Abstract
The management of the arthritic anterior cruciate ligament (ACL)-deficient knee remains a significant challenge to surgeons, as these patients are often young with high functional demands. While unicompartmental knee arthroplasty (UKA) would appear to have significant advantages, it has historically been considered contraindicated due to an increased incidence of aseptic loosening.
Around one-third of knees undergoing arthroplasty have been reported as being ACL deficient, with half of these being contraindicated for UKA, based on the pathoanatomy of disease, most commonly due to involvement of both tibiofemoral compartments.
Ten case series, 308 knees, have reported the outcomes of UKA implanted in the setting of ACL deficiency (four series without, six series with ACL reconstruction) at a mean follow-up of 5 years. Overall, the results of UKA alone are inferior to UKA with ACL reconstruction, with the results of UKA with ACL reconstruction being comparable to published outcomes of UKA in the ACL-intact knee.
In arthritic ACL-deficient knees who meet indications for UKA, good outcomes following UKA with ACL reconstruction are seen, with this choice of management having significant advantages in this challenging patient group.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Unicompartmental knee arthroplasty
- Anterior cruciate ligament
- Patient selection
- Clinical outcomes
- Implant survival
Introduction
While the anterior cruciate ligament (ACL) is intact in the majority of knees undergoing knee arthroplasty, the management of the arthritic ACL-deficient knee remains a significant challenge to surgeons. Often, patients with ACL deficiency and knee osteoarthritis (OA) are young, with high functional demands meaning unicompartmental knee arthroplasty (UKA) may represent the ideal treatment. Currently, however, there is a lack of consensus about whether UKA is indicated in the ACL-deficient knee and, if it is indicated, whether it should be performed concurrently with ACL reconstruction.
This chapter first reviews the role of the ACL, the incidence of ACL deficiency in OA and suitability for UKA in this population before focusing on how to determine ACL deficiency preoperatively and the treatment options in the ACL-deficient knee. The chapter concludes by reviewing the operative technique when performing UKA and concurrent ACL reconstruction before presenting a case study to illustrate relevant aspects of the management of the arthritic ACL-deficient knee.
Role of the Anterior Cruciate Ligament
In the native knee, the ACL is important for femoral rollback, the screw-home mechanism and maintenance of normal gait [1]. ACL deficiency is associated with instability, abnormal knee kinematics and a decline in activity [2, 3]. In addition, ACL deficiency is associated with loss of knee proprioception, provided in part by mechanoreceptors within the ACL, which is independently associated with poor knee function [4].
ACL deficiency in the setting of knee OA can be considered either primary or secondary. Primary ACL deficiency, where ACL deficiency typically occurs due to significant trauma, is an established risk factor for the development of secondary OA of the knee and following nonsurgical management of ACL rupture, the reported rates of OA range from 11% to 73% [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Conversely, secondary ACL deficiency occurs in the setting of established knee OA and is typically insidious in nature. Patients with a primary ACL deficiency are typically younger, and more active, with a more focal disease pattern, whereas in secondary ACL deficiency, patients are typically older and less active with a more extensive pattern of disease [19,20,21,22,23,24].
In an arthritic knee with functionally intact ligaments, the wear pattern on the tibial plateau is anteromedial. As the ACL degenerates, the wear pattern on the tibial plateau increases in size, but so long as the ACL remains intact, it rarely extends to the posterior quarter of the plateau and never to the posterior joint margin [20, 21, 25, 26]. With an intact ACL, posterior bone (and cartilage) within the medial compartment is preserved, as in flexion, the femur rolls back on the tibia ensuring that the medial collateral ligament (MCL) length is maintained and limb alignment restored (varus deformity disappears in flexion). Rupture of the ACL results in the wear pattern on the tibial plateau to extend posteromedially, which can be associated with posterior subluxation of the femur on the tibia, structural shortening of the MCL and structural damage to the lateral compartment [27].
Incidence of ACL Deficiency and Suitability for Unicompartmental Knee Arthroplasty
The ACL has been reported to be intact in around two-thirds of patients undergoing knee arthroplasty for OA (mean 69%, range 25–86%) [19, 28,29,30,31,32,33,34] (Table 12.1).
