Abstract
Coenzyme Q10 (CoQ10) is a vitamin-like substance which functions as an electron carrier within the mitochondrial respiratory chain, as well as serving as an important intracellular antioxidant. Most of the body’s CoQ10 requirements are met by endogenous synthesis, although the capacity for CoQ10 production decreases substantially with increasing age. In this article we have reviewed the potential role of CoQ10 supplementation in the treatment of tissue fibrosis, which has been implicated in the age-related loss of function of various organs including the heart. Clinical studies have indicated that CoQ10 supplementation may decrease the level of cardiovascular fibrosis to which older individuals are subjected, and thereby improve cardiovascular function and reduce the risk of cardiovascular associated mortality. Although the factors responsible for the anti-fibrotic action of CoQ10 have yet to be fully elucidated, its antioxidant and anti-inflammatory functions are thought to be major contributors to its clinical efficacy in the treatment of this age-related disorder.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Coenzyme Q10 (CoQ10) is a naturally occurring vitamin-like substance, first characterised in 1957 by Professor Fred Crane at the University of Wisconsin, USA [1]. CoQ10 is a lipophilic molecule which is a member of a group of compounds known as ubiquinones because of their ubiquitous distribution in nature, being found in animal, plants and microorganisms [1]. Ubiquinones are composed of a benzoquinone nucleus and an isoprenoid side chain, which varies in length among the different ubiquinone species with CoQ10 having a side chain composed of ten isoprenoid subunits (Fig. 6.1) [1]. CoQ10 is the predominant ubiquinone species found in human tissues [1]. CoQ10 plays an essential role in cellular energy generation within the mitochondrial respiratory chain (MRC) . The role of CoQ10 is of particular importance in tissues with a high energy requirement, such as cardiac muscle. In addition to its role in cellular energy generation, CoQ10 also serves as an important lipid soluble antioxidant and anti-inflammatory agent within the body [1,2,3]. The objective of this article is to review the potential role of CoQ10 as a biomarker of aging, specifically with regards to the prevention of tissue fibrosis in the heart, which has been implicated in age-related dysfunction of this organ.
2 Functions of CoQ10
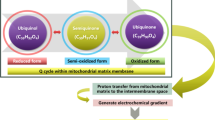
CoQ10 serves as an electron carrier within the MRC (Fig. 6.2), where it is involved in the transfer of electrons derived from complex I (NADH:ubiquinone reductase) and complex II (succinate dehydrogenase) to complex III (ubiquinol cytochrome c reductase), allowing a continuous passage of electrons within the MRC, which is required in the process of oxidative phosphorylation and the concomitant production of ATP, the energy currency of the cell [1]. Tissues with a high energy requirement, especially the heart and skeletal muscles, contain higher numbers of mitochondria within their cells and are particularly reliant on maintaining adequate tissue CoQ10 levels for normal functioning [2]. Thus, the heart and skeletal muscles typically contain about 1000 mg of CoQ10, out of a total body pool of 1500–2000 mg. CoQ10 occurs in cells in two closely related forms, oxidised (ubiquinone) and fully reduced (ubiquinol) [1]. The interconversion between these two forms is essential for the normal functioning of CoQ10, and this interconversion is principally mediated by the selenium-containing enzyme, thioredoxin reductase [4].
CoQ10 is also important within the body serving as a major fat-soluble antioxidant, protecting cell membranes (particularly those of the mitochondria) and circulatory lipoproteins from the damaging effects of free radical species [e.g., reactive oxygen species (ROS)] [1]. CoQ10 is the only lipid soluble antioxidant produced within the body [5]. The antioxidant function of CoQ10 is attributed to its ubiquinol form, which must be constantly regenerated from CoQ10 to maintain its antioxidant capacity [5]. In addition to directly preventing lipid peroxidation, ubiquinol is able to enhance the cellular antioxidant capacity by maintaining the antioxidants, vitamin C and E, in their active fully reduced forms [6]. Most recently, a gene expression profiling study showed that CoQ10 influences the expression and regulation of hundreds of genes in the human body [7]. In cell culture, CoQ10 has been shown to reduce the activity of inflammatory markers, suggesting CoQ10 may have anti-inflammatory action via gene expression modification, most notably in elderly individuals [8, 9].
