Abstract
Pantepui is an archipelago of sky islands formed by the flat summits of the Neotropical Guiana table mountains (tepuis) situated between the Orinoco and Amazon basins. Pantepui is a virtually pristine land and a natural laboratory to study the origin and evolution of Neotropical biodiversity. This review aims to synthesize the existing biological knowledge of Pantepui, with an emphasis on the latest developments in biogeographical, ecological and evolutionary studies. Biogeographically, Pantepui is a province of the Guiana region, within the Neotropical realm, but the precise definition of this province varies according to the taxonomic group studied. Here we adopt a definition based on elevation, with a diffuse lower boundary at 1200–1500 m and an upper boundary at the uppermost elevations of the Guiana Highlands (ca. 3000 m). The biodiversity and endemism patterns of Pantepui are outstanding. With almost 2600 known species (>5000 species/10,000 km2), plants are the most diverse organisms and situate Pantepui among the most diverse regions of the world. Endemism usually ranges from 30 to 40% but may reach 55% in amphibians. Ecology is poorly known. Autecological studies are lacking, and community studies are available only for vegetation and solely in descriptive terms. Paleoecological studies have shown that plant communities have changed through time under the action of Holocene climatic changes and fire. Glacial-interglacial alternation has deeply modified the Pantepui biota and this biogeographical unit has been recurrently disassembled during glaciations and reassembled during interglacials. The origin and evolution of the Pantepui biota has been explained by diverse evolutionary processes involving a variety of environmental drivers and diversification mechanisms. Most of these hypotheses emerged from the study of extant biogeographical patterns and geological-paleoecological reconstructions. The inception of molecular phylogenetics, albeit still incipient in Pantepui, has provided evidence useful for testing these proposals. Taken individually, none of the proposed hypotheses can explain the evolution of the whole Pantepui biota, whose proper understanding requires complex thinking and the consideration of multiple drivers and a diversity of ecological and evolutionary processes and mechanisms acting together across spatiotemporal scales. Pantepui pristinity could be threatened by direct human disturbance and global warming. Preliminary estimates suggest that, under the worst warming scenario, >80% (including >50% of endemics) of the unique vascular flora could lose their habitat by the end of this century. In situ conservation actions are difficult to implement, and ex situ strategies (germplasm banks, botanical gardens, managed relocation) should thus be considered. More systematic and target-focused, rather than exploratory, approaches are needed for future research on Pantepui. International cooperation and the improvement of bureaucratic facilities are required to preserve the still-pristine Pantepui biota and ecosystems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biogeography
- Climate change
- Conservation
- Ecology
- Evolution
- Glacial cycles
- Global warming
- Guiana highlands
- Neogene
- Paleoecology
- Quaternary
1 Introduction
Pantepui is one of the few pristine areas remaining in the world and constitutes a unique laboratory for studying the origin and evolution of tropical biodiversity (Rull 2010). Pantepui is located on the Guiana Highlands, one of the most spectacular Neotropical landscapes, characterized by remote table mountains (the tepuis) with flat and largely inaccessible summits (Fig. 15.1) that form an archipelago of sky islands (McCormack et al. 2009) of approximately 1500–3000 m in elevation and from <1 to approximately 1000 km2 in surface area, separated from the surrounding lowlands and uplands by vertical cliffs up to 1000 m high (Huber 1995a) (Fig. 15.1). The assemblage of these tepui summits is known as Pantepui (from the Greek pan, meaning “all”, and the local Pemon word tepui, meaning “table mountain”). Pantepui pristinity is guaranteed by its remoteness, difficulty of access due to the complex topography and the lack of natural resources to exploit, as well as the fact that indigenous peoples inhabiting the surrounding uplands and lowlands do not climb to these summits because of religious constraints (Huber 1995d). The unique features of the Pantepui biota and the communities it forms, together with the amazing levels of biodiversity and endemism, are the basis for the definition of the Pantepui biogeographical province (Berry et al. 1995a).
The Guiana Highlands landscape and examples of tepuis (see Fig. 15.2 for location). (1) Roraima-Kukenán massif; from left to right: Kukenán-tepui (summit area of 20 km2 and 2650 m of maximum elevation), Roraima-tepui (35 km2 and 2720 m) and Wei-Assipu-tepui (3 km2 and 2260 m). (2) Vertical cliffs of the Chimantá massif. (3) General view of the NE sector of the Chimantá massif. (4) Tirepón-tepui (2600 m) in the Chimantá massif. (5) Gran Sabana uplands with Angasima-tepui (2 km2 and 2250 m) (left) and the Upuigma-tepui (>1 km2 and 2100 m) (right) in the background. (6) Landing at the Eruoda-tepui summit in the Chimantá massif. (7) Angasima-tepui. (8) Ilú-Tramen massif (5.6 km2 and 2700 m): Tramen-tepui (left), Ilú-tepui (right) and the Karaurín-tepui (2 km2 and 2500 m) in the front. (9) Upuigma-tepui. (10) Los Testigos massif. From left to right: Aparamán-tepui (1 km2 and 2100 m), Murisipán-tepui (5 km2 and 2350 m), Tereké-yurén-tepui (<1 km2 and 1900 m) and Kamarkawarai-tepui (5 km2 and 2400 m). Photos: V. Rull
The scientific exploration of the tepuis began in the mid-nineteenth century, when the German brothers Robert and Richard Schomburgk collected plant and animal specimens on the southern slopes of the Roraima-tepui, between 1838 and 1842. Approximately 40 years later (1881–1883), British ornithologist Henry Whitely explored the upper slopes of the Roraima-Kukenán massif but did not reach its summits. Only a few years later (1884 and 1898), British botanist Everard im Thurn and his colleagues climbed to the summit of the Roraima-tepui (Fig. 15.1) and collected the first rare plants and animals from this hitherto new and strange life zone (Huber 1995b). The oddity of the biological specimens collected during these first expeditions suggested that the tepui summits were a separate world different from what was known at that time and inspired the famous Arthur Conan Doyle’s fantastic novel entitled “The Lost World” (Doyle 1912). The first scientific explorers managed to access some tepui summits by foot after long and difficult trips but this was only possible—and still is today—on a few of these table mountains. The exploration of Pantepui underwent a decisive bourgeoning after the Second World War, with the use of helicopters, which are still the preferred—and in many cases, the only—means to reach the tepui summits. A detailed account of the history of Pantepui scientific exploration can be found in Huber (1995b).
With time and the intensification of scientific exploration of the tepui summits, the perception of the rarity of the Pantepui biota has decreased, but its uniqueness still captivates the most experienced researchers and has contributed to the progress of general disciplines, notably biogeography and evolution (Rull 2010). Unfortunately, the ecological study of Pantepui is still embryonic, likely because of the difficulty in conducting regular and extended field studies atop the tepuis. This is due in part to the remoteness and inaccessibility of the tepui summits in addition to the permanent problems in regard to obtaining official fieldtrip permits for scientific collection, especially for genetic studies (Rull and Vegas-Vilarrúbia 2008). This not only hinders the progress of ecological and evolutionary study but also delays the adoption of conservation measures to protect the Pantepui biota from eventual extinction by habitat loss under the action of ongoing global warming (Rull et al. 2016). Another handicap is that until recently, knowledge of Pantepui biota was dispersed among hundreds of publications, including a significant amount of grey literature, and a synthesis of the state of the art was lacking. Recently, Rull et al. (2019a) summarized the most relevant information available for biodiversity and endemism patterns of the better-known taxonomic groups in Pantepui. Although the available information is largely descriptive, it constitutes the first empirical basis for a still-inexistent integrative biogeographical, ecological and evolutionary perspective of the unique Pantepui region.
This review is a first trial towards such integrative view aimed at developing a preliminary holistic framework encompassing biogeographical patterns, ecological and evolutionary processes and mechanisms, and environmental drivers such as past climatic changes and paleogeographical reorganizations. The paper is subdivided into three main sections. The first section briefly describes the main physical and environmental features of Pantepui. The second section is a synthesis of the existing knowledge of its biogeography, ecology, evolution and conservation. The third part suggests future research directions in the same fields. It is hoped that this review will help set the stage for further studies, hopefully leading to an integrative understanding of the biological features of the unique Pantepui region and, eventually, the general progress of tropical ecology, evolution and biogeography, especially in relation to the origin of Neotropical biodiversity and latitudinal biodiversity gradients (Rull 2019a). It is also hoped that the ensuing research will contribute to the establishment of a more suitable framework for the preservation of Pantepui biota and ecosystems in the face of ongoing threats derived from human actions.
2 Pantepui
2.1 Physical Setting
Physiographically, the Guiana region has been subdivided into three main altitudinal levels, characterized by different physical, environmental and biotic features to define six main life zones (Fig. 15.3). The lowlands (<500 m elevation) are characterized by premontane and the basimontane life zones, the uplands (500–1500 m) are characterized by lower montane and montane life zones, and the highlands, where the upper montane and high-tepui life zones develop (Fig. 15.2). The tepui summits are part of the highlands. Approximately 70 tepuis and high mountains have been recognized, attaining a total surface area of >5000 km2; most of them are located in Venezuela, with a few representatives in Brazil and Guyana (Table 15.1, Fig. 15.3). In Venezuela, the term “Guayana” is frequently used instead of “Guiana” but the meaning is exactly the same (Berry et al. 1995b). Care should be taken to not confound these terms with the country name “Guyana”.
Physiographic sketch of the Guiana region around Pantepui indicating the elevational belts (lowlands, uplands, highlands) and the altitudinal sequence of life zones (piedmont, slopes, summits). Redrawn from Huber (1995c)
Topographic map of the Guiana Highlands, with the locations of its tepuis and tepuian massifs (Rull et al. 2019b). Abbreviations in Table 15.1. Base map: radar image courtesy of NASA/JPL/SRTM, Feb 2000 (freely available at https://photojournal.jpl.nasa.gov/catalog/pia03388)
Geologically, the Guiana region lies on the igneous-metamorphic Guiana Shield, one of the oldest South American rock complexes formed by Archaeo-Proterozoic granites and gneisses (Huber 1995a). This is the basement on which the quartzites/sandstones of the Roraima Group sedimented during the Precambrian. Further erosion of the Roraima Group caused the development of several planation surfaces from which the typical tabular physiography of the Guiana Highlands originated (Fig. 15.4). The tepui summits that constitute the Pantepui archipelago of sky islands are the isolated remnants of the highest (and oldest) of these erosion surfaces (Briceño and Schubert 1990). Details on the geochemical weathering of the Roraima sandstones can be found in Mecchia et al. (2019) and the literature therein.