Thus, in one-third of knees with OA that are ACL deficient, it is important to establish which knees may be suitable for UKA, and have a focal disease pattern, and which knees have a more extensive disease pattern in which TKA would be the most appropriate treatment option [35].
In a series of 46 consecutive knees (42 patients) with ACL deficiency listed for medial UKA at the time of surgery, it was found that half had partial or focal full-thickness cartilage loss on the lateral femoral condyle, seven times higher than that seen in a matched control group with an intact ACL [36]. These data are supported by a radiographic cross-sectional study of almost 500 consecutive knees undergoing arthroplasty where of the 23% of knees (107 of 457 knees) that were identified as having radiographic evidence of ACL deficiency, based on a posterior wear pattern, half (51%; 55 of 107 knees) of these knees had evidence of lateral compartment disease, based on valgus stress radiograph [37] (Fig. 12.1).
In this radiographic cross-sectional study in knees with radiographic ACL deficiency, 53% (57 of 107 knees) were considered contraindicated for medial UKA based on radiographic assessment (medial partial-thickness cartilage loss seven knees (6.5%), lateral compartment disease 55 knees (51%), bone loss with grooving at the lateral patella facet 8 knees (7.5%) and functionally abnormal MCL one knee (1%)) [37].
Knees with radiographic ACL deficiency that retained suitability for UKA, based on the pathoanatomy of disease, had better preoperative Knee Society Functional Scores (mean 58.1 vs. 47.9, p = 0.01), Lower Extremity Activity Scores (mean 10.5 vs. 9.1, p = 0.05) and flexion (mean 113.5° v 107.7°, p = 0.01) compared tothose knees that had a more extensive disease pattern that did not meet criteria for UKA. Thus, those knees that retain suitability for UKA, based on the pathoanatomy of disease, may be more likely to benefit from UKA given their better level of preoperative function.
Determining ACL Deficiency Preoperatively
As a third of knees undergoing arthroplasty are ACL deficient, and around half of these may be eligible for UKA (primary ACL deficiency with secondary OA), it is important to consider, in the workup for UKA, how best to assess for ACL deficiency.
Based on patient history, it is often not possible to reliably identify ACL deficiency, as whilst a half of patients with knee OA and ACL deficiency recall a significant knee injury, around a quarter of patients with knee OA and an intact ACL also recall such an event [31]. Clinical examination using the Lachman test can be useful to screen for ACL deficiency; however, in the setting of osteoarthritis, findings can be misleading due to the presence of osteophytes and joint contracture [30, 34]. The ability of the Lachman test to exclude ACL deficiency (negative predictive value) is low (84%); however, its positive predictive value is high (94%; 95% CI 70–100%) [34]. As such, the presence of anterior tibial translation during Lachman test must alert the surgeon to high probability of ACL deficiency. The Pivot-Shift Test has not been found to be useful in the arthritic knee [30].
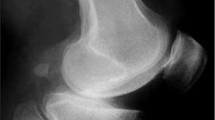
Arguably, the most useful preoperative test to assess for ACL deficiency is the true lateral radiograph of the knee. On the true lateral radiograph where the ACL is functionally abnormal or absent, the tibial erosion extends to the back of the tibial plateau and may be accompanied by posterior femoral subluxation. If the tibial erosion cannot be seen or does not extend to the back of the tibia, there is a 95% chance that the ACL is functionally normal [38, 39] (Fig. 12.2).
MRI has also been used to assess the status of the ACL. The sensitivity and specificity of MRI at detecting ACL tears has been reported as 87% (95% CI 77–94%) and 93% (95% CI 91–96%), respectively, although its performance is known to be lower in older patients, possibly due to the higher number of chronic, as opposed to acute ruptures in this group [40]. Whilst MRI has benefits in terms of providing morphological information about the status of the ACL, it has been demonstrated that provided the ACL is intact, the macroscopic status of the ACL does not influence outcomes after UKA. Based on this, it is the authors’ practice to rely on a combination of clinical and radiographic findings to determine ACL integrity preoperatively with a final assessment being made at the time of arthrotomy by passing a tendon hook around the native ACL and give a hard pull to assess its integrity [41].
