Abstract
In developed countries, blindness and visual impairment are caused mainly by diseases affecting the retina. These retinal degenerative diseases, including age-related macular dystrophy (AMD) and inherited retinal diseases such as retinitis pigmentosa (RP), are the predominant causes of human blindness worldwide and are responsible for more than 1.5 million cases in France and more than 30 million cases worldwide. Global prevalence and disease burden projections for next 20 years are alarming (Wong et al., Lancet Glob Health 2(2):e106–e116, 2014) and strongly argue toward designing innovative eye-care strategies. At present, despite the scientific advances achieved in the last years, there is no cure for such diseases, making retinal degenerative diseases an unmet medical need.
The majority of the inherited retinal disease (IRD) genes codes for proteins acting directly in photoreceptors. Yet, a few of them are expressed in the retinal pigment epithelium (RPE), the supporting tissue necessary for proper functioning of the photoreceptors. Among retinal degenerative diseases, impairment of some RPE genes engenders a spectrum of conditions ranging from stationary visual defects to very severe forms of retinal dystrophies in which the RPE dysfunction leads to photoreceptors cell death and consecutive irreversible vision loss. The accessibility of the eye and the immune privilege of the retina, together with the availability of noninvasive imaging technologies, make such inherited retinal dystrophies a particularly attractive disease model for innovative cell therapy approaches to replace, regenerate, and/or repair the injured RPE tissue. Proof-of-concept studies in animal models have demonstrated the safety and efficacy of the engraftment of therapeutic cells either to support RPE cell functions or to provide a trophic support to photoreceptors. These different approaches are now in the pipeline of drug development with objective to provide first cell-based treatments by 2020.
This chapter will focus on the different cell-based strategies developed in the past and current approaches to prevent photoreceptor death in RPE-associated degenerative eye diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Retinopathies Induced by a Primary RPE Defect
The retina is a 500 μm-thick light-sensitive tissue lining the inner surface of the eye. It is formed by multiple layers of interconnected neurons and is in charge of the first steps of visual processing (Fig. 3.1). The photoreceptors (rods and cones) are, together with the ganglion cells, the photosensitive cells of the retina. Rods and cones initiate the conversion of light energy into electrical signals through a process called phototransduction. Retinal interneurons (amacrine, bipolar, and horizontal cells) further codify the electrical signals into optic nerve impulses (through ganglion cells), which are subsequently interpreted by the brain as visual images [2]. Underlying the photoreceptors layer, we could find the RPE, constituted by a polarized monolayer of epithelial cells, which are real babysitters for photoreceptors. The RPE cells display a hexagonal shape and form a pigmented epithelium that lies between the choroid and the neural retina (Fig. 3.1). It is flanked by the Bruch’s membrane on its basal surface and by the outer segments of the photoreceptors on its apical portion. The cells in this layer are connected by tight junctions and constitute the outer components of the blood–retinal barrier [3, 4]. In the subretinal space (SRS) filled with the interphotoreceptor matrix, microvilli from RPE cells appose to the photoreceptor outer segments, forming the physical components that can contribute to the maintenance of retinal adhesion [5].
RPE cells perform a number of functions that are critical for the survival and proper functioning of the photoreceptors [6]. RPE cells, among other functions, are involved in the recycling of the vitamin A, buffer ion composition in the SRS, secrete growth factors, regulate T-cell activation in the eye, and phagocytize shed outer segment of photoreceptors [7, 8]. All these functions are essential, and a failure of any of them can lead to photoreceptors death, degeneration of the retina, loss of visual function, and ultimately blindness. Several disease conditions specifically alter RPE cells with major consequences in vision.
3.1.1 Retinitis Pigmentosa
RP is a highly heterogeneous group of retinal diseases affecting either photoreceptors or RPE or both [4, 9, 10] with incidence of 1/3500–4000 [11]. These diseases are rare monogenic dystrophies. Some mutations specifically affect RPE genes (https://sph.uth.edu/retnet/disease.htm) such as lecithin retinol transferase (LRAT) , retinal pigment epithelium-specific 65-kDa protein (RPE65), the MER tyrosine kinase proto-oncogene (MERTK) and bestrophin (BEST1) [4]. They are mainly inherited autosomal recessive, except for some dominant mutations on the bestrophin gene [12]. Though RP has a highly variable clinical presentation and progression, the majority of patients initially experience problems in night vision, since the rod photoreceptors are typically damaged first (Fig. 3.1). Then the peripheral vision is progressively lost leading to tunnel vision. In many cases, this can progress to include the central visual field and blindness [11].
Impairment of some RPE genes involved in the visual cycle leads to a spectrum of conditions ranging from stationary visual defects to very severe forms of retinal dystrophies. Additionally, in RPE disorders, pigment deposits in retina are scarce, even at late disease stages. This feature could reflect impaired RPE migration and proliferation, while with primary defects in photoreceptor cells, the RPE could be healthier. RPE65 was the first RPE gene reported to be involved in human retinal dystrophy. Mutations in RPE65 are responsible for 5–10% of the cases of Leber congenital amaurosis (LCA) [13] and a few cases (1–2%) of recessive RP [14,15,16,17]. The condition is usually severe and congenital. However, milder phenotypes could be encountered and reported as RP or retinal dystrophy [17, 18]. In contrast to RPE65, LRAT mutations are a very rare cause of severe retinal dystrophy. The phenotypes of CRALBP (RLBP1) and RDH5 mutations are quite similar, although with some differences. For both genes, night blindness is present from the first years of life and dark adaptation thresholds are elevated [19, 20].
One of the most important functions of the RPE is to participate actively in the photoreceptor membrane turnover. It has been calculated that each rod regenerates its outer segment (ROS) within 7–12 days [21]. Photoreceptor membrane turnover is composed of two phases, necessarily balanced in order to preserve cellular integrity: an anabolic aspect involving RNA transcription, protein synthesis, transport, and generation of new membranes; and a catabolic aspect consisting of exhausted membrane removal, digestion by RPE cells (phagocytosis), and recycling of some components to the photoreceptors [22]. Different receptors were identified to have a role in photoreceptor membrane phagocytosis by RPE cells: a mannose-6-phosphate receptor [23], CD36 [24, 25], αVβ5 integrin [26], and a number of glycoproteins expressed on the RPE surface [27]. A real breakthrough was the identification of the c-mer transmembrane tyrosine kinase (MERTK) receptor as responsible for photoreceptor outer segment (POS) internalization [28, 29]. Mutations in the MERTK gene have been linked to subgroups of patients with inherited retinal degeneration [30, 31].
Macular dystrophies are inherited retinal dystrophies in which various forms of deposits; pigmentary changes and atrophic lesions are observed in the macula lutea, the cone-rich region of the human central retina . Mutations in several genes, expressed in RPE cells (BEST1, CDH3, TIMP3), are associated with these conditions [14].
3.1.2 Age-Related Macular Degeneration
AMD is the leading cause of visual impairment in western countries in patients over 50 years of age. The prevalence of 0.05% before the age of 50 years increases to about 12% in population older than 80 years old. While the disease is particularly prevalent in developed countries, it is becoming a global health concern due to the worldwide increase in life expectancies [1]. Besides age, family history has become the second largest risk factor, and smoking, diet, obesity, hypertension, and chronic inflammation have been reported as other environmental risk factors. Although the primary site of the pathologic insult in AMD is not completely determined, different observations support the involvement of oxidative damages, inflammatory changes, and the gradual accumulation of deposits within the RPE cells [32].
Early AMD is characterized by the thickening of Bruch’s membrane, deposits under the RPE layer, and by pigmentation changes in the macula. More advanced stages of the disease demonstrate either subretinal neovascularization or atrophy of the retina and RPE. Based on the absence or presence of abnormal choroidal blood vessels’ growth below the RPE layer, two different forms of AMD can be distinguished; (1) the ‘dry’ or geographic atrophy form, the most frequent one (around 90% of AMD patients), resulting from RPE dysfunction leading to accumulation of pigment debris and drusen (deposits of proteins and lipids) and slowly progressing photoreceptor cell death, and (2) the ‘wet’ or choroidal neovascular form arising from a more rapid and destructive process of choroidal neovascularization through the Bruch’s membrane and the RPE in the SRS accompanied by exudation and hemorrhage [33]. The resulting damage to the macular region leads to a loss of vision in the central visual field.
With age, the eye undergoes several changes, some of which affect the RPE. The accumulation of lipofuscin, a reduction in melanin, diminished antioxidant capacity, and the progressive accumulation of deposits underlying Bruch’s membrane are hallmarks of aging eyes [3, 34]. Acute and chronic progressive dysfunctions of RPE cells and the age-related deterioration of this tissue have been shown to play a relevant role in AMD [5]. The inflammatory responses observed in AMD retinas are similar but more severe than those observed in normal aging [35]. Moreover, different lifestyle factors, environmental conditions, and gene alterations are likely to explain why certain individuals develop AMD with age, while others do not [36].
In both the atrophic and neovascular forms of AMD, the mutualistic and symbiotic relationship between the photoreceptors, RPE, Bruch’s membrane, and choriocapillaris is lost, which results in the death and dysfunction of all the components in the complex [37, 38]. Emerging evidences show that both “dry” AMD and “wet” AMD involve a common pathophysiological background and share similar initiating molecular and cellular alterations [39]. However, the differentiation of dry AMD and wet AMD is clinically relevant because of their distinct clinical presentation and therapeutic options.
3.1.3 Global Burden and Treatments
Altogether, diseases caused by RPE malfunction affect at least 30 millions of people worldwide, a figure that will triple with the increase in the aging population in the next 30–40 years [1]. In addition, as the population is growing older and higher expectations of better quality of life including ability of driving and reading are being asked by patients, morbidity resulting from AMD is becoming increasingly significant. A recent systematic review and meta-analysis has shown that 8.7% of the worldwide population has age-related macular degeneration, and the projected number of people with the disease is around 196 million in 2020, increasing to 288 million in 2040 [1].
Despite a crucial need for therapies, there is no treatment approved for RP. For AMD, due to the complex multifactorial nature of the disease, therapeutic options have evolved slowly. Lifestyle modifications, including smoking cessation and high-dose antioxidant supplements, were shown to decrease disease progression rate. Photocoagulation ablation of extrafoveal new vessels was the first treatment proposed by clinicians for wet AMD and is still in used in selected cases [40]. Photodynamic therapy with systemic administration of a photosensitizing agent and more selective ablation of abnormal vessels were also tested, but recovery was not complete and high rate of recurrence was observed [41]. Anti-VEGF (Vascular Epithelial Growth Factor) agents, which can prevent new vessel development, have significantly improved the prognosis of wet AMD. Repeated injections of these agents formulated by different companies, such as Pegaptanib (Macugen; Eyetech/Pfizer) [42], Ranibizumab (Lucentis; Genentech) [43,44,45], Aflibercept (Eylea; Regeneron) [46], or Bevacizumab (Avastin; Genentech) [47,48,49] show the capacity to slow the rate of vision loss but have no more than a 30% rate of effectiveness in all AMD cases.
Emerging treatment modalities include RNA interferences and attempt to block pathways down- or upstream of VEGF to prevent new vessel growth. Anti-PDGF (Platelet-Derived Growth Factor) agents (FovistaTM; Ophthotech, USA) that block pericytes’ recruitment were tried in combination with anti-VEGF medications [50].
While promising, gene therapy aiming at restoring gene defects in the RPE (like RPE65) shows limitations. Indeed, the prerequisite to this type of gene therapy is to know precisely the defective gene, and a second limitation is to develop a gene delivery candidate drug for each one of the defective genes. Moreover, recent data from preclinical and clinical studies show that, despite stabilization of vision during the follow-up period, patients treated by RPE65 gene therapy still undergo photoreceptor degeneration [51].
Although prognosis of wet AMD has been improved during the last decade, many patients may lose their sight because of late diagnosis or inadequate treatment. For dry AMD, no treatments are available. Different treatment strategies for both AMD could be envisioned: (1) preventing RPE dysfunction or death [52, 53], (2) providing support to stressed RPE [10], and finally (3) replacing RPE and eventually photoreceptors [54].
3.2 Therapeutics Based on Fetal or Adult Cells
The treatment of RPE-associated degenerative eye diseases by cell therapy was first proposed by Gouras and collaborators in early 1980s [55, 56]. This group successfully transplanted RPE cells in a monkey model, demonstrating the feasibility of such approach. By the end of the 1980s, it was well established that RPE cells can not only be transplanted in degenerated retina but also can delay photoreceptor degeneration in a rat model of retinal degeneration [57], opening the path to the development of therapeutic cell-based strategies. Laboratories from all over the world developed and tested methods to produce cells that can replace defective or degenerated endogenous RPE cells.
3.2.1 Use of Fetal-Derived Cells to Replace RPE Cells
One of the first sources identified was the fetal RPE cells. However, the use of such cells required ethical agreement from the civil society and is legally regulated in each country. Researchers and physicians agree to the ethical tenets that regulate experimentation requiring human material including informed consent, no incentive for abortion, procurement of human material that, if not used for research, would be discarded, and review of the protocol from ethical committees. In addition, in case of a clinical use of fetal cells, a detailed medical history of the donor and a complete separation of donor and recipient are required. This set of principles was adopted by the World Medical Association in the declaration of Helsinki (now in its seventh revision) [58].
Human fetal RPE cells from eyes of 10–21 weeks gestational age are separated from the anterior segment and the neural retina by dissection [59,60,61]. Then RPE cells can be either mechanically dissected or enzymatically separated in order to have an enriched cell culture [60, 61]. After 1 week, a mean of 1.4 × 106 RPE cells can be obtained and passaged for up to ten times [61]. Another protocol from the National Eye Institute (NIH, Bethesda, MA) allows obtaining between 160 and 250 millions of fetal RPE at Passage 1 (P1) per eye, increasing thus the yield [60].