Formation of the different erosion surfaces (Auyán-tepui, Wonkén and Imataca) on the Precambrian Roraima Group and on the igneous-metamorphic basement (Caroní-Aro erosion surface) from the Precambrian to the Pleistocene. Redrawn from Briceño and Schubert (1990)
The tepui summits are a mixture of bare rock, extensive peat blankets approximately 3–4 m deep and occasional diabase outcrops. Peatland soils (histosols) are highly acidic and poor in nutrients, which largely constrains the type of plant communities growing on them and has fostered particular morphological and ecophysiological adaptions to such substrates (Cuevas 1992; Zink and Huber 2011). Soils developed on diabase outcrops (entisols) are slightly more fertile, which promotes the establishment of biotic communities that cannot grow on other substrates (Huber 1995c). The tepui summits have been included in the so called “old, climatically buffered, infertile landscapes” (OCBILs), to differentiate them from the “young, often disturbed, fertile landscapes” (YODFELs), on which conventional ecological and evolutionary theories have developed. OCBILs are rare—other examples are the Southwest Australian Floristic Region and the South Africa Greater Cape—but it is believed that their extreme environmental conditions and the peculiar ecological traits and adaptions of their biotas will be able to provide new and interesting ecological and evolutionary insights (Hopper 2009).
2.2 Climate
The scarce meteorological data available for Pantepui is insufficient for a sound climatic characterization of the vast and topographically complex Pantepui area. However, the available data—12 years (1997–2009) of measurements from three weather stations situated between 1750 m and 2600 m elevation—enable some preliminary insights (Huber and García 2011). The annual average temperature ranges between 16.5 °C and 11.4 °C with a general elevational decrease (adiabatic lapse rate) of approximately −0.6 °C/100 m. This is in agreement with the general topographic temperature decrease for the whole Guiana region, which largely determines the altitudinal distribution of the above-mentioned life zones (Huber 1995c). Minimum air temperatures are always above 0 °C and frost has never been reported (Huber 1995a). Total annual precipitation increases with elevation from 2800 to 5300 mm per year, at a rate of approximately 30 mm/100 m. Additional moisture is provided by frequent dense mists. Seasonality in temperature is negligible and rainfall may vary throughout the year (rainfalls may be less common between December and March) but there is not a true dry season (<60 mm/month), especially on higher tepuis, where rainfall seasonality is minor. Winds and thunderstorms are frequent, especially during the rainier season (March to November). These data agree with previous inferences regarding mesothermic (lower tepui summits) to microthermic (higher tepui summits) ombrophilous climates, based on altitudinal inferences (Huber 1995a).
3 Existing Knowledge
The term “Pantepui” was coined by Mayr and Phelps (1967) to designate the assemblage of “…sandstone tabletop mountains in the Venezuelan Territorio Amazonas and Estado Bolivar and in the adjacent border regions of Brazil and Guyana”, whose avifauna is highly endemic and strikingly different from that in the surrounding lowlands. These authors considered Pantepui as an artificial unit rather than a biogeographical entity and did not specify any altitudinal limits to define this unit, although they considered that only the uppermost parts of the tepuis (>1500 m elevation) were suitable for the typical Pantepui avifauna. Further studies on insects, amphibians, reptiles and mammals adopted this concept with some modifications (review in Huber 1987), but Steyermark (1979) introduced a radically different idea of Pantepui. This botanist considered that Pantepui was a plant refugial complex including not only the Guiana Highlands but also some uplands and lowlands from the Central and Western Guiana provinces. This expanded Pantepui would have been a refugial area for rainforests and other tropical plant communities to persist and diversify during the assumedly arid glacial phases of the Pleistocene (Steyermark 1979). Sometimes, the terms “Guiana Highlands” and “Pantepui” are used interchangeably but it should be noted that the concepts behind them are different, as the first is a physiographical setting whereas the second is a formal biogeographical subdivision.
3.1 Biogeography
Pantepui has been formally defined as a biogeographical province by both botanists and zoologists but neither the definitions themselves, in terms of elevation and biotic characterization, nor the underlying biogeographical classification coincide. A recent update of the main plant and animal groups from Pantepui has provided a more comprehensive and integrative view.
3.1.1 Phytogeography
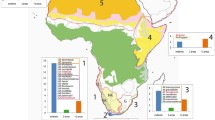
The Pantepui province was first defined in botanical terms, as part of the Guiana region, within the Neotropical realm (Fig. 15.5). The Guiana region has been subdivided into four provinces: Central Guiana, Eastern Guiana, Western Guiana and Pantepui (Fig. 15.3). Geographically, the Pantepui province was considered to be located within the Central Guiana province but restricted to the highlands (1500–3000 m elevation), where unique biotic and abiotic features occur that justify its biogeographical separation from the surrounding uplands and lowlands (Huber 1994). The higher limit of the Pantepui phytogeographical province is the maximum elevation of the Guiana Highlands (Sierra de la Neblina; 2994 m) but the lower limit has been modified over time. Huber (1987) set this lower limit at 1200/1500 m elevation. Later, the lower boundary was considered to coincide with the base of the Guiana Highlands, that is, 1500 m (Huber 1994; Huber and Riina 1997). The latest botanical characterization returned to a less rigid lower boundary situated between 1300 and 1500 m elevation (Huber et al. 2018).
Graphical representation of the phytogeographical (a) and zoogeographical (b) definitions of the Pantepui province. (a) Phytogeographical Pantepui. The Guiana region (green) and its four biogeographical provinces. The Pantepui province is represented as a discontinuous surface of red spots representing the major tepuis and tepuian massifs. Redrawn from Berry et al. (1995a). (b) Zoogeographical Pantepui. Zoogeographical provinces of northern South America. Colors represent the domains (pink—Pacific, orange—Boreal Brazilian, yellow—South Brazilian, blue—South-eastern Amazonian). G—Guajira, V—Venezuela, P—Pará. Modified from Morrone (2014)
According to Huber et al. (2018), the most characteristic features of the Pantepui province are the typical broadleaved meadows and hardleaved (sclerophyllous) woodlands growing on peat, the pioneering plant communities living on bare rocks (saxicolous) and the comparatively small patches of low forests growing in depressions and crevasses and along water courses (Fig. 15.6). The most characteristic plant families of Pantepui are the herbaceous Rapateaceae, the woody Bonnetiaceae and the pitcher plant family Sarraceniaceae, with their Pantepui endemic genus Heliamphora (Fig. 15.7). In general, montane shrublands, meadows and pioneer communities dominate the landscape, whereas forests play a subordinate role and are typically low and relatively species-poor. Shrublands are highly varied, ranging from dense high-tepui scrub to paramoid (for their similarity with the Andean paramo biome) and ericoid (i.e., dominated by plants of the family Ericaceae) scrub. Meadows are well-differentiated into broad-leaved, tubuliform and rosette meadows with several areas of grasslands (Berry et al. 1995b). Using the compilations of Berry and Riina (2005) and Riina et al. (2019), it has been estimated that vascular plant richness of Pantepui is approximately 5000 species per 10,000 km2, making it one of the most biodiverse areas worldwide (Mutke and Bathlott 2005), and endemism is 35–40%, which is also very high and comparable to that of many oceanic archipelagos.
Vegetation types representative of Pantepui (see Fig. 15.6 for representative species). (1) Bonnetia roraimae forest stands on protected sites of the Eruoda-tepui (Chimantá massif). (2) Aerial view of the Chimantá summit showing the typical gallery forests of Bonnetia roraimae (Bonnetiaceae) along a water course surrounded by broad-lived meadows dominated by Stegolepis ligulata (Rapateaceae). (3) General view of a paramoid shrubland on the Apakará summit of the Chimantá massif, dominated by Chimantaea mirabilis (Asteraceae) with an herbaceous layer dominated by Myriocladus steyermarkii (Poaceae, Bambusoideae). (4) Broad-leaved meadows of Stegolepis guianensis (Rapateaceae) with Brocchinia tatei (Bromeliaceae) rosettes on the summit of the Uei-tepui. (5) Peat bog on the summit of the Eruoda tepui (Chimantá massif) dominated by tubular rosettes of Brocchinia hechtioides (Bromeliaceae), with emergent caulirossulae of Chimantaea lanocaulis (Asteraceae). (6) Aerial view of the Chimantá summit showing wide extensions of cracked and bare rock with scattered stands of pioneer vegetation. (7) Closer view of the initial stages of rock colonization by pioneer plant communities (Eruoda summit, Chimantá massif). Photos: V. Rull
Representative vascular plants of Pantepui. (1) Bonnetia roraimae (Bonnetiaceae), Eruoda-tepui (Chimantá massif). (2) Chimantaea mirabilis (Asteraceae), Apakará-tepui (Chimantá massif). (3 and 4) Stegolepis ligulata (Rapateaceae), Apakará-tepui (Chimantá massif). (5) Chimantaea lanocaulis (Asteraceae), Eruoda-tepui (Chimantá massif). (6) Brocchinia tatei (Bromeliaceae), Uei-tepui. (7) Brocchinia hechtioides (Bromeliaceae), Eruoda-tepui (Chimantá massif). (8 and 9) Heliamphora minor (Sarraceniaceae), Eruoda-tepui (Chimantá massif). Photos: V. Rull
3.1.2 Zoogeography
Some zoologists have used the phytogeographical concept as a working framework (e.g., McDiarmid and Donnelly 2005), whereas others have included both lowlands and uplands when defining Pantepui (e.g., Kok et al. 2016; Borges et al. 2018). A number of zoologists believe that the concept of Pantepui as a biogeographical province should be reconsidered and redefined (e.g., Costa et al. 2013). A recent revision of the Neotropical biogeography by Morrone (2014) used primarily zoological criteria to formally define the Pantepui province (Fig. 15.5). This definition was explicitly considered by the author to be analogous to the informal Pantepui concept of Mayr and Phelps (1967) and the formal Pantepui phytogeographical province definition of Huber (1994). In this case, however, no distinction was made among lowlands, uplands and highlands; therefore, elevation was not used as a criterion to differentiate Pantepui from the surrounding terrains that, in the phytogeographical classification, define the Central Guiana province (Fig. 15.1). Another significant difference is that, whereas the phytogeographical definition places Pantepui within the Guiana region of the Neotropical realm, the zoogeographical conception does not define a Guiana unit and Pantepui is placed within the Boreal Brazilian dominion. In addition, the Neotropics is considered to be a biogeographical region, rather than a realm (Morrone 2014). The zoogeographical Pantepui was based on an extensive list of endemics, including a single plant genus, and 100 genera/species of arthropods and vertebrates.