Treatment Options in the ACL-Deficient Knee
For patients with ACL deficiency and bone-on-bone arthritis that does not respond to nonoperative treatment strategies, arthroplasty treatment options include the following:
-
Total knee arthroplasty (TKA).
-
Unicompartmental knee arthroplasty without ACL reconstruction.
-
Unicompartmental knee arthroplasty with ACL reconstruction.
In those knees, around a half, with ACL deficiency and both medial and lateral tibiofemoral disease, best identified on stress radiographs, TKA is recommended [35, 37]. In knees with ACL deficiency that are suitable for UKA, as they tend to be younger with higher levels of function, UKA represents a good treatment option. Whether in this scenario the ACL should be reconstructed or not remains a significant debate [23, 24].
Reviewing the literature, there have been ten case series (308 knees) of UKA implanted in the setting of ACL deficiency (Table 12.2). Four of these series (169 knees) have included knees where UKA has been performed without ACL reconstruction and six series (139 knees) where UKA has been performed in conjunction with ACL reconstruction . The mean follow-up of these series is 5 years (range 1.7–16 years).
Little information is provided in included studies to indicate why UKA was performed with or without ACL reconstruction and significant differences in the mean age of cases where UKA has been performed alone (65 years) and in cases where UKA was performed in conjunction with ACL reconstruction (50 years), indicating that there is likely selection bias with regard to the choice of management (Table 12.2). In addition, the mean follow-up where UKA has been performed alone was 6 years (range 3–16 years), compared to that of 4 years (range 1.7–5 years) in series where UKA was performed in conjunction with ACL reconstruction, and as such, longer term data are required to fully evaluate outcomes in these cohorts.
Functional outcomes following UKA with and without ACL reconstruction are outlined in Table 12.3. Given the heterogeneity of treatment groups, and paucity of data, aside from acknowledging that UKA, regardless of whether it is implanted with or without concurrent ACL reconstruction, appears to improve function, it is not possible to determine whether outcomes are superior in one group over the other.
In the four series, 169 knees, where UKA was performed without ACL reconstruction, there were 19 revisions. In 12 cases (63%), there was aseptic loosening of the tibial plateau , four cases (21%) lateral compartment disease progression, one case (5%) unexplained pain, one case (5%) of bearing dislocation and one case (5%) the indication for revision was unknown. Ten of these cases were converted to TKA, one converted to bi-unicompartmental arthroplasty and one arthrodesis. In seven cases, the revision procedure was not specified. No other complications were reported (Table 12.4).
In the six series, 139 knees, where UKA was performed with ACL reconstruction, there were five revisions. In three cases where bearing dislocation occurred (60%), requiring open reduction, in one case (20%), there was lateral compartment disease progression with conversion to TKA, and in one case (20%) conversion to TKA following two-stage revision for infection. There were no cases of aseptic loosening. In addition to the revision procedures, there were three complications. One arthroscopy and loose body removal was performed, and there was one case of stiffness managed with arthroscopy and manipulation under anaesthetic.
Overall, the revision rate in the UKA without ACL reconstruction series was 1.81 per 100 observed component years (95% CI 0.54–3.69), equivalent to a 10-year survival of 82% (95% CI 63–95%), whereas the revision rate in the UKA with ACL reconstruction series was 0.19 per 100 observed component years (95% CI 0–1.06), equivalent to a 10-year survival of 98% (95% CI 89–100%) (Fig. 12.3).
While the differences in implant survival between UKA without and with concurrent ACL reconstruction do not differ significantly (p = 0.17), it is the authors’ view that this is due to inadequate sample size and until further, longer term data are available, the authors would recommend that when UKA is performed, it should be done with concurrent ACL reconstruction.
