Abstract
Grafting of the male reproductive tract is an exciting new area of tissue engineering which may allow natural conception for patients with significant lengths of obstructed vas deferens. While stents had a significant and important role in increasing patency and pregnancy rates in the pre-microsurgical era, their role in the modern era of microsurgical two-layered anastomosis remains to be defined. To date, if the vasal obstruction is amenable to a primary watertight, tension-free anastomosis, microsurgical non-stented techniques remain the gold standard. Cases where a tension-free anastomosis is not possible because of the physical length of the obstruction remain problematic, but further research into tissue engineering in the form of implantable conduits holds much promise.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Partial vasal agenesis
- Congenital prostatic cysts
- Obstruction of the vas deferens
- Two-layered vasovasostomy
- Microsurgical two-layered anastomosis
- Spontaneous recanalization
- Tuberculous epididymitis
- Gonorrheal urethritis
- Obstructive epididymitis
- Chlamydial epididymitis
- Vas deferens autograft
-
Microsurgical two-layered anastomosis to correct vasal obstruction remains the gold standard.
-
The role of vasal stenting in the era of microsurgical two-layered anastomosis is limited.
-
Biodegradable conduits to bridge segments of obstructed vas deferens where a primary microsurgical two-layered anastomosis is impossible remain investigational but hold significant long-term promise.
1 Introduction
Surgical reconstruction of the vas deferens is performed to remove an obstructive lesion that is present along its course. Obstruction can exist at various parts of the vas deferens and can be the result of a prior vasectomy, congenital anomaly, inflammation secondary to a urogenital tract infection, trauma, or a surgical misadventure during prior inguinal, pelvic, or scrotal surgery. While no official reporting system exists in the United States to monitor the number of vasectomies performed each year, a survey in 2002 estimated this to be 526,501, which is approximately consistent with data reported in 1991 and 1995 [1, 2]. An estimated 2–6% of all men, and up to 11% of men aged 20–24 at the time of vasectomy, request a vasectomy reversal [3]. It has been estimated that between 30,000 and 80,000 vasectomy reversals are performed annually in the United States, though as with vasectomies, reporting requirements are not standardized so the exact number is unknown [4].
Congenital anomalies which can lead to obstruction of the vas deferens include congenital absence of the vas deferens, which is commonly associated with cystic fibrosis [5]. Partial vasal agenesis and congenital prostatic cysts can also lead to obstruction of the vas deferens [6, 7]. One example of a genetic disorder which can lead to obstructive azoospermia is Young’s syndrome, which is characterized by chronic sinusitis and bronchiectasis as well as obstructive azoospermia [8]. In Young’s syndrome , the obstruction usually occurs at the junction of the caput to corpus epididymis due to inspissated secretions. Inflammatory causes of obstruction are rare in the antibiotic era but include tuberculous epididymitis , gonorrheal urethritis progressing to obstructive epididymitis, and chlamydial epididymitis [9].
The success of a vasectomy reversal depends on several factors, only some of which can be controlled at the time of surgery. Factors which are independent of the method of reversal but which may influence subsequent conception include age and fertility potential of the patient’s partner, length of obstructive interval, presence of antisperm antibodies, and high intravasal and epididymal pressure after the original obstruction [10,11,12,13]. Some factors which influence the success of the reversal are directly related to the technique chosen and include rate of stricture or scar development and granuloma formation [14]. The most commonly cited reason for these specific complications is an anastomosis made under tension, devascularization of the wall of the vas deferens, or a technical problem with the anastomosis leading to sperm leakage [15].
The current gold standard to surgically correct an obstructed vas deferens is a microscope-assisted two-layered vasovasostomy, but this has not always been so [16, 17]. Given the complex and time-consuming nature of this operation, new surgical techniques including robotics, modifications, and tools are continuously being explored. Some of these techniques include the use of fibrin glue, laser soldering, absorbable and nonabsorbable stents, and artificial conduits with or without specific growth factors added [18]. This chapter highlights the development of surgical grafting techniques for vasectomy reversal, including the use of stents and grafts, as well as the current clinical application of these devices and areas where further research is required.