3.1.3 An Integrative View
The latest comprehensive review based on the main plant and animal groups studied to date in Pantepui (Rull et al. 2019a) is used here as the basis for a more holistic definition of the Pantepui province in biotic terms. Table 15.2 summarizes the documented richness and endemism of each taxonomic group studied to date in Pantepui, as well as the lower boundary of this biogeographical province considered for each of these groups. It should be highlighted that this information is still far from being complete and that some groups are better known than others. The most studied groups are vascular plants, amphibians, reptiles, birds and mammals and the poorly known groups are algae, bryophytes, insects, arachnids and snails. Fishes, a group that is especially diverse in the Neotropics, is absent on Pantepui. No typically highland fish species have been found to date and lowland/upland species rarely cross the lower elevational boundary of Pantepui, with very few exceptions (Lasso et al. 1989). Some examples of emblematic and representative species of groups displayed in Table 15.2 are depicted in Figs. 15.8 and 15.9.
Examples of Pantepui bryophytes, algae and animals (aquatic insects, butterflies and scorpions). (1) Cushion-like Sphagnum colonies widespread in wetlands on the Apakará-tepui (Chimantá massif) (Photo: V. Rull). (2) Algal mats colonizing bare rock surfaces on the Eruoda-tepui (Chimantá massif) (Photo: V. Rull). (3) Enderleina preclara, found on Akopán-tepui (Chimantá massif) and Kukenán-tepui (Photo: T. Derka). (4) Parakari churiensis, endemic to Churí-tepui (Chimantá massif) (Photo: T. Derka). (5) Entophysalis arboriformis endemic to the Eastern District (Photo: J. Kaštovský). (6) Ekerewekia churicola, endemic to Churí-tepui (Photo: J. Kaštovský). (7) Hydrolutos breweri, endemic to Pantepui (Photo: T. Derka). (8) Heliconius elevatus roraima, endemic to Pantepui (Photo: M. Costa). (9) Mesotaenia vaninka delafuentei, endemic to Pantepui (Photo: M. Costa). (10) Callithomia lenea bella, endemic to Pantepui (Photo: M. Costa). (11) Oxeoschistus romeo, endemic to Pantepui (Photo: M. Costa). (12) Taurepania porosa from Roraima-tepui, endemic to Pantepui (Photo: F. Rojas-Runjaic). (13) Vachoniochactas amazonicus from Sierra de la Neblina, endemic to Pantepui (Photo: F. Rojas-Runjaic)
Examples of Pantepui animals (vertebrate parasites, snails, amphibians reptiles, birds and mammals). (1) Laelaps conula found on the mouse Rhipidomys macconnelli, from Auyán-tepui. The only parasite considered to be endemic to Pantepui (Photo: R. Guerrero). (2) Plekocheilus (Eurytus) juliani from the Chimantá massif, endemic to Pantepui (Photo: A. Breure). (3) Plekocheilus (Eurytus) sophiae from Yuruaní-tepui, endemic to Pantepui (Photo: A. Breure). (4) Oreophrynella vazquezi, endemic to Ilú and Tramen tepuis (Photo: J. Mesa). (5) Anomaloglossus rufulus, endemic to Eruoda-tepui (Chimantá massif) (Photo: J. M. Rojas-Runjaic). (6) Tepuiphyla edelcae, endemic to Auyán-tepui and Los Testigos massif (Photo: J. M. Rojas-Runjaic). (7) Oreosaurus mcdiarmidi, endemic to the Chimantá massif (Photo: J. M. Rojas-Runjaic). (8) Anolis carlostoddi, endemic to Abakapá-tepui (Chimantá massif) (Photo: J. Mesa). (9) Thamnodynastes chimanta, endemic to the Chimantá massif (Photo: J. M. Rojas-Runjaic). (10) Campylopterus hyperythrus, endemic to the Eastern District (Photo: D. Southall). (11) Diglossa major, endemic to the Eastern District (Photo: D. Southall). (12) Myioborus castaneocapilla, endemic to Pantepui (Photo: D. Southall). (13) Marmosops pakaraimae, endemic to the Eastern tepui chain (Photo: B. Lim). (14) Platyrrhinus aritus, endemic to Pantepui (Photo: B. Lim)
From approximately half of the cases (bryophytes, vascular plants, aquatic insects, land snails and mammals), the lower Pantepui phytogeographical boundary of 1500 m is explicitly adopted, whereas for others, lower limits of 1200–1400 m are preferred. No explanation is provided in the case of algae (1300 m) and scorpions (1400 m) but in the case of butterflies, amphibians, reptiles and birds (1200–1300 m), it is explicitly argued that the 1500 m boundary is not appropriate, as tepui summits below this elevation hold faunas characteristic of Pantepui. Butterflies could be considered as an outlier, as the proposed boundary at ~1000 m includes most of the Gran Sabana uplands, which are biogeographically very different for all other organisms. However, an artifact due to flying ability could not be disregarded (Viloria and Costa 2019). The same could hold true for birds. According to these numbers, the former phytogeographical definition of Huber (1987), who situated the lower boundary between 1200 and 1500 m, would be adopted, and the specific elevation for each taxonomic group may be defined on the basis of its particular biogeographical patterns. Following these criteria, based on the widest range of taxonomic groups available to date, it is recommended that the zoogeographical definition of the Pantepui province (Morrone 2014), which does not explicitly consider altitudinal boundaries and hence implicitly includes lowlands, uplands and highlands, is revised on the basis of the most recent updates.
Regarding richness and endemism, it is necessary to distinguish between the better-known Pantepui organisms, whose numbers may be considered to be more stable, and the less-known groups, for which studies are still incipient and reliable estimates require further evaluation. Considering only the first group, plants are by far the more diverse group with almost 2600 known species, whereas the number of animal species ranges from approximately 60 to 100 (Table 15.2). Endemism ranges from 14% in mammals to 55% in amphibians, with intermediate values for birds (29%), plants (34%) and reptiles (44%). The percentage of single-tepui endemics is also high for amphibians and reptiles (42% and 35%, respectively), intermediate for plants (25%) and negligible for birds and mammals. In general, further studies are expected to increase richness and decrease endemism estimates, which is common in biogeographical studies.
3.2 Ecology
Ecology is the Cinderella of Pantepui research. Autecological studies of plant and animal species are almost nonexistent except for scattered observations of apparent preferences of certain species for specific substrates (bare rocks, peat bogs, sandstones, diabases, caves) or assumed biotic associations such as symbiotic or pollination relationships. However, these are sporadic field annotations that eventually appear in the description of certain species, and no systematic autecological studies exist for any Pantepui species. The most complete information regarding habitat preferences is the record of the elevational ranges of occurrence of all known vascular plant species available in the outstanding Flora of the Venezuelan Guayana (Steyermark et al. 1995–2005), which represents a milestone of Guianan biological research. Further updates are available from Berry and Riina (2005) and Riina et al. (2019). Similar information is available for amphibians, reptiles, birds and mammals (Señaris and Rojas-Runjaic 2019; Pérez-Emán et al. 2019; Lew and Lim 2019). Ecological studies at community level are better developed for plants but they are rather descriptive (review in Huber and Rull 2019), investigations of internal ecosystem dynamics and their relationships with external drivers are absent. Information regarding long-term ecological processes, such as community assembly and the potential role of Quaternary climatic changes in the shaping of present-day ecological communities, has also been provided by paleoecological studies.
3.2.1 Present-Day Patterns
The more systematic ecological studies have involved the identification, description and characterization of Pantepui vegetation types. Whereas most classical botanists have been primarily interested in its floristic aspects, Huber (1992, 1995c) developed an intensive and extensive record of plant associations that culminated in the definition of five main vegetation types: forests, shrublands, grasslands, meadows and pioneer communities. Within these general types, 40 plant communities have been defined, including 12 types of forests, 10 types of shrublands, 2 types of grasslands and 16 types of meadows (Huber and Rull 2019). Pioneer communities have not yet been classified. Based on spatial vegetation patterns, Vareschi (1992) speculated about the potential successional trends leading to the extant vegetation and considered that forests were the climax communities toward which natural succession was directed. However, no empirical evidence supporting or dismissing this interpretation has been found thus far.
3.2.2 Long-Term Processes
Paleoecological records have shown that present-day Pantepui communities originated following the complex succession of climatic shifts that fostered up-and-down migrations of sensitive species from the Last Glacial Maximum (LGM; ~21,000 years ago) to the present (see Rull et al. 2019c for a detailed review). Overall, this process can be viewed as a millennial-scale upward migration trend, punctuated with minor downward displacements driven by smaller temperature and moisture oscillations that occurred during the Holocene (the last 11,700 years). Species that migrated downwards during the LGM could have spread across the surrounding lowlands and uplands thus having the possibility of climbing to several tepuis during the Holocene warming (Fig. 15.10). However, not all tepui summits could have had the possibility of sharing species in this way, as a number of them are higher than the magnitude of the estimated downward biotic displacement, are totally isolated by vertical cliffs preventing elevational migrations, or both. The irregularity of climatic oscillations, combined with the complicated topography of the Guiana Highlands and the fact that species’ responses to climatic shifts are not homogeneous but idiosyncratic—i.e., dependent on the particular climatic requirements and tolerances of each involved species—makes the general upward migration since the LGM—and, therefore, the assembly of extant Pantepui communities—a very complex and unpredictable process. A geographical information systems (GIS) modelling approach has been used to reconstruct the potential routes and barriers for upward migration, and their changes over time and could be considered as a hypothetical framework to be tested in future studies (Rull and Nogué 2007).
Present (black) and simulated Last Glacial Maximum (LGM) extent of typical Pantepui climates (white). Lowlands below 400 m elevation are in grey. LGM simulation performed assuming a downward migration of climatic conditions up to 1100 m below the present lower boundary of Pantepui (1500 m) on a digital elevation model from the Shuttle Radar Topography Mission (USGS/NGA/NASA) of 5 arcsec (90 m) precision. Modified from Rull and Nogué (2007)
Fire seems to have played some role in vegetation shifts, as major community replacements coincided with Holocene fires, especially those that occurred between 6000 and 5000 years ago and approximately 3500 years ago (Rull and Montoya 2017). Thus far, it is not possible to know whether these fires were natural or anthropogenic, but the lack of evidence regarding human presence and the coincidence of charcoal peaks with climate shifts, especially in terms of moisture, suggest that humans were not involved. During the last millennium, fire, likely of human origin, has deeply modified the ecosystems of a tepui summit (Uei-tepui), which is connected to the adjacent Gran Sabana uplands by gradual and fully vegetated slopes (Safont et al. 2016). No similar fire events have been recorded on other tepui summits, during historical times.