To test the potential for cell therapy of fetal cells, the most useful model is the Royal College of Surgeons (RCS) rat. This model is characterized by a mutation in the MERTK gene coding for the transmembrane receptor tyrosine kinase MERTK [62]. In RCS rats, RPE are not able to phagocytosis photoreceptor outer segments [63]. This dysfunction leads to an accumulation of debris between RPE and photoreceptors. As the mutation affects primarily RPE cells, the RCS rat is a good model for both AMD and RP in which the mutation affects RPE cell functions [28, 30]. The photoreceptors begin to die from the third postnatal week. The degeneration progresses quickly, and by 2 months of age, the electroretinogram (ERG) is almost flat in response to light. It indicates that the retina is no more sensitive to light due to the photoreceptors’ degeneration. In addition, the decline in vision of RCS rats is also measured in the optokinetic paradigm. In that test, the animal is placed in a platform and exposed to moving stripes. When the rat detects the stimulus, a reflex movement of the head is observed. By modulating the size of stripes, a visual acuity is measured corresponding to the limit of the stimulus detection. In RCS rat, the visual acuity decreases drastically by 2 months of age.
In 1996, Little and collaborators have clearly demonstrated in RCS rats that human fetal RPE cells harvested from enucleated eyes of 10–16 weeks gestational age fetuses have a rescue effect [64]. Indeed 24-day-old rats were grafted with these fetal RPE cells and were sacrificed 1 month later. They reported, after histological examination, that photoreceptors were preserved in the areas surrounding the grafting site compared to sham injected site. They confirmed the presence of the cells by analysis of pigmented cells in the nonpigmented rat eye. RCS rats were immunocompromised by a daily injection of cyclosporine. This treatment was sufficient to prevent rejection during the follow-up.
Another strategy consisted of grafting small patches of fetal RPE still organized as a monolayer (1–5 mm2) [65]. Transplanted fetal RPE was rejected within 1 month into the rabbit eye but survived at 3 months in the monkey retina without immunosuppression. Moreover grafted RPE is able to phagocytosis outer segments as demonstrated by the presence of phagosomes in electron micrographies of rabbit retinas [65]. Berglin and collaborators had grafted human fetal retinas in six monkeys without immunosuppression. Integrity of local photoreceptors and survival of RPE patch transplants were effective for at least 6 months [66]. Interestingly rejections occur more frequently around fovea (60%) than at the periphery (30%). Prevention of rejection can be managed by Cyclosporin A treatment into the vitreous [67]. Lai’s report compared intravitreal release of Cyclosporine A from a capsule sutured in the vitreous or through weekly injections in rabbits transplanted with fetal RPE cell suspensions. The mean survival of the human fetal RPE xenograft was about 10 weeks with local immunosuppression compared to 4 weeks without Cyclosporin A [67]. In order to evaluate long-term effect of the transplant into the retina, Aramant and Seiler grafted a fetal sheet containing both RPE and neural retina into an immunocompromised model, the nude rat [68]. In that context, all xenografts survived an average of 40 weeks, indicating that the transplant can achieve long survival periods.
Fetal allogenic RPE transplantation showed no visual improvement and signs of rejection in nonimmunosuppressed AMD patients [69, 70]. Another group grafted a suspension of healthy fetal RPE in a 65-year-old legally blind women without systemic immunosuppressant. The patient did not show any visual improvement [71]. In that case, signs of immune rejection were also observed. Radtke and collaborators used intact sheets of fetal neural retina with RPE for grafting into five patients with RP without immune suppression [72]. There was no sign of rejection at 6 months despite HLA mismatch of the patient with the fetal tissue. However, no visual improvement was reported using multifocal ERG, probably due to advanced retinal degeneration. Following these results, a phase II clinical trial was conducted in ten patients (six RP and four AMD) between 2002 and 2005 [73]. Despite no immune suppression, investigators did not report any signs of rejection at 1 year post surgery. However, the pigmentation had decreased over time and was lost in eight out of ten patients at 3 months post operation, and there was no control group. They reported improvement in seven out of ten patients in the ETDRS scale but no improvement using other measures like multifocal ERG [73].
3.2.2 RPE Cells from Adult Eyes as a Source for Cell Therapy
Several cell sources were evoked for RPE cell therapy rising from the adult eye: autologous RPE cells harvested from the peripheral retinal, allogenic RPE graft harvested from cadavers, and recently a new potential source has been identified with the discovery of human RPE stem cells (RPESC).
The group of Suzanne Binder has proposed autologous RPE cell sources. Indeed they demonstrated in 14 human eye cadavers that RPE can be gently harvested and aspirated from the nasal area with a constant efficacy and a sufficient number [74]. The functionality of such cells was previously demonstrated in the RCS rat [75]. A research group of the University of Rochester has used human eye cadavers of 10- and 49-year-old for the isolation of adult RPE cells. Then these cells were grafted in 4-week-old immune-suppressed RCS rats (a time where photoreceptors are still present) [75]. After 1 month, rats were sacrificed and histological evaluation revealed that photoreceptors were preserved. Interestingly RPE from a 49-year-old donor was as effective as the one from a 10-year-old donor in rescuing rat photoreceptors [75]. Phillips and collaborators demonstrated in rabbit the feasibility of harvesting RPE in a subretinal nasal localization and graft the harvested RPE cells to another localization where the RPE cells were previously mechanically debrided [76]. At 30 days post surgery, debrided area grafted with autologous RPE decreases choriocapillaris atrophy and photoreceptors loss compared to debrided areas with no graft. In a prospective pilot study, 13 patients with AMD and foveal choroidal neovascularization (CNV) were operated in order to remove this foveal CNV and were grafted at the same time with autologous adult RPE freshly harvested from the nasal subretinal area [77]. Visual outcomes were encouraging after 17 months with visual acuity improvement in eight eyes without major adverse effects, and a prospective trial with a larger cohort was analyzed. Over a 36 months period, visual gain remained limited, probably due to the poor prognosis expected from these patients [74].
The human adult RPE cell line ARPE-19 was derived from a 19-year-old man who died from a head trauma following motor vehicle accident in 1986. This cell line is easy to grow and retains characteristics of RPE cells through the expression of specific markers and the ability to form cobblestone monolayers [78]. These cells were extensively used to characterize human RPE functions and were used for proof of concept of the cell transplantation approach in the RCS rat. Indeed Pinilla and collaborators had grafted ARPE-19 cells at postnatal day 22 (P22) [79]. Retinas had a preserved connectivity, and photoreceptors were preserved by the treatment. Moreover, ERG recording later during the follow-up (at P90 and P120) demonstrated that transplanted rats maintain their ERG responses compared to control condition, indicating that photoreceptors were still functional [79]. In addition Coffey and collaborators have developed a system to analyze cortically mediated vision following ARPE-19 cell transplantation in RCS rats [80]. In this study, they demonstrated that the primary visual cortex of transplanted rats was responsive to visual stimulation while the one of nonoperated rats was not at 6 months of age.
These different studies demonstrated the feasibility of the use of human adult RPE cells harvested from cadavers to prevent photoreceptors from death. The major limitation for the use of allogeneic RPE from cadavers and autologous RPE is the yield of cells obtained, limiting thus large-scale use due to low amplification potential. For the millions of patients to be treated, a more industrialized manufacturing process is required. To achieve this aim, other cell sources should be investigated.
Recently a new potential source of human RPE came from the discovery of human RPE stem cells (RPESCs) [81]. It was known that RPE cells retain some plasticity like in amphibians where RPE can regenerate retinal cells in vivo upon injury [82]. However, this is not the case in vivo for human RPE cells. In 2012, Salero and collaborators identified a subpopulation of human RPE cells (about 2–3%) that have the potential to be activated upon culture in vitro, acquiring self-renewing properties that they named RPESCs [81]. These cells were harvested from human eyes of 22- to 99-year-old cadavers. They highly express c-Myc and Klf4 indicating stem cell-like properties. RPESC multipotentiality was illustrated through the ability to differentiate into neural or mesenchymal lineages but not into the endoderm lineage. RPESCs are cultured clonally at low density and form nonadherent spheres in a medium containing knockout serum replacement (KSR) supplemented with FGF2 (Fibroblast Growth Factor). Finally, these cells, if they are proven to rescue classical animal models of RPE pathology, might be another potential source of RPE cells for cell therapy. In 2014, Stanzel and collaborators brought the first evidence that these RPE cells obtained from human RPESCs retain fundamental characteristics of RPE when grafted as a monolayer into the rabbit eye [83]. Moreover, these cells survive (95%) for at least 1 month in this model and maintain their monolayer and polarity (ezrin protein localized at the apical side). However, their isolation and amplification remain difficult, and alternative cell types might be better choices.
3.2.3 Alternative Cell Types
The use of functional RPE cells to replace the defective ones is the best treatment to give to patients. However, the sources for RPE cells (essentially fetal or adult RPE sources) were still limited at the beginning of the twenty-first century. The cell transplantation, independently of the cell type, produces a trophic effect that is beneficial for photoreceptor survival. Moreover, some RPE functions like phagocytosis are not restricted to RPE cells. That is why, it is not surprising that several laboratories have developed cell-based therapeutics with different cell types.
3.2.3.1 Iris Pigment Epithelium
RPE and Iris pigment epithelium (IPE) share a common embryonic origin. IPE cells possess phagocytic properties, as demonstrated by the ability of porcine IPE cells to phagocytize rod outer segments [84]. IPE cells form a monolayer with tight junctions suitable to form a de novo blood–retinal barrier. Rat IPE cells were isolated from 20- to 26-day-old and grafted into RCS rats through a transcleral route [85]. At 2 months post surgery, rat IPE was localized between host RPE and retina. They contained phagosomes from rod outer segment uptake. Photoreceptors were found preserved near IPE cells and at some distance from them in transplanted eyes. This indicates that phagocytosis uptake is not the only rescuing mechanism, and trophic factors are probably also released [85]. Another study by Thumann et al. confirmed these results with both transplantation of human and rat IPE in RCS rats [86]. Photoreceptors were preserved at 3 months post surgery to a similar level of ARPE-19 cell-transplanted eyes.
A strategy of autologous human IPE transplantation was tested in 2000 [87]. Human IPE are relatively easy to obtain from patients with a local anesthesia in the same eye than the one to be transplanted. IPE were dissociated and cultured with 15% of autologous serum for 1–2 months. Seven AMD patients with neovascular membranes from 49 to 85 years old were transplanted subretinally. Neovascular membranes were removed through retinotomy just before the transplantation of an IPE cell suspension. Fifteen control patients underwent only neovascularization removal [87]. The majority of operated patients improved their best-corrected visual acuity by two lines or more; however, no statistical difference was found between control and IPE-transplanted patients at up to 13 months of follow-up. The same group followed 56 consecutive patients for at least 2 years using the same procedure [88]. They reported also a statistically improved visual acuity until at least 48 months compared to pretransplantation.
Semkova and collaborators proposed to engraft IPE genetically modified to overexpress the PEDF. The idea was that PEDF would act as an antiangiogenic and a neuroprotective agent to potentiate the effect of transplanted IPE [89]. Indeed, rat IPE was transduced with a high-capacity adenovirus vector having both low toxicity and low immunogenicity. Thereafter RCS rats were transplanted with these IPE cells. The authors found that, at 2 months post surgery, photoreceptors in IPE expressing PEDF-transplanted eyes were better preserved when compared to IPE-transplanted and nonoperated eyes. In addition in a rat model of laser-induced CNV, IPE overexpressing PEDF prevented the formation of new vessels within the laser burns [89]. Another strategy of growth factor overexpression was proposed based on the effect of Brain-Derived Neurotrophic Factor (BDNF) [90]. IPE was transduced with an adeno-associated virus 2 (AAV2) containing the BDNF gene and grafted in a model of phototoxicity. Rats were exposed to constant light for 3 months after the transplantation. The measurement of the outer nuclear layer thickness demonstrated that photoreceptors were preserved in eyes transplanted with IPE overexpressing BDNF compared to normal IPE [90].
The concern with this approach, beside no proven efficacy in human, is the comparability with different batches of IPE from different donors. This strategy is not compatible with the industrial process required to treat at large-scale the millions of patients.
3.2.3.2 Schwann Cells
The Schwann cells produce trophic factors necessary for the survival of photoreceptors, including basic fibroblast growth factor (bFGF), ciliary neurotrophic factor (CNTF), and BDNF. Lawrence and collaborators proposed to graft Schwann cells into the SRS of RCS rats in order to compensate for defective RPE cells. These grafted cells are supposed to provide support for both RPE and photoreceptors improving thus their functionality and their survival [91]. In this study, the supply of the Schwann cells came from the sciatic nerves of congenic neonatal rats. When transplanted in RCS rats, the Schwann cells allowed photoreceptors to survive around the site of injection. Transplanted animals were then analyzed 2 and 3 months after surgery for their visual performances . Using the optokinetic paradigm, the authors demonstrated that transplanted rats were more prone to detect moving stripes compared to control animals up to 3 months post surgery. Keegan and collaborators showed that Schwann cells transplanted in the SRS of rhodopsin knockout (rho−/−) mice; another model of acute retinal degeneration preserved the photoreceptor survival until 1 month [92]. They addressed the release of specific factors using reverse transcriptase polymerase chain reaction (RT-PCR) assay for precise measurement of RNA production. BDNF, CNTF, and glial-derived neurotrophic factor (GDNF) transcripts were found, but there was no evidence of NGF and bFGF in Schwann cell culture. The same group demonstrated later that Schwann cells engineered to express GNDF or BNDF improve the survival and function of photoreceptors, when transplanted into the SRS of RCS rat, compared to the same cells not transfected [93]. This highlights the role of these two trophic factors secreted by Schwann cells. For a cell therapy strategy, the supply of Schwann cells could originate directly from the recipient’s own cells (e.g., from the sural nerve). This approach might reduce the risk of disease transfer and of immune response.
