Abstract
Chemoprevention of cancer is an emerging field: using well-known supplements or medications to prevent carcinoma before invasion. Numerous agents are being studied to try to prevent esophageal malignancy given its high burden of mortality and late stage at diagnosis.
Proton pump inhibitors (PPIs), widely used in symptom management, have a role in controlling the progression of Barrett’s esophagus. Studies have moved towards defining optimum dosing required for PPIs as a chemopreventive agent.
Aspirin is implicated in preventing esophageal cancer via pathways including COX inhibition and beta-catenin. Its use for colorectal cancer prevention is already clear, and its use in cardiovascular disease gives it promise as a cheap, multi-disease agent.
Statins hold promise as an agent that can both benefit cardiovascular disease and prevent cancer. Studies show links with esophageal cancer, although safety concerns and numbers needed to treat are so far high.
Metformin is linked with reduced numbers of all cancers in diabetic patients. Its role in esophageal chemoprevention is still being explored, but helping to prevent other factors such as diabetes and obesity could augment this.
Further high-quality studies are required, and risk stratification of patients at higher cancer potential is likely to shape the use of chemoprevention in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

Keywords
An emerging focus over the last few decades has been into cancer chemoprevention, using supplements or medication to avoid or delay the potential medical and psychological catastrophe of a cancer diagnosis. The idea is to take a safe, economically viable, well-tolerated, and well-understood medication which, given to a group in the population, could prevent carcinoma before invasion or at least delay the premalignant process to a later time point.
Esophageal cancer carries a huge burden of morbidity and mortality to patients around the world, with the UK having one of the worst rates of adenocarcinoma [1]. At diagnosis the disease is often at an advanced stage, surgery is extremely invasive, chemotherapy and radiotherapy treatments are aggressive, and endoscopic treatments limited to tertiary centers in areas of higher socioeconomic strength. Chemoprevention is an exciting prospect for this condition given the potential impact on patients and the potential relief to healthcare systems as populations age. Chemoprevention has been a key focus in other areas of medicine and has been extremely effective in reducing the burden of disease in cardiology, and many medications used in large populations for this purpose hold promise in cancer chemoprevention as will be described. The challenge going forward is narrowing down which agents can be attractive to large populations of essentially healthy patients in the hope of preventing malignancy. Described below is an overview of the evidence for a few of the key areas of interest for esophageal chemoprevention, with an exploration of the associated side effects and some considerations for the future.
Proton Pump Inhibitors
Acid exposure plays an important role in the initiation of Barrett’s esophagus (BE) and its progression to esophageal adenocarcinoma; therefore, proton pump inhibitors (PPIs) have been historically used as the backbone of medical treatment for the symptoms of gastroesophageal reflux disease (GERD). Several studies have investigated the role of proton pump inhibitors in the prevention of progression from BE to esophageal adenocarcinoma. In a large prospective cohort study, 75% of patients known to have BE and taking PPI had a reduction in the risk of neoplastic progression, independent of age, gender, BE length, esophagitis, histology, and use of other medications [2].
Other studies have shown that despite PPI use, 20% of BE patients experience pathological reflux, hence none of the PPIs have been proven to completely prevent neoplastic progression [3]. Maintenance of normal epithelial differentiation and cell proliferation is an important goal in cancer chemoprevention. Bearing in mind that intermittent esophageal acid exposure enhances cell proliferation, which is well correlated with the development of dysplasia, this may explain why BE patients remain at a certain risk for neoplastic progression during PPI use.
Several studies have hypothesized that effective intra-esophageal acid suppression may be beneficial in the long-term treatment of BE patients, due to the theoretical and logical concept that acid suppression should lead to well-differentiated BE epithelia while also minimizing cell proliferation, and thus should reduce the likelihood of progression to dysplasia or adenocarcinoma [4]. A systematic review that pooled the results of several trials investigating chemoprevention of esophageal adenocarcinoma reported mixed results [5]; some studies reported that PPIs cause regression of BE [6, 7], while others failed to reach statistical significance [8, 9]. These discrepancies in the literature at that stage resulted mainly from a lack of standardized method for measuring the length and distribution of Barrett’s [10]. A lack of correlation between the acid suppression and symptom relief might also mean higher doses of PPI are required to achieve therapeutic acid suppression [11]. This concept has been investigated in a study which reported that standard doses of PPIs administered to BE patients could relieve symptoms of GERD after a 6-month period, but many participants continued to have pathological acid reflux as measured by 24-h pH monitoring, and remained, therefore, at risk of developing adenocarcinoma [4].
