Abstract
Water is a solvent that is absolutely essential to sustain life. It is one of the world’s most precious resources. The uniqueness consists in its polarity, high boiling point, and some other important properties. Several of the water parameters, for example, alkalinity or redox potential, were discussed in this chapter. The environmental significance of these basic parameters is enormous because they are affecting the stability and mobility of substances in the ecosystem, and other parameters are either directly determined or strongly influenced by them. In addition, further important geochemical aspects, presented, for example, in the graphical form, i.e., the pε-pH diagram (Pourbaix diagram) or redox ladder, were thoroughly explained in this chapter. Subsequently, the in situ chemical reduction (ISCR) techniques were discussed with the special emphasis on the nano zero-valent iron (nZVI). The nZVI reactions and thermodynamics behind them were also reviewed here.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Water is the carrier of the entire geochemical system of natural exogenous processes. Water has a number of unique properties that determine the behavior of the rock matrix and biogenic components. Its basic properties are a high dielectric constant (Malmberg and Maryott 1956), high surface tension, and high heat capacity. Natural water contains dissolved substances, some of which have reduction or oxidation properties. This creates a geochemical balance between the solvent (water), the dissolved substance, and the rock matrix. Altering these conditions also leads to changes in the water chemistry. A significant change in the environment is alkalinity, which in natural waters is mainly a function of the sum of carbonate species and OH−. Therefore, the geochemical system has a certain inertia and, to some degree, can buffer natural oscillations or cause anthropogenically induced changes.
The basic chemical processes used in remediation are mainly changes in acid-alkaline conditions, redox conditions, or the concentration of species causing precipitation. Since the two basic components of chemical reactions are protons and electrons, their exchanges in redox processes and acid-alkaline reactions are fundamental in nature. These processes, which are observed as a reaction of mineral precipitation, weathering, dissolution, or organic matter decomposition, etc., are fundamental reactions occurring on the surface of the earth’s crust because of the contact between rock, water, and the atmosphere, and determine the parameters of the surrounding environment. While the significance of pH for the toxicity and mobility of hazardous substances (heavy metals, radionuclides, organic substances, etc.) has been known for a long time, the importance of the redox potential has been significantly underestimated or even neglected except for the oxidation zone of ore deposits. It is commonly assumed that pH is a critical parameter for the state of the natural environment so the other parameters (redox potential, content of dissolved solids in the aqueous environment, concentration of gases) play just a minor role. However, the results of systematic studies on the natural environment and natural processes show that the decisive factors in most cases are redox processes and pH, and the other parameters are either directly determined or strongly influenced by these processes.
2 Stability of Redox Conditions
The stable redox environment in the geosphere is caused by biota that enables photosynthetic processes. This biota synthesizes complex organic substances from carbon dioxide using solar energy according to the following simplified equation of photosynthesis (Eq. 1.1)
where CH2O is a general formula for organic matter (like glucose C6H12O6). During the process, 472 kJ/mol of solar energy is consumed (Stumm and Morgan 1995) and the energy is “stored” and subsequently used to secure the life processes of the biota. When looking at the oxidation state (formal charge, valence) of the substance or compound, reduction of carbon occurs through photosynthesis from oxidation state (+IV) to (0) and an oxidation of oxygen from oxidation state –II to 0:
Photosynthesis ensures a constant renewal of elemental oxygen, the main oxidant in the geosphere, and produces organic compounds, which enter the soil and water after the biota dies. These organic substances then act as reducing agents through respiration or decay (natural decomposition of organic substances). This process is actually the above-mentioned photosynthetic reaction (Eq. 1.2) in reverse; oxygen is consumed releasing carbon dioxide, water, and energy.