2 Grafting Techniques in Reconstruction of the Male Reproductive Tract
2.1 Stents
As previously stated, several factors related to the success of a vasectomy reversal can be controlled at the time of surgery. From the 1950s to the mid-1970s, macrosurgical techniques for vasovasostomy were common and accepted as the gold standard. This technique allowed a primary anastomosis of the vas deferens to be created but was plagued with, by today’s standards, low patency and pregnancy rates. According to a survey of the American Urological Association (AUA) members published in 1973, members at that time practicing vasovasostomy reported a 38% patency rate and a 19.5% pregnancy rate [19]. It should be remembered that the microsurgical techniques which are common today were not developed until the mid-1970s, and as such, stricture resulting in either partial or complete vasal obstruction was the most pressing technical complication of that period. To address this common complication, approximately 90% of urologists performing vasovasostomies at that time employed stents, with either silver wire or nylon suture being the most commonly reported [19]. The reason for the widespread use of stents can also be found in the 1973 AUA survey. Members reported that the pregnancy rate for non-stented reversals was significantly lower, at 10.9% compared to 19.9–26% for stented procedures, depending on the stent used. Numerous techniques had been developed in 1973 which maximized both patency and pregnancy rates. The main variation between these techniques was the use of loupes for magnification and/or the use of stent [20].
In the lexicon of the modern urologist, a stent most commonly refers to the hollow silicon tube that is used in the ureter for treatment of either intrinsic or extrinsic ureteral obstruction. A vasovasostomy stent, as it was originally used, was quite different. A stent in that sense was any foreign body, usually a piece of suture, the purpose of which was to maintain patency of the lumen of the vas deferens during and immediately after a macrosurgical (either with or without the supplemental use of loupes) primary anastomosis of the vas deferens. The simple goal of the stent was to prevent obstruction at the anastomosis site either because of a poorly placed suture at the time of surgery or as prevention of stricture or scar formation in the immediate postoperative period. In one example of this technique, a short section of 2-0 nylon suture is used as a stent to bridge the anastomosis, while 6-0 Prolene is used to actually complete the anastomosis [21]. In this technique, the nylon suture is removed before the operation is completed, and its purpose is to ensure that the vas deferens lumen remains patent during the procedure. In another variation on this theme, described by Dorsey, a zero monofilament suture is fed through a hollow needle introduced approximately 1 cm proximal to the site of the intended anastomosis [22]. This suture is then fed into the distal vas deferens. The anastomosis is then completed using 6–0 Ethiflex, and the proximal end of the stenting suture is brought through the scrotal skin and removed in 12–14 days. The goal of this stent in this technique is to ensure patency of the anastomotic site both during the procedure and in the immediate postoperative healing period. The success rates of these procedures were reported to be over 80% patency, which contrasts with the success rates reported in the 1973 AUA survey.
Even with the improvement in both patency and pregnancy rates reported by clinicians using stents during vasovasostomy, there were numerous known disadvantages of stents, especially with the use of exteriorized stents such as described by Dorsey [20]. The point of exit for the stent is a theoretical source of infection as well as a location where sperm can leave the lumen of the vas [20, 23]. Additionally, the location where the exteriorized stent left the lumen of the vas was identified in a publication by Fernandes as a common site of subsequent luminal obstruction (often instead of the primary anastomosis itself) [24]. To avoid the problem of an exteriorized stent, some groups have experimented with absorbable intravasal suture as a stent to bridge the anastomosis—the theoretical advantage being that these stents would slowly dissolve, maintaining the patency of the anastomosis both during the procedure itself and the postoperative healing period without need to be removed. In one experiment in a canine model, Montie et al. compared three groups: no stent, a Dexon intravasal stent, and a chromic intravasal stent [23]. Three to six months after the vasectomy reversal procedure, retrograde vasography was used to identify patency rates. Both absorbable stent groups had higher patency rates than the control (no stent) group, with the chromic group having the highest overall patency at 70% vs. 60% for the Dexon group and 50% for the no stent group. This concept was tested in a human clinical model by Rowland and colleagues a few years later, who found that intravasal absorbable stents (using 3-0 chromic) had higher patency rates than a group using exteriorized silkworm gut stents (86% vs. 67%, respectively) [25].