If the two studies in which UKA were performed in an era where ACL was not recognised as a potential risk factor, Goodfellow et al. (1988) and Hernigou et al. (2004) are excluded, the failure rate in ACL-deficient knees decreases to 0.90 per 100 observed component years (95% CI 0.26–1.85; 10-year survival of 91% (95% CI 82–97%)), which is improved, but remains higher than that in those series in which the ACL had been reconstructed. This suggests that, during the period between these initial studies and more recent ones, there have been improvements in patient selection, surgical technique, implant design or perhaps changes to the revision threshold. Nonetheless, until such a time as clear selection criteria for performing UKA without ACL reconstruction are developed, based on long-term data, the current literature does not support performing isolated UKA in the ACL-deficient knee.
Conversely, where concurrent ACL reconstruction is performed, the revision rates of UKA are low and equivalent to a 10-year survival of 98% (95% CI 89–100%), which compared favourably to the rates in the literature, which reports a 10-year survival of 94% (95% CI 92–95%) in series of mobile-bearing UKA in the knee with an intact ACL and minimum 10-year follow-up [50]. Therefore, based on these data, the authors would conclude that in the ACL-deficient knee, UKA and ACL reconstruction represents good treatment, provided the patient meets indications for UKA.
Why there is a higher failure rate in UKA compared with UKA plus ACL reconstruction, with aseptic loosening of the tibial component being the predominant failure mechanism, may relate to the biomechanics of the knee following surgery. Kinematic assessment of the ACL-deficient knee has demonstrated that, compared to the ACL-intact knee, there is abnormal knee kinematics and bearing movement following mobile-bearing UKA [51, 52] (Fig. 12.4). This abnormal kinematics, and bearing movement, may increase shear forces on the tibial component and, consequently, result in aseptic loosening of the prosthesis.
Relationship between bearing position and knee flexion angle for the step-up and forward lunge exercises following mobile-bearing UKA; results for the ACLD and ACLI patient groups. The shaded areas indicate the 95% confidence intervals. Positive BP denotes anterior bearing position, and negative BP denotes posterior positioning. (From Pegg EC et al. [53])
In ACL-deficient knees undergoing osteotomy, it is known that tibial slope modification affects ACL strain and knee stability, and similarly, in cadaveric studies of fixed-bearing UKA, tibial tray slope modifications have been demonstrated to reduce anterior tibial translation in ACL-deficient knees [54]. In the case series by Hernigou, a tibial slope of more than 7° was associated with an increase in the rate of aseptic loosening, and thus in fixed-bearing UKA, it is not recommended to exceed this limit [42]. Whilst in mobile-bearing UKA, modification of tibial slope is not advised, overall, as outcomes of UKA are worse in the ACL-deficient knee without reconstruction, in both fixed and mobile-bearing designs, than in the reconstructed knee. Modification of the tibial slope without reconstruction of the ACL cannot be recommended.
Whether to use a fixed or mobile-bearing UKA remains an area of debate. Of the series in which UKA and ACL reconstructions were performed, three studies, 50 knees, assessed fixed-bearing designs and three studies, 89 knee, mobile-bearing designs. No failures were reported in the series reporting the outcomes of fixed-bearing designs at a mean of 3.2 years (range 2.0–4.2 years) compared to five failures in the series reporting the outcomes of mobile-bearing designs at a mean of 4.4 years (range 1.7–5.0 years). In the series reporting the outcomes of mobile-bearing design, there were three bearing dislocations, managed with bearing exchange, one lateral compartment disease progression managed with TKA and one infection managed with two-stage conversion to TKA.
No statistical difference was seen in implant survival between fixed and mobile-bearing UKA in ACL-reconstructed knees (p = 0.79), although the number of knees is too small to make an accurate comparison. The revision rate in fixed-bearing UKA with ACL reconstruction series was 0 per 100 observed component years (95% CI 0–0.70), equivalent to a 10-year survival of 100% (95% CI 93–100%). The revision rate in mobile-bearing UKA with ACL reconstruction series was 0.62 per 100 observed component years (95% CI 0–2.01), equivalent to a 10-year survival of 94% (95% CI 80–100%) (Fig. 12.5).
