Abstract
Heavy metal (Pb, Cd, Cr, Ni, As, Se, etc.) contaminations in fertile soils and fresh water are one of the worldwide growing issues along with the modernization of the life style. Contamination in natural resources due to heavy metals is a serious threat to sustainability of ecosystems and human life. A special urge is needed to restore the natural resources in its natural state. Based on the contamination type, various site-specific physical, chemical, and biological bioremediation strategies could be applied. However, the major limitation of physicochemical approaches is its higher cost and relatively low competence. Conversely, the biotic action of contaminated environment is slightly economical and ecologically attractive alternative to the present physicochemical methods of treatment. Among different bioremediation techniques, phytoremediation and mycoremediation are having its merit of eco-friendliness. Microorganisms play an important role in heavy metal bioremediation from contaminated resources attributed to its easy operation, without any secondary pollution and showing higher efficiency at low metal concentrations. Mycoremediation is the utilization of fungi for remediation of the contaminated natural resources. Unlike bacteria, the fungal phytoremediation does not require absolute water phase as fungus can grow of air-water interface. The pH, moisture, substrate, and species specificity are the important factors which highly influence the fungal phytoremediation. This chapter mainly emphasizes the detailed mechanism of fungal phytoremediation. Some potential species are provided for abatement of heavy metals from contaminated water and soil. The heavy metals toxicity, stress response and their impact on humans as well as on plants are described in brief. Further, it also highlights the utilization efficiency of fungal phytoremediation for sustainable removal of toxic heavy metals from contaminated natural soil and water resources.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
Phytoremediation is the technique in which living plants are used for remediation of the contaminated soils, water, sediment, and ecosystem (Cunningham and Ow 1996). The utilization of fungus for remediation of the contaminated resources is fungal phytoremediation. Fungi survive about 5300 years (Gams and Stalpers 1994). Armillaria bulbosa is the longest and largest living fungal species in the world (Smith et al. 1992). Fungi play vital role in all ecosystems and are capable of regulating the nutrient as well as energy flow through their mycelial networks, and hence, they are considered as natural and true ecosystem engineers (Lawton and Jones 1995). The ecological and biochemical capacity of fungi to degrade environmental chemicals and decrease the risk associated with metals and metalloids through chemical modification or its bioavailability makes them as a potent bioremediation agent. However, to date, the ecological demands and ecophysiological strengths of fungi in bioremediation have not been potentially explored. Unlike bacteria, the fungal phytoremediation does not require absolute water phase as fungus can grow in the air-water interface. However, the water phase acts as a carrier for nutrient transport for hydrophobic organic contaminants.
Interaction of fungi with metals includes mobilization and immobilization in the mycosphere, sorption to cell walls, and uptake into fungal cell. Thereafter, chemical transformation, translocation, and metabolization along with reactions of pollutants on fungal enzymes such as extracellular oxidoreductases/cell-bound enzymes allow fungi to act on various metal pollutants (Harms et al. 2011; Prakash 2017). Hence, the role of filamentous fungi becomes important where translocation of essential factors necessitates for the transformation or detoxification of environmental chemicals. Conversely, requirement of fungal degradation is needed for pollutant classes, i.e., dioxins, 2, 4, 6-trinitrotoluene, synthetic drugs, or endocrine-disrupting chemicals found in medium as these are inefficiently degraded by bacteria (Harms et al. 2011; Macellaro et al. 2014; Mnif et al. 2011). Fungi can be used in the treatment of contaminated soil surface with organic/metal contaminants, water streams with trace organic contaminants and removal of metals from water stream, VOCs from air, and organic pollutants using isolated extracellular enzymes instead of whole fungi (Nguyen 2015; Pinedo-Rivilla et al. 2009).
Conversely, an increasing trend toward energy- and cost-efficient passive phytoremediation methods for the reclamation of contaminated natural resources, i.e., land, water, and air is the need of hour. The low degree of mechanical intervention in natural attenuation of natural resources especially soils favors the importance of filamentous fungi in sustainable fungal phytoremediation (Harms et al. 2011). Another aspect involves arbuscular mycorrhizal (AM) fungal association with plants, as these are integral, functioning parts in plant roots and enhance plant growth even under highly contaminated soils with heavy metals. AM fungi play an important role in metal tolerance and accumulation of heavy metals in the plants root growing on heavy metal-contaminated soils. Hence, isolation of stress-adapted indigenous AM fungi could be targeted as a potential biotechnological tool for inoculation of plants for degraded ecosystems. Major role of AM fungi attributed to the secretion of glomalin (a glycoprotein), stabilizing the aluminum in soil and in the roots of Gmelina plants, has been reported (Dudhane et al. 2012). There are several fungal species such as A. niger, A. pullulans, C. resinae, F. trogii, G. lucidum, Penicillium sp. (Loukidou et al. 2003; Say et al. 2003), R. arrhizus, and T. versicolor, which efficiently recover heavy metals from the contaminated environment. Heavy metal bioaccumulation potential of A. versicolor was observed 6 for 50 mg/L Cr (VI) and Ni (II) and 5 for Cu (II) ions with the 99.89, 30.05, and 29.06% removal yield, respectively at optimal pH by Taştan et al. (2010). Kumar Ramasamy et al. (2011) found that Aspergillus fumigates is very suitable for removal of Pb (II) ions from the electronic waste aqueous solution (containing Pb 100 mg/L) through batch sorption with adsorption capacity of 85.41%. El Zeftawy and Mulligan (2011) found that micellar-enhanced ultrafiltration MEUF could treat phosphorous-rich heavy metal wastewater with a transmembrane pressure of 69 kPa, at 25 °C and pH 6.9. Häyrynen et al. (2012) observed that pressure and cross-flow velocity significantly affects the flux of Cd and Cu in MEUF purification methods, while P was not retained (Landaburu-Aguirre et al. 2012). Thus, potential application of MEUF for heavy metal decontamination of nutrient-rich wastewaters has been recently justified (Mani and Kumar 2014).