In 1975, Silber reported the first use of microsurgical vasovasostomy in humans [26]. His work, along with independent work by Owen, led the way to the modern microsurgical two-layered anastomosis [26, 27]. From a historical standpoint, it should be noted that it was Silber and his group who found through histologic and electron microscopic work that stricture was more common than originally thought with macrosurgical anastomosis techniques [28]. Silber also popularized the two-layered closure based on his observation that this technique provided a better watertight mucosal approximation given the common discrepancies between proximal and distal luminal diameters of the vas deferens [29]. The techniques developed by these investigators have allowed the microsurgical two-layered vasovasostomy anastomosis to become the gold standard for vasectomy reversal, with success rates dependent on time since obstruction. These success rates may be as high as 97% patency and 76% pregnancy when the obstructive interval is less than 3 years and 71% patency and 30% pregnancy after 15 years of obstruction [13].
The reproducible success of this new technique resulted in a dearth of research into alternative techniques for many years. Even with its success, Silber’s microsurgical technique was not perfect. The downside was that microsurgical anastomosis was a time-consuming operation best done by surgeons with specialized training using expensive operating microscopes. This prompted research into new techniques that would simplify the technique while maintaining the high patency and pregnancy rates. In 1989, Flam et al. reported work in a rat model on a hollow, absorbable polyglycolic acid tube [30]. In their experiment, they inserted a 10-mm-long by 0.5-mm outer diameter hollow stent into the lumen of the vas at the site of anastomosis on one side and completed the anastomosis with a single layer of suture (Fig. 15.1). On the contralateral side, they performed standard microsurgical anastomosis. They showed a trend toward improved patency in the stented vas deferens. Flam emphasized in his paper that sperm leakage at the site of the anastomosis could lead to secondary stricture and should be avoided. This work led to a clinical trial using absorbable intravasal stents conducted by Rothman and colleagues in 1996 [31]. This randomized study compared conventional two-layered microsurgical anastomosis to a modified approach using an absorbable polyglycolic acid stent without intraluminal sutures (Fig. 15.2). While the operative time was significantly reduced in the stented group (118 min vs. 137 min), both the patency and the pregnancy rates were lower in the stented groups (81% vs. 89% and 22% vs. 51%, respectively), and the authors concluded that intravasal stents should not be used.
Hollow polyglycolic acid stent (0.5-mm outer diameter) shown on a dime. (Used with permission from Flam et al. [30]. With permission from Elsevier)
More recently, Vrijhof et al. reported on a nonabsorbable stent in a rabbit model [14]. They theorized that the absorbable nature of the previously reported stents allowed strictures to develop at the site of the anastomosis once the stent had dissolved and that a nonreactive nonabsorbable stent would bypass this problem while simplifying the operation. Their stent was made of a biocompatible material designed to have both hydrophilic and hydrophobic characteristics. The stent also had a transverse ridge which was designed to minimize migration from the anastomotic site (Fig. 15.3). This group reported that all vasa were patent at the end of their study (39–47 weeks) and that total sperm count was higher in the stented group. No human data is available on this type of stent.
Nonabsorbable polymeric stent with transverse ridge to minimize migration. (Reprinted from Vrijhof et al. [14]. With permission from Elsevier)
In the era of cost-conscious medicine, especially when many patients must pay out of pocket for vasectomy reversals, further research into efforts that simplify the present gold standard is appropriate with the caveat that patency and pregnancy rates should not be compromised. It is important to note that all of the absorbable and nonabsorbable stents which have been used to date in human studies have been well tolerated with no side effects and little to no inflammatory response.