Another very important component of the natural geochemical system is iron. Iron is the most widespread transient metal element and the second most widespread metal on Earth. The content of iron in the Earth’s crust is 62 g/kg. In nature, it is mainly found in valence states of Fe2+ and Fe3+. Under oxidation conditions, stable iron is in the valence state of Fe3+; under reducing conditions, it is in the valence state of Fe2+, and minerals containing Fe3+ are also common. Besides oxidation of organic matter, a change in the valence state of Fe is the main process that generates electron transfer in nature. Electrons are generated during oxidation and consumed during reduction. Fe4+ compounds are very unstable and have no practical significance, Fe5+ and Fe6+ compounds are used as very strong oxidizing agents but are not found in nature. Pure iron rarely occurs in nature (in volcanites). Iron in low valence states (Fe0) can be used as a reducing agent.
In the case of iron (ZVI nanoparticles), it is known that water and dissolved oxygen cause iron oxidation, which leads to corrosion (rusting) by the following Eq. (1.3):
The very name of the redox process—oxidation-reduction reaction—implies that there are two processes occurring—oxidation and reduction, which are inseparable, simultaneous, and dependent. This is a contrast to acid-base reactions, where protons (H+ ions) are released by, e.g., acid dissolution in water, solvated and stable in a water environment.
Oxygen participates in the above process in the reduction part in which electrons are consumed and the oxidation state of oxygen reduces according to the formal reaction (Eq. 1.4).
Reversely, iron participates in the oxidation process in which electrons are released and the iron oxidation state increases.
By combining these two equations—reduction (Eq. 1.4) and oxidation (Eq. 1.5) respectively—we obtain the final equation for iron rusting, where two electrons are exchanged between the iron and oxygen. For such a heterogeneous process, the surface area of the solid reagent is the key parameter for the reaction rate. This has a practical significance only in the case of systems described by a single process because comparison with a more complex process is ambiguous (Tiehm et al. 1997).
3 Quantitative Expression of Redox Potential
Redox potential is an intensive parameter of the environment and expresses the overall potential of the system for oxidation-reduction processes, just as pH expresses the acidity of acid-base reactions. Usually, the redox potential for a general oxidation-reduction reaction (Stumm and Morgan 1995).
is derived from the well-known Nernst equation (Eq. 1.7).
where Eh is the potential of the redox reaction (Eq. 1.6). E0 is the standard potential, i.e., potential in unit activities of oxidized (Ox) and reduced (Red) forms of the substance, R is the universal gas constant, T is thermodynamic temperature, F is Faraday’s constant, n is the number of electrons that are exchanged during the redox reaction between Ox and Red species of the redox pair, aOx and aRed are activities of oxidized and reduced species, respectively.
Analogically to pH, which expresses the activity of protons in a solution in the form of a negative decadic logarithm, it is possible to express Eh as the pε, negative decadic logarithm of the activity ae− of electrons in the environment:
with the relation
where ae− is the activity of electrons.
To measure the potential of electrochemical reactions, it is necessary to use two electrodes between which the potential is measured. One electrode measures the observed response, the other serves as a reference. Platinum is a good material for the electrodes (Schuettler 2007) because it is resistant in most environments and is not subject to its own redox reactions. The reference standard hydrogen electrode (SHE) is a plate coated with spongy black platinum, which is saturated by gaseous hydrogen H2 at a pressure of 101.325 kPa and is immersed in a solution with a unit activity of H+.
The electrochemical reaction.
on the reference electrode under standard conditions is arbitrarily given a potential (Eh) of 0 V. For practical reasons, redox potential is measured using other reference electrodes with a stable potential instead of SHE. The most commonly used reference electrodes are Ag/AgCl (silver chloride) (Ives and Janz 1961) and Hg/Hg2Cl2 (calomel) electrodes. Silver chloride electrodes consist of a silver wire coated with AgCl precipitate, which is immersed in a KCl solution with a specific concentration. Based on the KCl concentration in the solution, the potential of the electrode ranges from 197 mV (saturated KCl) to 288.1 mV (0.1 M KCl) and is temperature-dependent. The saturated calomel electrode whose potential is about 247 mV (at 20 °C) works in a similar way. The measured potential must then be recalculated to SHE by adding the above-mentioned values depending on the reference electrode used and the temperature. The values not corrected to SHE are sometimes reported as ORP (oxidation-reduction potential) compared to Eh or ORPH related to SHE.