Whilst at medium-term follow-up no difference in outcomes between fixed- and mobile-bearing designs is seen, proponents of mobile-bearing designs argue that, in the longer term, mobile-bearings may be less susceptible to wear and offer superior outcomes. Blunn et al. have reported higher polyethylene wear with cyclic sliding, as seen in fixed-bearing designs , compared with compression or rolling, seen in mobile-bearing designs, and multiple studies have shown that the wear rate with mobile-bearing UKA is significantly less than that with fixed-bearing UKA [55,56,57]. Thus, mobile-bearing designs may be more forgiving in the setting of minor ligamentous laxity seen in ACL deficiency and subsequent reconstruction. This is particularly relevant, as typically, these patients are younger than the usual population undergoing TKA, and as such, long-term implant survival with low wear is crucial for this patient group. At present, the authors cannot recommend one design over another without further longer term data assisting in determining whether there is superiority of either fixed- or mobile-bearing design UKA in the ACL-reconstructed knee.
Similarly, there is a paucity of evidence as to whether there is an optimum fixation type, cemented versus cementless prosthesis or ACL graft choice, with outcomes of hamstring and bone-patellar tendon-bone autograft both reported in the literature. Further work is required to identify whether there is any benefit of either type of fixation of the prosthesis or type of ligament reconstruction to inform future practice (Table 12.5).
Surgical Technique
ACL reconstruction can be performed simultaneously or in a staged procedure. In the authors’ practice, patients whose primary symptoms relate to instability receive an ACL reconstruction initially, and if the patient subsequently presents with pain, then a mobile-bearing UKA is implanted if they meet indications for UKA. In patients who meet indications for UKA and whose primary symptoms are pain, a simultaneous procedure is performed. In the published literature, three studies reported a staged procedure in some cases (total 46 knees), while a simultaneous UKA with ACL reconstruction was performed in all other cases.
Combined UKA and ACL reconstruction is a longer, more technically demanding procedure; however, it avoids the need for a protracted recovery due to reoperation. Performing UKA with concurrent ACL presents two technical challenges that are not encountered when the procedures are performed independent of each other. The first is avoiding impingement of the graft tunnel on the tibial component of the UKA, and the second is applying appropriate graft tension. To avoid impingement of the graft tunnel on the tibial component, which may also lead to tibial plateau fracture particularly with uncemented prosthesis, it is advised to place the tibial tunnel more vertically, with the entry point more lateral than normal (Fig. 12.6). In addition, if using cementless implants, the tibial tunnel should be drilled after positioning and impacting the tibial component to lower the risk of fracture during this manoeuvre. The graft tension should then be adjusted after implantation of the UKA and fixation of the femoral end of the ACL graft.
Placement of the tibial tunnel, lateralised and verticalised, when performing simultaneous UKA with ACL reconstruction . (From Mancuso et al. [58])
Whilst, as discussed above, there is a paucity of evidence as to the optimum ACL graft choice for simultaneous UKA with ACL reconstruction, the authors favour a bone–tendon–bone graft, as opposed to hamstring for three main reasons. First, we believe it provides stronger initial fixation (bone to bone rather than bone to tendon) permitting more aggressive early rehabilitation; second the tibial tunnel can be drilled through the donor site and is slightly lateralised, as previously mentioned. Third, the medial third of the patellar tendon may be harvested through the traditional UKA approach, thus reducing the operative morbidity.
Case Study
A 55-year-old teacher was referred to clinic with a two-year history of progressive, activity-related, right knee pain nonresponsive to nonsurgical management . His significant past medical history is that of ACL rupture, managed nonoperatively, which he sustained aged 45 playing soccer.
On clinical examination, he has a correctable 5° varus deformity of the right knee. Range of movement in the right knee is from 0° to 115° flexion. Lachman test is positive with no firm endpoint suggesting ACL deficiency.