3 Synthesis and Deficiency of CoQ10
Although some CoQ10 is obtained from the diet, most of the daily requirement is synthesized within the body, particularly by the liver, although all organs of the body have the capacity to synthesize CoQ10 [1]. Based on a total body pool of 2 g and an average tissue turnover time of 4 days, the human requirement for CoQ10 has been estimated at approximately, 500 mg/day [10]. A relatively small proportion of this daily requirement is obtained from the normal diet, typically up to 5 mg/day [10]. The synthesis of CoQ10 is a complex, multistage process requiring a number of amino acids, vitamins and trace element precursors and cofactors, and a deficiency in any of these can adversely affect the normal production of CoQ10 [1]. It is noteworthy that CoQ10 shares a common biosynthetic pathway with cholesterol [1]. As people age, it has been reported that the capacity of the body to synthesize CoQ10 decreases [5]. Optimal production of CoQ10 occurs around the mid-twenties, with a continual decrease thereafter to approximately 50% at age 70. CoQ10 levels can also be depleted by intense exercise, certain types of prescription medicines, and illnesses [1, 5]. Dietary supplementation with coenzyme CoQ10 therefore provides a mechanism to maintain adequate levels of CoQ10 within the body.
In humans, at least 13 genes are thought to be involved in the biosynthesis of CoQ10, and mutations in 10 of these genes have been associated with primary CoQ10 deficiency, a condition which results from a genetic defect in the CoQ10 biosynthetic pathway [11,12,13]. The first cases of primary CoQ10 deficiency were reported in 1989 by Ogasahara and colleagues [14]. The two patients were sisters born to unrelated parents and they presented with recurrent rhabdomyolysis, which was associated with developmental delay and seizures [14]. Subsequently, a number of other patients have been described with primary CoQ10 deficiency, which appears to have a heterogeneous clinical presentation. This can be divided into five distinct clinical phenotypes: (1) encephalomyopathic (as in the case of the two siblings described by Ogasahara et al. [14]; (2) cerebral ataxic; (3) infantile multisystem disease; (4) steroid resistant nephrotic syndrome; and (5) isolated myopathy [15].
In most cases of suspected primary CoQ10 deficiency a family history suggests an autosomal mode of inheritance, however, genetic diagnosis is complicated by the fact that the CoQ10 biosynthetic pathway has not been fully elucidated in humans, and diagnosis generally relies on the determination of the endogenous CoQ10 status of the patient [16]. Interestingly, a CoQ10 deficiency can also result from a disorder not associated with a genetic defect in the CoQ10 biosynthetic pathway [17]. This type of CoQ10 deficiency is known as a secondary CoQ10 deficiency which is thought to be more prevalent than a primary deficiency, and has been associated with diseases such as MRC disorders, cardiovascular disease, Parkinson’s disease and sepsis [1, 17]. The cause of secondary CoQ10 deficiency in disease has not yet been fully elucidated and a number of theories have been suggested to account for this loss of CoQ10 including increased oxidative stress-induced catabolism and/or ROS induced inhibition of the CoQ10 biosynthetic pathway enzymes [17].