Peters et al. performed a randomized double-blind study, in which participants were given 40 mg omeprazole twice a day and underwent pH esophageal monitoring to confirm adequate acid suppression. After 2 years, there was a statistically significant regression of BE [12]. There is a paucity of data investigating the cellular effects of PPI treatment. Absolute suppression of acid reflux has been shown to reduce cell proliferation [4, 13] and increase expression of the cyclin-dependent kinase inhibitors p16 and p21 [14]. This therefore suggests that aggressive acid suppression may influence the alterations in cell cycle control that occur during carcinogenesis; reducing risk and therefore also supporting the findings reported by Peters et al. [12].
Whether aggressive acid-lowering treatment can modify the risk of cancer development is still unconfirmed due to a lack of robust clinical trials investigating this question. It may be that transformation of Barrett’s to dysplasia is the most important step that should be focused upon rather than regression of Barrett’s epithelium. A prospective analysis of over 200 patients over a 20-year period has shown that PPIs significantly reduced risk of dysplasia in Barrett’s esophagus [15]. This study, unfortunately, remains in relative isolation; however, preliminary data is emerging from the Aspect trial, which is the largest randomized controlled trial looking at aspirin plus high or low dose omeprazole. Data presented at the ASCO annual conference at the time of writing showed high dose (40 mg BD) esomeprazole, in combination with aspirin, provided a significant effect on all-cause mortality in Barrett’s patients versus 20 mg once daily if taken for at least 7 years. These data are encouraging but it is important to note that the study enrolled Barrett’s confirmed cases and so this does not yet represent a course for all GERD cases, pending further information from the full dataset.
NSAIDs/Aspirin
Aspirin, a key agent in cardiovascular chemoprevention, has already been found to have a significant role in the prevention of colorectal cancer and is recommended for use in 50–59-year-olds with a significant cardiovascular risk profile (10% or more over 10 years) by the US Preventative Services Task Force [16]. Through evidence initially gathered in large cohort studies [17,18,19], this relationship was demonstrated in hereditary colorectal cancer patients in randomized controlled trials through the CAPP trial series [20]. The large cohorts also showed significant links with esophageal cancer and extensive work to define the biochemical process involved has been undertaken.
There are four main theories of why aspirin works in chemoprevention. Firstly, inflammation plays a significant role in the cancers that aspirin is considered to prevent and on one level it inhibits the release of inflammatory cytokines by immune cells, reducing downstream cellular changes, particularly through limiting release of TNF, INFy, WNT5A, IL-1, IL-6, and CXCL1 [21,22,23]. Platelet-mediated effects have also been described, linking reduced thromboxane production from platelets preventing cell proliferation that occurs as a reaction to neoplastic disruption to tissues [17].
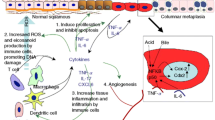
However, the main causative pathways in esophageal cancer that appear to relate to aspirin and NSAIDs are the COX mediated pathways and the subsequent effects on β-catenin [24] (Fig. 5.1). Cell migration and proliferation are stimulated by the shift of β-catenin to the nucleus of the cell where it causes a gene expression sequence, hence it has pro-neoplastic effects at higher concentrations in the cell [21]. β-catenin is usually ubiquitylated after being flagged by T41 and S45 amino acid residues; however, in the context of aspirin this process is emphasized by inactivation of protein phosphatase 2A which is responsible for breaking down T41 and S45 [21]. PGE2 produced via the COX pathway stimulates the migration of β-catenin via stimulation of the EP-2 receptors in the epithelial cell and WNT-signaling.