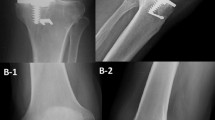
An anteroposterior standing radiograph demonstrates bone-on-bone arthritis in the medial compartment (Fig. 12.7a), and a true lateral radiograph demonstrates a posterior wear pattern on the tibial plateau (Fig. 12.7b), suggesting ACL deficiency. A skyline radiograph excludes bone loss with grooving to the lateral facet of the patella, which, whilst rare, represents a contraindication to medial UKA (Fig. 12.7c). To assess the lateral compartment and integrity of the MCL, a valgus stress radiograph is performed, which demonstrates full-thickness preserved cartilage in the lateral compartment (Fig. 12.7d).
Case Study. (a) Standing anteroposterior radiograph demonstrating bone-on-bone arthritis in the medial compartment, (b) Lateral radiograph demonstrating a posterior wear pattern, (c) Patellar view demonstrating preserved joint space, (d) Valgus stress radiograph demonstrating an intact lateral compartment, (e) AP postoperative radiograph demonstrating medial UKA with ACL reconstruction, (f) Lateral postoperative radiograph demonstrating medial UKA with ACL reconstruction
Based on clinical and radiographic assessment ACL deficiency is suspected. A structured radiographic assessment including valgus stress radiographs confirms suitability for medial UKA based on the pathoanatomy of disease . The decision is made to proceed with mobile-bearing UKA in conjunction with simultaneous ACL reconstruction. Following arthrotomy, ACL deficiency is confirmed and the joint is inspected to confirm suitability for medial UKA. ACL reconstruction is as describe above in the surgical technique using a lateralised and verticalised tibial tunnel placement (Fig. 12.7e, f).
Conclusion
-
Around one-third of knees undergoing arthroplasty are ACL deficient.
-
Around half of knees with ACL deficiency remain suitable for UKA based on the pathoanatomy of disease.
-
The most common reason that ACL-deficient knees are not suitable for UKA is lateral compartment disease.
-
A positive Lachman test is strongly associated with ACL deficiency; however, a negative Lachman test may be due to the presence of osteophytes and joint contracture.
-
A posterior wear pattern on the true lateral radiograph, which may be associated with posterior femoral subluxation, suggests ACL deficiency.
-
The results of UKA alone in ACL-deficient knees are inferior to UKA with ACL reconstruction.
-
The results of UKA and concurrent ACL reconstruction in ACL-deficient knees are comparable to outcomes of UKA in the ACL-intact knee.
-
While UKA with ACL reconstruction is a technically demanding operation both operations can be performed simultaneously and are associated with a low morbidity.
-
When performing UKA with ACL reconstruction, care must be taken to avoid impingement of the graft on the tibial prosthesis and tensioning of the ACL graft, which should be performed after UKA implantation.
References
McLean SG, Mallett KF, Arruda EM. Deconstructing the anterior cruciate ligament: what we know and do not know about function, material properties, and injury mechanics. J Biomech Eng. 2015;137(2):020906.
Chalmers PN, et al. Does ACL reconstruction alter natural history?: a systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292–300.
Smith TO, et al. Is reconstruction the best management strategy for anterior cruciate ligament rupture? A systematic review and meta-analysis comparing anterior cruciate ligament reconstruction versus non-operative treatment. Knee. 2014;21(2):462–70.
Relph N, Herrington L, Tyson S. The effects of ACL injury on knee proprioception: a meta-analysis. Physiotherapy. 2014;100(3):187–95.
Fowler PJ, Regan WD. The patient with symptomatic chronic anterior cruciate ligament insufficiency. Results of minimal arthroscopic surgery and rehabilitation. Am J Sports Med. 1987;15(4):321–5.
Giove TP, et al. Non-operative treatment of the torn anterior cruciate ligament. J Bone Joint Surg Am. 1983;65(2):184–92.
Hawkins RJ, Misamore GW, Merritt TR. Followup of the acute nonoperated isolated anterior cruciate ligament tear. Am J Sports Med. 1986;14(3):205–10.
Kannus P, Jarvinen M. Conservatively treated tears of the anterior cruciate ligament. Long-term results. J Bone Joint Surg Am. 1987;69(7):1007–12.
Kessler MA, et al. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–8.
Lohmander LS, et al. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–52.
McDaniel WJ Jr, Dameron TB Jr. The untreated anterior cruciate ligament rupture. Clin Orthop Relat Res. 1983;172:158–63.