4 Laboratory Measurement of CoQ10 Status
Quantification of CoQ10 is not usually included in routine biochemical analysis of blood by hospital pathology laboratories. The most common laboratory procedures used to assess CoQ10 status are based on high-pressure liquid chromatography (HPLC) with either ultraviolet (HPLC-UV) or electrochemical detection (HPLC-ED) [16]. CoQ10 levels are usually determined in plasma isolated from blood, with normal plasma levels typically in the range of 0.5–1.7 μM [16]. In view of the fact that CoQ10 levels are dependent upon the circulatory lipoprotein status (lipoproteins are the major carriers of CoQ10 in the circulation), it has been suggested that plasma CoQ10 levels should be expressed as a ratio to total plasma cholesterol status to take into account the lipoprotein status of the blood [1]. Furthermore, dietary intake has also been reported to influence plasma CoQ10 status, contributing up to 25% in some cases of the total amount of this isoprenoid in the circulation [1, 10]. Accordingly, it has been suggested that plasma may not be an appropriate surrogate for the assessment of endogenous CoQ10 status, and skeletal muscle is generally considered as the tissue of choice for this determination [16]. In addition to direct assessment in skeletal muscle, decreased activity of the linked MRC enzymes, NADH:cytochrome c reductase (complex I + III) and/or succinate:cytochrome c reductase (complex II + III) may also indicate evidence of a CoQ10 deficiency since the activity of these linked enzymes is dependent upon the endogenous CoQ10 status of the tissue [16]. However, in view of the invasive nature of a muscle biopsy, blood mononuclear cells or fibroblast skin cells have also been suggested as alternative surrogates to assess endogenous CoQ10 status [16]. With regards to the neurological dysfunction associated with CoQ10 deficiency, the potential to assess cerebral CoQ10 status would be of diagnostic value. However, since it is not possible to directly determine the CoQ10 status of brain tissue, cerebral spinal fluid (CSF) has been used as an alternative surrogate for this evaluation [16]. Tentative references ranges for CSF CoQ10 status have now been established at 1.18–4.91 nM, although highly sensitive mass spectrometry techniques are required for this determination, in view of the low levels of CoQ10 detected in this matrix [18]. Furthermore, considering the increasing number of reported cases of steroid resistant nephrotic syndrome associated with CoQ10 deficiency, Yubero et al. [19] have developed a reliable method for the determination of the CoQ10 status of kidney epithelial cells isolated from urine and it is hoped that such an approach will prevent the need for an invasive needle biopsy to assess the CoQ10 status of this organ.
5 Aging and Fibrosis
Fibrosis is the formation of fibrous connective tissue, particularly collagen, in response to an injury. Fibrosis is an adaptive response to tissue injury, and is an essential part of the normal processes of wound healing and tissue repair [20]. In younger individuals, such fibrous material is replaced over time by new functional tissue. However, in older people, tissue scarring tends to persist and may continue to form and accumulate. Uncontrolled continuation of fibrosis can result in scarring and the permanent remodelling of the organ which can result in an eventual loss of function [21]. Progressive fibrosis is a hallmark of the aging process and has been implicated in the pathogenesis of diseases of the heart, lungs, liver, kidneys and bone marrow [22]. It has been estimated that organ fibrosis may be the root cause of over 800,000 deaths per year, approximately 50% of the total number of human deaths [20]. However, at present there is no effective treatment for fibrosis or information available for designing appropriate therapeutic strategies [21, 22].
5.1 Fibrosis Mechanism
Fibrous connective tissue such as collagen is produced by specific types of cells called fibroblasts, which are present in most organs. Under normal circumstances, fibroblasts produce collagen in a controlled manner, to provide a “scaffold” for the structural support of the various types of functional cells in the different organs. Following tissue injury, fibroblasts are activated via inflammatory cytokines to produce collagen as part of the scar formation/healing process [23]. It is well established that fibrosis is linked to inflammation, and there is evidence that even low-grade but persistent inflammation is sufficient to promote, for example, cardiovascular fibrosis where an increased amount of collagen can contribute to the increased stiffness of both arteries and the heart wall [21].