A diagram showing the β-catenin pathways hypothesized to be affected by aspirin, modified from a diagram by Drew et al. in the 2016 paper “Aspirin and colorectal cancer: the promise of precision chemoprevention” Nature Reviews [21]. Green arrows represent stimulation pathways and red arrows inhibitory. Inhibiting the stabilization of β-catenin through inactivation of protein phosphatase 2A (PP2A)-promoting ubiquitylation; through inhibiting COX-2-mediated production of prostanoids by preventing COX-2 from converting arachidonic acid to prostaglandin E2 (PGE2) which in turn can stimulate the WNT signaling pathway
In the COX pathway, arachidonic acid is metabolized through COX enzymes, resulting in the production of prostaglandins such as PGE2, PGF2, and PGD2. NSAIDs and aspirin disturb this process through interfering with the action of the COX enzyme [25]. There are two types of COX, denoted COX1 and COX2: high levels of COX2 have been implicated in neoplastic conditions [26]. It was also noted that metaplastic cell progression through to dysplasia and adenocarcinoma was associated with increased levels of COX2 mRNA and protein [27]. Barrett’s esophagus and associated esophageal adenocarcinoma patients were found to have upregulation of COX2 mRNA expression, which occurs early in the neoplastic transformation process [28]. One of the studies carried out in the US concluded that inhibition of COX2 expression through using selective COX2 inhibitor has a chemopreventive effect in Barrett’s esophagus [29]. This was supported further by another study which showed that food-borne natural flavonoid quercetin and selective COX2 inhibitors hinder cell proliferation and induce apoptosis in esophageal adenocarcinoma in vitro [30].
Clinical trials are encouraging. A meta-analysis by Rothwell et al. found a significant reduction in 20-year cancer-related mortality for patients with all solid cancers and particularly GI cancers taking daily aspirin versus control [31]. Evidence from Parkin et al. suggested an all-cancer reduction of 7–10% with 10 years of regular aspirin use in 50–65-year-olds, with most clear associations in GI cancers with esophageal, colorectal and gastric cancers all reduced by up to one-third [32]. A large population-based case-control study of UK and Netherlands populations by Masclee et al. looked at esophageal adenocarcinoma risk in Barrett’s patients with concurrent use of PPI, NSAIDs, aspirin or statins and found no significantly significant associations [33]. However, a large case control study derived from Scotland-based general practice demonstrated decreased risk of upper aerodigestive tract cancer with usage of aspirin and not COX2 inhibitor [34]. Systematic review and meta-analysis supported the protective association between aspirin/NSAID and esophageal adenocarcinoma with more protection in patients with greater usage and longer duration [35].
The risks associated with long-term aspirin use are well understood, namely an increased risk of bleeding in general through unselective COX inhibition, which reduces thromboxane release, thus increasing the risk of platelet-mediated bleeding, and an increased risk of GI bleeding due to COX1 inhibition causing gastric ulceration through reduced production of prostaglandin E2 [17]. This creates concerns for many investigators regarding the use of aspirin in otherwise healthy populations; however encouraging data has come from Cuzick et al. suggesting a 10-year use of daily aspirin in 100 average > 55-year-olds would only produce 0.25 more GI bleeds in women and 0.49 in men for a benefit of 2.29 fewer cancers, strokes and MI in men and 1.32 in women over a 15-year period [36]. The use of combination therapy with PPI could ameliorate this risk further and we await data from the full publication of ASPECT [37]. Hur et al. assessed patient preferences for chemopreventive agents and found 76% of Barrett’s patients would be open minded to the use of aspirin in this context [38]. The familiarity of aspirin to both patients and clinicians, and its extensive use in cardiovascular disease as a secondary effect strongly support its potential, and further studies are required prior to its widespread use for esophageal cancer prevention.
Statins
Statins, as a widely used cardiovascular risk reduction treatment, were also found in large cohort studies [39] to show potential for chemoprevention of cancer. Since then, statins have been linked with prevention in many different cancer types including colorectal [40], advanced prostate [41], hepatocellular [42, 43], and esophageal [39] cancers. The proposed mechanism for this relates to how statins affect the RAF-MAPK-ERK pathway resulting in an anti-inflammatory and proapoptotic state, and also prevent problems with normal cell survival and differentiation through inhibition of HMG CoA’s conversion to mevalonate [44]. Activation of the mitogen-activated protein kinase (MAPK) signaling cascade was found to play a role in neoplastic progression of Barrett’s esophagus [45] which creates a possible route for neoplasia suppression by statins, although overall the mechanism is not completely understood.
A recent meta-analysis of 39 cohort and two case-control studies were conducted to evaluate the role of statins in influencing mortality in esophageal cancer patients. This concluded that using statins prediagnosis and postdiagnosis has a positive impact on survival rate [46]. One of the population-based cohort studies showed that patients on statins prior to diagnosis of esophageal cancer had 19% reduction in their mortality [47]; however another cohort study in the UK concluded that although patients with esophageal adenocarcinoma experienced reduced risk of cancer related mortality, this effect was not observed in patients with esophageal squamous cell cancer [48].
Statins, like aspirin, have a crossover effect with cardiovascular disease prevention which gives them potential for secondary morbidity reduction and they are well known to clinicians and patients, allowing for ease of counseling. Unfortunately some major concerns have been raised about possible problems with the elderly including an increased risk of cancer [49, 50]. The numbers needed to treat coming out of trials are extremely high—for esophageal cancer they have been quoted as high as 1266 and are offset dramatically by numbers needed to harm of 91 for myopathy in men (moderate-severe myopathy) and 136 for severe liver derangement [51]. The link to esophageal cancer prevention at this stage is too weak to recommend use for chemoprevention, especially in the context of the concerns raised above. Large randomized controlled trials would help to assess the value of statins for esophageal cancer chemoprevention.
Metformin
Studies have looked at the antineoplastic and chemopreventative effects of metformin in esophageal squamous cell carcinoma in vivo and in vitro. It was found that metformin selectively inhibits human esophageal squamous cancer cell growth and induces apoptosis and autophagy through inactivating Stat3 and repressing Bcl-2 [52]. Associations have also been made with metformin triggering an AMPK-related stress response reducing cancer cell survival via the AMPK/LKB1 pathway [53].
Randomization has not been utilized to study the effects of metformin in esophageal cancer. However, metformin has been shown to improve radiological and pathological response in established esophageal adenocarcinoma patients when used as a neo-adjuvant to chemoradiation; this effect is dose-dependent [54]. Though it is not yet clear from the current evidence base if we can associate metformin with esophageal cancer reduction, certainly some risk factors for all cancers—obesity, sedentary lifestyle, and diabetes—relate to the metabolic state and there is evidence to suggest a link to metformin reducing the rate of all cancers by 31% in diabetic patients in long-term use [55]. GI upset in many patients can make metformin prohibitive in healthy patients and the evidence is not strong enough here either; there is possible stronger evidence in hepatocellular prevention [56], 31% overall risk reduction of all cancers and colonic adenoma rates [57] (p = 0·034, risk ratio 0·67 [95% CI 0·47–0·97]) in nondiabetic populations also. Increasing need for this medication in the general population due to rising obesity levels and early-onset type II diabetes may allow for more large-scale trials.
Conclusions
Chemoprevention is an extremely exciting prospect overall; however, moving this approach into widespread use is still a long way away (Table 5.1). There is strong evidence for the use of chemoprevention in a few cancer areas—aspirin for colorectal cancer and tamoxifen for estrogen-receptor positive breast cancers, and aspirin is recommended in high-risk groups [16, 58, 59]. If it would be possible to slow or halt the progression of Barrett’s to dysplasia using a simple, cheap, readily available medication, combining this with improving our ability to perform targeted endoscopic assessment and build on the surveillance process could improve the incidence rates of esophageal cancer. The concern has been raised that preventing a curable malignancy by pushing the time to progression forward may result in patients being diagnosed too late for alternative modalities such as surgery, especially as many develop esophageal cancer in older age. Although some data support the concept of widespread aspirin or PPI chemoprevention, before the evidence is stronger, we would risk delaying a few cases while placing a healthy population at risk of adverse drug reactions. Further studies will help stratify these difficult decisions (Table 5.2). Genetic profiling trials are also underway looking for gene targets to risk stratify patients into chemoprevention programs. Certainly, until these genes can be defined, demographic risk stratification is likely to shape chemoprevention practice for esophageal cancer, as already occurs in cardiology.
References
Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. https://doi.org/10.1016/S0140-6736(12)60643-6.
Kastelein F, Spaander MC, Steyerberg EW, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11(4):382–8. https://doi.org/10.1016/j.cgh.2012.11.014.
Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285(18):2331–8. https://doi.org/10.1001/JAMA.285.18.2331.
Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology. 1999;117(2):327–35. https://doi.org/10.1053/gast.1999.0029900327.
Mehta S, Johnson IT, Rhodes M. Systematic review: the chemoprevention of oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2005;22(9):759–68. https://doi.org/10.1111/j.1365-2036.2005.02667.x.
GORE S, HEALEY CJ, SUTTON R, et al. Regression of columnar lined (Barrett’s) oesophagus with continuous omeprazole therapy. Aliment Pharmacol Ther. 1993;7(6):623–8. https://doi.org/10.1111/j.1365-2036.1993.tb00143.x.
El-Serag HB, Aguirre T, Kuebeler M, Sampliner RE. The length of newly diagnosed Barrett’s oesophagus and prior use of acid suppressive therapy. Aliment Pharmacol Ther. 2004;19(12):1255–60. https://doi.org/10.1111/j.1365-2036.2004.02006.x.
Sharma P, Sampliner RE, Camargo E. Normalization of esophageal pH with high-dose proton pump inhibitor therapy does not result in regression of Barrett’s esophagus. Am J Gastroenterol. 1997;92(4):582–5. http://www.ncbi.nlm.nih.gov/pubmed/9128303
Neumann CS, Iqbal TH, Cooper BT. Long term continuous omeprazole treatment of patients with Barrett’s oesophagus. Aliment Pharmacol Ther. 1995;9(4):451–4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8527623
Armstrong D. Review article: towards consistency in the endoscopic diagnosis of Barrett’s oesophagus and columnar metaplasia. Aliment Pharmacol Ther. 2004;20(Suppl 5):40–7.; discussion 61-2. https://doi.org/10.1111/j.1365-2036.2004.02132.x.
Yeh RW, Gerson LB, Triadafilopoulos G. Efficacy of esomeprazole in controlling reflux symptoms, intraesophageal, and intragastric pH in patients with Barrett’s esophagus. Dis Esophagus. 2003;16(3):193–8. https://doi.org/10.1046/j.1442-2050.2003.00327.x.
Peters FTM, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s oesophagus during omeprazole treatment; a randomised double blind study. Gut. 1999;45(4):489–94. https://doi.org/10.1136/gut.45.4.489.
Peters FT, Ganesh S, Kuipers EJ, et al. Effect of elimination of acid reflux on epithelial cell proliferative activity of Barrett esophagus. Scand J Gastroenterol. 2000;35(12):1238–44. http://www.ncbi.nlm.nih.gov/pubmed/11199360
Umansky M, Yasui W, Hallak A, et al. Proton pump inhibitors reduce cell cycle abnormalities in Barrett’s esophagus. Oncogene. 2001;20(55):7987–91. https://doi.org/10.1038/sj.onc.1204947.
El-Serag HB, Aguirre TV, Davis S, Kuebeler M, Bhattacharyya A, Sampliner RE. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2004;99(10):1877–83. https://doi.org/10.1111/j.1572-0241.2004.30228.x.
U.S. Preventive Services Task force. Recommendation Statement: Aspirin to prevent cardiovascular disease and cancer U.S. Preventive Services Task Force. U.S. Preventative Task Force Online. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer?ds=1&s=aspirin. Accessed May 6, 2018.
Chan AT, Arber N, Burn J, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev Res. 2012;5(2):164–78. https://doi.org/10.1158/1940-6207.CAPR-11-0391.
Vaughan LE, Prizment A, Blair CK, Thomas W, Anderson KE. Aspirin use and the incidence of breast, colon, ovarian, and pancreatic cancers in elderly women in the Iowa Women’s health study. Cancer Causes Control. 2016;27(11):1395–402. https://doi.org/10.1007/s10552-016-0804-8.
Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for Cancer. JAMA Oncol. 2016;2(6):762–9. https://doi.org/10.1001/jamaoncol.2015.6396.
Burn J, Gerdes AM, MacRae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081–7. https://doi.org/10.1016/S0140-6736(11)61049-0.
Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16(3):173–86. https://doi.org/10.1038/nrc.2016.4.
Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–7. https://doi.org/10.1016/S1470-2045(09)70035-X.
Jankowski JA, Hawk ET. A methodologic analysis of chemoprevention and cancer prevention strategies for gastrointestinal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3(2):101–11. https://doi.org/10.1038/ncpgasthep0412.
Jankowski JA, Anderson M. Review article: management of oesophageal adenocarcinoma –control of acid, bile and inflammation in intervention strategies for Barrett’s oesophagus. Aliment Pharmacol Ther. 2004;20(s5):71–80. https://doi.org/10.1111/j.1365-2036.2004.02143.x.
Vane JR, Botting RM. Anti-inflammatory drugs and their mechanism of action. Inflamm Res. 1998;47:78–87. https://doi.org/10.1007/s000110050284.
Tucker ON, Dannenberg AJ, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59(5):987–90. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10070951&retmode=ref&cmd=prlinks%5Cnpapers3://publication/uuid/B59E91AA-82A7-40F0-BFA6-7F772463C3C5
Lagorce C, Paraf F, Vidaud D, et al. Cyclooxygenase-2 is expressed frequently and early in Barrett’s oesophagus and associated adenocarcinoma. Histopathology. 2003;42(5):457–65. https://doi.org/10.1046/j.1365-2559.2003.01627.x.
Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s esophagus and associated adenocarcinomas. Cancer Res. 1998;58(14):2929–34.
Buttar NS, Wang KK, Anderson MA, et al. The effect of selective cyclooxygenase-2 inhibition in Barrett’s esophagus epithelium: an in vitro study. J Natl Cancer Inst. 2002;94(6):422–9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11904314
Cheong E, Ivory K, Doleman J, Parker ML, Rhodes M, Johnson IT. Synthetic and naturally occurring COX-2 inhibitors suppress proliferation in a human oesophageal adenocarcinoma cell line (OE33) by inducing apoptosis and cell cycle arrest. Carcinogenesis. 2004;25(10):1945–52. https://doi.org/10.1093/carcin/bgh184.
Rothwell PM, Fowkes FGR, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet 2011.
Parkin DM, Boyd L, Walker LC. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105:S77–81. https://doi.org/10.1038/bjc.2011.489.
Masclee GMC, Coloma PM, Spaander MCW, et al. NSAIDs, statins, low-dose aspirin and PPIs, and the risk of oesophageal adenocarcinoma among patients with Barrett’s oesophagus: a population-based case–control study. BMJ Open. 2015;5:e006640. https://doi.org/10.1136/bmjopen-2014-006640.
Macfarlane TV, Lefevre K, Watson MC. Aspirin and non-steroidal anti-inflammatory drug use and the risk of upper aerodigestive tract cancer. Br J Cancer. 2014;111(9):1852–9. https://doi.org/10.1038/bjc.2014.473.
Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124(1):47–56. https://doi.org/10.1053/gast.2003.50008.
Cuzick J, Thorat MA, Bosetti C, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26(1):47–57. https://doi.org/10.1093/annonc/mdu225.
Jankowski JA. Aspirin chemoprevention for Barrett’s and esophageal cancer: the AspECT trial. Cancer Prev Res. 2011;4(10). http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L71294276%5Cn;http://cancerpreventionresearch.aacrjournals.org/cgi/content/meeting_abstract/4/10_MeetingAbstracts/ED04-03?sid=3a6f865f-6a2d-447b-ab11-801a2038933a%5Cn
Hur C, Broughton DE, Ozanne E, Yachimski P, Nishioka NS, Gazelle GS. Patient preferences for the chemoprevention of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2008;103(10):2432–42. https://doi.org/10.1111/j.1572-0241.2008.02117.x.
Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. https://doi.org/10.1136/bmj.c2197.
Lytras T, Nikolopoulos G, Bonovas S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World J Gastroenterol. 2014;20(7):1858–70. https://doi.org/10.3748/wjg.v20.i7.1858.
Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–25. https://doi.org/10.1093/jnci/djj499.
Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):323–32. https://doi.org/10.1053/j.gastro.2012.10.005.
Tsan Y-T, Lee C-H, Wang J-D, Chen P-C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30(6):623–30. https://doi.org/10.1200/JCO.2011.36.0917.
Demierre MF, Higgins PDR, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5(12):930–42. https://doi.org/10.1038/nrc1751.
Souza RF, Shewmake K, Pearson S, et al. Acid increases proliferation via ERK and p38 MAPK-mediated increases in cyclooxygenase-2 in Barrett’s adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2004;287(4):G743–8. https://doi.org/10.1152/ajpgi.00144.2004.
Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41(6):554–67. https://doi.org/10.1016/j.ctrv.2015.04.005.
Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced Cancer-related mortality. N Engl J Med. 2012;367(19):1792–802. https://doi.org/10.1056/NEJMoa1201735.
Alexandre L, Clark AB, Bhutta HY, Chan SSM, Lewis MPN, Hart AR. Association between statin use after diagnosis of Esophageal Cancer and survival: apopulation-based cohort study. Gastroenterology. 2016;150(4):854–865.e1. https://doi.org/10.1053/j.gastro.2015.12.039.
Bonovas S, Sitaras NM. Does pravastatin promote cancer in elderly patients? A meta-analysis. CMAJ. 2007;176(5):649–54. https://doi.org/10.1503/cmaj.060803.
Weverling-Rijnsburger AW, Blauw GJ, Lagaay a M, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350(9085):1119–23. https://doi.org/10.1016/S0140-6736(97)04430-9.
Treatment C, Ctt T. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. https://doi.org/10.1016/S0140-6736(10)61350-5.
Feng Y, Ke C, Tang Q, et al. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5(2):e1088. https://doi.org/10.1038/cddis.2014.59.
Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74. https://doi.org/10.1172/JCI200113505.
Skinner HD, McCurdy MR, Echeverria AE, et al. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol. 2013;52(5):1002–9. https://doi.org/10.3109/0284186X.2012.718096.
DeCensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res. 2010;3(11):1451–61. https://doi.org/10.1158/1940-6207.CAPR-10-0157.
Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11(1):45–54. https://doi.org/10.1038/nrgastro.2013.143.
Higurashi T, Hosono K, Takahashi H, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17(4):475–83. https://doi.org/10.1016/S1470-2045(15)00565-3.
Cuzick J, DeCensi A, Arun B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol. 2011;12(5):496–503. https://doi.org/10.1016/S1470-2045(11)70030-4.
National Collaborating Centre for Cancer. Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. National Institute for Health and Clinical Excellence: Guidance, Clinical guideline [CG164]. Cardiff: NCC-C; 2013. p. 253.
Acknowledgement
Funding: Cancer Research UK, Royal College of Surgeons Ireland.
Declaration of Interests: Prof. Jankowski is the Chief Investigator for the Aspect trial.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ratcliffe, E.G., Shibeika, M., Higham, A.D., Jankowski, J.A. (2020). Chemoprevention of Esophageal Cancer. In: Saba, N., El-Rayes, B. (eds) Esophageal Cancer. Springer, Cham. https://doi.org/10.1007/978-3-030-29832-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-29832-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29831-9
Online ISBN: 978-3-030-29832-6
eBook Packages: MedicineMedicine (R0)