4 Stability of Water and Eh-pH Diagrams
The acidity and redox properties of an aqueous environment are the basic parameters affecting the stability and mobility of substances in this environment (Violante et al. 2010). These properties and their influence on the state of a substance can be summarized in the form of Eh-pH (or pε-pH) diagrams. The redox conditions (like pH conditions) of an aqueous environment cannot acquire unlimited values as they are limited by the reaction of water with other substances. Since water in most cases is in contact with the atmosphere (either with the normal atmosphere containing oxygen or with the soil atmosphere containing carbon dioxide or hydrogen), the processes limiting the Eh values are the oxidation or reduction reactions of water producing these gases. Both types of reactions are very similar in principle (Table 1.1). Oxidation can be expressed as a reaction in which water loses the electrons and oxygen gas is formed; and reduction as a reaction where water acquires electrons and hydrogen gas is formed.
The pε values were calculated by the following conditions: In both equations of equilibrium constants, there is a partial pressure of the gas involved. The partial pressure can be equal to 1 and its log = 0. The equilibrium constant for the oxidation of water at 25 °C and pressure 0.1 MPa has a value of log K = −41.56 and the water has a unit activity; for the reduction process the log K = 0 according to (Eq. 1.10). These curves are the limits of the pε-pH graph of water stability because higher and lower values for water oxidation and reduction, respectively, are not thermodynamically possible.
On the pε-pH diagram (Pourbaix diagram) these are the parallel lines with a slope − 1, which intersect the vertical axis at pH = 0 at a value of 20.78 (upper limit) and 0 (lower limit). In Fig. 1.1 these curves are shown on the Eh-pH and pε-pH diagrams and the areas of conditions characteristic of various natural environments are indicated. It should be noted that the value of the redox potential without expressing the pH cannot determine whether the conditions are oxidizing or reducing. For example, a redox potential of +400 mV in acidic water represents reducing conditions (peat bogs), but in alkaline water, it represents oxidizing conditions (saline lakes, saline soils).
pH values and redox potential in Eh and pε scales for various natural environments. Under conditions that lie outside the defined boundaries, the water is unstable, and oxidation occurs through the release of gaseous oxygen (upper limit) or reduction through the release of hydrogen gas (lower limit)
5 Problems of Measurement and Interpretation of Redox Potentials
The redox potential of an environment is not an absolutely specified property. It is determined by the substances present in the environment. Substances that can release (reduction agent) or accept electrons (oxidizing agent) change the electron activity in the environment, thus changing the Eh. The most important substances in the environment determining the redox potential are presented in Table 1.2. Taking into consideration the fact that many of these substances are multi-electron transition components or elements, the overall reaction occurs as a multistage cascade of partial reactions with equilibriums between forms of substances (species) in various stages of oxidation state. Calculation of the redox potential based on the analytical concentration of individual redox pairs is very difficult for many reasons (Pitter 2009):
-
the presence of several redox pairs simultaneously,
-
slow reaching thermodynamic equilibrium,
-
dynamic stationary state of the system with a low proportion of oxidized or reduced components,
-
multi-electron transition of electrons in redox pairs.
In many cases, the redox potential in natural water is controlled by a redox pair of divalent and trivalent iron
with the following relationship applied for the activity of the electrons
where log K = −13.01 (under standard conditions). The corresponding value of Eh can be expresses by Eq. (1.9). The analytically determined concentrations of Fe+II and Fe+III are usually taken for calculating Eh. However, under real conditions, both oxidation states of iron occur not only as free ions Fe2+ and Fe3+ but also as many hydroxo- and other complexes (sulfate, chloride, etc.). The activities of these ions are then significantly different from the analytical total concentrations (often almost negligible) of the valence forms and therefore the Eh calculation is incorrect.