5.2 Preclinical Studies on CoQ10 Supplementation and Fibrosis
A number of studies have demonstrated the beneficial effects of CoQ10 supplementation on fibrosis related parameters (including oxidative stress/inflammation) in various animal models of fibrosis. These include dimethylnitrosamine-induced liver fibrosis in mice [24]. In this model, CoQ10 treatment was found to block the activation of the stella cells (which is a central event in liver fibrosis) via activation of the nuclear factor, erythroid 2–like (Nrf2) which also resulted in an increased expression of the enzymes involved in the synthesis of the antioxidant, glutathione (GSH) in the hepatic cells. In sub-optimal nutrition-induced liver fibrosis in rats, CoQ10 supplementation was found to reverse fibrosis via suppression of the expression of cytokine transforming growth factor beta 1 (Tgfβ1) via a mechanism involving the upregulation of Nrf2-antioxidant response element (ARE)-associated genes [25]. In a study by Chen et al. which investigated the effect of CoQ10 supplementation on doxorubicin induced cardiac fibrosis in rats, it was found that CoQ10 was able to ameliorate the fibrosis by decreasing the expression of both Tgfβ1 and connective tissue growth factor (CTGF) [26].
In a study which assessed isoprenaline induced cardiac fibrosis in rats, CoQ10 was found to inhibit fibrosis in the heart as well as the kidney via its ability to reduce oxidative stress and prevent inflammatory cell infiltration in the tissues. However, the details of the mechanism of action of CoQ10 were not elucidated in the study [27]. In a recent study, Xue et al. [28] reported that CoQ10 supplementation was able to suppress the activation of mouse pancreatic stellate cells (PCSs) , which has been associated in the development of pancreatic fibrosis, by its ability to decrease intracellular ROS levels and induce the mammalian target of rapamycin (mTOR) cell signalling pathway. CoQ10 supplementation was also found to ameliorate methotrexate induced lung and liver fibrosis in rats by the attenuation of hepatic oxidative stress which, contrary to the previous study [28], was found to result in a down-regulation of mTOR expression and increased evidence of autophagy [29]. The reasons for the disparity between the two studies are uncertain, but may represent a species or organ specific mechanism of fibrosis induction. Interestingly, in the aforementioned studies, CoQ10 appears to attenuate fibrosis by its ability either directly or via activation of cell signalling pathways to ameliorate cellular oxidative stress.
5.3 Cardiovascular Fibrosis
In the normal heart, contractile cells (myocytes) occupy approximately 75% of the tissue volume, with the remainder comprising other cell types, which are predominatly fibroblasts [21]. Collagen secreted by fibroblasts forms a supportive scaffold for the myocytes, as well as providing a means of transmission for myocyte generated force within the heart [21]. With regard to the normal ageing process, post-mortem analysis of cardiac tissue from human subjects without cardiovascular disease found that the collagen content increased by approximately 50% between the third and seventh decades of life [30].
Unlike other organs, the heart has a limited regenerative capacity following injury, with the repair process involving the removal of necrotic cells, followed by fibrotic scar tissue replacement [21]. Two common aspects of cardiovascular disease in which fibrosis plays a major role are myocardial infarction and heart failure [20]. Myocardial infarction is caused by the blockage of the coronary arteries, which results in the death of the cardiomyocyte cells responsible for contraction. This in turn causes fibroblasts to produce excess collagen, which helps to preserve the structural integrity of the tissue, but which stiffens cardiac muscle, impairing heart contractility and relaxation, ultimately leading to impaired cardiac function and eventual heart failure [31]. Excessive production of collagen by cardiac fibroblasts can also cause fibrotic thickening of the heart valves, again ultimately resulting in heart failure [30, 31].