Meuffels DE, et al. Ten year follow-up study comparing conservative versus operative treatment of anterior cruciate ligament ruptures. A matched-pair analysis of high level athletes. Br J Sports Med. 2009;43(5):347–51.
Meunier A, Odensten M, Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17(3):230–7.
Myklebust G, et al. Clinical, functional, and radiologic outcome in team handball players 6 to 11 years after anterior cruciate ligament injury: a follow-up study. Am J Sports Med. 2003;31(6):981–9.
Neuman P, et al. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med. 2008;36(9):1717–25.
Noyes FR, et al. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154–62.
Pattee GA, et al. Four to ten year followup of unreconstructed anterior cruciate ligament tears. Am J Sports Med. 1989;17(3):430–5.
von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–73.
Harman MK, et al. Wear patterns on tibial plateaus from varus and valgus osteoarthritic knees. Clin Orthop Relat Res. 1998;352:149–58.
Rout R, et al. The pattern of cartilage damage in antero-medial osteoarthritis of the knee and its relationship to the anterior cruciate ligament. J Orthop Res. 2013;31(6):908–13.
White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582–6.
Pandit H, et al. Combined anterior cruciate reconstruction and Oxford unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2006;88(7):887–92.
Shelbourne KD, Benner RW. Isolated anterior cruciate ligament reconstruction in the chronic ACL-deficient knee with degenerative medial arthrosis. J Knee Surg. 2007;20(3):216–22.
Weston-Simons JS, et al. Outcome of combined unicompartmental knee replacement and combined or sequential anterior cruciate ligament reconstruction: a study of 52 cases with mean follow-up of five years. J Bone Joint Surg Br. 2012;94(9):1216–20.
Hasegawa A, et al. Anterior cruciate ligament changes in the human knee joint in aging and osteoarthritis. Arthritis Rheum. 2012;64(3):696–704.
Mullaji AB, et al. Cruciate ligaments in arthritic knees: a histologic study with radiologic correlation. J Arthroplasty. 2008;23(4):567–72.
Deschamps G, Lapeyre B. Rupture of the anterior cruciate ligament: a frequently unrecognized cause of failure of unicompartmental knee prostheses. Apropos of a series of 79 Lotus prostheses with a follow-up of more than 5 years. Rev Chir Orthop Reparatrice Appar Mot. 1987;73(7):544–51.
Cloutier JM. Results of total knee arthroplasty with a non-constrained prosthesis. J Bone Joint Surg Am. 1983;65(7):906–19.
Cloutier JM, Sabouret P, Deghrar A. Total knee arthroplasty with retention of both cruciate ligaments. A nine to eleven-year follow-up study. J Bone Joint Surg Am. 1999;81(5):697–702.
Dodd M, et al. The pivot shift test is of limited clinical relevance in the arthritic anterior cruciate ligament-deficient knee. J Knee Surg. 2010;23(3):131–5.
Hill CL, et al. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52(3):794–9.
Jenny JY, Jenny G. Preservation of anterior cruciate ligament in total knee arthroplasty. Arch Orthop Trauma Surg. 1998;118(3):145–8.
Lee GC, et al. Evaluation of the anterior cruciate ligament integrity and degenerative arthritic patterns in patients undergoing total knee arthroplasty. J Arthroplasty. 2005;20(1):59–65.
Johnson AJ, et al. The ACL in the arthritic knee: how often is it present and can preoperative tests predict its presence? Clin Orthop Relat Res. 2013;471(1):181–8.
Berend KR, et al. Consensus statement on indications and contraindications for medial unicompartmental knee arthroplasty. J Surg Orthop Adv. 2015;24(4):252–6.
Boissonneault A, et al. No difference in survivorship after unicompartmental knee arthroplasty with or without an intact anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2480–6.
Hamilton TW, et al. Radiological Decision Aid to determine suitability for medial unicompartmental knee arthroplasty: development and preliminary validation. Bone Joint J. 2016;98-B(10 Supple B):3–10.