This behavior can be documented using a simple example: in a solution at pH = 4.88 and Eh = 481.8 mV, there is 20 μmol/L of dissolved iron (1.12 mg/L). Analytically, the concentrations of dissolved iron in a divalent form of 17.4 μmol/L (87.2% of the total concentration of dissolved iron) and in a trivalent form of 2.4 μmol/L were determined. The redox potential calculated from these total concentrations using the relations (Eq. 1.12) and (Eq. 1.9) gives Eh = 720 mV, a significantly different value from the input value of 481.1 mV. This also shows an oxidizing environment, while the actual redox potential corresponds to anoxic or mildly reducing conditions (Fig. 1.1). Under the given conditions, both cations undergo a complexation reaction with the hydroxyl anion and form hydroxocomplexes. While free Fe2+ ions are prevalent, constituting 99.63% of its total Fe+II content (Fe(OH)+ being in the minority), for a trivalent iron hydroxocomplex, Fe(OH)2+ constitutes 92.96% of its overall content and free Fe3+ ions contribute only negligibly to the total content (less than 0.01%). If we use the free concentrations of the participating substances calculated by the speciation model, we obtain the true value of 481.8 mV for the redox potential.
6 Geochemical Processes in Water
The main geochemical parameters and the stability of the environment are determined by pH and the oxidative reduction potential of Eh. The stability of pH in natural waters is determined by the carbonate system and exchange reactions of clay minerals. The acidity of the natural environment is determined by the CO2 cycle and alkalinity by dissolving and weathering limestone and silicate rocks. In the case of Eh, the oxidation capacity reservoir is not only the oxygen itself (in the atmosphere and dissolved in the water), but also oxidized substances (e.g., nitrates). Reduction capacity is determined primarily by dead organic matter and also by reduced substances (e.g., sulfides). Putting aside the crucial redox reactions of biogenic processes (photosynthesis vs. respiration and decomposition), it is possible to describe the most important redox actions in exogenous processes by the following reactions:
-
Oxygen reduction/decomposition of water.
$$ \frac{1}{2}{\mathrm{O}}_2+2{\mathrm{e}}^{\hbox{--} }+2{\mathrm{H}}^{+}\leftrightarrow {\mathrm{H}}_2\mathrm{O} $$(1.13) -
Weathering/crystallization of pyrite.
$$ {\mathrm{Fe}\mathrm{S}}_2+8{\mathrm{H}}_2\mathrm{O}\leftrightarrow {\mathrm{Fe}}^{2+}+2{\mathrm{SO}}_4^{2\hbox{--} }+14{\mathrm{e}}^{\hbox{--} }+16{\mathrm{H}}^{+} $$(1.14) -
Oxidation/reduction of nitrogen.
$$ {\mathrm{NH}}_4^{+}+2{\mathrm{H}}_2\mathrm{O}\leftrightarrow {\mathrm{NO}}_2^{-}+6{\mathrm{e}}^{-}+8{\mathrm{H}}^{+} $$(1.15)$$ {\mathrm{NO}}_2^{-}+{\mathrm{H}}_2\mathrm{O}\leftrightarrow {\mathrm{NO}}_3^{-}+2{\mathrm{e}}^{-}+2{\mathrm{H}}^{+} $$(1.16) -
Oxidation/reduction of Fe2+ according to (Eq. 1.11) and subsequent hydrolysis
$$ {\mathrm{Fe}}^{3+}+3{\mathrm{H}}_2\mathrm{O}\leftrightarrow \mathrm{Fe}{\left(\mathrm{OH}\right)}_3+3{\mathrm{H}}^{+} $$(1.17) -
The dissolution of pyrite as an important reservoir of reduction capacity is illustrated in Fig. 1.2.
Changes in the pH of an environment may be caused by redox reactions but not vice versa. Nevertheless, changes in the pH are usually buffered by the carbonate system in the natural processes. The buffer capacity is the ability of the solution to withstand the addition of an acid or alkali and maintain a near constant pH. It is highest in situations where concentrations of acids and conjugated (linked) bases are comparable. A solution is least buffered in a situation where the concentration of the acid and the conjugated base differs most (one form is mostly present in the solution). Therefore, acidity and alkalinity are important parameters of the natural environment and can fundamentally influence the remedial action conducted by chemically supported technologies.
The Eh value determines the activity of electrons in the natural environment. The greatest electron activity (pε) is in a reducing environment and decreases in an increasing oxidation environment. In natural systems, the general sequence of reduction processes—the so-called redox ladder—applies when a tiered profile of the pε develops, which is valid for a certain time and position until the relevant oxidant is consumed.