6 CoQ10 and the KISEL-10 Study
In the KISEL-10 randomised controlled clinical trial , normal elderly individuals were supplemented with coenzyme Q10 (200 mg/day) and selenium (200 μg/day) for 4 years. The supplemented individuals showed a 53% reduction in cardiovascular related mortality risk compared to placebo [32]. Supplemental selenium was included in this study as this trace metal functions as a co-factor for the enzyme thioredoxin reductase, which is responsible for the interconversion of the ubiquinone and ubiquinol forms of CoQ10. In addition, there is evidence suggesting that a selenium deficiency may be prevalent within the general populations of a number of European countries, including Sweden [37]. Furthermore, selenium is also required for the biological activity of the antioxidant enzyme, glutathione peroxidase which together with ubiquinol contributes to the cellular antioxidant defense [38]. A more recent study by Alehagen et al. [33] using data derived from the KISEL-10 study has now identified significant reductions in the blood levels for a range of biochemical markers of fibrosis, including cathepsin S, endostatin, galectin-3, growth differentiation factor 15 (GDF-15), matrix metalloproteinase-1 (MMP1), MMP9 and tissue inhibitor of metalloproteinase 1 (TIMP1). It is therefore concluded that the improvement in heart function and reduced risk of cardiovascular mortality following supplementation with coenzyme Q10 and selenium results from a reduction in cardiovascular fibrosis. It is of note that the reduction in systemic fibrosis markers may also help to prevent fibrotic degeneration of other tissues such as the lungs and liver [33].
7 Safety of Coenzyme Q10 Supplementation
The safety of CoQ10 has been investigated by Hidaka et al. [34] and Hosoe et al. (2007) [35]. These authors reported that CoQ10 is generally well tolerated, with no serious adverse effects being detected in long term use. Rarely, some individuals may experience mild gastrointestinal disturbances, although this is not dose related. There are no known toxic side effects, and CoQ10 cannot be overdosed. CoQ10 is well tolerated in healthy adults at an intake of 900 mg/day, and in rats at a dose of up to 1200 mg/kg/day. In addition, Yamaguchi et al. [36] reported that CoQ10 had no genotoxic activity. The safety of CoQ10 has been confirmed in more than 200 randomised controlled trials, over a wide range of disorders including cardiovascular disease, Parkinson’s diseases and mitochondrial disease [1].
8 Requirements for Coenzyme Q10 Supplementation
CoQ10 is a lipid soluble substance absorbed from the digestive tract in the same manner as other dietary fats. Because of its hydrophobicity and large molecular weight, absorption of CoQ10 is in general slow and somewhat limited. Oil based formulations show the highest bioavailability. Absorption of CoQ10 is non-linear, with increasing doses absorbed to a decreasing degree. CoQ10 is therefore best administered in split doses (typically 100 mg two or three times daily).
When first manufactured, CoQ10 is produced in a crystalline form which cannot be absorbed from the digestive tract. In CoQ10 supplements, this crystalline form must be further treated to break it down into individual molecules to enable absorption and, most importantly, crystals should not re-form within the capsule. Supplement manufacturers vary in their ability to fulfil these requirements, and previous clinical trial studies reporting lack of benefit in a variety of disorders may have failed because of insufficient dosage and/or lack of bioavailability of the particular supplement used.
9 Conclusions
Oral supplementation with CoQ10 and selenium provides a means of correcting dietary deficiencies to which older individuals may be subject. In addition, supplementation significantly reduces the levels of fibrotic markers in elderly individuals, thereby reducing the extent of cardiovascular fibrosis to which older individuals are subjected, and improving cardiovascular function and reducing risk of cardiovascular associated mortality. Although the factors responsible for the anti-fibrotic action of CoQ10 have yet to be fully elucidated, its antioxidant and anti-inflammatory functions are thought to be major contributors to its clinical efficacy. Further studies may lead to its use as an intervention to promote healthy aging. This is particularly pertinent to the treatment of idiopathic pulmonary fibrosis (IPF) , a progressive and ultimately fatal lung disorder disproportionately affecting the elderly, with two-thirds of patients who present with IPF being older than 60 [39]. The management of IPF via prescription-type drugs has proved to be particularly refractory, and alternative therapeutic strategies for the treatment of this disorder are clearly warranted and CoQ10 may be an appropriate consideration.