18.2 Potential Sources of Heavy Metal Contamination and Associated Risks
18.2.1 Anthropogenic
Based on the relative higher densities (3.5–7.0 g/cm3), atomic weights, or atomic numbers (>20), metals are termed as heavy metals. Some heavy metals are essential nutrients (Fe, Co, Zn), relatively harmless (Ru, Ag, and Id), but potentially can be toxic in larger amounts or certain forms. Conversely, heavy metals, such as Cd, Hg, and Pb, are highly poisonous. The common source of heavy metals is antiseptics, fertilizers, sedimentation, cars, golf clubs, mobile phones, plastics, self-cleaning ovens, solar panels, and particle accelerators (Gupta et al. 2018; Singh et al. 2013; Hübner et al. 2010; Singh et al. 2017; Yadav et al. 2018b, c) (Table 18.1). The potential sources are atmospheric deposition; automobile exhausts, metal industries, mine spoils, river dredging and urban refuse disposal, pyrometallurgical industries, and fossil fuel combustion are also the main sources of heavy metals (Lottermoser 2010a, b; Matta et al. 2018; Prasad 2001) (Table 18.1). Industries such as microelectronics, plastics, refinery textiles, wood preservatives, agrochemicals (fertilizers and pesticides), sugar-based industries and waste disposal sewage sludge, landfill leachate, and fly ash disposal are also some of the chief sources of the heavy metals (Bhatia et al. 2015; Gupta et al. 2018; Singh et al. 2013a; Kumar et al. 2016; Singh and Kumar 2006; Yadav et al. 2018b, c) (Fig. 18.1).
Overview of sources of heavy metal pollution and its agroecological consequences. (Source: Srivastava et al. (2017))
18.2.2 Water Resources
Water contamination due to heavy metals is a known threat and has been attributed to anthropogenic sources involving untreated domestic and industrial wastewater discharges, chemical spills, and agricultural residues (Malyan et al. 2014; Tchounwou et al. 2012). The outcome is poor water quality, degradation, and water borne-human health risks even at lower doses of heavy metals (Kumar et al. 2014; Micó et al. 2006; Wongsasuluk et al. 2014). Major heavy metals such as lead, mercury, chromium, cadmium, copper, and aluminum for water contaminations are originated through anthropogenic activates and natural incidents like seepage from rocks, volcanoes, and forest fires. Over a time period, heavy metals enter in the food chain through water, and there chronic effects could be manifested for many years and may exert several threats such as mental disorders, pain in joints, gastric disorders, and even cancer. Human population living near industries are more susceptible to heavy metal toxicity. Along with that, pregnant women and malnutritioned children are more vulnerable to heavy metal toxicity. Freshwater bodies are heavily affected by pathogens from untreated wastewater and heavy metals from mining and industrial release (Caravanos et al. 2016). It has been reported that more than 80% of the world’s wastewater is released to the environment without treatment, which is the major cause of nearly 58% diarrheal disease (major cause of child mortality) (Connor et al. 2017). Hence, it is of utmost importance in the coming future to mitigate this global threat of water toxicity with proper remediation measure, and techniques are required for the treatment of water. In that context, fungal phytoremediation serves as an environment-friendly, pocket-friendly, and reliable technique.
18.3 Role of Heavy Metals in Living Beings
Heavy metals such as chromium (glucose metabolism), cobalt (metabolism), copper and iron (oxygen and electron transport), zinc (hydroxylation reactions) (Nieboer and Richardson 1978), manganese and vanadium (enzyme regulation), nickel (cell growth), and selenium (antioxidant and hormone production) (Emsley 2011) are important for certain biological processes. Molybdenum (catalysis of redox reactions), cadmium (in marine diatoms), tin (growth in a few species), and tungsten (metabolic processes of archaea and bacteria) may be required for growth of different species (Emsley 2011). A deficiency and excess of any of these above-discussed heavy metals may impart heavy metal poisoning of living beings (Venugopal and Luckey 1978). Hence, excess amount of heavy metals could dysfunction various physiological and biological effects in the human beings which have been elaborated in next sections.