The general classification of a redox environment is usually determined by the source of oxygen. In an oxic environment, the source of the oxidation capacity is O2 or NO3−. In a suboxic environment, it is MnO2 or Fe(OH)3, and in a reducing environment it is SO42− or CO2 (Fig. 1.3).
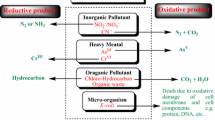
7 The Principle of Remedial Reduction Technologies
For reduction methods, reagents that easily release electrons are used as a reduction agent, thus adjusting the conditions in the groundwater on the lower limit of water stability or even below it (Fig. 1.1). If there are substances that are capable of accepting electrons in the system, oxidation-reduction processes are then in progress. The most commonly used reagents are sodium dithionites (Na2S2O4) (Chung 1981), calcium polysulfide (CaSx) (Wazne et al. 2007), sodium metabisulfite (Na2S2O5) (Chang 2003), sodium hydrosulfide (NaHS), sodium sulfite (Na2SO3) (Bianco Prevot et al. 2018), ferrous sulfate (Fe2SO4) (Mončeková et al. 2016), and currently metallic elements (zero-valent), namely Fe0 (Tosco et al. 2014) iron, in the form of chips or nanoparticles. In addition, organic waste from food production, e.g., whey, which contains the anion of lactic acid (CH3CH(OH)COO−), can be biologically degraded to CO2 with the release of electrons. The reaction is similar to the general degradation of organic compounds caused by oxygen, which is the opposite of photosynthesis expressed by Eq. (1.1). The oxidation reaction for the above-mentioned reducing agents can be expressed by the following equations
Electrons are released in all of these reactions, causing a reduction of the target contaminant. An example can be a reduction of chlorinated hydrocarbon of general formula RX
e.g., of TCE
As opposed to oxidation, the product is not carbon dioxide but non-chlorinated ethylene.
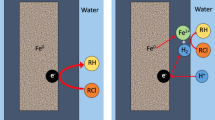
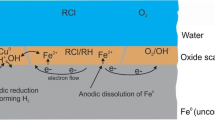
8 Principle of nZVI Application
Iron in a metallic form is not stable in contact with atmosphere and water (Torrey et al. 2015) and undergoes chemical oxidation (rusting). Figure 1.4 shows the Eh-pH stability diagram for dissolved iron of activity 10−6, which corresponds to a concentration of about 0.06 mg/L. As the figure shows, under these conditions, the solution only retains divalent iron because during its oxidization to trivalent iron, the Fe3+ is precipitated in the form of goethite, oxyhydroxide (FeOOH), or magnetite (ferrous-ferric oxide, Fe3O4). Figure 1.4b shows that the area of the elementary iron Fe0 stability lies below the lower limit of the water stability and the iron will always oxidize in contact with oxygenated water according to Eq. (1.3). The area of the Fe2+ stability and solid iron oxyhydroxide (goethite) overlaps and the redox equilibrium in the solution will be set by the reaction.
It is clear that a detailed thermodynamic analysis of a specific environment together with the analytical data on the composition of water, substrate, and contaminants can significantly contribute to determining the optimum remedial strategy.