References
Hargreaves IP (2003) Ubiquinone: cholesterol’s reclusive cousin. Ann Clin Biochem 40(Pt3):207–218
Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20(6):591–598
Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH (2003) Effects of coenzyme Q10 supplementation on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 119:128–136
Xia L, Nordman T, Olsson JM, Damdimopoulos A, Björkhem-Bergman L, Nalvarte I et al (2003) The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J Biol Chem 278(4):2141–2146
Mantle D (2015) Coenzyme Q10 and cardiovascular disease: an overview. Br J Cardiol 22(4):1–7
Navas P, Villalba JM, de Cabo R (2007) The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion 7(Suppl):S34–S40
Gutierrez-Marisca FM, Yubero-Serrano EM, Villalba JM, Lopez-Miranda J (2018) Coenzyme Q10: from bench to clinic in aging diseases, a translational review. Crit Rev Food Sci Nutr 16(1):1–18
Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Döring F (2008) Functions of coenzyme Q10 in inflammation and gene expression. Biofactors 32(1–4):179–183
Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuñiga O, Delgado-Lista J, Gutierrez-Mariscal FM, Perez-Martinez P et al (2012) Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci 67(1):3–10
Weber C, Bysted A, Hłlmer G (1997) The coenzyme Q10 content of the average Danish diet. Int J Vitam Nutr Res 67(2):123–129
Doimo M, Desbats MA, Cerqua C, Cassina M, Trevisson E, Salviati L (2014) Genetics of coenzyme q10 deficiency. Mol Syndromol 5(3–4):156–162
Awad AM, Bradley MC, Fernández-Del-Río L, Nag A, Tsui HS, Clarke CF (2018) Coenzyme Q10 deficiencies: pathways in yeast and humans. Essays Biochem 62(3):361–376
Yubero D, Montero R, Santos-Ocaña C, Salviati L, Navas P, Artuch R et al (2018) Molecular diagnosis of coenzyme Q10 deficiency: an update. Expert Rev Mol Diagn 18(6):491–498
Ogasahara S, Engel AG, Frens D, Mack D (1989) Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc Natl Acad Sci U S A 86(7):2379–2382
Emmanuele V, López LC, Berardo A, Naini A, Tadesse S, Wen B et al (2012) Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch Neurol 69(8):978–983
Yubero D, Montero R, Artuch R, Land JM, Heales SJ, Hargreaves IP (2014) Biochemical diagnosis of coenzyme Q10 deficiency. Mol Syndromol 5(3–4):147–155
Neergheen V, Hargreaves IP (2018) Secondary coenzyme Q10 deficiency: causes and consequence. In: Grigoryeva S (ed) Coenzyme Q10- uses, health effects and role in disease. Nova Science Publishers, New York, pp 89–111. ISBN-10: 1536132845
Duberley KE, Hargreaves IP, Chaiwatanasirikul KA, Heales SJ, Land JM, Rahman S et al (2013) Coenzyme Q10 quantification in muscle, fibroblasts and cerebrospinal fluid by liquid chromatography/tandem mass spectrometry using a novel deuterated internal standard. Rapid Commun Mass Spectrom 27(9):924–930
Yubero D, Montero R, Ramos M, Neergheen V, Navas P, Artuch R et al (2015) Determination of urinary coenzyme Q10 by HPLC with electrochemical detection: reference values for a paediatric population. Biofactors 41(6):424–430
Murtha LA, Schuliga MJ, Mabotuwana NS, Hardy SA, Waters DW, Burgess JK et al (2017) The processes and mechanisms of cardiac and pulmonary fibrosis. Front Physiol 8:777. https://doi.org/10.3389/fphys.2017.00777
Biernacka A, Frangogiannis NG (2011) Aging and cardiac fibrosis. Aging Dis 2(2):158–173
Jiang S, Li T, Yang Z, Yi W, Di S, Sun Y et al (2017) AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res Rev 38:18–27