In summary, stents were investigated as a method to improve patency rates in the era of macrosurgical vasovasostomy but were eclipsed by the application of the operating microscope to the field and the introduction of microsurgical two-layered vasovasostomy. Efforts to improve and simplify the microsurgical operation using absorbable stents have not improved overall patency or pregnancy rates. Recent efforts using nonabsorbable stents show promise in animal models but have not been tested in humans so their utility remains unproven. The ideal stent would, at a minimum, maintain the patency and pregnancy rates achieved through a conventional two-layered microsurgical anastomosis while decreasing the operative time, training, and cost required to achieve these results.
2.2 Conduits
The preferred method to bypass an obstructed portion of the vas deferens, regardless of the etiology of the obstruction, is surgical excision or exclusion of the obstructed segment and reanastomosis of the vas deferens using a microsurgical two-layered anastomosis . The goal of the operation is a watertight, tension-free, widely patent anastomosis. As described previously, numerous techniques have been suggested in an attempt to simplify this procedure while preserving its patency and pregnancy rates. The assumption in all of the previously described techniques is that the vas deferens could be sufficiently mobilized to allow a tension-free anastomosis. Unfortunately, cases exist where, due to the physical length of the obstruction, the vas deferens cannot be reconstructed in a watertight, tension-free manner. These cases present a clinical challenge because the resulting obstructive azoospermia is theoretically amenable to surgical correction. Presently, the only reproductive option available for these patients is surgical sperm retrieval. The technique of retrieving sperm from either the testicle or epididymis has been successfully reported in cases of obstructive azoospermia that is not surgically correctable but must be coupled with in vitro fertilization [32]. The hormonal manipulation, surgical interventions, risk of multiple gestations, and increased financial cost of in vitro fertilization make this solution less than ideal and create an intriguing field of research into reconstruction of the male reproductive tract.
Grafting of the male reproductive tract theoretically can take one of three forms. The first option is to use transplanted vas deferens with all of the complications, both technical and immunological, associated with such a procedure. The second option is to replace the obstructed segment of vas deferens with a tubular structure, the sole purpose of which is to simply allow passage of sperm in a distal direction. An analogous clinical problem can be found in vascular surgery where surgeons often replace diseased segments of vessels with either endogenous grafts such as the long saphenous vein or exogenous grafts such as a Teflon-coated endovascular stent. The third option involves tissue engineering. Tissue engineering as it applies to reconstruction of the male reproductive system involves the concept of creating an artificial conduit which serves as a scaffolding for the regrowth of the vas deferens itself. In a different biological system, polymer scaffoldings have been shown to facilitate peripheral nerve regeneration in segments as long as 1 cm [33]. Regardless of the method chosen to graft over the obstructed segment of vas deferens, the goal is to reestablish continuity of the male reproductive tract, allowing sperm to be present in the ejaculate and eliminating the need for assisted reproductive techniques (ART). It should be noted that even small amounts of ejaculated sperm could be a significant improvement, as this may allow for less invasive forms of ART [34].
The first reported experiment on the use of grafts in reconstruction of the male reproductive tract was by Romero-Maroto and colleagues in 1989 [35]. This group reported successfully autotransplanting a pediculated segment of vas deferens from one side to the contralateral in rabbits. They reported good patency rates, but no data on pregnancies was noted. The clinical use of this technique is likely limited, as these subjects would likely be candidates for crossover vasovasostomies, a rare procedure with a high reported success rate [36], and given the questionable feasibility of harvesting a long vasal segment for reconstruction of the contralateral side.
Regarding the second option for grafting the male reproductive tract, Carringer et al. in 1995 reported patency rates in rats after either a vasal or vascular graft obtained from either the contralateral side of the same animal or from female rats, respectively [37]. In this study, three different lengths of grafts were used (0.5, 1.0, and 1.5 cm), corresponding to approximately 10%, 20%, and 30% of the entire vas deferens length. Patency was confirmed by direct examination of the graft 4 weeks postoperatively. The authors found an overall patency rate of approximately 40% in both surgical groups (vasal and vascular graft) with higher rates in the shorter segments. Pregnancy rates were not evaluated. No human clinical trials have been reported using either of these techniques. Questions on long-term patency of extensive artificial grafts remain unanswered, even in animal models, and should be further investigated.