3.2.3 Biogeographical Implications
In addition to their relevance for community assembly and succession, Quaternary climate changes also affected the integrity of the Pantepui biogeographical province itself. The Pantepui concept has been subdivided into three main components: the orographic Pantepui, the climatic Pantepui and the biotic Pantepui. The first remained constant during the Quaternary but the other two underwent significant changes. The climatic Pantepui fluctuated approximately 50 times from glacial to interglacial conditions following a cyclicity of 41,000–100,000-year periods. During glacials, the biotic Pantepui disassembled as a result of the differential downward migration of sensitive species, according to their particular climatic requirements and tolerances. During interglacials, the biotic Pantepui reassembled as a result of upward migration but the species composition was not necessarily the same due to the species’ climatic idiosyncrasies and eventual extinctions of summit elements. This recurrence has been called the Pantepui oscillator (Fig. 15.11). Quaternary evolution could also have played a role in the modification of the Pantepui biota. This introduces a new dynamic concept of Pantepui, as a biogeographical province, allowing it to be viewed as a typical interglacial feature that could have adopted approximately 50 different states during the Quaternary (Rull and Vegas-Vilarrúbia 2019a).
The Pantepui oscillator. The orographic (solid lines), the climatic (dashed lines) and the biotic (dotted lines) Pantepui assemble during the interglacials and disassemble during glaciations, via the downslope migration of the latter two Pantepui components. The interglacial state is less stable, as it is maintained by external energy inputs (i.e., temperature maxima), whereas the glacial state is more stable, as entropy maximizes. Redrawn from Rull and Vegas-Vilarrúbia (2019a)
Glacial conditions have been the norm during the Quaternary, as they have persisted 80% of the time, whereas interglacials consisted of short warming peaks (Willis and Whittaker 2000; Bush et al. 2001). Therefore, glacial Pantepui disassembly could be considered the normal state, whereas the currently observed interglacial coincidence of the orographic, the climatic and the biotic pantepuis may be viewed as the exception. In addition, glacial-interglacial recurrence is typically asymmetric, with abrupt interglacial warming and gradual glacial cooling episodes. In thermodynamic terms, interglacials could be viewed as short unstable states maintained by external energy inputs that, once terminated, enable system relaxation and entropy maximization, which is characteristic of glaciations (Ellis and Palmer 2016). Therefore, glacial Pantepui disassembly could be viewed as the more stable state of Pantepui, with maximum entropy (disorder), whereas interglacial assembly (order) would be a transient condition maintained by the incoming of external energy inputs (Fig. 15.11).
3.3 Evolution
As has occurred in the Neotropics, in general, knowledge on the origin and evolution of Pantepui biodiversity can be subdivided into three main historical phases (Chap. 2). During the first phase, evolutionary inferences were based on biogeographical evidence, the second phase started with the advent of paleoecology and the third stage has been characterized by the recent remarkable development of molecular phylogenetic and phylogeographical studies (Rull 2019a).
3.3.1 The Biogeographical Phase
Mayr and Phelps (1967), the creators of the term “Pantepui”, summarized the first hypotheses that emerged from the study of birds and mammals, into five main categories (Table 15.3). The Plateau Theory (PT) proposes that the faunal differences among the tepui summits are due to vicariance by physical isolation, after a long-standing process of the maintained erosion of a former plateau formed by the Roraima quartzites/sandstones. The Cool Climate Theory (CCT) contends that the Pantepui fauna colonized the tepui summits from the surrounding lowlands during the Quaternary glaciations, which could have facilitated connectivity between the lowlands and summits thus allowing biotic interchange. According to the Habitat Shift Theory (HST), the Pantepui fauna is derived from long-ranging species (i.e., able to live from lowlands to highlands), after lowland extinction and highland survival and further differentiation (parapatric speciation). The Distance Dispersal Theory (DDT) maintains that the Pantepui fauna was derived from other montane regions (the Andes, Caribbean coastal ranges, the Brazilian Highlands) by island hopping, or jump dispersal using their flying capacity to cross inhospitable terrains. The Specialized Habitat Theory (SHT) proposes that Pantepui species are ecological specialists with very specific habitat requirements available only in Pantepui.
Other authors did not explicitly refer to the above hypotheses although they used similar or identical proposals. For example, Maguire (1970) implicitly favored the PT, although he did not mention it, for the origin of the Pantepui flora. In contrast, Steyermark and Dunsterville (1980) and Huber (1988) favored the idea that Pantepui plant species could have migrated up and down following the Quaternary glacial-interglacial cycles. These contrasting views were later called the Lost World Hypothesis (LWH) and the Vertical Displacement Hypothesis (VDH), respectively (Rull 2004). The VDH is sometimes confused with the CCT, likely due to the possibility of vertical migration, but they are actually opposite views. The CCT proposes upward migration of the lowland fauna to the tepui summits during glacials, with no reference to the interglacials and is based on theoretical considerations. On the contrary, the VDH proposes downward, rather than upward, glacial migrations and upward migrations during interglacials and is based on worldwide empirical evidence, including the Neotropics.
3.3.2 The Paleoecological Phase
Paleoecological studies have provided evidence for testing the LWH and VDH, and for suggesting new proposals. Past climatic and ecological records suggest that a combination of these two hypotheses is needed to explain the Pantepui biotic patterns. This is called the Vicariance-Migration Hypothesis (VMH) (Table 15.3). According to this proposal, glaciations are characterized by the biotic spread of Pantepui species across uplands and lowlands thus promoting hybridization and adaptive radiation (Fig. 15.12). Interglacial upward migration would have favored vicariance and extinction by habitat loss (Rull 2005). The classic debate between vicariance vs. dispersalism is not compatible with the VMH. This hypothesis was useful to evaluate Quaternary diversification but meta-analyses including a wide range of organisms have demonstrated that Neotropical biodiversity originated in a continuous manner during Neogene and Quaternary times with no diversification bursts at any particular time period (Rull 2008). Therefore, the analysis of pre-Quaternary diversification requires a different approach.
Graphical representation of the Vicariance-Migration Hypothesis (VMH) (Rull 2005) using the Upuigma-tepui. Photo: V. Rull
The apparent absence of pre-Quaternary sediments in Pantepui and surrounding areas prevented empirical evidence from illustrating biotic diversification and the drivers involved during that time. In contrast to other Neotropical areas (as for example the Andes or the Panama Isthmus), which were subjected to intense tectonically-driven orographic and paleogeographical changes, the Guiana region remained relatively stable during the Neogene, the major geological process being the continued erosion of the Roraima quartzites and sandstones leading to the formation of the tepuis (Fig. 15.4). However, the climate did not remain constant. During the Cenozoic, our biosphere has experienced a maintained million-scale global cooling since the Paleocene/Eocene boundary, when temperatures were approximately 14 °C higher than they are today on average (Hansen et al. 2013). Under these conditions, the early Cenozoic Pantepui biota was likely more similar, in bioclimatic terms, to the extant lowland biota. Therefore, the Cenozoic evolution of this biota took place on summits that were progressively smaller, lower, more isolated and cooler. Topographical isolation would have favored vicariance and extinction, whereas cooling would have fostered downward migration and species “pumping” to the surrounding lower terrains. This hypothesis has been called the Isolation-Cooling Hypothesis (ICH) (Table 15.3).
3.3.3 Molecular Phylogenetics and Phylogeography
Molecular phylogenetic studies and their geographical expression (phylogeography) are still scarce in Pantepui due to its remoteness and the difficulty of obtaining fieldwork permits for collecting genetic material (Rull and Vegas-Vilarrúbia 2008; Rull et al. 2009). However, the results obtained to date have been used to evaluate the existing hypotheses of Pantepui biotic evolution. The divergence time of the different taxa studied, inferred from time-calibrated phylogenetic trees, is a key parameter that has been used to support the different evolutionary hypotheses and theories, mainly for vascular plants, amphibians, birds and mammals. The main results obtained are as follows.
In plants, most studies of this type correspond to the families Rapateaceae and Bromeliaceae. The genus Stegolepis (Rapateaceae) would have invaded Pantepui at ~12 Ma and started to diversify at ~6 Ma by a combination of vicariance and bird-mediated dispersal (Givnish et al. 2000, 2004). In the Bromeliaceae, the Brocchinia species studied would have originated between 12 and 17 Ma, whereas the Lindmannia species would have started to diverge by 2.5 Ma, at the beginning of the Pleistocene (Givnish et al. 2007). Although the ICH was not yet proposed when these taxa were studied, the above results seem to be consistent with this proposal.
Among amphibians, the species of the genus Tepuiphyla were proposed to have originated between ~5 and ~2 Ma (Pliocene to Pleistocene), which was considered to support a combination of the HST and the VDH, thus dismissing the DDT (Salerno et al. 2012, 2014). Using the same genus and two other amphibian genera (Oreophrynella and Stefania), Kok et al. (2012) favored active/passive dispersal mechanisms, rather than ancient endemism, for the explanation of the lower than expected genetic variability. The genus Stephania, whose diversification occurred during the Miocene, was used by Kok et al. (2017) to support the PT, rather than the Disturbance-Vicariance Hypothesis (DVH) (Table 15.3), according to which current Neotropical biogeographical patterns could be explained by a combination of LGM cooling, a moderate precipitation reduction and atmospheric CO2 depletion (Bush 1994; Colinvaux 1998). A combination of the DDT and PT was proposed by Kok et al. (2018) after studying the genera Oreophrynella and Atelopus, whose divergence occurred ~40 Ma (Eocene) in the proto-Andes, after which they reached Pantepui by jump dispersal and diversified there by vicariance.
Regarding birds, a study of the genus Myoborus (redstars) was used to support the DVH, after initial dispersal from the Andes and/or the Venezuelan Coastal Range and further in situ diversification on the tepui summits (Pérez-Emán 2005). The Pantepui species of the genus Aulacorhynchus (toucanets) emerged during the Pliocene, which was used to support the DDT followed by Pleistocene population differentiation (Bonaccorso and Guayasamin 2013). Studies on other Neotropical bird genera with representatives on Pantepui also favored dispersal from either the Andes or the surrounding Guiana lowlands (Smith et al. 2014; Berv and Prum 2014).