The reaction of ZVI (and especially nZVI) in water has several steps due to the change in oxidation-reduction conditions of water. Initially, dissolved oxygen causes iron oxidation by Eq. (1.3). In the case of nZVI, which has an extremely large specific surface, the reaction (Eq. 1.3) is rapid. Iron precipitates in the form of ferric oxyhydroxide (FeOOH) or directly as ferric hydroxide Fe(OH)3. The solubility of oxygen depends on the concentration of dissolved substances and decreases as the dissolved substances increase (Pitter and Chudoba 1990). At 10 °C and atmospheric pressure (101.3 kPa) the solubility of oxygen in water is 11.3 mg/L. After consumption of all the oxygen present and a decrease in the redox potential, groundwater reacts with the iron under anoxic conditions through the process of corrosion with hydrogen production
Since the reaction (Eq. 1.28) is slower, the loss of elemental iron is not as intense as it is during aerobic corrosion (Eq. 1.3). On the other hand, hydrogen generated during the anaerobic corrosion can promote the growth of anaerobic microorganisms that can also dehalogenate chlorinated hydrocarbons. The growth of microorganisms on the surface of the iron is influenced by the porosity of the material, hence the size of the reactive surface. In strongly reducing environments consisting of cinder pig, there is often an increase in concentrations of methane and light gaseous hydrocarbons (e.g., propane, butane) (Pitter and Chudoba 1990). This may be caused by either a reduction of the CO2 present in the groundwater or by the hydrogenation of carbon, which is present in the iron as an admixture produced by hydrogen during anaerobic corrosion.
Because of iron corrosion under both aerobic and anaerobic conditions, there is an increase in the OH− concentration resulting in an increase in the pH value of the system. This often rises to values of around 10 depending on the buffer capacity of the groundwater (carbonate content in particular). Dissolved carbon dioxide and bicarbonate also inhibit the increase in pH by the following reactions
and help to buffer the system, which is important for both chemical and biological reduction processes.
In the real environment of natural water, other complexes (sulfate, carbonate, chloride, etc.) are present and must be included in the balance calculation, and therefore the speciation model is essential for an estimation of redox conditions.
Zero-valent iron can reduce chlorinated compounds to harmless components by a combination of Eqs. (1.25) and (1.28). The oxidation-reduction reaction is:
This process can be accomplished in several ways by nZVI. The major processes are as follows (Fig. 1.5):
-
1.
R-Cl reduction at Fe surface
$$ {\mathrm{Fe}}^0+\mathrm{R}-\mathrm{Cl}+{\mathrm{H}}^{+}\to {\mathrm{Fe}}^{2+}+\mathrm{R}-\mathrm{H}+{\mathrm{Cl}}^{\hbox{--} } $$(1.32) -
2.
R-Cl reduction by Fe2+ oxidation to Fe3+
$$ 2{\mathrm{Fe}}^{2+}+\mathrm{R}-\mathrm{Cl}+{\mathrm{H}}^{+}\to 2{\mathrm{Fe}}^{3+}+\mathrm{R}-\mathrm{H}+{\mathrm{Cl}}^{\hbox{--} } $$(1.33) -
3.
Fe0 reaction with water and hydrogen production
$$ {\mathrm{Fe}}^0+2{\mathrm{H}}_2\mathrm{O}\to {\mathrm{Fe}}^{2+}+{\mathrm{H}}_2+2{\mathrm{OH}}^{\hbox{--} } $$(1.34) -
4.
R-Cl reduction by hydrogen
$$ {\mathrm{H}}_2+\mathrm{R}-\mathrm{Cl}\to \mathrm{R}-\mathrm{H}+{\mathrm{H}}^{+}+{\mathrm{Cl}}^{\hbox{--} } $$(1.35)
All these processes play a role in R-Cl reduction.
From a thermodynamic point of view, a transfer of electrons according to Eq. (1.31) in one step is not very probable and is possible only if the R-Cl molecule remains on the iron surface for a sufficiently long period of time. There are two reaction paths considered—sequential hydrogenolysis and β-elimination (Janda et al. 2004; Liu et al. 2005).
8.1 Sequential Hydrogenolysis
During sequential hydrogenolysis, C-Cl bonds in halogenated hydrocarbons are gradually replaced by the C-H bonds under sufficiently strong reducing conditions to form Cl− and less chlorinated hydrocarbon according to Eq. (1.25). The feasibility of hydrogenolysis depends on the type of hydrocarbon and decreases from aliphatic to aromatic hydrocarbons. Hydrogenolysis is also influenced by the presence and location of substituents and heteroatoms.
8.2 Reductive β-Elimination
During reductive β-elimination of halogenated organic substances, multiple bonds (e.g., triple) between carbon atoms are created, and halogens are simultaneously removed from molecules in the form of halides. For the multiple bond formation, the halogen atoms must be bound to two adjoining carbon atoms. The reaction of halogenated ethene (e.g., 1,2-DCE) can be generally described by the equation
where acetylene is the final product.