18.4 Possible Impacts of Heavy Metal Contaminations
18.4.1 On Humans
Non-essential metals can escape control mechanisms such as binding to specified cell constituents, cellular processes malfunctioning, compartmentalization, homeostasis, oxidative deterioration, and transport, and therefore, they have toxic and further lethal effects (Gupta et al. 2018). The important health symptoms of heavy metal toxicity in human are central nervous system disorders, dementia in adults, emotional instability, insomnia, intellectual disability in children, kidney diseases, liver diseases, depression, vision disturbances, and increased morbidity and mortality rate (Jain et al. 2015; Yadav et al. 2018b, c). The metal toxicity depends on the generation of oxidative stress (increased reactive oxygen species (ROS) and reactive nitrogen species (RNS) production; depletion of intracellular antioxidant stores and free radical scavengers) (Jan et al. 2015). Heavy metals toxicity due to occupational exposure mainly responsible for multiple organ systems and toxicity levels mainly depends on the form and type of the heavy element, on route and duration of the exposure, and, to a greater extent, on a person’s individual susceptibility (Jan et al. 2015) (Fig. 18.2).
Trophic transfer of toxic HMs from soil to plants to humans and organism’s food to humans and their toxicity. (Adapted with permission from Saxena et al. (2019))
18.4.2 On Plants
Heavy metal contamination in soil and water resources affects growth and yield performance as well as nutritional quality of plants to a great extent. For the plants which are grown in close vicinity to the contaminated soil and water or at the contaminated site, metals cause physiological dysfunctioning and biochemical alterations (Sharma et al. 2012a; 2012b). In case of vegetables requiring high moisture percentage, the use of heavy metal-contaminated irrigation water is one of the major causes for high metal toxicity in plants. Some of the heavy metals at a lower concentration are required for optimum performance of plants; however, excess amount may cause toxicity, e.g., chromium (Yadav et al. 2018b, c). Common features pertaining to metal toxicity are reduced biomass reduction, leaf chlorosis, and root growth and seed germination inhibition (Ghani 2011). Cr toxicity considerably affects the physio-biochemical processes in barley, cauliflower, citrullus maize, wheat, and vegetables (Ghani 2011). ROS signalling and oxidative damage affect enzymes like catalase; cytochrome oxidase and peroxidase with iron as their component are affected by chromium toxicity. The catalase activity stimulated with an excess supply of chromium-inducing toxicity has been studied, concerning nitrate reductase activity, photosynthesis, photosynthetic pigments, and protein content in algae (Nath et al. 2008). Pb and Cd also affect the gas exchange attributes, ROS system, cause chlorophyll deterioration, and ultimately the overall performance of major agricultural crop worldwide (Anjum et al. 2015; Mobin and Khan 2007; Pinho and Ladeiro 2012; Zhu et al. 2007). The microbes are ubiquitous in nature and have been reported from diverse sources including extreme habitats (Yadav et al. 2015a, b, c, 2017b) and as plant microbiomes (Kour et al. 2019b; Yadav 2018; Yadav et al. 2016). These microbes have potential applications in agriculture, industry, pharmaceutical, and environment (Kour et al. 2019a; Yadav et al. 2017a, 2018a, 2019a, b).
18.5 Phytoremediation of Contaminated Soils/Water Resources
In general, phytoremediation is the process of bioremediation using plant species called hyperaccumulators to reduce the toxic contaminants in the environment. This is a novel advanced technology, considered as eco-friendly having lesser investment cost. Current scenario explains the feasibility and accountability of this technique. Many plant species are being used as hyperaccumulators, and new species are being explored (Ali et al. 2013). Eventually, phytoremediation is an interdisciplinary branch that requires knowledge for soil composition, soil microbiology and environment engineering, plant physiological processes, and in recent development use of lower plant groups as a sustainable system for the bioremediations of toxic heavy metals (Pisani et al. 2011). Some of the species of the plants used in phytoremediation are Robinia pseudoacacia and Sesbania drummondii for Pb, Stanleya pinnata for Se, etc. (Yang et al. 2016).
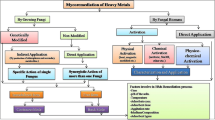
18.6 Fungal Phytoremediation
As its name explains, fungal phytoremediation or mycoremediation is a form of bioremediation where the degradative abilities of fungi are utilized to remove or neutralize the harmful contaminants present in soil and water. It is a relatively new form of bioremediation where its use only spans a few decades, beginning as early as 1966 (Matsumura and Boush 1966), but it is known or being practiced to a lesser extent. Malathion (an insecticide and neurotoxin) breakdown was successfully done using Trichoderma viride and Pseudomonas (Matsumura and Boush 1966). There are several mushroom species identified till date to remove the heavy metals from the contaminated resources. The important species are Galerina vittiformis (Cu, Cd, Cr, Pb, and Zn), Hypholoma capnoides (Ti, Sr, and Mn), and Marasmius oreades (bismuth and titanium). The other important fungal species which are having high fungal phytoremediation potentials are Agaricus bisporus, Lentinus squarrosulus, Phanerochaete chrysosporium, Pleurotus ostreatus, Pleurotus tuber-regium, P. ostreatus, P. pulmonarius, and Trametes versicolor (Adenipekun and Lawal 2012; D’Annibale et al. 2005).
In this chapter, the sources of different heavy metals (HMs) with adverse effects in major countries on human health along with the permissible limits of HMs has been highlighted to have the understanding on the current scenario of fungal phytoremediation works (Table 18.2). Similarly, the different groups of fungus having remediation potential for the most potent heavy metals have been highlighted in Table 18.3. Further, the categorical classification of different fungus and their importance in particular metal have been worked out with extensive literature survey in order to target potential fungal phytoremediation techniques for the metal contamination in soil. In addition to that for the mechanistic understanding on growth conditions, enzyme production, type of compound degradation has been explored (Table 18.4). The bioconversion efficiency of wastes by some fungal species has been reported worldwide (Table 18.5).