The representatives of Pantepui mammal fauna studied to date using molecular methods seem to favor the DDT from a variety of Neotropical areas, including the Andes, the Amazon lowlands and the Brazilian Shield. For example, the ancestor of the opossum Monodelphis reigi was proposed to have dispersed to Pantepui from the Andes during the Miocene (Velazco and Patterson 2008). A further study of the whole genus situated the origin of M. reigi in the Amazon lowlands and its establishment on the tepui summits in the Quaternary, between 2.4 and 1.2 Ma (Pavan et al. 2016). A similar situation was proposed for the opossum Marmosops pakaraimae (Voss et al. 2013). A mouse endemic to the Roraima-tepui, Podoxymys roraimae, diverged from its closest relative at the end of the Pliocene, between 3.7 and 2.5 Ma, and dispersed to Pantepui from the Brazilian Shield. In this case, the CCT and the HST were dismissed, and the DDT was favored (Leite et al. 2015).
A preliminary conclusion is that most hypotheses proposed to date (Table 15.3), as well as combinations of them, have been supported by one or more case studies; therefore, based on the available evidence, none of the hypotheses can be dismissed. Different taxonomic groups seem to have acquired their current biodiversity and endemism patterns by diverse processes and mechanisms under the action of different drivers in different Pantepui areas and at different times. Therefore, single case studies based on particular taxonomic groups and/or specific geographical areas cannot be generalized to all of Pantepui, which is also true for the entire Neotropics (Rull 2013). As occurs in the Neotropics, in general, a proper understanding of the origin of the Pantepui biota requires complex thinking and the consideration of multiple drivers (tectonics, paleogeographical reorganizations, marine incursions, climatic changes) as well as a diversity of ecological and biogeographical processes (migration, long-distance dispersal, in situ evolution, microrefugia, extinction) and evolutionary mechanisms (vicariance, gene flow, hybridization, adaptive radiation) acting together across spatial and temporal scales (Rull 2011, 2015). This has been called the Continuum Multifactor Hypothesis (CMH) (Rull 2019b and Table 15.3).
3.4 Conservation
Due to its remoteness and pristinity, it could be expected that the biota and ecosystems of Pantepui are not threatened by human activities. On the tepui summits, activities such as hydrocarbon exploitation, mining, hydroelectricity production, forestry and farming are not possible due to the special geological, edaphic and vegetation features (Huber 1995d). The main activities atop the tepuis are tourism and scientific exploration, although permanent facilities for such tasks (hotels, scientific stations, etc.) are nonexistent and these activities are carried out by camping. Only a few tepui summits, notably the Roraima-tepui and Auyán-tepui, are accessible by foot, while others can be reached by helicopter. The tepuis have been declared natural monuments and some Pantepui areas are under other designations, such as national parks, biosphere reserves and a human heritage site (Huber 1995d; Bevilacqua et al. 2019). However, the available resources for effective control and surveillance of such a huge and remote area are insufficient (Rull et al. 2016; Rull and Vegas-Vilarrúbia 2017). In 1989, official permits to visit the tepui summits were suspended. Since then, a long, complex, erratic and uncertain process was established to obtain permits for visiting the tepuis that makes scientific research virtually impossible (Rull and Vegas-Vilarrúbia 2008; Rull et al. 2009). In spite of this, illegal touristic and scientific activities have not stopped and are difficult to control. Other threats for Pantepui are human activities that occur in the surrounding uplands and lowlands (notably fire), which can eventually affect the tepui summits and their slopes. This is especially true for the Eastern Tepui District, where the tepuis are surrounded by the Gran Sabana uplands, the most populated and active area in the region. In addition to direct human impacts, the ongoing anthropogenic global warming may also affect the Pantepui biota, as occurs in other Neotropical and worldwide mountain areas. This section briefly discusses the potential direct and indirect consequences of local and global human activities on Pantepui.
3.4.1 Direct Impacts
Humans visit the tepui summits for a variety of purposes including scientific collection, recreation, adventure tourism, extreme sports (climbing, paragliding, skydiving), photography, documentary filming and biopiracy (illegal biodiversity trade and commercial exploitation of biochemical and genetic resources), among others. Presently, most of these activities are carried out without the corresponding official permits, likely due to the difficulty of obtaining them. It should be noted that indigenous people do not choose to reach these summits on their own because they consider them to be sacred places, although they are often hired by tourists as guides and/or porters. The main impacts of these activities are vegetation trampling, plant/animal extraction and garbage accumulation. These impacts are most significant on the only two tepuis that are reachable by foot and open to touristic visits, the Auyán-tepui and the Roraima-tepui, especially on the second, which is the most visited by far. The summit of the Roraima-tepui (~34 km2) receives 3000–4000 tourists each year and vegetation trampling/damage is a common problem, together with the presence of garbage, food scraps, toilet paper, human excrement and graffiti (Safont et al. 2014). Recently, two novel threats have been identified: invasive plants and water contamination.
The latest botanical survey of the Roraima summit, conducted with the corresponding official permits, documented the presence of 13 plant species introduced by humans (Safont et al. 2014). Most of these species are cultivated or occur as small and localized populations and, therefore, do not represent an immediate menace. However, two of these introduced species, the grasses Polypogon elongatus and Poa annua, may be more dangerous. P. elongatus is a well-known invasive elsewhere and its abundance and patterns of spatial occurrence on the Roraima summit fulfil the criteria to be considered naturalized and invasive (Richardson et al. 2000). P. annua is of cosmopolitan distribution and is considered to be one of the most aggressive weeds (Holm et al. 1997). The eventual invasion of the Roraima and other summits by these plants could lead to large-scale degradation of the vegetation and the eventual extinctions of the autochthonous biota (Rull et al. 2016). Another recent study documented the presence of Helicobacter pylori—a well-known dweller of the human gastrointestinal tract that causes gastritis, ulcers and cancer—in the Roraima freshwaters near the usual camp sites (Fernández-Delgado et al. 2016). This bacterium may be easily transported by water to the uplands and lowlands and spread across the Guiana region through the fluvial network (Rull et al. 2016).
Human fires also constitute a direct threat, especially in the Gran Sabana tepuis (Eastern District). Currently, summit fires do not occur, but it is well known that Gran Sabana fires are active deforestation agents in gallery forests and on tepui slopes (Fig. 15.13). These fires are frequent and recurrent, and it has been estimated that more than 10,000 fires are lit by humans each year and that a given Gran Sabana area is recurrently burnt every 2–3 years (Huber 1995d). The danger of these fires reaching a tepui summit is latent. This fact has already been documented on the Uei-tepui summit, which is not totally isolated by vertical cliffs from the surrounding uplands (Safont et al. 2016) (Fig. 15.13). As we have seen above, many tepui summits are connected to uplands and lowlands by ridges and extended valleys (Huber 1988) through which fires could propagate to the highlands.
Examples of Gran Sabana fires and their effects on tepuis in the Eastern District. (1 and 2) Active Gran Sabana fires near Santa Elena de Uairén. (3) Gallery forest reduced by recent fires near Wonkén. (4) General view of the southern Gran Sabana showing a recent fire (brow patch in the front) and the mostly deforested landscape. In the background, the Kukenán-tepui (left) and the Roraima-tepui (right). (5 and 6) SE flanks of the Chimantá massif near Yunek, showing the slopes extensively deforested by fire. The brown area corresponds to a major recent fire that reached the cliffs. (7 and 8) The summit of the Uei-tepui, showing standing charred trunks of the former Bonnetia forests removed by fire and the secondary broad-leaved meadows of Stegolepis and Brocchinia with the fire-tolerant shrub Cyrilla. Photos: V. Rull
3.4.2 Global Warming
Global warming is considered to be a significant threat to mountain biota worldwide, as species may respond by shifting their altitudinal distributions, leading to changes in the diversity and composition of their communities and reductions, fragmentation or loss of their habitat. Tropical mountains are of particular concern because of their high degree of biodiversity and endemism (Safont et al. 2014 and literature therein). In Pantepui, several studies have been conducted to quantitatively estimate the potential extinction of vascular plant species by GW-induced habitat loss and to evaluate the possible conservation actions (Rull and Vegas-Vilarrúbia 2006, 2017; Nogué et al. 2009; Safont et al. 2012, 2014; Vegas-Vilarrúbia et al. 2012). These estimates have been based on three complementary methods, namely the altitudinal range shift (ARS) method, the species-area relationship (SAR) method and the climate envelope distribution model (CEDM) method (see Rull and Vegas-Vilarrúbia 2019b for more details). The climatic scenarios used as a reference correspond to the IPCC (Intergovernmental Panel on Climate Change) projections of a 2–4 °C increase for northern South America, by the end of the twenty-first century (Houghton et al. 2001; Solomon et al. 2007; Stocker et al. 2013).
The first projections estimate a plant diversity reduction of 75% (28% Pantepui endemics) for the more optimistic scenario (2 °C) and of 83% (54% endemics) for the more pessimistic scenario (4 °C) (Nogué et al. 2009). Further research did not improve expectations as the estimates for endemic extinction by habitat loss ranged from approximately 30% to 50% for the best and the worst-case scenarios, respectively (Safont et al. 2012). These preliminary studies were conducted on the basis of the available data, which consisted of only presence/absence records for the studied tepui summits and altitudinal ranges of occurrence for the known species. The lack of autecological knowledge on Pantepui species prevented the consideration of idiosyncratic features such as differential phenotypic plasticity among species and the resulting acclimation capacity, as well as the potential for rapid genetic change and the ensuing evolutionary adaption to novel environments. Therefore, the first estimates should be considered preliminary and likely inflated, and should be revised when more autecological knowledge is available. Rull and Vegas-Vilarrúbia (2017) suggested future studies that should be developed for more realistic estimates of the potential effect of global warming on Pantepui species.
Several in situ and ex situ actions have been discussed to minimize extinction by habitat loss due to global warming. In situ actions are problematic because warming is a global phenomenon and, hence, difficult to palliate at the local and regional levels. Under the current estimates, most tepui summits are set to totally lose their climatic Pantepui envelope by 2100 except the Chimantá massif and Cerro Marahuaka, which would retain the current Pantepui climates across approximately 40% of their surface, and the Roraima-Kukenán massif, with a typical Pantepui climate remaining across approximately 20% of its surface by 2100 (Vegas-Vilarrúbia et al. 2012) (Fig. 15.14). Therefore, eventual in situ conservation efforts should be focused on these summits. Regarding ex situ possibilities, the creation of germplasm banks and botanical gardens, as well as the viability of managed relocation practices, have been discussed (Safont et al. 2012). The first two possibilities are common and would be relatively easy to implement. However, managed relocation is a highly controversial conservation option, especially in the case of Pantepui, where ecological knowledge on its biota is lacking. Much more ecological and evolutionary research is still needed to determine whether the managed relocation of Pantepui species is a feasible and convenient option.