References
Bianco Prevot A, Ginepro M, Peracaciolo E, Zelano V, De Luca DA (2018) Chemical vs bio-mediated reduction of hexavalent chromium. An in-vitro study for soil and deep waters remediation. Geoderma 312:17–23. https://doi.org/10.1016/j.geoderma.2017.09.032
Chang L-Y (2003) Alternative chromium reduction and heavy metal precipitation methods for industrial wastewater. Environ Prog Sustain Energy 22(3):174–182. https://doi.org/10.1002/ep.670220315
Chung SK (1981) Mechanism of sodium dithionite reduction of aldehydes and ketones. J Org Chem 46(26):5457–5458. https://doi.org/10.1021/jo00339a057
Ives DJG, Janz GJ (eds) (1961) Reference electrodes: theory and practice. Academic, New York
Janda V, Vasek P, Bizova J, Belohlav Z (2004) Kinetic models for volatile chlorinated hydrocarbons removal by zero-valent iron. Chemosphere 54(7):917–925. https://doi.org/10.1016/j.chemosphere.2003.08.033
Liu Y, Majetich SA, Tilton RD, Sholl DS, Lowry GV (2005) TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ Sci Technol 39(5):1338–1345. https://doi.org/10.1021/es049195r
Malmberg CG, Maryott AA (1956) Dielectric constant of water from 0° to 100° C. J Res Natl Bur Stand 56(1):2641
Mončeková M, Novotný R, Koplík J, Kalina L, Bílek V, Šoukal F (2016) Hexavalent chromium reduction by ferrous sulphate heptahydrate addition into the Portland clinker. Proced Eng 151:73–79. https://doi.org/10.1016/j.proeng.2016.07.382
Pitter P (2009) Hydrochemie, 4th edn. VŠCHT Praha, Prague
Pitter P, Chudoba J (1990) Biodegradability of organic substances in the aquatic environment. CRC Press, Boca Raton
Schuettler M (2007) Electrochemical properties of platinum electrodes in vitro: comparison of six different surface qualities. In: 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 22–26 Aug. 2007, pp 186–189. https://doi.org/10.1109/IEMBS.2007.4352254
Stumm W, Morgan JJ (1995) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York
Tiehm A, Stieber M, Werner P, Frimmel FH (1997) Surfactant-enhanced mobilization and biodegradation of polycyclic aromatic hydrocarbons in manufactured gas plant soil. Environ Sci Technol 31(9):2570–2576. https://doi.org/10.1021/es9609967
Torrey JD, Killgore JP, Bedford NM, Greenlee LF (2015) Oxidation behavior of zero-valent iron nanoparticles in mixed matrix water purification membranes. Environ Sci Water Res Technol 1(2):146–152. https://doi.org/10.1039/C4EW00068D
Tosco T, Petrangeli Papini M, Cruz Viggi C, Sethi R (2014) Nanoscale zerovalent iron particles for groundwater remediation: a review. J Clean Prod 77:10–21. https://doi.org/10.1016/j.jclepro.2013.12.026
Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10(3):268–292. https://doi.org/10.4067/S0718-95162010000100005
Wazne M, Jagupilla SC, Moon DH, Jagupilla SC, Christodoulatos C, Kim MG (2007) Assessment of calcium polysulfide for the remediation of hexavalent chromium in chromite ore processing residue (COPR). J Hazard Mater 143(3):620–628. https://doi.org/10.1016/j.jhazmat.2007.01.012
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Černík, M., Zeman, J. (2020). Geochemical Principles of Reductive Remediation Processes. In: Filip, J., Cajthaml, T., Najmanová, P., Černík, M., Zbořil, R. (eds) Advanced Nano-Bio Technologies for Water and Soil Treatment. Applied Environmental Science and Engineering for a Sustainable Future. Springer, Cham. https://doi.org/10.1007/978-3-030-29840-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-29840-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29839-5
Online ISBN: 978-3-030-29840-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)