Nanthakumar CB, Hatley RJ, Lemma S, Gauldie J, Marshall RP, Macdonald SJ (2015) Dissecting fibrosis: therapeutic insights from the small molecule toolbox. Nat Rev Drug Discov 14(10):693–720
Choi HK, Pokharel YR, Lim SC, Han HK, Ryu CS, Kim SK et al (2009) Inhibition of liver fibrosis by solubilized coenzyme Q10: role of Nrf2 activation in inhibiting transforming growth factor-beta1 expression. Toxicol Appl Pharmacol 240(3):377–384
Tarry-Adkins JL, Fernandez-Twinn DS, Hargreaves IP, Neergheen V, Aiken CE, Martin-Gronert MS et al (2016) Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am J Clin Nutr 103(2):579–588
Chen PY, Hou CW, Shibu MA, Day CH, Pai P, Liu ZR et al (2017) Protective effect of Co-enzyme Q10 On doxorubicin-induced cardiomyopathy of rat hearts. Environ Toxicol 32(2):679–689
Ulla A, Mohamed MK, Sikder B, Rahman AT, Sumi FA, Hossain M et al (2017) Coenzyme Q10 prevents oxidative stress and fibrosis in isoprenaline induced cardiac remodeling in aged rats. BMC Pharmacol Toxicol 18(1):1–12
Xue R, Wang J, Yang L, Liu X, Gao Y, Pang Y et al (2019) Coenzyme Q10 ameliorates pancreatic fibrosis via the ROS triggered mTOR signalling pathway. Oxidative Med Cell Longev 2019:8039694. https://doi.org/10.1155/2019/8039694
Mohamed D, Khairy E, Tawfek SS, Habib EK, Fetouh MA (2019) CoQ10 attenuates lung and liver fibrosis via modulation of autophagy in methotrexate treated rat. Biomed Pharmacother 109:892–901
Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R (2001) Age related changes in collagen network of the human heart. Mech Ageing Dev 122(10):1049–1058
Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE (2011) Left ventricular remodelling in heart failure: current concepts in clinical significance and assessment. JACC Cardivasc Imaging 4(1):98–108
Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U (2013) Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol 167(5):1860–1866
Alehagen U, Aaseth J, Alexander J, Svensson E, Johansson P, Larsson A (2018) Less fibrosis in elderly subjects supplemented with selenium and CoQ10- a mechanism behind reduced cardiovascular mortality. Biofactors 44(2):137–147
Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K (2008) Safety assessment of coenzyme Q10 (CoQ10). Biofactors 32(1–4):199–208
Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M (2007) Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol 47(1):19–28
Yamaguchi N, Nakamura K, Oguma Y, Fujiwara S, Takabe M, Sono A et al (2009) Genotoxicity studies of ubidecarenone (coenzyme Q10) manufactured by bacteria fermentation. J Toxicol Sci 34(4):389–397
Mantle D, Hargreaves I (2019) Coenzyme Q10 and degenerative disorders affecting longevity: an overview. Antioxidants (Basel) 8(2). pii: E44. https://doi.org/10.3390/antiox8020044
Stepien K, Heaton R, Rankin S, Murphy A, Bentley J, Sexton D (2017) Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J Clin Med 6(7). pii: E71. https://doi.org/10.3390/jcm6070071
Hecker L (2018) Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging population. Am J Phys Lung Cell Mol Phys 314(4):L642–L653
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hargreaves, I.P., Mantle, D. (2019). Coenzyme Q10 Supplementation in Fibrosis and Aging. In: Guest, P. (eds) Reviews on Biomarker Studies in Aging and Anti-Aging Research. Advances in Experimental Medicine and Biology(), vol 1178. Springer, Cham. https://doi.org/10.1007/978-3-030-25650-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-25650-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25649-4
Online ISBN: 978-3-030-25650-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)