ARS-GIS simulation (see text) of the Pantepui area at the top of the Chimantá massif for the end of the present century under the more optimistic (2 °C increase) and the more pessimistic (4 °C increase) IPCC scenarios (Solomon et al. 2007). Redrawn from Nogué et al. (2009) and Vegas-Vilarrúbia et al. (2012)
4 Future Research
The potential of Pantepui to provide relevant insights on the origin and evolution of the Neotropical biota has not been fully exploited, due to the still incipient and biased research conducted in this biogeographical province. Most studies have been aimed at reporting and documenting the biodiversity and endemism patterns of different taxonomic groups and have been carried out by specialists on these groups. General ecological, evolutionary and conservation studies are still lacking. This is likely due to three main factors. First, the uniqueness of the species and life forms has fostered the interest of specialists avid in identifying new rare species and documenting high levels of endemism. This has largely led research regarding Pantepui since its discovery. Second, the remoteness and inaccessibility of the Guiana Highlands, together with the lack of in situ facilities, make any study based on periodic and/or systematic campaigns using complex logistics and equipment difficult and expensive. Therefore, research on these mountains has largely been based on sporadic studies consisting of short and occasional visits. It seems that, more than a century and a half after its scientific discovery, Pantepui is still in an exploratory phase. Third, the difficulty—and sometimes the impossibility—of obtaining official permits has aggravated this situation, as it not only makes biological sampling difficult but also prevents the establishment of permanent or semipermanent recording instruments. These handicaps should be circumvented to exploit the full ecological and evolutionary potential still hidden in Pantepui. After the review presented here, some major knowledge gaps have been identified that should be urgently addressed. Suggestions for future studies can be classified into four broad categories: (1) biodiversity and endemism, (2) ecology and paleoecology, (3) genomics and evolution and (4) conservation.
4.1 Biodiversity and Endemism
Despite the numerous visits to the tepui summits during the last century and a half, knowledge on the diversity and endemism patterns of Pantepui organisms is only fragmentary. On the one hand, research on some organism groups is still incipient, and no general insights on their diversity or geographical patterns are yet possible. This is the case, for example, for bryophytes, algae, aquatic insects, butterflies, arachnids and snails when considering only to refer only the organisms included in this review. Other groups are even less known or completely unknown. Even within the better known organisms (vascular plants, amphibians, reptiles, birds and mammals) there are important gaps that prevent robust generalizations. Therefore, taxonomic research should continue on Pantepui for a sound understanding of its biodiversity and endemism patterns. Such research, however, should leave behind the exploratory mindset and open a new research era based on more specific aims and more systematic studies according to taxonomic group, geographical area, or both. Pioneers of Pantepui scientific exploration were fundamental for acquiring current knowledge on this unique biogeographical province, which is the seed from which all research has emerged and will continue to develop. However, the existing biodiversity background for major groups seems already sufficient to advance towards a more systematic and target-oriented research framework, including not only biodiversity but also ecological, evolutionary and conservation studies. For example, the theory of island biogeography (Whittaker and Fernández-Palacios 2007) could be a suitable framework for conducting Pantepui research. Some attempts have been made using species-area relationships and the distance to possible sources of Pantepui species, notably the Andes (Michelangeli 2000; Berry and Riina 2005; Nogué et al. 2009; Riina et al. 2019; Pérez-Emán et al. 2019), but the potential of this approach has not been fully exploited.
4.2 Ecology and Paleoecology
Ecology is perhaps the most overlooked discipline in Pantepui research. Incipient ecological studies are available for plants after the definition of the main vegetation types and plant communities, with indication of their preferred substrates (bare rock, peat, sandstone, gneiss). However, the ecological preferences and tolerances of individual species are completely unknown due to the lack of autecological studies and the scarcity of environmental data to define species niches. This is true not only for plants but also for all taxonomic groups reported here. Sometimes, the elevational ranges of species have been used as a proxy for temperature tolerance, but this is not enough. Autecological and ecophysiological research on Pantepui species, with emphasis on the most relevant elements, is mandatory and urgent. From a synecological point of view, knowledge on community assembly and the main internal and external ecological drivers is still insufficient. The available paleoecological studies useful for this purpose are restricted to the Holocene and should be extended backwards. To date, it has not been possible to find LGM and earlier sediments but this is fundamental to advance the ecological knowledge. Functional ecology is also completely unknown and multidisciplinary studies on the composition and ecological dynamics (biotic and abiotic interactions) should be prioritized to obtain sound ecological knowledge.
4.3 Genomics and Evolution
Molecular phylogenetic and phylogeographical research on Pantepui organisms has already begun but it should be continued and focused on specific problems. Genetic analyses not only resolve group-specific taxonomies but also contribute to inferring evolutionary relationships. As we have seen above, evolutionary inference based solely on biogeographical patterns and geological/paleoecological data are necessary but insufficient, as they provide circumstantial evidence. Molecular phylogenies render direct evolutionary evidence and their geographical expression contributes to causal inferences regarding diversification processes and their mechanisms. It is noteworthy, however, that molecular studies conducted to date have been aimed at testing the existing hypotheses on the origin and evolution of Pantepui biota, which are still largely based on pioneer biogeographical studies. It could be expected that genomic research—with the corresponding biogeographical, geological and paleoecological inputs—may provide new possible explanations but this has not yet occurred. For this, it is necessary that molecular phylogenetic and phylogeographical research is more target-focused and based on fully sampled taxonomic and/or geographical evolutionary units, rather than on partial evidence. This could help circumvent generalizations from single case studies thus favoring complex thinking, which seems mandatory to properly understand Neotropical biotic patterns and processes.
4.4 Conservation
Robust biogeographical, ecological and evolutionary knowledge is mandatory for estimating the potential effects of direct and indirect threats to the Pantepui biota and ecosystems, as well as to inform conservation strategies and, eventually, restoration options. Autecological knowledge, notably regarding species tolerances to environmental parameters, is fundamental for forecasting the potential responses—i.e., in situ survival, migration, and extinction—of each species to future environmental change, which affects not only biodiversity but also the composition of future communities and therefore intra-community ecological dynamics. In addition, surviving climate change depends not only on the phenotypic plasticity of species but also on their capacity to undergo short-term evolutionary adaption, which is largely dependent on their intra- and interpopulation genetic variability. Therefore, genomic studies can also contribute to estimating the capacity of species to resist environmental change. In summary, ecological and evolutionary research can be useful for both understanding the origin and evolution of the Pantepui biota and conserving it in the face of future global warming. The existing studies on the subject have already provided a list of the most endangered plant species sorted by risk, which could be useful for establishing priorities for more detailed autecological and evolutionary studies. Similar studies may be developed for other organisms. International worldwide initiatives, such as GLORIA (GLobal Observation Research Initiative in Alpine environments; www.gloria.ac.at), which has a network of stations to record changes in mountain vegetation driven by global warming, should be considered for Pantepui. Regarding restoration practices, paleoecology may be useful to identify better communities for present-day environmental conditions thus avoiding eventual unrealistic or nonviable combinations, such as LGM or Late Glacial communities.
4.5 Summary
A general message from the above considerations is that future Pantepui research should be more systematic and target focused. The available knowledge seems sufficient to consider the pioneering and exploratory times to have already finished, and a new mentality should be adopted based on more scientific procedures. For this to occur, it is essential that the three main handicaps mentioned above are circumvented. Only one of these handicaps—i.e., the new/rare species approach as the only target—depends on scientists’ ability to shift the goal of research, whereas the other two—i.e., the difficulty of access and of obtaining official permits—are beyond the control of researchers. However, nothing on Earth seems more remote and inaccessible than the poles, which are now a global priority for research. Perhaps it would be possible to convince the countries sharing the Guiana Highlands of the importance of knowing and preserving Pantepui as one of the few remaining pristine areas on Earth. The Guiana Shield Facility (www.guianashield.org) could be a suitable platform, provided it is able to secure funds from international agencies and provide a suitable political environment for conducting systematic and target-focused scientific research on Pantepui.
5 Conclusions
-
Pantepui is a highly fragmented biogeographical province with a >5000 km2 total surface area, formed by the assemblage of remote and pristine flat summits (up to approximately 3000 m elevation) of tabular Guiana mountains (tepuis) situated between the Orinoco and Amazon basins. The lower elevational boundary of the Pantepui province varies according to the taxonomic group considered. After the analysis of the better-known Pantepui floral and faunal groups and their respective distribution patterns, a lower boundary between 1200 m and 1500 m elevations seems more appropriate than a rigid boundary situated at 1500 m, as was usual in former classifications.
-
The Pantepui biodiversity and endemism patterns are outstanding and vary across taxonomic groups. The most diverse organisms are plants, with almost 2600 known species (>5000 species/10,000 km2), which situates Pantepui among the most diverse regions of the world. Endemism usually ranges from 30 to 40% in birds, plants and reptiles and may reach 55% in amphibians, which are similar to the percentages on many oceanic islands. The frequency of local endemics—i.e., species restricted to a single tepui summit—is high (25%) for plants and very high (35–40%) for amphibians and reptiles.
-
Ecological knowledge of Pantepui biota and the communities it forms is almost inexistent. There are no autecological studies useful for defining the niche preferences of the different species and their tolerance ranges to biotic and abiotic variables. Only preliminary elevational ranges are available for the species of some taxonomic groups. Regarding communities, only descriptive studies of vegetation types are available, and almost nothing is known about community assembly and ecosystem dynamics. The situation is even worse for animals, where synecological studies are absent.
-
Long-term ecological studies using paleoecological methods have shown that Pantepui plant communities have changed through time under the influence of Holocene climatic changes (notably temperature and moisture balance) and fire events. The biotic responses, notably in the form of altitudinal range displacements, to these environmental shifts have been idiosyncratic—i.e., dependent on the particular tolerances of each species—rather than at the community level. This has led to continuous changes in community composition that have shaped the present communities.