3.2.3.3 Fetal Brain-Derived Neural Progenitors
Human cortical progenitor cells were proposed and tested in vivo in animal models of RP [94, 95]. Here the idea was not to replace defective RPE or even photoreceptors. Human cortical progenitors are rather expected to produce trophic factors that might preserve photoreceptors. This is the mode of action of such cells when grafted in neurodegenerative disease models [94]. The human fetal cortex could be obtained from a brain tissue at 21 weeks of gestation and cultured as neurospheres for several passages [95]. When such cells were grafted into the SRS of RCS rats, they showed the potential to sustain the vision of RCS rats for a long period of time (280 days) as evaluated by the optokinetic paradigm. Interestingly, histological analysis revealed that grafted cells formed an “RPE-like layer” into the SRS, with some cells being pigmented [94]. However, they did not express typical RPE markers, and the cells were still proliferating into the SRS until at least P150 [95]. The human cortical progenitors cells were also injected into the SRS of six normal rhesus monkeys [96]. In that context, cells were transduced with Green Fluorescent Protein (GFP) to follow them. Monkeys recovered well from the surgical procedure, and there was no disturbance in multifocal ERG. Cells survived as a monolayer at 39 days post operation even if treated only with 5 days of topical steroids [96]. The authors concluded that the surgery might be safe for human, and that the cells should survive without need of systemic immunosuppression.
Human central nervous system stem cells (HuCNS-SCs) were purified by StemCells, Inc. in 2000 [97]. A single fetal brain tissue (16–20 weeks of gestation) was exposed to monoclonal antibodies for cell-surface markers and cell sorted. The HuCNS-SC express high levels of CD133 and low levels of CD24. In addition, they do not express antigens of the hematopoietic lineage CD45 or CD34. These cells can self-renew, form neurospheres, and can be banked. They are developed for the treatment of central nervous system diseases and were tested for retinal protection also in AMD. When HuCNS-SCs were transplanted into the SRS of the RCS rat, photoreceptors were protected and the vision was preserved [98]. Donor cells remain immature and do not express retinal markers during at least 7 months. In addition, the authors reported limited proliferation. StemCells, Inc. launched an ongoing phase I/II clinical trial in US in 16 patients with geographic atrophy secondary to AMD in order to investigate safety and preliminary efficacy [99].
3.2.3.4 Bone Marrow Mesenchymal Stem Cells
Bone marrow mesenchymal stem cells (MSCs) are precursors of bone, cartilage, and adipocytes in vivo, and they provide a suitable environment for the hematopoietic stem cell niche [100]. These cells have the major advantage to be harvested from bone marrow and expanded, thus allowing autologous transplantation. They are also poorly immunogenic, and it was demonstrated that MSCs secrete trophic factors that promote neuronal survival, like BDNF or bFGF [101]. Arnhold and collaborators tested the potential of MSCs for the rescue of retinal degeneration in RCS rats [102]. The authors used rat MSCs transduced with GFP in order to follow their fate in the rat eye. Transplanted MSCs adopted, in that context, an RPE-like morphology at 2 months post surgery. This was also demonstrated in vitro by cocultures of MSCs and RPE, where MSCs adopted a typical RPE morphology (intracellular pigment granules, hexagonal morphology, and cytokeratin expression). Photoreceptors of transplanted retinas were still numerous compared to the control ones at 2 months post operation [102]. Inoue and collaborators transplanted mouse MSCs into the SRS of RCS rats. It resulted in the slowdown of retinal degeneration with a drastic reduction of photoreceptor loss at 8 weeks after surgery [103]. In addition, the authors analyzed retinal functions and found that transplanted animals had a better ERG preservation compared to SHAM-treated eyes at both 4 and 8 weeks post operation . As a conditioned MSCs medium is able to promote survival of mouse retinal cultures, the authors suggested that the rescue effect observed in RCS rats might be mediated by the release of trophic factors that improve the environment [103]. In this study, MSCs remained in the SRS without any migration to the retinal layers. By contrast to Arnhold and collaborators, the authors did not report any signs of transdifferentiation of MSCs into RPE-like cells. Human adult bone marrow-derived somatic cells (derived using a proprietary process) were finally tested in RCS rats in 2010 [104]. The authors showed that transplanted RCS rats had a better visual acuity in the optokinetic test up to 3 months postnatal despite the absence of cell at that time. The survival of cells was not improved with a cyclosporine A treatment. Another report stated that human bone marrow MSCs grafted into the SRS of RCS rats did not survive more than 2 weeks [105]. They demonstrated also that the effects were sustained for 20 weeks, and that a second transplantation did not improve the phenotype.
3.2.3.5 Retinal Neurospheres
In the adult mammalian eye, neural progenitors are located in the pigment ciliary bodies [106]. These cells are rare, quiescent but can be stimulated to proliferate in vitro with bFGF. They can be amplified as neurospheres, and they are named retinal neurospheres (RNS) [107]. RNS retain their pigmentation and express markers like the neuroectodermal marker nestin. They are multipotent as they give rise to neurons, astrocytes, and oligodendrocytes. Rat or mouse RNS express also Chx10, a homeobox gene that is a retinal progenitor marker [106, 108]. In human, RNS were derived from the pars plicata and pars plana of the retinal ciliary margin [109]. These cells were isolated from early postnatal to 70-year-old postmortem donors. When cultured under specific differentiation media, they are able to give rise to different retinal cell types and to RPE. Human RNS were transplanted into immunodeficient NOD/SCID mice of 1-day-old. The authors showed that the cells survived at 28 days and gave rise to retinal progeny. RNS stem cell nature is controversial; some scientists stated that the pigmented ciliary epithelium contains differentiated cells that ectopically express markers upon specific culture conditions [110, 111]. Finally, RNS cells should be tested into animal models of retinal degeneration to demonstrate their therapeutic potential.
3.2.3.6 Umbilical-Cord Stem Cells
Human umbilical tissue-derived cells (hUTCs) are a promising cell source as it can be amplified to at least 1 × 1017 cells from a single donor without genetic abnormalities or changes in phenotype [112]. Cells are obtained by a simple enzymatic digestion of the human umbilical cord following normal births. When grafted into the SRS of RCS rats, hUTCs improved ERG responses to light compared to SHAM-treated animals. In addition, visual acuity evaluated by the optokinetic response to moving stripes was preserved in hUTCs-treated rats at 2.5 months post surgery [112]. Neurotrophins secreted by hUTCs, like BNDF, are suggested to mediate the preservation of photoreceptors.
3.3 RPE Derived from Pluripotent Stem Cells
Human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs) represent the two principal types of pluripotent cells. These unspecialized cells are able to self-renew and could give rise to all somatic cell types. Compared to the other cell sources described earlier, banking of these cells might be up scaled at an industrial level to treat millions of AMD and RP patients.
In 1998, the first hESC lines were derived from the blastocyst inner cell mass of human embryos produced by in vitro fertilization. These hESC lines showed unlimited proliferation ability in vitro and maintained the potential to form derivatives of the three embryonic germ layers: endoderm, mesoderm, and ectoderm [113].
The reprogramming of adult somatic cells, into artificial pluripotent stem cells, called induced pluripotent stem cells (iPSCs) by Yamanaka lab allowed to overcome ethical constraints associated to the use of human embryos [114]. Indeed human adult somatic cells, typically human fibroblasts, were forced to express pluripotency genes by retroviral transduction of four transcription factors: Oct3/4, Klf4, Sox2, and c-Myc [114]. This was reproduced by Thomson’s lab using a different cocktail of factors [115].
The hiPSCs are comparable to hESCs in terms of morphology, cell growth, surface markers, gene expression, in vitro differentiation, and teratoma formation when transplanted into immunocompromised animals [114]. The potential to generate autologous cell sources from reprogrammed patient’s somatic cells make hiPSCs very attractive for clinical applications [116]. These two cell sources (hESCs and hIPSCs) were used to produce RPE cells that might treat RPE-associated degenerative eye diseases.
3.3.1 Clinically Compatible Protocols to Obtain and Bank RPE Cells
Significant research efforts have focused on finding the ideal method for efficiently deriving RPE from embryonic and induced pluripotent stem cells. It has been well demonstrated that hPSCs have the potency to “spontaneously” differentiate into RPE using the continuous adherent culture method or the embryoid body method [117]. Protocols published in the literature could be gathered in three main methods: spontaneous differentiation, intermediate embryoid body (EB) formation, and 3-D cellular aggregates. In recent clinical trials, the first two types were used in order to generate GMP (Good Manufacturing Process)-compatible RPE cells. In 2004, Klimanskaya and collaborators reported the first spontaneous differentiation of hESCs into RPE cells [118]. To achieve this differentiation, the hESCs were cultured on mouse feeder cells in absence of FGF2. After 6–8 weeks, the authors observed the presence of brown-pigmented regions in over-confluent cultures. Pigmented regions were isolated and subcultured to generate the RPE cell population. The limitation with this protocol was the low efficiency regarding the number of cells obtained and the requirement of the manual selection of pigmented areas.
To improve the efficacy of RPE differentiation protocols, several teams developed protocols based on developmental studies made in xenopus, zebrafish, and mouse. This approach has led to the use of an increasing number of growth factor and small-molecule supplements in the culture media [117]. The pathways targeted vary from protocol to protocol, but the inhibition of TGF-ß pathway using noggin, LDN-193189, and/or SB-431542 is commonly used to engage neuroectodermal differentiation [119]. Neuroectodermal progenitors are then specified toward an anterior fate using inhibitors of the WNT signaling pathway (Dkk1/Nodal and Lefty A) to obtain eye field progenitors [120]. Using this method, Lamba’s team showed that it was possible to obtain nearly 80% of retinal progenitors [121]. For now, one of the quicker and the more efficient protocol was published by Buchholz and collaborators [122]. This team combined Lamba’s protocol with already described RPE-inducing factors such as nicotinamide and activin A in order to obtain 78% of RPE cells at day 14 [122]. One year later, the same team published that the RPE differentiation is even better with the use of activator of the WNT pathway such as CHIR99021 at the end of the differentiation process [123]. RPE cells could be obtained without these cytokines, but the efficiency is much lower [124]. Whatever the protocol used, the RPE obtained from pluripotent stem cells are similar to primary human fetal RPE [125]. Recently, a quantitative real-time PCR-based high-throughput screen was used to establish a new differentiation protocol with small-molecule treatment and that is suitable for a clinical application. Using this strategy, Chetomin, an inhibitor of hypoxia-inducible factors, was found to strongly increase RPE differentiation. The combination with nicotinamide resulted in conversion of over one-half of the differentiating cells into RPE. Single passage of the whole culture yielded a highly pure hPSC-RPE cell population that displayed many of the morphological, molecular, and functional characteristics of native RPE [126].
The second strategy to generate RPE cells involves an intermediate step: the embryoid body (EB) formation. The pluripotent stem cells, when cultured onto nonadherent culture dishes form aggregated structures that resemble early postimplantation embryos and that are therefore called EBs [127]. Pluripotent stem cells derived in EB structures acquire markers specific to the three embryonic germ layers [128] and could differentiate into multiple cell lineages. To induce the neuroectodermal lineage, the EBs obtained are then cultured in adherence, and after 4 additional weeks, pigmentation starts rising. These pigmented regions are expanded and ultimately form a monolayer of hexagonal pigmented cells [129]. This kind of differentiation can also be improved by the sequential use of nicotinamide and activin A, although the mechanism by which nicotinamide improves neural and eye field differentiation remains elusive [130].
In the last years, many research teams have explored new approaches to increase further the efficacy of RPE differentiation protocols. The most innovating approaches are represented by the generation of RPE cells from tridimensional structures. Gamm and collaborators developed a protocol where the pluripotent stem cells are differentiated into neural rosette, a primitive anterior neuroepithelium structure [131]. The neural rosette structures were isolated and cultured in suspension. After 20 days of culture, 3-D cell aggregates appeared and formed an optical vesicle-like (OV) shape. These tridimensional structures contained photoreceptors and RPE cell progenitors. To generate RPE cells, OV-like structures were cultured in presence of activin A for 20 days. Pigmented OV-like structures were plated onto coated dishes in order to promote RPE cell proliferation. This protocol was used to generate RPE cells from hESCs and hiPSCs in a reproducible manner [131]. Recently, Goureau and collaborators developed a new protocol of self-forming neural retina structures containing RPE and retinal progenitor cells including precursors of photoreceptors, without addition of exogenous molecules [132, 133]. In this study, hiPSCs were cultured in a serum-free medium containing N2 supplement. To generate RPE cells, the FGF was removed from culture medium to promote the neuroectoderm induction. After 14 days, neuroepithelial-like structures with pigmented areas were observed. These pigmented patches were mechanically isolated and cultured on gelatin-coated dishes. The pigmented patches proliferated and formed the typical cobblestone epithelial structure of RPE [133].
Recently, automated retinal differentiation including RPE differentiation was reported by Duncan E. Crombie and collaborators [134]. They showed the ability to maintain and subculture pluripotent stem cells using a customized automated platform. Then, they differentiated these cells into RPE cells using a directed protocol and reported the apparition of pigmented areas at day 35. Although, this is a promising step toward the scale up of the RPE differentiation that will be required for future applications; further works are necessary to obtain a pure population of cells with this method [134].
For the clinical translation step of RPE cell differentiation protocols, all parameters need to be evaluated with utmost rigor. According to European and US regulatory guidelines, the production of RPE cells requires to be reproducible and to use a fully defined medium. Thus, the constituents of differentiation and culture medium, cytokines and the matrix used to coat cell dishes, are selected based on their origin; their certificates indicating that they are pathogen-free and produced in a sterile environment. Moreover, the manufacturer should provide a full traceability of their starting and raw materials. All this selection increases the translation from the initial research protocol to the first clinical trial. This optimization process faces challenges due to the risk of inconsistency between the non-GMP starting/raw material and the GMP one and due to the batch to batch variability of a same product.