Keyes GW, et al. The radiographic classification of medial gonarthrosis. Correlation with operation methods in 200 knees. Acta Orthop Scand. 1992;63(5):497–501.
Mullaji AB, Marawar SV, Luthra M. Tibial articular cartilage wear in varus osteoarthritic knees: correlation with anterior cruciate ligament integrity and severity of deformity. J Arthroplasty. 2008;23(1):128–35.
Phelan N, et al. A systematic review and meta-analysis of the diagnostic accuracy of MRI for suspected ACL and meniscal tears of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1525–39.
Hamilton TW, et al. Unicompartmental knee replacement: does the macroscopic status of the anterior cruciate ligament affect outcome? Knee. 2016;23(3):506–10.
Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506–11.
Engh GA, Ammeen DJ. Unicondylar arthroplasty in knees with deficient anterior cruciate ligaments. Clin Orthop Relat Res. 2014;472(1):73–7.
Goodfellow JW, et al. The Oxford Knee for unicompartmental osteoarthritis. The first 103 cases. J Bone Joint Surg Br. 1988;70(5):692–701.
Krishnan SR, Randle R. ACL reconstruction with unicondylar replacement in knee with functional instability and osteoarthritis. J Orthop Surg Res. 2009;4:43.
Tinius M, Hepp P, Becker R. Combined unicompartmental knee arthroplasty and anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):81–7.
Ventura A, et al. Medial unicondylar knee arthroplasty combined to anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):675–80.
Dervin GF, Conway AF, Thurston P. Combined anterior cruciate ligament reconstruction and unicompartmental knee arthroplasty: surgical technique. Orthopedics. 2007;30(5 Suppl):39–41.
Tian S, et al. Combined unicompartmental knee arthroplasty and anterior cruciate ligament reconstruction in knees with osteoarthritis and deficient anterior cruciate ligament. BMC Musculoskelet Disord. 2016;17:327.
Hamilton TW, et al. The interaction of caseload and usage in determining outcomes of unicompartmental knee arthroplasty: a meta-analysis. J Arthroplasty. 2017;32(10):3228–3237.e2.
Pegg EC, et al. Behaviour of Anterior Cruciate Ligament (ACL) deficient knees after Unicompartmental Knee Arthroplasty (UKA), in American Academy of Orthopaedic Surgeons. 2015: Las Vegas.
Pandit H, et al. Combined anterior cruciate reconstruction and Oxford unicompartmental knee arthroplasty: in vivo kinematics. Knee. 2008;15(2):101–6.
Pegg EC, et al. Sagittal kinematics of mobile unicompartmental knee replacement in anterior cruciate ligament deficient knees. Clin Biomech. 2016;31:33–9.
Suero EM, et al. Effects of tibial slope changes in the stability of fixed bearing medial unicompartmental arthroplasty in anterior cruciate ligament deficient knees. Knee. 2012;19(4):365–9.
Brown TD, Bartel DL, G. Implant Wear Symposium Engineering Work. What design factors influence wear behavior at the bearing surfaces in total joint replacements? J Am Acad Orthop Surg. 2008;16(Suppl 1):S101–6.
Simpson DJ, et al. The effect of bearing congruency, thickness and alignment on the stresses in unicompartmental knee replacements. Clin Biomech (Bristol, Avon). 2008;23(9):1148–57.
Blunn GW, et al. Wear in retrieved condylar knee arthroplasties. A comparison of wear in different designs of 280 retrieved condylar knee prostheses. J Arthroplasty. 1997;12(3):281–90.
Mancuso F, et al. Medial unicompartmental knee arthroplasty in the ACL-deficient knee. J Orthop Traumatol. 2016;17(3):267–75.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hamilton, T.W., Pandit, H. (2020). Unicompartmental Knee Arthroplasty and Anterior Cruciate Ligament Deficiency. In: Gerlinger, T. (eds) Unicompartmental Knee Arthroplasty. Springer, Cham. https://doi.org/10.1007/978-3-030-27411-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-27411-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27410-8
Online ISBN: 978-3-030-27411-5
eBook Packages: MedicineMedicine (R0)