-
The recurrent glacial-interglacial cycles and the idiosyncratic responses of the different species would have affected the taxonomic composition of the tepui summits, turning Pantepui into a dynamic biogeographical unit. In this framework, Pantepui seems to have been a typically interglacial feature that has adopted different states during the different interglacials and has been disassembled during glacials, when highland species migrated to the surrounding lowlands and uplands. Pantepui assembly is viewed as a less stable state in comparison with glacial disassembly, as the latter has been much more frequent during the Quaternary and does not need external energy inputs to persist.
-
The origin and evolution of the Pantepui biota has been explained by diverse evolutionary processes involving a variety of environmental drivers (notably climatic changes and paleogeographical rearrangements) and diversification mechanisms (vicariance, jump dispersal and allopatry, parapatry, adaptive radiation). Most of these hypotheses emerged from the study of extant biogeographical patterns and geological-paleoecological surveys. The inception of molecular phylogenetics and phylogeography, albeit still incipient in Pantepui, has provided evidence useful for testing these hypotheses. Taken individually, none of the proposed explanations can explain the evolution of the whole Pantepui biota, whose proper understanding requires complex thinking and the consideration of multiple drivers and a diversity of ecological and evolutionary processes and mechanisms acting together across spatiotemporal scales.
-
In spite of their current pristinity, the Pantepui biota and ecosystems are threatened by direct (mechanical damage, introduction of invasive species, garbage accumulation, water contamination, fire) and indirect (global warming) consequences of human activities. Direct threats might be addressed by in situ actions (access control, surveillance), but unfortunately, the resources available are insufficient considering the huge size, the topographical complexity and the remoteness of the Guiana Highlands. The main consequence of global warming may be habitat loss for a large number of Pantepui species (up to 85% of vascular plant species, including 55% of endemics), which can be combatted only by ex situ actions such as the creation of germplasm banks and botanical gardens and, eventually, managed relocation.
-
At present, Pantepui research is at an impasse that slows advancement towards a biological synthesis of this singular Neotropical “lost world” and prevents gathering the information necessary to inform the conservation of its unique biota. The main drawback seems to be the current permitting policy, which directly affects research tasks, especially by blocking fieldwork. However, there is another handicap that is under the control of researchers. After more than a century and a half of research on Pantepui, the pioneering and exploratory times should be declared over, and a different mentality should be adopted based on more scientific procedures, including more systematic and target-focused activities.
References
Berry PE, Riina R (2005) Insights into the diversity of the Pantepui flora and the biogeographical complexity of the Guayana Shield. Biol Skrift 55:145–167
Berry PE, Huber O, Holst BK (1995a) Floristic analysis and phytogeography. In: Berry P, Holst BK, Yatskievych K (eds) Flora of the Venezuelan Guayana, Introduction, vol 1. Missouri Bot Gard Press, St. Louis, pp 161–191
Berry PE, Holst BK, Yatskievych K (1995b) Introduction. In: Berry P, Holst BK, Yatskievych K (eds) Flora of the Venezuelan Guayana, Introduction, vol 1. Missouri Botanical Garden Press, St. Louis, pp xv–xx
Berv JS, Prum RO (2014) A comprehensive multilocus phylogeny of the Neotropical cotingas (Cotingidae, Aves) with a comparative evolutionary analysis of breeding system and plumage dimorphism and a revised phyologenetic classification. Mol Phylogenet Evol 81:120–136
Bevilacqua M, Señaris JC, Huber O (2019) Conservation of Pantepui. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 391–402
Bonaccorso E, Guayasamin JM (2013) On the origin of Pantepui montane biotas: a perspective based on the phylogeny of Aulancorhynchus tocanets. PLoS One 8:6e67321
Borges SH, Santos MPD, Moreira M et al (2018) Dissecting bird diversity in the Pantepui area of endemism, northern South America. J Ornithol 159:1073–1086
Breure ASH (2019) Land snalis. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 247–261
Briceño HO, Schubert C (1990) Geomorphology of the Gran Sabana, Guayana Shield, southeastern Venezuela. Geomorphology 3:125–141
Bush MB (1994) Amazonian speciation: a necessarily complex model. J Biogeogr 21:5–17
Bush M, Stute M, Ledru M-P et al (2001) Palaeotemperature estimates for the lowland Americas between 30°S and 30°N at the Last Glacial Maximum. In: Margkgraf V (ed) Interhemispheric climate linkages. Academic, San Diego, pp 293–306
Chapman J (1931) The upper zonal bird-life of Mts. Roraima and Duida. Bull Am Mus Nat Hist 63:1–135
Colinvaux PA (1998) A new model for Amazon endemics. Glob Ecol Biogeogr Lett 7:95–96
Costa M, Viloria AL, Huber O et al (2013) Lepidoptera del Pantepui. Parte I: Endemismo y caracterización biogeográfica. Entomotropica 28:193–217
Cuevas E (1992) Relaciones nutricionales de la vegetación de turberas alto-tepuyanas. In: Huber O (ed) El Macizo del Chimantá. Oscar Todtmann, Caracas, pp 203–218
Derka T, Zamora-Muñoz C, Tierno de Figueroa JM (2019) Aquatic insects. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 167–192
Désamoré A, Vanderpoorten A, Laenen B, Gradstein SR, Kok PJR (2010) Biogeography of the lost world (Pantepui region, northeastern South America): insights from bryophytes. Phytotaxa 9:254–265
Doyle AC (1912) The lost world. Hodder & Stoughton, London
Ellis R, Palmer M (2016) Modulation of ice ages via precession and dust-albedo feedbacks. Geosci Front 7:891–909
Fernández-Delgado M, Giarrizzo JG, García-Amado MA et al (2016) Evidence of Helicobacter spp. in freshwaters from Roraima Tepui, Guayana Shield, South America. Antoine van Leeuwenhoeck 109:529–542
Givnish TJ, Evans TM, Zjhra ML et al (2000) Molecular evolution, adaptive radiation, and geographic diversification in the amphiatlantic family Rapateaceae: edidence from ndhF sequences and morphology. Evolution 54:1815–1937
Givnish TJ, Millam KC, Evans TM et al (2004) Ancient vicariance or recent long-distance dispersal? Inferences about phylogeny and South American-African disjunctions in Rapateaceae and Bromeliaceae based on ndhF sequence data. Int J Plant Sci 165:S35–S54
Givnish TJ, Millam KC, Berry PE, Sytsma KJ (2007) Phylogeny, adpative radiation, and historical biogeography of Bromeliaceae inferred from sdhF sequence data. Aliso 23:3–26
Guerrero R (2019) Vertebrate parasites. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 373–385
Hansen J, Sato M, Russell G, Kharecha P (2013) Climate sensitivity, sea level and atmospheric carbon dioxide. Philos Trans R Soc A 371:20120294
Holm LG, Holm E, Pancho JV, Herberger JP (1997) World weeds: natural histories and distribution. Wiley, New York
Hopper SD (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322:49–86
Houghton JT, Ding Y, Griggs DJ et al (2001) Climate change 2001: the scientific basis. Cambridge University Press, Cambridge
Huber O (1987) Consideraciones sobre el concepto de Pantepui. Pantepui 1:2–10
Huber O (1988) Guayana highlands versus Guayan lowlands, a reappraisal. Taxon 37:595–614
Huber O (1992) La vegetación. In: Huber O (ed) El Macizo del Chimantá. Oscar Todtmann, Caracas, pp 161–178
Huber O (1994) Recent advances in the phytogeography of the Guayana region, South America. Mém Soc Biogéogr 4(3ème sèr):53–63
Huber O (1995a) Geographical and physical features. In: Berry PE, Holst BK, Yatskievych K (eds) Flora of the Venezuelan Guayana, Introduction, vol 1. Missouri Bot Gard Press, St. Louis, pp 1–61
Huber O (1995b) History of botanical exploration. In: Berry PE, Holst BK, Yatskievych K (eds) Flora of the Venezuelan Guayana, Introduction, vol 1. Missouri Bot Gard Press, St. Louis, pp 63–95
Huber O (1995c) Vegetation. In: Berry PE, Holst BK, Yatskievych K (eds) Flora of the Venezuelan Guayana, Introduction, vol 1. Missouri Bot Gard Press, St. Louis, pp 97–160
Huber O (1995d) Conservation of the Venezuelan Guayana. In: Berry PE, Holst BK, Yatskievych K (eds) Flora of the Venezuelan Guayana, Introduction, vol 1. Missouri Bot Gard Press, St. Louis, pp 193–208
Huber O, García P (2011) The Venezuelan Guayana region and the study areas: geo-ecological characteristics. In: Zink A, Huber O (eds) Peatlands of the Western Guayana highlands, Venezuela. Springer, Berlin, pp 29–89
Huber O, Riina R (1997) Glosario fitoecológico de las Américas. In: América del Sur, países hispanoparlantes, vol 1. Tamandúa, Caracas
Huber O, Rull V (2019) Plant communities. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 149–164
Huber O, Prance GT, Kroonenberg S, Antonelli A (2018) The tepuis of the Guiana highlands. In: Hoorn C, Perrigo A, Antonelli A (eds) Mountains, climate and biodiversity. Wiley, Hoboken, NJ, pp 339–353
Kaštovský J, Fučiková K, Veselá J, Brewer Carías C, Vegas-Vilarrúbia T (2019) Algae. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 95–120
Kok PJR, MacCulloch RD, Means DB et al (2012) Low genetic diversity in tepui summit vertebrates. Curr Biol 22:R589–R590
Kok PJR, Russo VG, Ratz S, Aubret F (2016) On the distribution and conservation of two “lost world” tepui summit endemic frogs, Stefania ginesi Rivero, 1968 and S. satelles Señaris, Ayarzagüena, and Gorzula, 1997. Amphibian Reptile Conserv 10:5–12
Kok PJR, Russo VG, Ratz S et al (2017) Evolution in the South American ‘lost world’: insights from multilocus phylogeography of stefanias (Anura, Hemiphractidae, Stefania). J Biogeogr 44:170–181
Kok PJR, Ratz S, MacCulloch RD et al (2018) Historical biogeography of the palaeoendemic toad genus Oreophrynella (Amphibia: Bufonidae) sheds a new light on the origin of the Pantepui endemic terrestrial biota. J Biogeogr 45:26–36
Lasso C, Machado-Allison C, Pérez R (1989) Consideraciones zoogeográficas del los peces de la Gran Sabana (Alto Caroní) Venezuela, y sus relaciones con las cuencas vecinas. Mem Soc Cien Nat La Salle 49:109–129