18.6.1 Mechanistic Approach of Fungal Phytoremediation
In fungal phytoremediation, mechanism of fungal partner is very important to understand. Fungal phytoremediation has got several mechanistic pathways for bioremediation process. In general, fungus increases the ability of roots to absorb more heavy metals. Its mechanism could be devised as (i) avoidance and (ii) sequestration mechanisms. Avoidance ameliorates the metal toxicity though decreasing the concentration of metal by biosorption, precipitation, and uptake or efflux. Conversely, sequestration involves the formation of compounds for intracellular chelation (−) and further dilution in plant tissues due to plant growth, exclusion from uptake through precipitation, and chelation in the rhizosphere (Danesh et al. 2013). Both of these mechanisms may play part or even could counteract. Overall, the reduction in absorption owing to retention and immobilization takes part in fungal structures or mycorrhizal roots. The activation of specific/nonspecific transporters and pores play the part in the plasma membrane in plants and fungi, chelation in the cytosol and the sequestration into the vacuoles of plants as well as in fungal cells. Further, transportation and exportation occur through the fungal hyphae, involving both active and passive transportation into the mycorrhizae (Fig. 18.3).
Mechanisms involved in remediation of HM-contaminated soil by HMT-PGP microbes-plant interaction. (Sources: Mishra et al. (2017))
Fungal phytoremediation is proven to be efficient, where the abilities of hyperaccumulators diminished. One of the limitations of hyperaccumulators is to accumulate less concentration of contaminants due to their small biomass while fungi can accumulate more due to their some molecular mechanisms. Hence, intervening the interaction of hyperaccumulator plant with fungi and other legume plant and herbs could help us to use it as a potent strategy for phytoremediation (Yang et al. 2016). Therefore, further exercise is required for explaining the molecular mechanisms underlying.
18.6.2 Factors Influencing the Fungal Phytoremediation
Several factors influencing the fungal phytoremediation include species of plant and fungi, their association strength, plant-soil interaction, physical and chemical properties of soil, and biophysical aspects such as temperature, pH, salinity, soil microbes, and metal characteristics (Fig. 18.4).
Relationships among the factors affecting phytoremediation efficiency. (Adapted with permission from Saxena et al. (2019))
18.6.2.1 Temperature
The fungi are having their different temperature range for growth based on different habitat, such as mesophilic (5–35 °C), psychrophilic (below °C), thermophilic (above 40 °C), etc. With the change in the temperature, the bioavailability of the heavy metals is also changed. An increase in soil temperature tends to speed up the concentration of metals in the soil due to increase rate of organic matter degradation. It was observed that high temperature is favorable for the absorption of heavy metals. However, the temperature also affects the growth of fungi. So, fungi with high temperature tolerance will be beneficial for the bioremediation process (Yadav et al. 2018b, c). Fe and Mn are mobile in alternating in dry and wet conditions (Boisselet 2012).
18.6.2.2 pH
pH is an important parameter which controls the availability of heavy metals to get remediated. Heavy metals are present in a dissolved state if the pH of the solution is at 2–3. However, the bioavailability, dissolution, and precipitation of each metal have its own intrinsic capacity along with the pH range.
18.6.2.3 Redox Potential
The redox potential affects the state of oxidation of the metals, as different forms show different behaviors in solubility. Anaerobic conditions in deeper parts of the soil for oxidoreductive reactions of microorganisms can accelerate the heavy metal degradation. Redox potential along with pH affects the fungal-phyto interactions with the soil components by altering the sorption capacity and influencing stability of complexes.
18.6.2.4 Heavy Metals Bound with Hydrocarbon
Some of the heavy metals are present in the bound form of the other compounds such as polycyclic aromatic hydrocarbons (PAH). The remediation of such metals can be achieved only after degradation of the host compound. Some fungal species such as Agaricus bisporus, Pleurotus ostreatus, and Ganoderma lucidum are observed to degrade the hydrocarbons in petroleum. Pleurotus ostreatus is beneficial in degrading the PAH (García-Delgado et al. 2015).
18.6.2.5 Other Growth Requirements
Apart from the temperature, other factors such as moisture percentage, sugar and other organic materials, oxygen, amino acids, vitamins, fatty acids, etc. are also important for fungal growth. The change in these requirements can also enhance/limit the fungal phytoremediation potential.
18.6.2.6 Fungal Species
Different fungal species are having different capacity to remediate the heavy metals from the soil and water based on their internal genetic constitutes and external growth and environmental factors. To check the any new/existing species remediation potential, the arsenic test (preliminary assessment) will serve as good choice. Later on, the heavy metals-based potential check can be made and compared with the existing data. However, some of the fungal species can serve as a bioindicator of particular heavy metals. In this case, these species serve as the reference species for the remediation potential. For example, Lycoperdon perlatum may be employed as a bioindicator of heavy metals and selenium in soil pollution (Quinche 1990).
Filamentous fungi are known to possess higher adsorption capacities for heavy metal removal (Singh and Gauba 2014). Trichoderma and Mortierella species isolated from the soil and Aspergillus and Penicillium species isolated from marine and terrestrial environments, respectively, have the high ability to remediate contaminated environment (Thenmozhi et al. 2013). Arbuscular mycorrhizal fungus Glomus mosseae formed a symbiotic associate of P. vittata L. and possessed substantial resistance to arsenic toxicity by increasing the plant biomass, and this mycorrhiza can enhance the arsenic sink. Mycorrhiza can be a potential tool for fungal phytoremediation by choosing the native species of fungi/host and alteration in the association by changing any of the fungi/host or controlling factors or inoculation of the new fungal strains. This can be achieved through re-vegetation on the contaminated sites such as mine areas.
18.7 Precaution Prerequisite
Some prerequisite precautions are needed for successful achievement of fungal phytoremediation which involves selection of correct fungal species for targeted metal contamination for developing a screening protocol (Matsubara et al. 2006). Among these precautions, major points have been prescribed in general which should be considered. These involve as follows:
-
The catabolic activity and capacity of organisms involved to transform the target compound(s) and bring the concentrations to levels that meet regulatory standards
-
The rate of bioremediation
-
The possible production of toxic by-products at dangerous levels during the remediation process
-
Adaptability of the process to site conditions (environmental and anthropogenic)
-
Economic viability of the process
18.8 Conclusion and Future Prospects
As explained above, fungal phytoremediation is a very potent technology for sustainable bioremediation of contaminated soils and water. In general, it is still in infancy in laboratory conditions and greenhouse, which limits the outcome to the actual field condition pertaining to multiple factors. Hence, the assessment of the efficiency rate of fungal phytoremediation must be tested in field condition in order to commercialize this green technology by evaluating the different plant for targeted heavy metal. Similarly, there are few lags in this eco-friendly remediation technology such as to increase the growth rate of plants, increase the biomass of such plants for maximum absorption of heavy metals, and take a look on possible hazards on food chain. Field experiments should be devised to explore the hyperaccumulators from where these metals can be harvested easily and feasible techniques to harvest these metals without exerting a negative impact on environment. Also, the key to mycoremediation is determining the right fungal species to target a specific pollutant. Desirable traits should be identified from the hyperaccumulator and fungi genome. Such gene can be selected by the conventional techniques or new technologies of hybridization such as protoplast fusion. Identification of genes coding for different toxicants from different hyperaccumulators and their transformation in same plant can develop SUPERBUG plant for phytoremediation. Besides the several constraints and limitations, fungal phytoremediation appears to be the most potent, eco-friendly, economical, and environmentally attractive option of bioremediation in heavy metal-contaminated soils and water resources. Many fungal species can grow under various contaminated conditions, thus enabling remediation in the contaminated environment that may not be suitable for other organisms. Based on our review of the subject and key questions raised on the concerned topic, we do not conclude that it could solve the issues of metal or hydrocarbon contamination completely. Conversely, a synergistic approach involving proactive policy designing in the field of fungal phytoremediation ranging from lab-based desirable trait based targeted metal contaminations with a particular fungal species, after testing it in green houses it has a potential to be replicated in the field environment for the future safety of soil, plant, water resources and rising human population prone to future heavy metal contaminations.
References
Adenipekun C, Lawal R (2012) Uses of mushrooms in bioremediation: a review. Biotechnol Mol Biol Rev 7:62–68
Adeyemi AO (2009) Bioaccumulation of arsenic by fungi. Am J Environ Sci 5:364–370
Akhtar K, Akhtar MW, Khalid AM (2008) Removal and recovery of zirconium from its aqueous solution by Candida tropicalis. J Hazard Mater 156:108–117
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Alpat S, Alpat SK, Çadirci BH, Özbayrak Ö, Yasa İ (2010) Effects of biosorption parameter: kinetics, isotherm and thermodynamics for Ni (II) biosorption from aqueous solution by Circinella sp. Electron J Biotechnol 13:4–5
Amatussalam A, Abubacker M, Rajendran RB (2011) In situ Carica papaya stem matrix and Fusarium oxysporum (NCBT-156) mediated bioremediation of chromium. Indian J Exp Biol 49:925–931
Anjum SA, Tanveer M, Hussain S, Bao M, Wang L, Khan I, Ullah E, Tung SA, Samad RA, Shahzad B (2015) Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res 22:17022–17030
Arbanah M, Najwa MM, Halim KK (2012) Biosorption of Cr (III), Fe (II), Cu (II), Zn (II) ions from liquid laboratory chemical waste by Pleurotus ostreatus. Int J Biotechnol Wellness Ind 1:152–162
Archana, Jaitly AK (2015) Mycoremediation: utilization of fungi for reclamation of heavy metals at their optimum remediation conditions. Biolife 3(1):77–106
Arıca M, Arpa C, Kaya B, Bektaş S, Denizli A, Genç Ö (2003) Comparative biosorption of mercuric ions from aquatic systems by immobilized live and heat-inactivated Trametes versicolor and Pleurotus sajur-caju. Bioresour Technol 89:145–154
Bai S, Abraham TE (2001) Biosorption of Cr (VI) from aqueous solution by Rhizopus nigricans. Bioresour Technol 79:73–81
Bajgai RC, Georgieva N, Lazarova N (2012) Bioremediation of chromium ions with filamentous yeast Trichosporon cutaneum R57. J Biol Earth Sci 2:70–75
Bhatia A, Singh S, Kumar A (2015) Heavy metal contamination of soil, irrigation water and vegetables in peri-urban agricultural areas and markets of Delhi. Water Environ Res 87:2027–2034
Boisselet T (2012) Chemical and biological factors influencing heavy metal mobilisation in the rhizosphere implications for phytoremediation. Ph.D. Thesis in Institute of Earth Sciences of the Friedrich Schiller Universtity in Jena, Germany
Çabuk A, Ilhan S, Filik C, Çalişkan F (2005) Pb2+ biosorption by pretreated fungal biomass. Turk J Biol 29:23–28
Caravanos J, Carrelli J, Dowling R, Pavilonis B, Ericson B, Fuller R (2016) Burden of disease resulting from lead exposure at toxic waste sites in Argentina, Mexico and Uruguay. Environ Health 15:72
Connor R, Renata A, Ortigara C, Koncagül E, Uhlenbrook S, Lamizana-Diallo BM, Zadeh SM, Qadir M, Kjellén M, Sjödin J (2017) The united Nations World Water Development Report 2017. Wastewater: The untapped resource. The United Nations World Water Development Report
Cunningham SD, Ow DW (1996) Promises and prospects of phytoremediation. Plant Physiol 110:715
D’Annibale A, Ricci M, Leonardi V, Quaratino D, Mincione E, Petruccioli M (2005) Degradation of aromatic hydrocarbons by white-rot fungi in a historically contaminated soil. Biotechnol Bioeng 90:723–731
Danesh YR, Tajbakhsh M, Goltapeh EM, Varma A (2013) Mycoremediation of heavy metals. In: Fungi as bioremediators. Springer, Heidelberg, pp 245–267
Dudhane M, Borde M, Jite PK (2012) Effect of aluminium toxicity on growth responses and antioxidant activities in Gmelina arborea Roxb. inoculated with AM fungi. Int J Phytoremediation 14:643–655
Ekmekyapar F, Aslan A, Bayhan Y, Cakici A (2012) Biosorption of Pb (II) by nonliving lichen biomass of Cladonia rangiformis Hoffm. Int J Environ Res 6:417–424
El Zeftawy MM, Mulligan CN (2011) Use of rhamnolipid to remove heavy metals from wastewater by micellar-enhanced ultrafiltration (MEUF). Sep Purif Technol 77:120–127
Emsley J (2011) Nature’s building blocks: an AZ guide to the elements. Oxford ; New York : Oxford University Press
Fourest E, Roux J-C (1992) Heavy metal biosorption by fungal mycelial by-products: mechanisms and influence of pH. Appl Microbiol Biotechnol 37:399–403
Fulekar M, Sharma J, Tendulkar A (2012) Bioremediation of heavy metals using biostimulation in laboratory bioreactor. Environ Monit Assess 184:7299–7307
Gadd GM, de Rome L (1988) Biosorption of copper by fungal melanin. Appl Microbiol Biotechnol 29:610–617
Galun M, Galun E, Siegel B, Keller P, Lehr H, Siegel S (1987) Removal of metal ions from aqueous solutions by Penicillium biomass: kinetic and uptake parameters. Water Air Soil Pollut 33:359–371
Gams W, Stalpers J (1994) Has the prehistoric ice-man contributed to the preservation of living fungal spores? FEMS Microbiol Lett 120:9–10
García-Delgado C, Alfaro-Barta I, Eymar E (2015) Combination of biochar amendment and mycoremediation for polycyclic aromatic hydrocarbons immobilization and biodegradation in creosote-contaminated soil. J Hazard Mater 285:259–266
Ghani A (2011) Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egypt Acad J Biol Sci 2:9–15
Ghosh P, Ghosh U (2018) Bioconversion of agro-waste to value-added product through solid-state fermentation by a potent fungal strain Aspergillus flavus PUF5. In: Utilization and management of bioresources. Springer, Singapore, pp 291–299
Gupta N, Yadav KK, Kumar V, Kumar S, Chadd RP, Kumar A (2018) Trace elements in soil-vegetables interface: translocation, bioaccumulation, toxicity and amelioration: a review. Sci Total Environ 651:2927–2942
Harms H, Schlosser D, Wick LY (2011) Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat Rev Microbiol 9:177–192
Häyrynen P, Landaburu-Aguirre J, Pongrácz E, Keiski RL (2012) Study of permeate flux in micellar-enhanced ultrafiltration on a semi-pilot scale: Simultaneous removal of heavy metals from phosphorous rich real wastewaters. Separation and Purification Technology 93:59–66
Hübner R, Astin KB, Herbert RJ (2010) “Heavy metal”—time to move on from semantics to pragmatics? J Environ Monit 12:1511–1514
Ianis M, Tsekova K, Vasileva S (2006) Copper biosorption by Penicillium cyclopium: equilibrium and modelling study. Biotechnol Equip 20:195–201
Ikeda Y, Park EY, Okuda N (2006) Bioconversion of waste office paper to gluconic acid in a turbine blade reactor by the filamentous fungus Aspergillus niger. Bioresour Technol 97:1030–1035
Ita B, Ebong G, Essien J, Eduok S (2008) Bioaccumulation potential of heavy metals in edible fungal sporocarps from the Niger Delta Region of Nigeria. Pak J Nutr 7:93–97
Jain N, Johnson TA, Kumar A, Mishra S, Gupta N (2015) Biosorption of Cd (II) on jatropha fruit coat and seed coat. Environ Monit Assess 187:411. https://doi.org/10.1007/s10661-015-4658-4
Jan A, Azam M, Siddiqui K, Ali A, Choi I, Haq Q (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16:29592–29630
Kantifedaki A, Kachrimanidou V, Mallouchos A, Papanikolaou S, Koutinas A (2018) Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J Clean Prod 185:882–890
Kiran I, Akar T, Tunali S (2005) Biosorption of Pb (II) and Cu (II) from aqueous solutions by pretreated biomass of Neurospora crassa. Process Biochem 40:3550–3558
Kour D, Rana KL, Yadav N, Yadav AN, Rastegari AA, Singh C, Negi P, Singh K, Saxena AK (2019a) Technologies for biofuel production: current development, challenges, and future prospects. In: Rastegari AA, Yadav AN, Gupta A (eds) Prospects of renewable bioprocessing in future energy systems. Springer International Publishing, Cham, pp 1–50. https://doi.org/10.1007/978-3-030-14463-0_1
Kour D, Rana KL, Yadav N, Yadav AN, Singh J, Rastegari AA, Saxena AK (2019b) Agriculturally and industrially important fungi: current developments and potential biotechnological applications. In: Yadav AN, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through fungi, Volume 2: Perspective for value-added products and environments. Springer International Publishing, Cham, pp 1–64. https://doi.org/10.1007/978-3-030-14846-1_1
Kulshreshtha S, Mathur N, Bhatnagar P, Kulshreshtha S (2013) Cultivation of Pleurotus citrinopileatus on handmade paper and cardboard industrial wastes. Ind Crop Prod 41:340–346
Kumar Ramasamy R, Congeevaram S, Thamaraiselvi K (2011) Evaluation of isolated fungal strain from e-waste recycling facility for effective sorption of toxic heavy metal Pb (II) ions and fungal protein molecular characterization—a mycoremediation approach. Asian J Exp Biol Sci 2:342–347
Kumar SS, Celin SM, Bishnoi NR, Malyan SK (2014) Phytoremediation of HMX contaminated soil through Jatropha curcas International Journal of Recent Scientific Research Research 5(8):1444–1450
Kumar SS, Malyan S, Kumar A, Bishnoi NR (2016) Optimization of Fenton’s oxidation by Box-Behnken design of response surface methodology for landfill leachate. J Mater Environ Sci 7:4456–4466
Lamrood P, Ralegankar S (2013) Biosorption of Cu, Zn, Fe, Cd, Pb and Ni by non-treated biomass of some edible mushrooms. Asian J Exp Biol Sci 4:190–195
Landaburu-Aguirre J, Pongrácz E, Sarpola A, Keiski RL (2012) Simultaneous removal of heavy metals from phosphorous rich real wastewaters by micellar-enhanced ultrafiltration. Sep Purif Technol 88:130–137
Lawton JH, Jones CG (1995) Linking species and ecosystems: organisms as ecosystem engineers. In: Linking species & ecosystems. Chapman & Hall, New York, pp 141–150
Levinskaite L (2001) Simultaneous effect of Ni, Cd and Cr on soil micromycetes. Biologija 4:13–15
Lottermoser BG (2010a) Introduction to mine wastes. In: Mine wastes. Springer, Berlin, Heidelberg, pp 1–41
Lottermoser BG (2010b) Tailings. In: Mine wastes. Springer, Berlin, Heidelberg, pp 205–241
Loukidou MX, Matis KA, Zouboulis AI, Liakopoulou-Kyriakidou M (2003) Removal of As (V) from wastewaters by chemically modified fungal biomass. Water Res 37:4544–4552
Luo J-M, Xiao X (2010) Biosorption of cadmium (II) from aqueous solutions by industrial fungus Rhizopus cohnii. Trans Nonferr Metal Soc 20:1104–1111
Macellaro G, Pezzella C, Cicatiello P, Sannia G, Piscitelli A (2014) Fungal laccases degradation of endocrine disrupting compounds. Biomed Res Int 2014:1–8. https://doi.org/10.1155/2014/614038
Mahurpawar M (2015) Effects of heavy metals on human health. Int J Res Granthaalayah 1:2394–3629
Majumdar SS, Das SK, Chakravarty R, Saha T, Bandyopadhyay TS, Guha AK (2010) A study on lead adsorption by Mucor rouxii biomass. Desalination 251:96–102
Mäkinen MA, Risulainen N, Mattila H, Lundell TK (2018) Transcription of lignocellulose-decomposition associated genes, enzyme activities and production of ethanol upon bioconversion of waste substrate by Phlebia radiata. Appl Microbiol Biotechnol 102:5657–5672
Malyan SK, Kumar J, Kumar SS (2014) Assessment of groundwater pollution of Saharanpur district, western Uttar Pradesh, India International Journal of Recent Scientific Research Research 5(6):1112–1115
Mamun A, Alam Z, Mohd NAN, Rashid SS (2011) Adsorption of heavy metal from landfill leachate by wasted biosolids. Afr J Biotechnol 10:18869–18881
Mani D, Kumar C (2014) Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation. International Journal of Environmental Science and Technology 11(3):843–872
Matsubara M, Lynch J, De Leij F (2006) A simple screening procedure for selecting fungi with potential for use in the bioremediation of contaminated land. Enzyme Microb Technol 39:1365–1372
Matsumura F, Boush G (1966) Malathion degradation by Trichoderma viride and a Pseudomonas species. Science 153:1278–1280
Matta G, Kumar A, Naik PK, Kumar A, Srivastava N (2018) Assessment of heavy metals toxicity and ecological impact on surface water quality using HPI in Ganga river. INAE Lett 3:123–129
Micó C, Recatalá L, Peris M, Sánchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 65:863–872
Mishra J, Singh R, Arora NK (2017) Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front Microbiol 8:1706. https://doi.org/10.3389/fmicb.2017.01706
Mnif W, Hassine AIH, Bouaziz A, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8:2265–2303
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Muraleedharan T, Iyengar L, Venkobachar C (1995) Screening of tropical wood-rotting mushrooms for copper biosorption. Appl Environ Microbiol 61:3507–3508
Nagy B, Măicăneanu A, Indolean C, Mânzatu C, Silaghi-Dumitrescu L, Majdik C (2014) Comparative study of Cd (II) biosorption on cultivated Agaricus bisporus and wild Lactarius piperatus based biocomposites. Linear and nonlinear equilibrium modelling and kinetics. J Taiwan Inst Chem Eng 45:921–929
Nasseri S, Mazaheri A, Noori S, Rostami K, Shariat M, Nadafi K (2002) Chromium removal from tanning effluent using biomass of Aspergillus oryzae. Pak J Biol Sci 5:1056–1059
Nath K, Singh D, Shyam S, Sharma YK (2008) Effect of chromium and tannery effluent toxicity on metabolism and growth in cowpea (Vigna sinensis L. Saviex Hassk) seedling. Res Environ Life Sci 1:91–94
Nguyen LN (2015) Trace organic contaminant removal by fungal membrane bioreactors and enzymatic membrane reactors. Doctor of Philosophy thesis, School of Civil, Mining and Environmental Engineering, University of Wollongong. https://ro.uow.edu.au/theses/4540
Nieboer E, Richardson D (1978) Lichens and “heavy” metals. Int Lichenol Newsl 11:1–3
Pal TK, Sauryya B, Arunabha B (2010) Cellular distribution of bioaccumulated toxic heavy metals in Aspergillus niger and Rhizopus arrhizus. Int J Pharma Biosci 1(2):1–6
Paszczynski A, Crawford RL (1995) Potential for bioremediation of xenobiotic compounds by the white-rot fungus Phanerochaete chrysosporium. Biotechnol Prog 11:368–379
Pattanapipitpaisal P, Brown N, Macaskie L (2001) Chromate reduction by Microbacterium liquefaciens immobilised in polyvinyl alcohol. Biotechnol Lett 23:61–65
Pinedo-Rivilla C, Aleu J, Collado I (2009) Pollutants biodegradation by fungi. Curr Org Chem 13:1194–1214
Pinho S, Ladeiro B (2012) Phytotoxicity by lead as heavy metal focus on oxidative stress. J Bot 2012:1–10. https://doi.org/10.1155/2012/369572
Pisani T, Munzi S, Paoli L, Bačkor M, Loppi S (2011) Physiological effects of arsenic in the lichen Xanthoria parietina (L.) Th. Fr. Chemosphere 82:963–969
Prakash V (2017) Mycoremediation of environmental pollutants. Int J Chemtech Res 10:149–155
Prakasham R, Merrie JS, Sheela R, Saswathi N, Ramakrishna S (1999) Biosorption of chromium VI by free and immobilized Rhizopus arrhizus. Environ Pollut 104:421–427
Prasad MNV (2001) Metals in the environment: analysis by biodiversity. Marcel Dekker, New York
Prasad AA, Varatharaju G, Anushri C, Dhivyasree S (2013) Biosorption of lead by Pleurotus florida and Trichoderma viride. Br Biotechnol J 3:66–78
Prasenjit B, Sumathi S (2005) Uptake of chromium by Aspergillus foetidus. J Mater Cycles Waste Manag 7:88–92
Quinche J-P (1990) Lycoperdon perlatum, a fungus accumulating heavy metals and selenium. Mycol Helv 3:477–486
Rajendran P, Muthukrishnan J, Gunasekaran P (2003) Microbes in heavy metal remediation. Indian J Exp Biol 41:935–944
Rao KR, Rashmi K, Latha J, Mohan PM (2005) Bioremediation of toxic metal ions using biomass of Aspergillus fumigatus from fermentative waste. Indian J Biotechnol 4:139–143
Rhodes CJ (2014) Mycoremediation (bioremediation with fungi)–growing mushrooms to clean the earth. Chem Spec Bioavailab 26:196–198
Saxena G, Purchase D, Mulla SI, Saratale GD, Bharagava RN (2019) Phytoremediation of heavy metal-contaminated sites: eco-environmental concerns, field studies, sustainability issues, and future prospects. In: Reviews of environmental contamination and toxicology (continuation of residue reviews). Springer, New York. https://doi.org/10.1007/398_2019_24
Say R, Yilmaz N, Denizli A (2003) Removal of heavy metal ions using the fungus Penicillium canescens. