic enzymes in adult rats. Asian J Androl. 2001;3(2):135–8.
Saradha B, Vaithinathan S, Mathur PP. Lindane alters the levels of HSP70 and clusterin in adult rat testis. Toxicology. 2008;243(1–2):116–23.
Kasahara E, et al. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J. 2002;365(Pt 3):849–56.
Li D, Yin D, Han X. Methyl tert-butyl ether (MTBE)-induced cytotoxicity and oxidative stress in isolated rat spermatogenic cells. J Appl Toxicol. 2007;27(1):10–7.
Tay TW, et al. Phagocytosis plays an important role in clearing dead cells caused by mono(2-ethylhexyl) phthalate administration. Tissue Cell. 2007;39(4):241–6.
Gong Y, Han XD. Nonylphenol-induced oxidative stress and cytotoxicity in testicular Sertoli cells. Reprod Toxicol. 2006;22(4):623–30.
Qian J, et al. Octylphenol induces apoptosis in cultured rat Sertoli cells. Toxicol Lett. 2006;166(2):178–86.
Sen Gupta R, et al. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells. 2004;17(1):132–9.
Zamzami N, et al. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182(2):367–77.
Bortner CD, Oldenburg NB, Cidlowski JA. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995;5(1):21–6.
Kohler C, et al. Protease activation in apoptosis induced by MAL. Exp Cell Res. 1999;249(2):260–8.
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–12.
Tripathi P, Hildeman D. Sensitization of T cells to apoptosis—a role for ROS? Apoptosis. 2004;9(5):515–23.
Krammer PH. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210.
Scaffidi C, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17(6):1675–87.
Sanchez-Gomez MV, et al. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci. 2003;23(29):9519–28.
Henkler F, et al. The extracellular domains of FasL and Fas are sufficient for the formation of supramolecular FasL-Fas clusters of high stability. J Cell Biol. 2005;168(7):1087–98.
Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5(11):897–907.
Um HD, Orenstein JM, Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156(9):3469–77.
Chiba T, et al. Fas-mediated apoptosis is modulated by intracellular glutathione in human T cells. Eur J Immunol. 1996;26(5):1164–9.
Chainy GB, Samantaray S, Samanta L. Testosterone-induced changes in testicular antioxidant system. Andrologia. 1997;29(6):343–9.
Acknowledgments
P. P. Mathur acknowledges the receipt of financial support from the Department of Science and Technology, Government of India, under the projects (1) SP/SO/B-65/99, (2) DST-FIST-2009.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Latchoumycandane, C., Vaithinathan, S., D’Cruz, S.C., Mathur, P.P. (2020). Apoptosis and Male Infertility. In: Parekattil, S., Esteves, S., Agarwal, A. (eds) Male Infertility. Springer, Cham. https://doi.org/10.1007/978-3-030-32300-4_37
Download citation
DOI: https://doi.org/10.1007/978-3-030-32300-4_37
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32299-1
Online ISBN: 978-3-030-32300-4
eBook Packages: MedicineMedicine (R0)