In 2012, Astellas Pharma (previously named OCATA Therapeutics) announced the first preliminary data of the clinical application of RPE derived from hESCs [135]. In this phase I/II clinical trial, a hESC line derived from in vitro fertilization embryo named MA09 was used. The MA09 cell line was cultured and amplified on Mitomycin C-treated mouse embryonic fibroblast (MEF) in GMP facility to generate a clinical Master Cell Bank. This manufacturing process used the EB formation process to generate RPE cells. hESCs were treated with 0.05% trypsin-EDTA to detach from feeder cells and subsequently plated into low-attachment cell dishes to promote the EB formation in MEM medium containing the B27 supplement. The EBs were maintained in culture for 1 week and seeded on gelatin-coated plates until the pigmented colonies appeared. Using enzymatic and mechanical methods, the pigmented RPE cells were isolated and cultured in Endothelial Growth Basal Medium on gelatin-coated cell dishes. The RPE cells were amplified for two passages, before being frozen to create the RPE clinical bank. The hESC-derived RPE cells were thawed and injected as a cell suspension into the SRS of patients suffering from dry AMD and Stargardt’s disease. The RPE cryovials could be sent frozen to different hospitals without the requirement of specific cell culture materials at the site of surgery. This allows a large-scale use of such cell product.
A second clinical trial using hPSCs-derived RPE for retinal degenerative diseases started in September 2014 in Japan. This study represents the first clinical assay using autologous iPSC therapy for the exudative form of AMD. In their study, Dr. Takahashi and her team have transplanted sheets of hiPSCs-derived RPE cells without any artificial matrix or scaffold ([116, 136]; Press Release, RIKEN July 30, 2013). hiPSCs were cultured and amplified on mouse embryonic fibroblast (MEF) feeder cells. To generate RPE cells from hiPSCs in xeno-free conditions, a direct differentiation protocol was used, in which there was no intermediate stage of EB formation. That process used two differentiation media and two different coatings. The undifferentiated hiPSCs were cultured in feeder-free conditions using gelatin-coated plates. During the differentiation process, the cells were cultured with Glasgow Minimum Essential Medium (GMEM) in presence of decreasing percentage of Knockout Serum Replacement (KSR) (from 20% to 10% during 20 days of culture). To induce RPE differentiation, some molecules were added in the culture medium during the first 18 days like ROCK inhibitor (Y-27632), a TGFβ inhibitor (SB 431542), and a protein kinase inhibitor (CK17) [137]. After 3 weeks of culture, pigmented cells appeared and the differentiation media was replaced with DMEM/F12 supplemented with B27 [116].
Our lab, in collaboration with the Institut de La Vision (Paris, France) is developing another protocol based on hESCs using a spontaneous differentiation protocol without growth factors or small molecules [138, 139]. The clinical grade hESC line, named RC-09 (Roslin Cell Laboratory, Edinburgh), was amplified in feeder-free conditions, in a GMP facility, to create a clinical hESC Master Bank. To differentiate the hESCs into RPE cells, the RC-09 are cultured in DMEM medium supplemented with 10% of KSR in the absence of FGF2. After 4–5 weeks of cultures, the presence of pigmented regions in the overgrowth colonies of RC-9 could be observed. These pigmented cluster cells are then mechanical isolated and cultured in culture dishes in presence of 4% of KSR (Passage P0). The RPE cells are amplified and frozen at passage P1, to create the RPE Bank.
All these culture methods will be useful for the demonstration of the safety and efficacy of hPSCs-derived RPE in ongoing and future clinical trials. The next challenges will be the development of commercially viable sources of RPE that are low cost, reliable, and robust.
3.3.2 Quality Controls of Differentiated RPE Cells
In most countries, the use of cellular products for medical use is regulated by governmental agencies to ensure the protection of patients, so that novel therapies will be the most widely beneficial for the population.
Many technical and regulatory breakthroughs in the last few years have made stem cell-based treatment for retinal degeneration more plausible. During the manufacturing process of hPSCs-derived RPE cells, several important limiting key points need to be carefully evaluated according to regulatory guidelines. It includes safety, purity, identity, potency, and stability of differentiated cells [135, 140]. Even minimal manipulation of the cells outside the human body introduces a risk of contamination with pathogens, and prolonged passages in cell culture carries the potential for genomic and epigenetic instabilities that could lead to cell dysfunctions or frank malignancy.
The purpose of preclinical studies is to (1) provide evidence of product safety and (2) establish proof of principle for therapeutic effects. International research ethic policies, such as the Declaration of Helsinki and the Nuremberg Code, strongly encourage the performance of animal studies prior to clinical trials. Before initiating clinical studies with stem cells in humans, researchers should have persuasive evidence of clinical promise in appropriate in vitro and/or animal models.
3.3.2.1 Safety of Therapeutic Cells
All reagents should be subjected to quality control systems to ensure the quality of the reagents (raw and starting materials) prior to introduction into the manufacturing process. For extensively manipulated stem cells intended for clinical application, GMP procedures should be strictly followed [141].
The RPE cells should be checked for safety, RPE identity, potency, and functional activity at various steps of the manufacturing process (in-process testing during the manufacturing, after RPE thawing and in the final clinical product) [135]. The absence of bacterial and mycoplasma contamination all along the process and in the final product in its final formulation should also be tested. Components of animal origin may present a risk of transferring pathogens or unwanted biological material. For example, when cultured onto MEF (Mouse embryonic Fibroblasts), the cells should also be monitored for the absence of residual murine viruses. In some circumstances, it may not be possible or optimal to substitute these components. Researchers need to demonstrate that the risk to use such product is mitigated by the different additional safety tests of these animal-based components.
hPSCs, regardless of particular cell type, carry additional risks due to their pluripotency. These include the ability to acquire mutations when maintained for prolonged periods in culture, to grow and differentiate into inappropriate cellular phenotypes, to form benign teratomas or malignant outgrowths, and to fail to mature in the cell type of interest. Teratomas are tumors of multiple lineages, containing tissues derived from the three germ layers [142]. It confers additional risk to patients, and appropriate tests must be planned to ensure safety of stem cell-derived products [143]. When undifferentiated, hPSCs are transplanted into immunocompromised animals; they might give rise to these teratomas. For hPSCs-derived products, a quantification strategy needs to be developed to evaluate the risk of remaining undifferentiated cells in the final product. The level of impurities in the final product should be enough low to prevent teratoma formation in long-term animal studies (over 6 months). Tumorigenicity assays should actually include groups of animals transplanted with undifferentiated hPSCs and other groups injected with serial dilutions (spiking studies) of undifferentiated hPSCs with hPSCs-derived RPE cells to set a contamination limit that is susceptible to induce a teratoma and that should not be reached in the cell therapy product. The cells used in tumorigenic test (regulatory toxicity studies) should be produced with a manufacturing process similar to the one used for the clinical application [143]. Several immunodeficient animal models are now available for such studies: NOD/SCID (NOG) mice, and NOD/SCID/IL-2rg KO (NSG) mice, Rag2-yC double-knockout (DKO) mice. These animal models are T-cell, B-cell, and NK-cell-deficient and show very high engraftment potential of human cells compared to nude mice (T-cell-defective) [142].
Other important issues to consider in safety assays are related to the biodistribution of grafted cells. The stem cells and their differentiation derivatives may have the potential to migrate from the injection site to other organs. Careful evaluation of biodistribution, assisted by sensitive techniques of imaging and monitoring of homing, retention, and subsequent migration of transplanted cell populations, is crucial for measuring cell dispersion. While rodents or other small animal models are typically a necessary step in the development of stem cell-based therapies, they are likely to reveal only major toxic events [142]. Biodistribution tests are performed in injured or healthy animals using the route of administration of the cell therapy product. The proximity of many physiological functions between large mammals and humans may favor testing the biodistribution and toxicity of a novel cell therapy in at least one large animal model. Additional histological analyses or banking of organs for such analysis at late time points is recommended. Depending on the laws and regulations of the specific country, biodistribution and toxicity studies often need to be performed in a GLP (Good Laboratory Practice)-certified animal facility.
In Astellas Pharma preclinical toxicity studies, the hESC-derived RPE was injected in the SRS of NIH III mice, a typical nude mice characterized by the absence of thymus and T-cell function and by complications in the maturation of T-independent B cells. The absence of human cells in the eyes and others organs of these mice was determined by RT-qPCR and immunostaining at 4, 12, and 40 weeks after grafting. All tests showed no safety issues in any animal even if hESCs-derived RPE cells were spiked with 0.01% of undifferentiated cells [135].
Similar results were obtained with hiPSCs-derived RPE cell sheets transplanted into the SRS. Kamao and collaborators did not observe any tumor formation in various animal models (RCS rat, immunodeficient mouse and monkey) [116, 136].
Simplified culture conditions are essential for large-scale drug screening and medical applications of hPSCs. However, hPSCs are prone to genomic instability, a phenomenon that is highly influenced by the culture conditions (various culture media, passaging techniques, and culture feeders/matrices) that may differently affect genetic stability or impart different selective pressure on cells [144, 145]. For example, enzymatic dissociation, a cornerstone of large-scale hPSCs culture systems, is deleterious, but the extent and the timeline of the genomic alterations induced by this passaging technique are still unclear [144]. For the karyotype analysis of undifferentiated hPSCs and their derivatives, the G-banding and FISH methods are used. In the protocol developed by Astellas Pharma, the chromosomal integrity of hESC Master Cell Bank was evaluated by FISH and G-Banding methods [135]. The reprogramming process of somatic cells into pluripotent stem cells can induce chromosomal abnormalities, as highlighted recently. The Japanese clinical trial team has identified the presence of mutation in the hiPSCs generated from the second patient [136]. Three single-nucleotide variations and three copy-number variants were detectable in the hiPSCs while were absent in the original patient fibroblasts. For this reason, it was decided not to treat the second patient with autologous hiPSCs-derived RPE [146].
3.3.2.2 Purity of the Differentiation Process
To evaluate the purity of hPSCs-derived cells, a panel of RPE-specific markers should be used, like ZO-1 (tight junction marker), MITF, and PAX-6 (eye field specification), RPE-65 and CRALBP (retinoid cycle), Bestrophin (chloride channels), and MERTK (phagocytosis function) [116, 138].
In the protocol developed by Astellas Pharma, the hESC-derived RPE was characterized by immunofluorescence. hESCs-derived RPE cells should coexpress more than 95% of PAX-6 and MITF, 95% of PAX-6 and Bestrophin and express more than 95% of ZO-1 [135]. The purity of RPE cells generated from autologous hiPSCs, in the Takahashi’s protocol, was characterized by the quantification of PAX-6 or Bestrophin-positive cells [116, 136].
Gene expression of pluripotency and RPE-specific genes could be monitored by quantitative PCR. The absence of contaminating hPSCs in the RPE population might be evaluated by the downregulation of OCT-4, NANOG, and SOX-2 mRNA expression compared to undifferentiated pluripotent stem cells. The monitoring of the upregulation of some specific RPE markers, like RPE-65, PAX-6, CRALBP, and BEST1, indicates the RPE identity [116, 136, 138].
3.3.2.3 Functionality of the RPE Obtained from Differentiation
The functional assessment is based on typical characteristics of RPE cells as the phagocytosis activity, the polarized secretion of growth factors, and the barrier function of epithelial cells. To maintain the excitability of photoreceptors, POS undergo a constant renewal process [6, 31]. In the protocol developed by Astellas Pharma, the phagocytosis activity of hESCs-derived RPE, was monitored through the uptake of fluorescent bioparticles. The internalization of bioparticles is measured in this approach by flow cytometry [135]. Other systems use porcine POS labeled with fluorescent markers. When RPE cells are exposed to such POS, they are able to phagocyte them [133, 139].
Maintaining the polarized secretion of growth factors is a crucial parameter for restoring some RPE functions after the graft. Two growth factors are particularly important: PEDF (apical secretion) essential for the structural integrity of the retina, and the VEGF (basal secretion) essential for the integrity of choriocapillaries. The concentrations of PEDF and VEGF in a conditioned RPE medium could be measured by ELISA assays and reflect functional properties of RPE cells [116].
The measure of RPE epithelial resistance is an additional method to evaluate the potency of hPSCs-derived RPE cells. Tight junctions are a type of cell–cell adhesion that has a fundamental role in the functionality of the RPE layer [147]. Tight junctions form a partially occluding seal that retards diffusion of solutes across the paracellular space. This barrier permits the RPE layer to establish and maintain concentration gradients between its apical and basal environments [148]. The strength of this barrier may be measured by the transepithelial electrical resistance in vitro [136].
3.3.3 Injection Strategy of the Cell Therapy Product
Significant research efforts are focusing on finding the ideal method for transplanting hPSCs-derived RPE cells into the subretinal space. Optimizing RPE transplantation procedures resulted in the development of two different therapeutic strategies: (1) introducing a cell suspension of nonpolarized RPE cells into the subretinal space and allowing the donor cells to integrate within the host retina, and (2) transplanting polarized sheets of RPE to allow for improved safety and better clinical outcomes, since normal RPE functions are dependent on specific cellular features of its apical and basal domains [125, 149].
In phase I/II clinical trials sponsored by Astellas Pharma, the RPE cells derived from hESCs were delivered as a cell suspension into SRS of dry AMD and Stargardt’s patients [135]. 5 × 104 hESCs-derived RPE cells in 150 μL were injected into a preselected region of the pericentral macula that was not completely damaged [125]. Three cell doses were initially proposed for injections: 5 × 104, 1 × 105, and 1.5 × 105 RPE cells in 150 μL in order to evaluate the safety of the cell therapy. To avoid the dispersion of donor RPE cells in other site than the retina, the injection site was carefully chosen based on the presence of native RPE layer albeit damaged, to improve the transplant integration [140].
The most important point of success for cell transplantation into SRS region is the integrity of the implanted region. Retinal degenerative diseases, like AMD may lead to the destruction of RPE-Brüch’s membrane, compromising the integrity of the blood–retinal barrier. Thus, if RPE cells are transplanted as a cell suspension, their chance to survive and integrate correctly might be compromised. To overcome this problem, a RPE monolayer that exhibits the physiology of its natural counterpart represents a favorable alternative to RPE cell suspension [83].
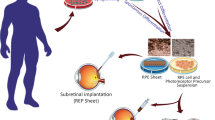
Takahashi and collaborators developed a strategy based on RPE sheets transplantation without any matrix or scaffold using an ingenious system [116]. Indeed, hiPSCs-derived RPE are cultured onto Transwell inserts coated with collagen type I. When the RPE cells reach confluency and form a typical cobblestone pigmented epithelium, the RPE sheet is removed from the insert by a collagenase treatment [116, 136]. The size of the RPE sheet is then adjusted to 1.3 × 3 mm using a laser microdissection system.