Leite YLR, Kok PJR, Weksler M (2015) Evolutionary affinities of the ‘lost world’ mouse suggest a late Pliocene connection between the Guiana and Brzailian shields. J Biogeogr 42:706–715
Lew D, Lim B (2019) Mammals. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 333–356
Maguire B (1970) On the flora of the Guayana highland. Biotropica 2:85–100
Mayr E, Phelps WH (1967) The origin of the bird fauna of the South Venezuela highlands. Bull Am Mus Nat Hist 136:273–327
McCormack JE, Huang H, Knowles LL (2009) Sky islands. In: Gillespie R, Clague D (eds) Encyclopedia of islands. University of California Press, Berkeley, pp 839–843
McDiarmid RW, Donnelly MA (2005) The herpetofauna of the Guayana highlands: amphibiands and reptiles of the lost world. In: Donnelly MA, Crother BI, Guyer C, Marvalee H, White ME (eds) Ecology and evolution in the tropics. University of Chicago Press, Chicago, pp 461–560
Mecchia M, Sauro F, Piccini L, Columbu A, De Waele J (2019) A hybrid model to evaluate subsurface chemical weathering and fractures kartsification in quartz sandstone. J Hydrogeol 572:745–760
Michelangeli F (2000) Species composition and species-area relationships in vegetation isolates on a summit of a sandstone mountain in southern Venezuela. J Trop Ecol 16:69–82
Morrone JJ (2014) Biogeographical regionalisation of the Neotropical region. Zootaxa 3782:1–110
Mutke J, Bathlott W (2005) Patterns of vascular plant diversity at continental to global scales. Biol Skr 55:521–531
Nogué S, Rull V, Vegas-Vilarrúbia T (2009) Modelling biodiversity loss by global warming on Pantepui, northern South America: projected upward migration and potential habitat loss. Clim Chang 96:77–85
Ochoa JA, Rojas-Runjaic F (2019) Scorpions. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 223–241
Pavan SE, Jansa SA, Voss RS (2016) Spatiotemporal diversification of a low-vagility Neotropical vertebrate clade (short-tailed opossums, Didelphidae: Monodelphis). J Biogeogr 43:1299–1309
Pérez-Emán JL (2005) Molecular phylogenetics and biogeography of the Neotropical redstars (Myoborus miniatus). Ornitol Monogr 67:511–528
Pérez-Emán JL, Lentino M, Bonaccorso E (2019) Birds. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 299–332
Richardson DM, Pyšek P, Remánek M et al (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107
Riina R, Berry PE, Huber O, Michelangeli F (2019) Vascular plants and bryophytes. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 121–147
Rull V (2004) Biogeography of the “lost world”: a paleoecological perspective. Earth-Sci Rev 67:125–137
Rull V (2005) Biotic diversification in the Guayana highlands: a proposal. J Biogeogr 32:921–927
Rull V (2008) Speciation timing and neotropical biodiversity: the Tertary-Quaternary debate in the light of molecular phylogenetic evidence. Mol Ecol 17:2722–2729
Rull V (2009) Pantepui. In: Gillespie R, Clague D (eds) Encyclopedia of islands. University of California Press, Berkeley, pp 717–720
Rull V (2010) The Guayana highlands: a natural laboratory for the biogeographical and evolutionary study of the neotropical flora. In: Sanchez-Villagra MM, Aguilera O, Carlini AA (eds) Urumaco and Venezuelan Paleontology. The fossil record of the northern neotropics. Indiana University Press, Bloomington, pp 84–102
Rull V (2011) Neotropical biodiversity: timing and potential drivers. Trends Ecol Evol 26:508–513
Rull V (2013) Some problems in the study of the origin of neotropical biodiversity using palaeoecological and molecular phylogenetic evidence. Syst Biodivers 11:415–423
Rull V (2015) Pleistocene speciation is not refuge speciation. J Biogeogr 42:602–609
Rull V (2019a) Neotropical diversification: historical overview and conceptual insights. In: Rull V, Carnaval A (eds) Neotropical diversification: patterns and processes. Springer, Berlin
Rull V (2019b) Origin and evolution of the Pantepui biota. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 69–91
Rull V, Montoya E (2017) Holocene vegetation dynamics on the Apakará summit of the neotropical Guayana highlands and potential environmental drivers. Rev Palaeobot Palynol 240:22–32
Rull V, Nogué S (2007) Potential migration routes and barriers for vascular plants of the neotropical Guyana highlands during the quaternary. J Biogeogr 34:1327–1341
Rull V, Vegas-Vilarrúbia T (2006) Unexpected biodiversity loss under global warming in the neotropical Guayana highlands: a preliminary appraisal. Glob Chang Biol 12:1–9
Rull V, Vegas-Vilarrúbia T (2008) Biopiracy rules hinder conservation efforts. Nature 453:26
Rull V, Vegas-Vilarrúbia T (2017) Potential responses of vascular plants from the pristine ‘lost world’ of the neotropical Guayana highlands to global warming: review and perspectives. Front Plant Sci 8:81
Rull V, Vegas-Vilarrúbia T (2019a) Pantepui as a dynamic biogeographical concept. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 55–67
Rull V, Vegas-Vilarrúbia T (2019b) Pantepui and global warming. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 403–417
Rull V, Vegas-Vilarrúbia T, Nogué S, Montoya E (2009) Bureaucratic obstruction of conservation science in the Guayana highlands. Conserv Biol 22:508–509
Rull V, Vegas-Vilarrúbia T, Safont E (2016) The lost world’s pristinity at risk. Divers Distrib 22:995–999
Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (2019a) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London
Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (2019b) Definition and characterization of the Pantepui biogeographical province. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 3–32
Rull V, Montoya E, Nogué S, Safont E, Vegas-Vilarrúbia T (2019c) Climatic and ecological history of Pantepui and surrounding areas. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 33–54
Safont E, Vegas-Vilarrúbia T, Rull V (2012) Use of environmental impact assessment (EIA) tools to set priorities and optimize strategies in biodiversity conservation. Biol Conserv 149:113–121
Safont E, Rull V, Vegas-Vilarrúbia T et al (2014) Establishing a baseline of plant diversity and endemism on a neotropical mountain summit for future comparative studies assessing upward migration: an approach from biogeography and nature conservation. Syst Biodivers 12:292–314
Safont E, Rull V, Vegas-Vilarrúbia T et al (2016) Late-Holocene vegetation and fire dynamics on the summits of the Guayana highlands: the Uei-tepui palynological record. Palaeogeogr Palaeoclimatol Palaeoecol 455:33–43
Salerno PE, Ron SR, Señaris JC et al (2012) Ancient tepui summits harbor young rather than old lineages of endemic frogs. Evolution 66:3000–3013
Salerno PE, Señaris JC, Rojas-Runjaic FJM, Cannatella DC (2014) Recent evolutionary history of lost world endemics: population genetics, species delimitation, and phylogeography of sky-island treefrogs. Mol Phylogenet Evol 82:314–323
Señaris JC, Rojas-Runjaic F (2019) Amphibians and reptiles. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 263–297
Smith BT, McCormack JE, Cuervo AM et al (2014) The drivers of tropical speciation. Nature 515:406–409
Solomon S, Qin D, Manning M (2007) Climate change 2007: the physical science basis. Cambridge University Press, Cambridge
Steyermark JA (1979) Plant refuge and dispersal centres in Venezuela: their relict and endemic element. In: Larsen K, Holm-Nielsen L (eds) Tropical botany. Academic, New York, pp 185–221
Steyermark JA, Dunsterville GCK (1980) The lowland floral element of the summit of Cerro Guaiquinima and other cerros of the Guayana Highlands of Venezuela. J Biogeogr 7:285–303
Steyermark JA, Berry PE, Holst BK (1995–2005) Flora of the Venezuelan Guayana, vol 1–9. Missouri Bot Gard Press, St. Louis
Stocker TF, Qin D, Plattner G-K et al (2013) Climate change 2013: the physical science basis. Cambridge University Press, Cambridge
Tate GHH (1938) A new ‘lost world’. Nat Hist 2:107–120
Vareschi V (1992) Observaciones sobre la dinámica vegetal en el Macizo del Chimantá. In: Huber O (ed) El Macizo del Chimantá. Oscar Todtmann, Caracas, pp 179–188
Vegas-Vilarrúbia T, Nogué S, Rull V (2012) Global warming, habitat shifts and potential refugia for biodiversity conservation in the neotropical Guayana highlands. Biol Conserv 152:159–168
Velazco PM, Patterson BD (2008) Phylogenetics and biogeography of the broad-nosed bats, genus Platyrrhinus (Chiroptera: Phyllostomidae). Mol Philogenet Evol 49:749–759
Viloria AL, Costa M (2019) Butterflies. In: Rull V, Vegas-Vilarrúbia T, Huber O, Señaris C (eds) Biodiversity of Pantepui, the pristine “lost world” of the neotropical Guiana highlands. Elsevier, London, pp 193–222
Voss RS, Lim BK, Díaz-Nieto JF, Jansa SA (2013) A new species of Marmosops (Marsupialia: Didelphidae) from the Pakaraima Highlands of Guyana, with remarks on the origin of the endemic Pantepui Mammal fauna. Am Mus Novit 3778:27
Whittaker RJ, Fernández-Palacios JM (2007) Island biogeography: ecology, evolution and conservation. Oxford University Press, Oxford
Willis KJ, Whittaker RJ (2000) The refugial debate. Science 287:1406–1407
Zink JA, Huber O (2011) Peatlands of the Western Guayana Highlands, Venezuela. Springer, Berlin
Acknowledgements
This paper is a homage to the pioneers of Pantepui scientific exploration, without whom nothing reported here would have been possible (“we stand on the shoulders of giants”). The first author is especially grateful to Otto Huber, who introduced him to Pantepui research in 1984 and led many of the expeditions in which he has participated. The authors are grateful to Abrahram Breure, Mauro Costa, Tomas Derka, Ricardo Guerrero, Jan Kaštovský, Burton Lim, Javier Mesa, José Ochoa, Jorge-Pérez-Emán, Fernando Rojas-Runjaic, Celsa Señaris, David Southall and Ángel Viloria for providing photographs and information for Figs. 15.8 and 15.9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rull, V., Vegas-Vilarrúbia, T. (2020). The Pantepui “Lost World”: Towards a Biogeographical, Ecological and Evolutionary Synthesis of a Pristine Neotropical Sky-Island Archipelago. In: Rull, V., Carnaval, A. (eds) Neotropical Diversification: Patterns and Processes. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-31167-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-31167-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31166-7
Online ISBN: 978-3-030-31167-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)