The lack of a suitable allograft in humans for vasal reconstruction has led to research on the potential for biocompatible degradable polymer scaffolding for tissue engineering. As mentioned earlier, this model has been successfully applied to the clinical problem of peripheral nerve regeneration [33]. Additions to this technology, including micro-patterned (grooved) inner lumens as well as target-specific growth factors, can increase the efficacy of this technology [38, 39]. The vas deferens is a good target for investigation because it has been shown to undergo spontaneous recanalization at the site of vasectomy [40].
Further evidence that may support tissue engineering of the vas deferens is the demonstration of elevated levels of selected growth factors at the vasectomy site in an animal model. Previous examination of vasectomy sites in rats using real-time polymerase chain reaction, enzyme-linked immunosorbent assay, and histopathological analysis demonstrated a 12-fold increase in platelet-derived growth factor beta and a ninefold increase in transforming growth factor beta [41].
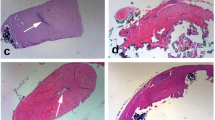
Using the peripheral nerve regeneration model as a guide, biodegradable conduits made of d,l-lactide were studied for reconstruction of the reproductive tract in a rat model [42]. Biodegradable conduits with micro-patterned grooves on the inner surface were implanted in 47 rats following vasectomy (Fig. 15.4, scanning electron microscopy image). At 8 weeks postimplantation of the conduits, no evidence of recanalization was found. However, at 12 weeks, evidence of recanalization was noted in three of the remaining rats, with one showing a microcanal spanning the entire 0.5-cm conduit and the other two showing distinct epithelialized vas deferens microcanals at the conduit edges (Fig. 15.5) [42].
Scanning electron microscope image of PDLA conduit . Bar = 200 μm. (Reprinted from Simons et al. [42]. With permission from Creative Commons License 4.0: https://creativecommons.org/licenses/by-nc-sa/4.0/)
(a) Evidence of microrecanalization at the midpoint of a 0.5-cm poly-(d,l-lactide) (PDLA) graft (magnification ×40). Bar = 1 mm. (b) Microcanal at the midpoint of a 0.5-cm PDLA graft (magnification ×200) Bar = 0.5 mm. (c) Microcanal at the interface zone of a 0.5-cm PDLA graft (magnification ×40). Bar = 1 mm. All panels: white arrows microcanals, black arrows graft. (Reprinted from Simons et al. [42]. With permission from Creative Commons License 4.0: https://creativecommons.org/licenses/by-nc-sa/4.0/)
Following the demonstration of microrecanalization of the vas deferens in this biodegradable graft model, attempts were made to identify ways to maximize this response (unpublished data). Based on the identification of elevated growth factor levels at the site of vasectomy, the effect of local microparticle-delivered growth factors on the rate of vasal recanalization in a biodegradable conduit model was examined. Delivering growth factors to a specific location in the body over a sustained period of time is not a simple task. Effective supplementation of growth factors selectively at the site of the grafted vas deferens may be compromised by the fact that the ability of growth factors to perform their function depends on their tertiary structure, which is susceptible to degradation if it is not protected from the local environment. Thus, delivery of a locally sustained concentration of growth factors requires the use of microspheres. The goal of a microsphere is to sequester the biologically active molecule and allow a controlled, sustained release of the molecule. The exact timing of the sustained release is a function of the characteristics of the microsphere into which it is placed. With these considerations in mind, a poly-(d,l-lactide) material was chosen for construction of the microspheres. As the biodegradable conduits used in this study were constructed of the identical material, noncovalent binding was assumed to keep the microspheres near the conduit. Reconstruction of surgically induced vasal gaps using biodegradable conduits soaked in microspheres containing TGF-beta and PDGF revealed an increase in the number of new microcanals in the graft but not in their length at 12 weeks postoperatively.
In an effort to further optimize the conditions for vasal recanalization, methods to increase the vascularity of the reconstructed vas deferens were investigated, based on an observation suggesting that neovascularization increased with time at the conduit to vasal border (unpublished data). To bolster this neovascularization and potentially increase the rate of recanalization, the effect of oral sildenafil citrate on recanalization in the biodegradable graft model was examined. Sildenafil citrate is a type 5 phosphodiesterase inhibitor that has been shown to promote neovascularization in other systems [43]. Rats received a daily dose of 5 mg/kg of oral sildenafil citrate following reconstruction of the vas deferens with a biodegradable graft. At 16 weeks, the rats on sildenafil citrate had a significantly increased number of microcanals (29 vs. 4) though the average length of the canals was constant at 2 mm. This observation was confirmed by an increase in staining for CD31, an endothelial marker. An ongoing study involves combining both oral sildenafil citrate with increasing the local concentration of TGF-beta and platelet-derived growth factors via microspheres. Areas of future research into this field include examining different substrates of which the conduit itself is composed and embedding the growth factors directly into the conduit to maximize local concentration.
2.3 Autografts
In addition to the earlier studies of allografts, vascular autografts, and vascularized (pediculated) vasal autografts for vasal reconstruction, more recent research has been conducted on the possible utilization of non-vascularized vas deferens autografts.
Kadioglu et al. [44] evaluated the possible utilization of a non-vascularized vas deferens autograft in a rat model. In this study, segments of isolated vas deferens, 2.5 cm in length, were used as bilateral autografts in 15 rats. Each autograft was implanted between the two transected ends of vas deferens using end-to-end anastomosis. Unlike the earlier study by Carringer [37], this study also assessed pregnancy outcomes. Fertility, sperm motility, and graft survival were evaluated and compared with a control group. After 3 months, 9 of the 15 (60%) rats were able to breed successfully and 24 (80%) vas grafts were patent and viable. Large granulomata were reported to be present at the proximal anastomosis sites in six (20%) autografts that failed. Unilateral minimal fluid leakage was reported in six (20%) of the proximal (testicular end) anastomosis sites in those rats that were able to breed. On semen analysis, forward motility was noted in 76% of sperm in the experimental group, compared to 78% in the control group (p > 0.05). The authors of this study concluded that vas deferens autograft can be successfully performed in a rat model with subsequent breeding capability [44].
However, these results were contradicted by a study by Nasir et al. that compared different autogenous graft materials for reconstruction of large segment vas deferens defect in a rat model. In this study, vas deferens, artery, and vein grafts were used to reconstruct 30% and 50% defects of the total vas deferens length. No patency was found in any of the grafts [45].
In addition to avoiding immunological difficulties, the utilization of isolated, non-vascularized vas deferens autografts may also allow more flexibility in the possible location of the implantation site along the reproductive tract, which might be limited when vascularized (pediculated) vasal grafts are used. More research would be required regarding the possible implementation of this technique for the reconstruction of long vas deferens defects.
3 Conclusions
Grafting of the male reproductive tract is an exciting new area of tissue engineering which may allow natural conception for patients with significant lengths of obstructed vas deferens. While stents had a significant and important role in increasing patency and pregnancy rates in the pre-microsurgical era, their role in the modern era of microsurgical two-layered anastomosis remains to be defined. To date, if the vasal obstruction is amenable to a primary watertight, tension-free anastomosis, microsurgical non-stented techniques remain the gold standard. Cases where a tension-free anastomosis is not possible because of the physical length of the obstruction remain problematic, but further research into autografts and tissue engineering in the form of implantable conduits holds much promise.
4 Review Criteria
An extensive search of studies examining grafting techniques for vasectomy reversal was performed using search engines such as ScienceDirect, OVID, Google Scholar, PubMed, and MEDLINE. The start and end dates for these searches were 1973 and January 2019, respectively. The overall strategy for study identification and data extraction was based on the following keywords: “grafting techniques,” “obstruction of vas deferens,” “vasectomy reversal,” “epididymal obstruction,” “partial vasal agenesis,” and “vas deferens autograft.” Articles published in languages other than English were also considered. Data that were solely published in conference or meeting proceedings, websites, or books were not included. Websites and book chapter citations provide conceptual content only.
References
Barone MA, Hutchinson PL, Johnson CH, et al. Vasectomy in the United States, 2002. J Urol. 2006;176:232–6.
Magnani RJ, Haws JM, Morgan GT, et al. Vasectomy in the United States, 1991 and 1995. Am J Public Health. 1999;89:92–4.
Holman CD, Wisniewski ZS, Semmens JB, et al. Population-based outcomes after 28,246 in-hospital vasectomies and 1,902 vasovasostomies in Western Australia. BJU Int. 2000;86:1043–9.
Schiff J, Li PS, Goldstein M. Toward a sutureless vasovasostomy: use of biomaterials and surgical sealants in a rodent vasovasostomy model. J Urol. 2004;172:1192–5.
Castaldo G, Tomaiuolo R, Vanacore B, et al. Phenotypic discordance in three siblings affected by atypical cystic fibrosis with the F508del/D614G genotype. J Cyst Fibros. 2006;5:193–5.
Stricker H, Kunin J, Faerber G. Congenital prostatic cyst causing ejaculatory duct obstruction: management by transrectal cyst aspiration. J Urol. 1993;149:1141–3.
Engin G, Kadioglu A, Orhan I, et al. Transrectal US and endorectal MR imaging in partial and complete obstruction of the seminal duct system. A comparative study. Acta Radiol. 2000;41:288–95.
Handelsman DJ, Conway AJ, Boylan LM, et al. Young’s syndrome. Obstructive azoospermia and chronic sinopulmonary infections. N Engl J Med. 1984;310(1):3–9.
Thomas AH, Sabanegh ES Jr. Microsurgical treatment of male infertility. In: Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the male. 4th ed. New York: Cambridge University; 2009. p. 392–406. A very well written summary of current microsurgical surgical techniques.
Kolettis PN, Woo L, Sandlow JI. Outcomes of vasectomy reversal performed for men with the same female partners. Urology. 2003;61:1221–3.
Silber SJ. Microscopic vasectomy reversal. Fertil Steril. 1977;28:1191–202.
Vrijhof HJ, Delaere KP. Vasovasostomy results in 66 patients related to obstructive intervals and serum agglutinin titres. Urol Int. 1994;53:143–6.
Belker AM, Thomas AJ Jr, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol. 1991;145:505–11.
Vrijhof EJ, de Bruine A, Zwinderman A, et al. New nonabsorbable stent versus a microsurgical procedure for vasectomy reversal: evaluating tissue reactions at the anastomosis in rabbits. Fertil Steril. 2005;84:743–8.
Carbone DJ Jr, Shah A, Thomas AJ Jr, et al. Partial obstruction, not antisperm antibodies, causing infertility after vasovasostomy. J Urol. 1998;159:827–30.
Practice Committee of the American Society for Reproductive Medicine. Vasectomy reversal. Fertil Steril. 2008;90:S78–82.
Lipshultz LI, Rumohr JA, Bennett RC. Techniques for vasectomy reversal. Urol Clin North Am. 2009;36:375–82. The most recent definitive treatise on surgical techniques.
Kolettis PN. Restructuring reconstructive techniques—advances in reconstructive techniques. Urol Clin North Am. 2008;35:229–34.
Derrick FC Jr, Yarbrough W, D’Agostino J. Vasovasostomy: results of questionnaire of members of the American Urological Association. J Urol. 1973;110:556–7.
Kim HH, Goldstein M. History of vasectomy reversal. Urol Clin North Am. 2009;36:359–73. A concise and fascinating summary of the history of the vasectomy reversal.
Amelar RD, Dubin L. Vasectomy reversal. J Urol. 1979;121:547–50.
Dorsey JW. Surgical correction of post-vasectomy sterility. J Urol. 1973;110:554–5.
Montie JE, Stewart BH, Levin HS. Intravasal stents for vasovasostomy in canine subjects. Fertil Steril. 1973;24:877–83.
Fernandes M, Shah KN, Draper JW. Vasovasostomy: improved microsurgical technique. J Urol. 1968;100:763–6.
Rowland R, Nanninga JB, O’Connor VJ. Improved results in vasovasostomies using internal plain catgut stents. Urology. 1977;10:260–2.
Silber SJ. Microsurgery in clinical urology. Urology. 1975;6:150–3.
Owen ER. Microsurgical vasovasostomy: a reliable vasectomy reversal. Aust N Z J Surg. 1977;47:305–9.
Silber SJ, Galle J, Friend D. Microscopic vasovasostomy and spermatogenesis. J Urol. 1977;117:299–302.
Silber SJ. Vasectomy and vasectomy reversal. Fertil Steril. 1978;29:125–40.
Flam TA, Roth RA, Silverman ML, et al. Experimental study of hollow, absorbable polyglycolic acid tube as stent for vasovasostomy. Urology. 1989;33:490–4.
Rothman I, Berger RE, Cummings P, et al. Randomized clinical trial of an absorbable stent for vasectomy reversal. J Urol. 1997;157:1697–700.
Nudell DM, Conaghan J, Pedersen RA, et al. The mini-micro-epididymal sperm aspiration for sperm retrieval: a study of urological outcomes. Hum Reprod. 1998;13:1260–5.
Miller C, Shanks H, Witt A, et al. Oriented Schwann cell growth on micropatterned biodegradable polymer substrates. Biomaterials. 2001;22:1263–9.
Kamischke A, Nieschlag E. Analysis of medical treatment of male infertility. Hum Reprod. 1999;14(Suppl 1):1–23.
Romero-Maroto J, Escribano G, Egea L, et al. Transplant of a pediculate segment of vas deferens. Experimental study. Eur Urol. 1989;16:133–7.
Gilis J, Borovikov AM. Treatment of vas deferens large defects. Int Urol Nephrol. 1989;21:627–34.
Carringer M, Pedersen J, Schnurer LB. Experimental vas replacement by either vas or a vascular graft. Scan J Urol Neprhol. 1995;29:97–102.
Rutkowski GE, Miller CA, Jeftinija S, et al. Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration. J Neural Eng. 2004;1:151–7.
Miller C, Jeftinija S, Mallapragada S. Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 2002;8:367–78.
Labrecque M, Hays M, Chen-Mok M, et al. Frequency and patterns of early recanalization after vasectomy. BMC Urol. 2006;6:25.
Stahl BC, Ratliff TL, De Young BR, et al. Involvement of growth factors in the process of post-vasectomy micro-recanalization. J Urol. 2008;179:376–80.
Simons CM, De Young BR, Griffith TS, et al. Early microrecanalization of vas deferens following biodegradable graft implantation in bilaterally vasectomized rats. Asian J Urol. 2009;11:373–8.
Koneru S, Varma Penumathsa S, Thirunavukkarasu M, et al. Sildenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: role of VEGF/angiopoietin-1. J Cell Mol Med. 2008;12:2651–64.
Kadioglu CK, Temple-Smith PD, Southwick G. Interpositional substitution of free vas deferens segment autografts in rat: feasibility and potential implications. BMC Urol. 2014;14:61.
Nasir S, Soyupek S, Altuntas S, Konas E, Roach EC, Özorak A, Bircan S. Comparison of different autogenous graft materials for reconstruction of large segment vas deferens defect: experimental study in rat. Urol J. 2014;11(2):1457–64.
Acknowledgment
We would like to thank Kris Greiner for her editorial assistance in preparing this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rosevear, H.M., Wald, M. (2020). Grafting Techniques for Vasectomy Reversal. In: Parekattil, S., Esteves, S., Agarwal, A. (eds) Male Infertility. Springer, Cham. https://doi.org/10.1007/978-3-030-32300-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-32300-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32299-1
Online ISBN: 978-3-030-32300-4
eBook Packages: MedicineMedicine (R0)