We developed an alternative approach based on a natural scaffold to graft an RPE sheet. Our cell therapy product is constituted of a three dimensional (3D) patch of hESCs-derived RPE cultured on a human amniotic membrane (hAM) [138]. hAMs are obtained from cesarean sections of normal births [150]. The hAM consists of an epithelial monolayer, a thick basement membrane, and a multilayer of collagen. The components of the hAM create an interesting native scaffold for cell seeding in tissue engineering. Moreover, the hAM presents significant biological advantages like anti-inflammatory, healing, and antimicrobial properties [151]. For all these reasons, the hAM is commonly used in clinical applications for ocular surface reconstruction [152]. In this approach, the hAM is treated in order to remove the native epithelia cell layer, and the denuded hAM is attached to a culture insert, which allows the culture of RPE cells. The hESCs-derived RPE cultured on this natural scaffold, form a typical cobblestone pigmented epithelial layer, with polarized secretion of VEGF (Fig. 3.2).
Synthetic scaffolds may also be used to transplant RPE cells. Several parameters must be taken into consideration, as thickness, mechanical properties, and biodegradation, to prevent additional damage of the retina, and improve the interactions between the retina and RPE [153]. The transplantation of such polarized RPE monolayer on ultrathin parylene substrates has proven its safety and efficacy [154].
Coffey and collaborators developed a porous polyester scaffold who serves as a matrix for the transplantation of the hESCs-derived RPE. The targeted patients for this cell therapy suffer from wet AMD (Press Release UCL, Sept 29, 2015; ClinicalTrials.gov NCT01691261) [155].
3.4 Clinical Trials for the Treatment of AMD and RP
3.4.1 Adult and Fetal RPE
Use of adult and fetal RPE cells demonstrated the potential of the cell therapy approach for treating RPE-associated diseases [74, 156]. However, their reduced availability has limited their potential for large-scale use in clinic. One of the last strategies taking advantage of adult RPE cells is the one of the group of Sally Temple (Neural Stem Cell Institute, NY) who had discovered and isolated RPESCs from adult RPE cell culture [81]. They are now developing cGMP optimization of allogeneic cells from adult RPE in order to target AMD patients with geographic atrophy [157].
3.4.2 hiPSCs/hESCs-Based Clinical Trials
Several phase I/II clinical trials were launched by Astellas Pharma (Table 3.1) using allogeneic hESCs-derived RPE as cell suspension both in USA and UK for Stargardt’s macular dystrophy (SMD) and AMD. The same cells were also used by CHABiotech CO., Ltd. in the Republic of Korea to treat patients with AMD. Preliminary reported data indicated no major safety issue [135, 140, 158]. Astellas Pharma is moving forward with a phase II clinical trial, which is about to be launched (NCT02563782). Recently, the London Project to Cure Blindness and Pfizer has started a phase I/II clinical trial with the first wet AMD patient being treated in 2015. Here the transplant is composed of RPE derived from allogeneic hESCs cultured over a polyester membrane [159]. Other clinical trials based on allogeneic hESCs-derived RPE are currently recruiting AMD patients in California (NCT02590692) and Israel (NCT02286089). In France, a consortium composed of I-Stem and Institut de la Vision is currently optimizing a cell therapy with allogeneic hESCs-derived RPE over a biological substrate in order to launch a phase I/II clinical trial targeting RP in 2019 [138].
As an alternative to allogeneic cells, other laboratories develop clinical trials based on autologous hiPSCs. The Riken Center for developmental biology in Japan has initiated in the end of 2014 the first phase I/II clinical trial based on autologous hiPSCs derived into RPE. The first patient had a wet AMD with CNV, and five other patients were planned. However, due to genetic defects found in the cells from the second patient, this first-in-man clinical trial was suspended [136, 146]. A Japan’s new law facilitates now the commercialization of hiPSCs. Indeed, regenerative therapy will be conditionally approved if they are demonstrated safe. Then they have up to 7 years of commercialization to demonstrate efficacy. New strategies have been adopted, and Riken now pushes toward the use of allogeneic hiPSCs to reach earlier the market. The National Eye Institute (NIH) and Cellular Dynamics International are developing autologous hiPSCs-derived RPE cultured on a biodegradable scaffold [157]. They are currently optimizing the cGMP process and expect to treat the first patient in 2018.
3.4.3 Other Stem Cell Trials
Autologous BM-derived stem cells, delivered intravitreally, are developed by many laboratories around the world (Table 3.2). The surgery is easier as a trophic effect is expected; the cells do not need to be located in the SRS like RPE cells.
Human central nervous system stem cells derived from fetal brain are under investigation for dry AMD patients. The phase I/II clinical trial was started in 2012, and preliminary results in 15 patients indicate no safety issues (Table 3.3). The Phase II study is currently recruiting patients. Other companies use human retinal progenitors injected either through an intravitreal (jCyte, Inc.) or subretinal route (ReNeuron Limited). jCyte, Inc. had currently treated 4 out of 16 patients at the 2015 summer in California. They will be followed for 12 months to report any safety issue. The effect of the cells is expected to be trophic.
An ongoing phase I/II clinical trial sponsored by Janssen Research & Development, LLC is based on a subretinal delivery of human umbilical tissue-derived stem cells. The cells are delivered through a catheter delivery system [159]. Finally, a clinical trial was launched based on autologous adipose-derived stem cells (NCT02024269) by the Hollywood Eye Institute in Florida and US Stem Cell, Inc. The cells are obtained via liposuction. After isolation of adipose-derived stem cells, they are injected into the vitreous [160]. However, recent results observed in three patients treated in a stem cell clinic with such cells raised concerns about their safety. Loss of vision acuity was reported, one patient been blind due to the treatment [161].
3.5 Management of the Graft Rejection
The eye is a prototypic immune-privileged tissue that resists immunogenic inflammation through multiple mechanisms [162, 163]. Inside the eye, the SRS is even a better transplantation site than the vitreous cavity. Indeed, cells grafted into the SRS demonstrated better survival than the ones transplanted into the vitreous cavity [105, 164]. Moreover, stem cells have low immunogenic capacities [164,165,167], reducing their chances of rejection. Thus, the eye appears as a good candidate organ for stem cell therapy. Despite these characteristics, most studies using stem cells for SRS transplantation faced poor survival rate of the graft [168, 169]. The potential for the stem cell-derived RPE cells to replace degenerated endogenous cells in retinal diseases is challenged by this threat of immune rejection. Although immunosuppressive agents were used to address the rejection, significant morbidity is associated with such treatments, especially in the elderly. More knowledge is then necessary about the immune characteristics of the SRS and RPE cells [149].
3.5.1 Immune Privilege of the Eye
The SRS, area between the RPE layer and the outer limiting membrane of the retina, is considered as an immune-privileged site within the eye [170] and thus a logical target for cell transplantation. The integrity of the RPE layer appears to be critical for the immune-privileged status of the SRS [171]. Some studies found that RPE cells in vitro can suppress T-cell activation by direct cell-to-cell contact [172] and by using supernatant of RPE eyecups; others demonstrated that RPE could secrete factors that suppress T-cell activation and production of interferon [173]. RPE possesses characteristics that promote its own survival even when transplanted to nonimmune-privileged site [174]. Indeed CD95-deficient RPE cells (cells lacking Fas Ligand) promote immune reaction leading to the rejection [174]. Transplantation of fetal retina/RPE tissue under the retina of patients suffering from RP or AMD was not associated with significant immune rejection reactions [73]. One explanation proposed by the authors is that the absence of detectable graft rejection, even in patients with donor-specific antibodies before implantation, may indicate that the blood–retinal barrier is restricting antibody access into the SRS. This mechanism could be parallel to the Anterior Chamber-Associated Immune Deviation (ACAID) process existing in the anterior chamber of the eye [73, 163]. Other studies proposed a regulation of T-cell differentiation through TGF-β secretion by RPE cells [175, 176] and/or Interleukin-10 (IL-10) secretion by macrophages [177]. Evidence suggests that innate and adaptive components of the immune system could be regulated through surface expression of molecules on RPE cells, as well as through autocrine and paracrine effects of cytokines and growth factors secreted from the basal and apical sides of the cells [172, 177,178,179,181]. The most important molecules secreted by RPE cells and identified to have a role in the regulation of the immune system are (1) TGF-β and thrombospondin (acting on adaptive immune system) and (2) PEDF (Pigment epithelium-derived growth factor) and somatostatin (for innate immune system) [173, 176].
Even if, RPE cells could secrete anti-inflammatory molecules, there are also some evidences that RPE cells could behave as antigen presenting cells to the T cells, stimulating their activation [182]. Regulatory T cells (Tregs) are part of intraocular immunosuppressive mechanisms [183], and their activation depends on TGF-β signaling. To the best of our knowledge, MHC-class II molecules (e.g., HLA-DR antigens) are not expressed on RPE cells [181], and there is little or no expression of positive costimulatory molecules (e.g., CD40, CD80, and CD86) by RPE cells under normal conditions [181, 184]. Moreover, RPE cells express negative costimulatory molecules such as B7-H1 (PD-L1), [181] suggesting that T cells infiltrating the graft site after transplantation might interact with these molecules and be inactivated.
Recent studies specifically addressed the capacities of hPSCs-derived RPE cells to regulate subretinal and retinal immune environments. These studies observed that hPSCs-derived RPE cells injected into the SRS survived short-term without evident immune inflammation [116, 184,185,187]. hiPSCs-derived RPE cell sheets exhibit the morphological properties, in vitro and in vivo function, gene expression, and immunogenicity of authentic RPE cells [116]. In addition, recent clinical trials presumed that hESCs-derived RPE transplants in patients with dry AMD and Stargardt’s diseases were not rejected [135, 140]. hiPSCs express low levels of MHC-class I (MHC-I) and β2-microglobulin (β2-MG) proteins, which increase upon differentiation [116]. Based on the findings that hiPSCs-derived RPE cells exclusively suppress T-cell activation (e.g., production of IFN-γ) through immunosuppressive factor(s), recent studies showed that cultured hiPSCs-derived RPE cells significantly inhibited cell proliferation and IFN-γ production by T cells when the target T cells were stimulated with anti-human CD3/CD28 antibodies, PHA-P, and recombinant IL-2. The hiPSCs-derived RPE cells constitutively expressed and secreted TGFβ, and TGFβ siRNA-transfected hiPSCs-derived RPE cells did not inhibit T-cell activation. Thus, cultured hiPSCs-derived RPE cells fully suppress T-cell activation in vitro, and hESCs-derived RPE does not stimulate Peripheral Blood Monocytes Cell (PBMC) proliferation (as shown by BrdU assay) nor cytotoxicity (as shown by Mixed Lymphocyte Reaction assay) [116, 188]. However, this is mitigated by a recent study that detected antibody-mediated rejection generated by B cells in allogeneic RPE transplantation in a monkey model [189]. Finally, the maturation status of RPE cells and their organization as polarized cells have effects on the immunosuppressive potential of these cells. Sonoda and collaborators demonstrated that the polarity is associated with a significant shift in growth factors and other cytokines production capacity of the cells [190]. In addition, RPE cells in suspension have a lower tolerance to oxidative stress than those in monolayers [191]. Whether hPSCs-derived RPE cells in suspension culture differ from sheets attached is not clearly determined, but polarized hPSCs-derived RPE might differ in the rate of secretion of molecules involved in immune suppression [116]. Many nonbiodegradable and biodegradable substrates are being tested as scaffold for hPSCs-derived RPE as an alternative to cell suspension. Cons and pros arise from these two strategies as cell suspension confers less surgical trauma but shows a lower tolerance to oxidative stress and a lower survival than epithelial RPE sheets [191].
Even if the SRS is largely an immune-privileged site, attention should be drawn toward the fact that surgical trauma during cell transplantation compromises the blood–ocular barrier and subjects surrounding cells to an increased level of recognition and reactions. Moreover, in the case of diseased patients and particularly in AMD patients where the deregulation of the complement system appears to be a major pathogenesis contributor, promoting the long-term survival of grafted cells in a proinflammatory microenvironment could be a real challenge [149].
In conclusion, the mechanisms conferring immune privilege to the SRS may include (1) suppression of T-cell activation by the release of cytokines from RPE or hPSCs-derived RPE [172, 179, 183, 184, 191,192,194]; (2) production of other immunosuppressive factors by RPE cells that suppress innate immune activity [173, 194]; (3) surface expression of program death-1 (PD-L1) and Fas ligand [179, 195, 196] by RPE cells; (4) conversion of CD8+ and CD4+ T cells into regulatory T cells [193]; and (5) the intact physical barrier of the RPE layer [171].
3.5.2 Current Immunosuppressive Treatment and Global Haplotype Cell Banking Initiative
Occurrence of chronic loss of transplanted RPE cells in the SRS indicates that the immune privilege is not perfect despite these immunosuppressive mechanisms [163, 197]. Several laboratories demonstrated that even with robust anti-inflammatory regimen, gradual loss of allogeneic or xenografted RPE could occur and starts 1–2 months after grafting into the SRS [83, 198, 199]. Local inflammation can be induced by numerous factors among which any artificial scaffold associated to the transplanted cells.
In order to obtain long-term survival and function of the transplanted cells, we might provide some protection against the inflammatory response that could be triggered at the time of surgery by using robust immunosuppressive regimen (mycophenolate mofetil and tacrolimus, [135, 140]) or intraocular corticosteroid capsules (intravitreal dexamethasone or fluocinolone acetonide implants [200, 201]).
Matching MHC, ABO blood groups and minor antigens improve the survival of hiPSCs-derived RPE grafts [116]. However, allofactors other than MHC antigens of the transplanted cells are recognized as a heterogeneous group of targets for immune recognition and the importance of such molecules is illustrated by the observation of transplant rejection in HLA identical siblings [202].
Generation of hPSCs-derived RPE with known HLA genotypes may reduce risks of rejection [141, 203]. This concept already exists for cord blood [204]. HLA genes are located on chromosome 6 and represent the most polymorphic system in the human genome. They are divided into two groups of antigens, HLA class I or HLA-I (HLA-A, HLA-B, and HLA-C) expressed by nearly all nucleated cells and HLA class II or HLA-II (HLA-DR, HLA-DP, and HLA-DQ), which are expressed by some specific cells, such as dendritic or B cells [205]. Studies are now conducted to determine whether allogeneic T cells can recognize hiPSCs-derived RPE cells from HLA-3 locus homozygote donors [206]. In solid organ transplantation, many studies have demonstrated the importance of HLA-A, HLA-B, and HLA-DR for the long-term graft survival in addition to immunosuppressive drugs. Matching for these loci not only reduces the allograft rejection but also diminishes the use of immunosuppressive drugs [207]. Sugita and collaborators demonstrated that hiPSCs-derived RPE from HLA homozygous did not elicit a T-cell response in vitro [208]. This group also demonstrated that MHC homozygous donor of iPSCs-derived RPE transplanted to a compatible recipient monkey did not induced the infiltration of inflammatory cells to the graft. By contrast, MHC mismatched donor and recipient monkeys had inflammatory cell infiltration into the transplanted RPE sheet [209]. Due to HLA diversity, the best possibility is a bank of HLA homozygous cell lines. Nakajima et al. calculated that if a bank possesses hPSC lines from 100 randomly healthy selected donated embryo, 19% of patients were expected to find at least one hiPS cell line with a complete matching for HLA-A, HLA-B, and HLA-DR in the Japanese population [210]. Taylor and collaborators showed that, in the UK, 150 donors would cover 18.5% of the population with a full match. Interestingly, using this number of 150 donors would also cover 21% of the Japanese population with a full match [203]. Gourraud and collaborators developed a probabilistic model and demonstrated that using a bank comprising the 100 iPSC lines with the most frequent HLA in each population would leave out only 22% of the European Americans, but 37% of the Asians, 48% of the Hispanics, and 55% of the African Americans [211]. International strategies started now in order to create such banks , which might be useful as a source of allografts in retinal disorders [141].
3.6 Conclusions
Human retinal degenerative diseases are currently incurable, and retinal degeneration, once initiated, is irreversible. As RPE cell defects are involved in some RP and AMD pathogenesis, reestablishment of a healthy RPE layer through implantation of hPSCs-derived RPE has created hope for preventing blindness of millions of patients. Recent US clinical trials demonstrated the safety of nonpolarized hESCs-derived RPE approaches, and other ongoing clinical trials are promising in evaluating functional outcomes for nonpolarized and polarized hPSCs-derived RPE transplantation. As pointed out by the NEI, technical and logistical roadblocks remain to be identified, and potential solutions will come from collaboration between academic labs and private companies [157]. We are now entering a very exciting era leading to different treatment options for patients.
References
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2(2):e106–e116
Kolb H, Nelson R, Fernandez E, Jones B (2012) WEBVISION, The Organization of the Retina and Visual System. http://webvision.med.utah.edu/
Nag TC, Wadhwa S (2012) Ultrastructure of the human retina in aging and various pathological states. Micron 43(7):759–781
Sparrow JR, Hicks D, Hamel CP (2010) The retinal pigment epithelium in health and disease. Curr Mol Med 10(9):802–823
Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I (2014) Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res 43:17–75
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85(3):845–881
Ben M’Barek K, Regent F, Monville C (2015) Use of human pluripotent stem cells to study and treat retinopathies. World J Stem Cells 7(3):596–604
da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P (2007) RPE transplantation and its role in retinal disease. Prog Retin Eye Res 26(6):598–635
Bird AC (1995) Retinal photoreceptor dystrophies LI. Edward Jackson Memorial Lecture. Am J Ophthalmol 119(5):543–562
Sahel JA, Marazova K, Audo I (2015) Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb Perspect Med 5(2):a017111
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368(9549):1795–1809
Berger W, Kloeckener-Gruissem B, Neidhardt J (2010) The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res 29(5):335–375
den Hollander AI, Roepman R, Koenekoop RK, Cremers FP (2008) Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res 27(4):391–419
Hamel CP (2014) Gene discovery and prevalence in inherited retinal dystrophies. C R Biol 337(3):160–166
Hamel CP, Griffoin JM, Lasquellec L, Bazalgette C, Arnaud B (2001) Retinal dystrophies caused by mutations in RPE65: assessment of visual functions. Br J Ophthalmol 85(4):424–427
Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP (1997) Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet 17(2):139–141
Marlhens F, Griffoin JM, Bareil C, Arnaud B, Claustres M, Hamel CP (1998) Autosomal recessive retinal dystrophy associated with two novel mutations in the RPE65 gene. Eur J Hum Genet 6(5):527–531
Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP (1998) Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc Natl Acad Sci U S A 95(6):3088–3093
Burstedt MS, Forsman-Semb K, Golovleva I, Janunger T, Wachtmeister L, Sandgren O (2001) Ocular phenotype of bothnia dystrophy, an autosomal recessive retinitis pigmentosa associated with an R234W mutation in the RLBP1 gene. Arch Ophthalmol 119(2):260–267
Maw MA, Kennedy B, Knight A, Bridges R, Roth KE, Mani EJ, Mukkadan JK, Nancarrow D, Crabb JW, Denton MJ (1997) Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet 17(2):198–200
Young RW, Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42(2):392–403
Bok D (1985) Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest Ophthalmol Vis Sci 26(12):1659–1694
Tarnowski BI, Shepherd VL, McLaughlin BJ (1988) Mannose 6-phosphate receptors on the plasma membrane on rat retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 29(2):291–297
Ryeom SW, Silverstein RL, Scotto A, Sparrow JR (1996) Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J Biol Chem 271(34):20536–20539
Ryeom SW, Sparrow JR, Silverstein RL (1996) CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci 109(Pt 2):387–395
Lin H, Clegg DO (1998) Integrin alphavbeta5 participates in the binding of photoreceptor rod outer segments during phagocytosis by cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci 39(9):1703–1712
Hall MO, Burgess BL, Abrams TA, Ershov AV, Gregory CY (1996) Further studies on the identification of the phagocytosis receptor of rat retinal pigment epithelial cells. Exp Eye Res 63(3):255–264
D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9(4):645–651
Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC (2004) Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med 200(12):1539–1545
Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D (2000) Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet 26(3):270–271
Mazzoni F, Safa H, Finnemann SC (2014) Understanding photoreceptor outer segment phagocytosis: use and utility of RPE cells in culture. Exp Eye Res 126:51–60
Zarbin MA, Rosenfeld PJ (2010) Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina 30(9):1350–1367
Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP (2003) Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 48(3):257–293
Boulton M, Dayhaw-Barker P (2001) The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (Lond) 15(Pt 3):384–389
Nowak JZ (2006) Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 58(3):353–363
Xu H, Chen M, Forrester JV (2009) Para-inflammation in the aging retina. Prog Retin Eye Res 28(5):348–368
Bhutto I, Lutty G (2012) Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med 33(4):295–317
Dunaief JL, Dentchev T, Ying GS, Milam AH (2002) The role of apoptosis in age-related macular degeneration. Arch Ophthalmol 120(11):1435–1442
Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, Igo RP Jr, Buitendijk GH, Sim X, Weeks DE, Guymer RH, Merriam JE, Francis PJ, Hannum G, Agarwal A, Armbrecht AM, Audo I, Aung T, Barile GR, Benchaboune M, Bird AC, Bishop PN, Branham KE, Brooks M, Brucker AJ, Cade WH, Cain MS, Campochiaro PA, Chan CC, Cheng CY, Chew EY, Chin KA, Chowers I, Clayton DG, Cojocaru R, Conley YP, Cornes BK, Daly MJ, Dhillon B, Edwards AO, Evangelou E, Fagerness J, Ferreyra HA, Friedman JS, Geirsdottir A, George RJ, Gieger C, Gupta N, Hagstrom SA, Harding SP, Haritoglou C, Heckenlively JR, Holz FG, Hughes G, Ioannidis JP, Ishibashi T, Joseph P, Jun G, Kamatani Y, Katsanis N, Keilhauer N, Khan JC, Kim IK, Kiyohara Y, Klein BE, Klein R, Kovach JL, Kozak I, Lee CJ, Lee KE, Lichtner P, Lotery AJ, Meitinger T, Mitchell P, Mohand-Said S, Moore AT, Morgan DJ, Morrison MA, Myers CE, Naj AC, Nakamura Y, Okada Y, Orlin A, Ortube MC, Othman MI, Pappas C, Park KH, Pauer GJ, Peachey NS, Poch O, Priya RR, Reynolds R, Richardson AJ, Ripp R, Rudolph G, Ryu E, Sahel JA, Schaumberg DA, Scholl HP, Schwartz SG, Scott WK, Shahid H, Sigurdsson H, Silvestri G, Sivakumaran TA, Smith RT, Sobrin L, Souied EH, Stambolian DE, Stefansson H, Sturgill-Short GM, Takahashi A, Tosakulwong N, Truitt BJ, Tsironi EE, Uitterlinden AG, van Duijn CM, Vijaya L, Vingerling JR, Vithana EN, Webster AR, Wichmann HE, Winkler TW, Wong TY, Wright AF, Zelenika D, Zhang M, Zhao L, Zhang K, Klein ML, Hageman GS, Lathrop GM, Stefansson K, Allikmets R, Baird PN, Gorin MB, Wang JJ, Klaver CC, Seddon JM, Pericak-Vance MA, Iyengar SK, Yates JR, Swaroop A, Weber BH, Kubo M, Deangelis MM, Leveillard T, Thorsteinsdottir U, Haines JL, Farrer LA, Heid IM, Abecasis GR, A. M. D. G. Consortium (2013) Seven new loci associated with age-related macular degeneration. Nat Genet 45(4):433–439, 439e431–432
Macular Photocoagulation Study Group (1991) Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Macular Photocoagulation Study Group. Arch Ophthalmol 109(9):1220–1231
Azab M, Boyer DS, Bressler NM, Bressler SB, Cihelkova I, Hao Y, Immonen I, Lim JI, Menchini U, Naor J, Potter MJ, Reaves A, Rosenfeld PJ, Slakter JS, Soucek P, Strong HA, Wenkstern A, Su XY, Yang YC, Visudyne in Minimally Classic Choroidal Neovascularization Study Group (2005) Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial. Arch Ophthalmol 123(4):448–457
Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial (2004) Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351(27):2805–2816
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, A. S. Group (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 116(1):57–65.e55
Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR, M. S. Group (2007) Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol 125(11):1460–1469
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, M. S. Group (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14):1419–1431
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ, Slakter JS, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Sowade O, Zeitz O, Norenberg C, Sandbrink R, Heier JS (2014) Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 121(1):193–201
CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364(20):1897–1908
Rosenfeld PJ (2006) Intravitreal avastin: the low cost alternative to lucentis? Am J Ophthalmol 142(1):141–143
Steinbrook R (2006) The price of sight—ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med 355(14):1409–1412
Tolentino MJ, Dennrick A, John E, Tolentino MS (2015) Drugs in Phase II clinical trials for the treatment of age-related macular degeneration. Expert Opin Investig Drugs 24(2):183–199
Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, Hauswirth WW, Aguirre GD (2013) Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A 110(6):E517–E525
Ali RR (2012) Gene therapy for retinal dystrophies: twenty years in the making. Hum Gene Ther 23(4):337–339
Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, de Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke FW, Weleber RG, Nardini M, Moore AT, Thompson DA, Petersen-Jones SM, Michaelides M, van den Born LI, Stockman A, Smith AJ, Rubin G, Ali RR (2015) Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med 372(20):1887–1897
Jha BS, Bharti K (2015) Regenerating retinal pigment epithelial cells to cure blindness: a road towards personalized artificial tissue. Curr Stem Cell Rep 1(2):79–91
Gouras P, Flood MT, Kjedbye H, Bilek MK, Eggers H (1985) Transplantation of cultured human retinal epithelium to Bruch’s membrane of the owl monkey’s eye. Curr Eye Res 4(3):253–265
Gouras P, Flood MT, Kjeldbye H (1984) Transplantation of cultured human retinal cells to monkey retina. An Acad Bras Cienc 56(4):431–443
Lopez R, Gouras P, Kjeldbye H, Sullivan B, Reppucci V, Brittis M, Wapner F, Goluboff E (1989) Transplanted retinal pigment epithelium modifies the retinal degeneration in the RCS rat. Invest Ophthalmol Vis Sci 30(3):586–588
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Liao JL, Yu J, Huang K, Hu J, Diemer T, Ma Z, Dvash T, Yang XJ, Travis GH, Williams DS, Bok D, Fan G (2010) Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet 19(21):4229–4238
Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS (2006) Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci 47(8):3612–3624
Zhu M, Provis JM, Penfold PL (1998) Isolation, culture and characteristics of human foetal and adult retinal pigment epithelium. Aust N Z J Ophthalmol 26(Suppl 1):S50–S52
Nandrot EF, Dufour EM (2010) Mertk in daily retinal phagocytosis: a history in the making. Adv Exp Med Biol 664:133–140
Mullen RJ, LaVail MM (1976) Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science 192(4241):799–801
Little CW, Castillo B, DiLoreto DA, Cox C, Wyatt J, del Cerro C, del Cerro M (1996) Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Invest Ophthalmol Vis Sci 37(1):204–211
Sheng Y, Gouras P, Cao H, Berglin L, Kjeldbye H, Lopez R, Rosskothen H (1995) Patch transplants of human fetal retinal pigment epithelium in rabbit and monkey retina. Invest Ophthalmol Vis Sci 36(2):381–390
Berglin L, Gouras P, Sheng Y, Lavid J, Lin PK, Cao H, Kjeldbye H (1997) Tolerance of human fetal retinal pigment epithelium xenografts in monkey retina. Graefes Arch Clin Exp Ophthalmol 235(2):103–110
Lai CC, Gouras P, Doi K, Tsang SH, Goff SP, Ashton P (2000) Local immunosuppression prolongs survival of RPE xenografts labeled by retroviral gene transfer. Invest Ophthalmol Vis Sci 41(10):3134–3141
Aramant RB, Seiler MJ (2002) Transplanted sheets of human retina and retinal pigment epithelium develop normally in nude rats. Exp Eye Res 75(2):115–125
Algvere PV, Berglin L, Gouras P, Sheng Y (1994) Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol 232(12):707–716
Algvere PV, Gouras P, Dafgard Kopp E (1999) Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur J Ophthalmol 9(3):217–230
Weisz JM, Humayun MS, De Juan E Jr, Del Cerro M, Sunness JS, Dagnelie G, Soylu M, Rizzo L, Nussenblatt RB (1999) Allogenic fetal retinal pigment epithelial cell transplant in a patient with geographic atrophy. Retina 19(6):540–545
Radtke ND, Seiler MJ, Aramant RB, Petry HM, Pidwell DJ (2002) Transplantation of intact sheets of fetal neural retina with its retinal pigment epithelium in retinitis pigmentosa patients. Am J Ophthalmol 133(4):544–550
Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ (2008) Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol 146(2):172–182
Binder S, Stanzel BV, Krebs I, Glittenberg C (2007) Transplantation of the RPE in AMD. Prog Retin Eye Res 26(5):516–554
Castillo BV Jr, del Cerro M, White RM, Cox C, Wyatt J, Nadiga G, del Cerro C (1997) Efficacy of nonfetal human RPE for photoreceptor rescue: a study in dystrophic RCS rats. Exp Neurol 146(1):1–9
Phillips SJ, Sadda SR, Tso MO, Humayan MS, de Juan E Jr, Binder S (2003) Autologous transplantation of retinal pigment epithelium after mechanical debridement of Bruch’s membrane. Curr Eye Res 26(2):81–88
Binder S, Stolba U, Krebs I, Kellner L, Jahn C, Feichtinger H, Povelka M, Frohner U, Kruger A, Hilgers RD, Krugluger W (2002) Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: a pilot study. Am J Ophthalmol 133(2):215–225
Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM (1996) ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 62(2):155–169
Pinilla I, Cuenca N, Sauve Y, Wang S, Lund RD (2007) Preservation of outer retina and its synaptic connectivity following subretinal injections of human RPE cells in the Royal College of Surgeons rat. Exp Eye Res 85(3):381–392
Coffey PJ, Girman S, Wang SM, Hetherington L, Keegan DJ, Adamson P, Greenwood J, Lund RD (2002) Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat Neurosci 5(1):53–56
Salero E, Blenkinsop TA, Corneo B, Harris A, Rabin D, Stern JH, Temple S (2012) Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 10(1):88–95
Chiba C (2014) The retinal pigment epithelium: an important player of retinal disorders and regeneration. Exp Eye Res 123:107–114
Stanzel BV, Liu Z, Somboonthanakij S, Wongsawad W, Brinken R, Eter N, Corneo B, Holz FG, Temple S, Stern JH, Blenkinsop TA (2014) Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Rep 2(1):64–77
Schraermeyer U, Enzmann V, Kohen L, Addicks K, Wiedemann P, Heimann K (1997) Porcine iris pigment epithelial cells can take up retinal outer segments. Exp Eye Res 65(2):277–287
Schraermeyer U, Kociok N, Heimann K (1999) Rescue effects of IPE transplants in RCS rats: short-term results. Invest Ophthalmol Vis Sci 40(7):1545–1556
Thumann G, Salz AK, Walter P, Johnen S (2009) Preservation of photoreceptors in dystrophic RCS rats following allo- and xenotransplantation of IPE cells. Graefes Arch Clin Exp Ophthalmol 247(3):363–369
Abe T, Yoshida M, Tomita H, Kano T, Sato M, Wada Y, Fuse N, Yamada T, Tamai M (2000) Auto iris pigment epithelial cell transplantation in patients with age-related macular degeneration: short-term results. Tohoku J Exp Med 191(1):7–20
Abe T, Yoshida M, Yoshioka Y, Wakusawa R, Tokita-Ishikawa Y, Seto H, Tamai M, Nishida K (2007) Iris pigment epithelial cell transplantation for degenerative retinal diseases. Prog Retin Eye Res 26(3):302–321
Semkova I, Kreppel F, Welsandt G, Luther T, Kozlowski J, Janicki H, Kochanek S, Schraermeyer U (2002) Autologous transplantation of genetically modified iris pigment epithelial cells: a promising concept for the treatment of age-related macular degeneration and other disorders of the eye. Proc Natl Acad Sci U S A 99(20):13090–13095
Hojo M, Abe T, Sugano E, Yoshioka Y, Saigo Y, Tomita H, Wakusawa R, Tamai M (2004) Photoreceptor protection by iris pigment epithelial transplantation transduced with AAV-mediated brain-derived neurotrophic factor gene. Invest Ophthalmol Vis Sci 45(10):3721–3726
Lawrence JM, Sauve Y, Keegan DJ, Coffey PJ, Hetherington L, Girman S, Whiteley SJ, Kwan AS, Pheby T, Lund RD (2000) Schwann cell grafting into the retina of the dystrophic RCS rat limits functional deterioration. Royal College of Surgeons. Invest Ophthalmol Vis Sci 41(2):518–528
Keegan DJ, Kenna P, Humphries MM, Humphries P, Flitcroft DI, Coffey PJ, Lund RD, Lawrence JM (2003) Transplantation of syngeneic Schwann cells to the retina of the rhodopsin knockout (rho(−/−)) mouse. Invest Ophthalmol Vis Sci 44(8):3526–3532
Lawrence JM, Keegan DJ, Muir EM, Coffey PJ, Rogers JH, Wilby MJ, Fawcett JW, Lund RD (2004) Transplantation of Schwann cell line clones secreting GDNF or BDNF into the retinas of dystrophic Royal College of Surgeons rats. Invest Ophthalmol Vis Sci 45(1):267–274
Gamm DM, Wang S, Lu B, Girman S, Holmes T, Bischoff N, Shearer RL, Sauve Y, Capowski E, Svendsen CN, Lund RD (2007) Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One 2(3):e338
Wang S, Girman S, Lu B, Bischoff N, Holmes T, Shearer R, Wright LS, Svendsen CN, Gamm DM, Lund RD (2008) Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci 49(7):3201–3206
Francis PJ, Wang S, Zhang Y, Brown A, Hwang T, McFarland TJ, Jeffrey BG, Lu B, Wright L, Appukuttan B, Wilson DJ, Stout JT, Neuringer M, Gamm DM, Lund RD (2009) Subretinal transplantation of forebrain progenitor cells in nonhuman primates: survival and intact retinal function. Invest Ophthalmol Vis Sci 50(7):3425–3431
Tsukamoto A, Uchida N, Capela A, Gorba T, Huhn S (2013) Clinical translation of human neural stem cells. Stem Cell Res Ther 4(4):102
McGill TJ, Cottam B, Lu B, Wang S, Girman S, Tian C, Huhn SL, Lund RD, Capela A (2012) Transplantation of human central nervous system stem cells - neuroprotection in retinal degeneration. Eur J Neurosci 35(3):468–477
Casarosa S, Bozzi Y, Conti L (2014) Neural stem cells: ready for therapeutic applications? Mol Cell Ther 2:31
Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR (2008) Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol 40(5):815–820
Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen S, Perez-Polo JR, Yang K (2005) Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res 80(5):611–619
Arnhold S, Heiduschka P, Klein H, Absenger Y, Basnaoglu S, Kreppel F, Henke-Fahle S, Kochanek S, Bartz-Schmidt KU, Addicks K, Schraermeyer U (2006) Adenovirally transduced bone marrow stromal cells differentiate into pigment epithelial cells and induce rescue effects in RCS rats. Invest Ophthalmol Vis Sci 47(9):4121–4129
Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y (2007) Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res 85(2):234–241
Lu B, Wang S, Girman S, McGill T, Ragaglia V, Lund R (2010) Human adult bone marrow-derived somatic cells rescue vision in a rodent model of retinal degeneration. Exp Eye Res 91(3):449–455
Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, Levkovitch-Verbin H, Barshack I, Rosner M, Rotenstreich Y (2014) Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Exp Eye Res 118:135–144
Ahmad I, Tang L, Pham H (2000) Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun 270(2):517–521
Ballios BG, Clarke L, Coles BL, Shoichet MS, Van Der Kooy D (2012) The adult retinal stem cell is a rare cell in the ciliary epithelium whose progeny can differentiate into photoreceptors. Biol Open 1(3):237–246
Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D (2000) Retinal stem cells in the adult mammalian eye. Science 287(5460):2032–2036
Coles BL, Angenieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic Y, van der Kooy D (2004) Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci U S A 101(44):15772–15777
Cicero SA, Johnson D, Reyntjens S, Frase S, Connell S, Chow LM, Baker SJ, Sorrentino BP, Dyer MA (2009) Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci U S A 106(16):6685–6690
Froen R, Johnsen EO, Nicolaissen B, Facsko A, Petrovski G, Moe MC (2013) Does the adult human ciliary body epithelium contain “true” retinal stem cells? Biomed Res Int 2013:531579
Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauve Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin FY, Gosiewska A, Mistry SK (2007) Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 25(3):602–611
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920
Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M (2014) Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep 2(2):205–218
Leach LL, Clegg DO (2015) Concise review: Making stem cells retinal: methods for deriving retinal pigment epithelium and implications for patients with ocular disease. Stem Cells 33(8):2363–2373
Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R (2004) Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 6(3):217–245
Choudhary P, Booth H, Gutteridge A, Surmacz B, Louca I, Steer J, Kerby J, Whiting PJ (2017) Directing differentiation of pluripotent stem cells toward retinal pigment epithelium lineage. Stem Cells Transl Med 6(2):490–501
Lidgerwood GE, Lim SY, Crombie DE, Ali R, Gill KP, Hernandez D, Kie J, Conquest A, Waugh HS, Wong RC, Liang HH, Hewitt AW, Davidson KC, Pebay A (2016) Defined medium conditions for the induction and expansion of human pluripotent stem cell-derived retinal pigment epithelium. Stem Cell Rev 12(2):179–188
Lamba DA, Karl MO, Ware CB, Reh TA (2006) Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A 103(34):12769–12774
Buchholz DE, Pennington BO, Croze RH, Hinman CR, Coffey PJ, Clegg DO (2013) Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med 2(5):384–393
Leach LL, Buchholz DE, Nadar VP, Lowenstein SE, Clegg DO (2015) Canonical/beta-catenin Wnt pathway activation improves retinal pigmented epithelium derivation from human embryonic stem cells. Invest Ophthalmol Vis Sci 56(2):1002–1013
Bharti K, Miller SS, Arnheiter H (2011) The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res 24(1):21–34
Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO (2009) Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells 27(10):2427–2434
Maruotti J, Sripathi SR, Bharti K, Fuller J, Wahlin KJ, Ranganathan V, Sluch VM, Berlinicke CA, Davis J, Kim C, Zhao L, Wan J, Qian J, Corneo B, Temple S, Dubey R, Olenyuk BZ, Bhutto I, Lutty GA, Zack DJ (2015) Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc Natl Acad Sci U S A 112(35):10950–10955
Odorico JS, Kaufman DS, Thomson JA (2001) Multilineage differentiation from human embryonic stem cell lines. Stem Cells 19(3):193–204
Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N (2000) Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 6(2):88–95
Carr AJ, Smart MJ, Ramsden CM, Powner MB, da Cruz L, Coffey PJ (2013) Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci 36(7):385–395
Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B (2009) Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 5(4):396–408
Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, Pattnaik B, Thomson JA, Gamm DM (2011) Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29(8):1206–1218
Reichman S, Slembrouck A, Gagliardi G, Chaffiol A, Terray A, Nanteau C, Potey A, Belle M, Rabesandratana O, Duebel J, Orieux G, Nandrot EF, Sahel JA, Goureau O (2017) Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in xeno-free and feeder-free conditions. Stem Cells 35(5):1176–1188
Reichman S, Terray A, Slembrouck A, Nanteau C, Orieux G, Habeler W, Nandrot EF, Sahel JA, Monville C, Goureau O (2014) From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A 111(23):8518–8523
Crombie DE, Daniszewski M, Liang HH, Kulkarni T, Li F, Lidgerwood GE, Conquest A, Hernandez D, Hung SS, Gill KP, De Smit E, Kearns LS, Clarke L, Sluch VM, Chamling X, Zack DJ, Wong RCB, Hewitt AW, Pebay A (2017) Development of a modular automated system for maintenance and differentiation of adherent human pluripotent stem cells. SLAS Discov 22(8):1016–1025
Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R (2012) Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379(9817):713–720
Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata KI, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M (2017) Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 376(11):1038–1046
Xu RM, Carmel G, Kuret J, Cheng X (1996) Structural basis for selectivity of the isoquinoline sulfonamide family of protein kinase inhibitors. Proc Natl Acad Sci U S A 93(13):6308–6313
Ben M’Barek K, Habeler W, Plancheron A, Jarraya M, Regent F, Terray A, Yang Y, Chatrousse L, Domingues S, Masson Y, Sahel JA, Peschanski M, Goureau O, Monville C (2017) Human ESC-derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci Transl Med 9(421):eaai7471
Lustremant C, Habeler W, Plancheron A, Goureau O, Grenot L, de la Grange P, Audo I, Nandrot EF, Monville C (2013) Human induced pluripotent stem cells as a tool to model a form of Leber congenital amaurosis. Cell Reprogram 15(3):233–246
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R (2015) Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385(9967):509–516
Andrews PW, Baker D, Benvinisty N, Miranda B, Bruce K, Brustle O, Choi M, Choi YM, Crook JM, de Sousa PA, Dvorak P, Freund C, Firpo M, Furue MK, Gokhale P, Ha HY, Han E, Haupt S, Healy L, Hei DJ, Hovatta O, Hunt C, Hwang SM, Inamdar MS, Isasi RM, Jaconi M, Jekerle V, Kamthorn P, Kibbey MC, Knezevic I, Knowles BB, Koo SK, Laabi Y, Leopoldo L, Liu P, Lomax GP, Loring JF, Ludwig TE, Montgomery K, Mummery C, Nagy A, Nakamura Y, Nakatsuji N, Oh S, Oh SK, Otonkoski T, Pera M, Peschanski M, Pranke P, Rajala KM, Rao M, Ruttachuk R, Reubinoff B, Ricco L, Rooke H, Sipp D, Stacey GN, Suemori H, Takahashi TA, Takada K, Talib S, Tannenbaum S, Yuan BZ, Zeng F, Zhou Q (2015) Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI). Regen Med 10(2 Suppl):1–44
Kuroda T, Yasuda S, Kusakawa S, Hirata N, Kanda Y, Suzuki K, Takahashi M, Nishikawa S, Kawamata S, Sato Y (2012) Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PLoS One 7(5):e37342
Kanemura H, Go MJ, Shikamura M, Nishishita N, Sakai N, Kamao H, Mandai M, Morinaga C, Takahashi M, Kawamata S (2014) Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One 9(1):e85336
Bai Q, Ramirez JM, Becker F, Pantesco V, Lavabre-Bertrand T, Hovatta O, Lemaitre JM, Pellestor F, De Vos J (2015) Temporal analysis of genome alterations induced by single-cell passaging in human embryonic stem cells. Stem Cells Dev 24(5):653–662
Weissbein U, Benvenisty N, Ben-David U (2014) Quality control: genome maintenance in pluripotent stem cells. J Cell Biol 204(2):153–163
Garber K (2015) RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol 33(9):890–891
Georgiadis A, Tschernutter M, Bainbridge JW, Balaggan KS, Mowat F, West EL, Munro PM, Thrasher AJ, Matter K, Balda MS, Ali RR (2010) The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS One 5(12):e15730
Kannan R, Zhang N, Sreekumar PG, Spee CK, Rodriguez A, Barron E, Hinton DR (2006) Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis 12:1649–1659
Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T, Clegg DO, Kashani AH, Hinton DR, Humayun MS (2015) Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retin Eye Res 48:1–39
Capeans C, Pineiro A, Pardo M, Sueiro-Lopez C, Blanco MJ, Dominguez F, Sanchez-Salorio M (2003) Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmol Scand 81(3):271–277
Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM (2008) Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater 15:88–99
Adds PJ, Hunt CJ, Dart JK (2001) Amniotic membrane grafts, “fresh” or frozen? A clinical and in vitro comparison. Br J Ophthalmol 85(8):905–907
Kador KE, Goldberg JL (2012) Scaffolds and stem cells: delivery of cell transplants for retinal degenerations. Expert Rev Ophthalmol 7(5):459–470
Hu Y, Liu L, Lu B, Zhu D, Ribeiro R, Diniz B, Thomas PB, Ahuja AK, Hinton DR, Tai YC, Hikita ST, Johnson LV, Clegg DO, Thomas BB, Humayun MS (2012) A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Res 48(4):186–191
da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr AF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, Feng G, Whitlock M, Robson AG, Holder GE, Sagoo MS, Loudon PT, Whiting P, Coffey PJ (2018) Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 36(4):328–337
Seiler MJ, Aramant RB (2012) Cell replacement and visual restoration by retinal sheet transplants. Prog Retin Eye Res 31(6):661–687
Bharti K, Rao M, Hull SC, Stroncek D, Brooks BP, Feigal E, van Meurs JC, Huang CA, Miller SS (2014) Developing cellular therapies for retinal degenerative diseases. Invest Ophthalmol Vis Sci 55(2):1191–1202
Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R (2015) Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients. Stem Cell Rep 4(5):860–872
Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ (2013) Stem cells in retinal regeneration: past, present and future. Development 140(12):2576–2585
Brandl C, Grassmann F, Riolfi J, Weber BH (2015) Tapping stem cells to target AMD: challenges and prospects. J Clin Med 4(2):282–303
Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE II, Parrott MB, Rosenfeld PJ, Flynn HW Jr, Goldberg JL (2017) Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med 376(11):1047–1053
Forrester JV (2009) Privilege revisited: an evaluation of the eye’s defence mechanisms. Eye (Lond) 23(4):756–766
Streilein JW, Ma N, Wenkel H, Ng TF, Zamiri P (2002) Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res 42(4):487–495
Sauve Y, Klassen H, Whiteley SJ, Lund RD (1998) Visual field loss in RCS rats and the effect of RPE cell transplantation. Exp Neurol 152(2):243–250
Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, Abe M (2013) Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 494(7435):100–104
Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N (2002) Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A 99(15):9864–9869
Wu KH, Wu HP, Chan CK, Hwang SM, Peng CT, Chao YH (2013) The role of mesenchymal stem cells in hematopoietic stem cell transplantation: from bench to bedsides. Cell Transplant 22(4):723–729
Sohn EH, Jiao C, Kaalberg E, Cranston C, Mullins RF, Stone EM, Tucker BA (2015) Allogenic iPSC-derived RPE cell transplants induce immune response in pigs: a pilot study. Sci Rep 5:11791
Xian B, Huang B (2015) The immune response of stem cells in subretinal transplantation. Stem Cell Res Ther 6:161
Zamiri P, Sugita S, Streilein JW (2007) Immunosuppressive properties of the pigmented epithelial cells and the subretinal space. Chem Immunol Allergy 92:86–93
Wenkel H, Streilein JW (1998) Analysis of immune deviation elicited by antigens injected into the subretinal space. Invest Ophthalmol Vis Sci 39(10):1823–1834
Sugita S, Futagami Y, Smith SB, Naggar H, Mochizuki M (2006) Retinal and ciliary body pigment epithelium suppress activation of T lymphocytes via transforming growth factor beta. Exp Eye Res 83(6):1459–1471
Zamiri P, Masli S, Streilein JW, Taylor AW (2006) Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci 47(9):3912–3918
Wenkel H, Streilein JW (2000) Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Invest Ophthalmol Vis Sci 41(11):3467–3473
Hirsch L, Nazari H, Sreekumar PG, Kannan R, Dustin L, Zhu D, Barron E, Hinton DR (2015) TGF-beta2 secretion from RPE decreases with polarization and becomes apically oriented. Cytokine 71(2):394–396
Zamiri P, Masli S, Kitaichi N, Taylor AW, Streilein JW (2005) Thrombospondin plays a vital role in the immune privilege of the eye. Invest Ophthalmol Vis Sci 46(3):908–919
Enzmann V, Stadler M, Wiedemann P, Kohen L (1998) In-vitro methods to decrease MHC class II-positive cells in retinal pigment epithelium cell grafts. Ocul Immunol Inflamm 6(3):145–153
Horie S, Sugita S, Futagami Y, Yamada Y, Mochizuki M (2010) Human retinal pigment epithelium-induced CD4+CD25+ regulatory T cells suppress activation of intraocular effector T cells. Clin Immunol 136(1):83–95
Sugita S, Horie S, Nakamura O, Maruyama K, Takase H, Usui Y, Takeuchi M, Ishidoh K, Koike M, Uchiyama Y, Peters C, Yamamoto Y, Mochizuki M (2009) Acquisition of T regulatory function in cathepsin L-inhibited T cells by eye-derived CTLA-2alpha during inflammatory conditions. J Immunol 183(8):5013–5022
Sugita S, Horie S, Yamada Y, Mochizuki M (2010) Inhibition of B-cell activation by retinal pigment epithelium. Invest Ophthalmol Vis Sci 51(11):5783–5788
Sugita S, Usui Y, Horie S, Futagami Y, Yamada Y, Ma J, Kezuka T, Hamada H, Usui T, Mochizuki M, Yamagami S (2009) Human corneal endothelial cells expressing programmed death-ligand 1 (PD-L1) suppress PD-1+ T helper 1 cells by a contact-dependent mechanism. Invest Ophthalmol Vis Sci 50(1):263–272
Osusky R, Dorio RJ, Arora YK, Ryan SJ, Walker SM (1997) MHC class II positive retinal pigment epithelial (RPE) cells can function as antigen-presenting cells for microbial superantigen. Ocul Immunol Inflamm 5(1):43–50
Imai A, Sugita S, Kawazoe Y, Horie S, Yamada Y, Keino H, Maruyama K, Mochizuki M (2012) Immunosuppressive properties of regulatory T cells generated by incubation of peripheral blood mononuclear cells with supernatants of human RPE cells. Invest Ophthalmol Vis Sci 53(11):7299–7309
Futagami Y, Sugita S, Vega J, Ishida K, Takase H, Maruyama K, Aburatani H, Mochizuki M (2007) Role of thrombospondin-1 in T cell response to ocular pigment epithelial cells. J Immunol 178(11):6994–7005
Diniz B, Thomas P, Thomas B, Ribeiro R, Hu Y, Brant R, Ahuja A, Zhu D, Liu L, Koss M, Maia M, Chader G, Hinton DR, Humayun MS (2013) Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci 54(7):5087–5096
Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO (2012) Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis 18:920–936
Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R (2009) Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 27(9):2126–2135
Hewitt Z, Priddle H, Thomson AJ, Wojtacha D, McWhir J (2007) Ablation of undifferentiated human embryonic stem cells: exploiting innate immunity against the Gal alpha1-3Galbeta1-4GlcNAc-R (alpha-Gal) epitope. Stem Cells 25(1):10–18
Sugita S, Makabe K, Fujii S, Iwasaki Y, Kamao H, Shiina T, Ogasawara K, Takahashi M (2017) Detection of retinal pigment epithelium-specific antibody in iPSC-derived retinal pigment epithelium transplantation models. Stem Cell Rep 9(5):1501–1515
Sonoda S, Sreekumar PG, Kase S, Spee C, Ryan SJ, Kannan R, Hinton DR (2010) Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany NY) 2(1):28–42
Hsiung J, Zhu D, Hinton DR (2015) Polarized human embryonic stem cell-derived retinal pigment epithelial cell monolayers have higher resistance to oxidative stress-induced cell death than nonpolarized cultures. Stem Cells Transl Med 4(1):10–20
Kawazoe Y, Sugita S, Keino H, Yamada Y, Imai A, Horie S, Mochizuki M (2012) Retinoic acid from retinal pigment epithelium induces T regulatory cells. Exp Eye Res 94(1):32–40
Sugita S, Horie S, Nakamura O, Futagami Y, Takase H, Keino H, Aburatani H, Katunuma N, Ishidoh K, Yamamoto Y, Mochizuki M (2008) Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. J Immunol 181(11):7525–7536
Sugita S, Kamao H, Iwasaki Y, Okamoto S, Hashiguchi T, Iseki K, Hayashi N, Mandai M, Takahashi M (2015) Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. Invest Ophthalmol Vis Sci 56(2):1051–1062
Ferguson TA, Griffith TS (2007) The role of Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) in the ocular immune response. Chem Immunol Allergy 92:140–154
Sugita S, Kawazoe Y, Imai A, Usui Y, Takahashi M, Mochizuki M (2013) Suppression of IL-22-producing T helper 22 cells by RPE cells via PD-L1/PD-1 interactions. Invest Ophthalmol Vis Sci 54(10):6926–6933
Zhang X, Bok D (1998) Transplantation of retinal pigment epithelial cells and immune response in the subretinal space. Invest Ophthalmol Vis Sci 39(6):1021–1027
Bhatt NS, Newsome DA, Fenech T, Hessburg TP, Diamond JG, Miceli MV, Kratz KE, Oliver PD (1994) Experimental transplantation of human retinal pigment epithelial cells on collagen substrates. Am J Ophthalmol 117(2):214–221
Crafoord S, Algvere PV, Kopp ED, Seregard S (2000) Cyclosporine treatment of RPE allografts in the rabbit subretinal space. Acta Ophthalmol Scand 78(2):122–129
Ahmad ZM, Hughes BA, Abrams GW, Mahmoud TH (2012) Combined posterior chamber intraocular lens, vitrectomy, Retisert, and pars plana tube in noninfectious uveitis. Arch Ophthalmol 130(7):908–913
Tomkins-Netzer O, Taylor SR, Bar A, Lula A, Yaganti S, Talat L, Lightman S (2014) Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology 121(8):1649–1654
Grafft CA, Cornell LD, Gloor JM, Cosio FG, Gandhi MJ, Dean PG, Stegall MD, Amer H (2010) Antibody-mediated rejection following transplantation from an HLA-identical sibling. Nephrol Dial Transplant 25(1):307–310
Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA (2005) Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet 366(9502):2019–2025
Copelan EA (2006) Hematopoietic stem-cell transplantation. N Engl J Med 354(17):1813–1826
de Rham C, Villard J (2014) Potential and limitation of HLA-based banking of human pluripotent stem cells for cell therapy. J Immunol Res 2014:518135
Okita K, Nagata N, Yamanaka S (2011) Immunogenicity of induced pluripotent stem cells. Circ Res 109(7):720–721
Opelz G, Dohler B (2010) Impact of HLA mismatching on incidence of posttransplant non-hodgkin lymphoma after kidney transplantation. Transplantation 89(5):567–572
Sugita S, Iwasaki Y, Makabe K, Kimura T, Futagami T, Suegami S, Takahashi M (2016) Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Rep 7(4):619–634
Sugita S, Iwasaki Y, Makabe K, Kamao H, Mandai M, Shiina T, Ogasawara K, Hirami Y, Kurimoto Y, Takahashi M (2016) Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Rep 7(4):635–648
Nakajima F, Tokunaga K, Nakatsuji N (2007) Human leukocyte antigen matching estimations in a hypothetical bank of human embryonic stem cell lines in the Japanese population for use in cell transplantation therapy. Stem Cells 25(4):983–985
Gourraud PA, Gilson L, Girard M, Peschanski M (2012) The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells 30(2):180–186
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ben M’Barek, K., Habeler, W., Regent, F., Monville, C. (2019). Developing Cell-Based Therapies for RPE-Associated Degenerative Eye Diseases. In: Bharti, K. (eds) Pluripotent Stem Cells in Eye Disease Therapy. Advances in Experimental Medicine and Biology, vol 1186. Springer, Cham. https://doi.org/10.1007/978-3-030-28471-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-28471-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28470-1
Online ISBN: 978-3-030-28471-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)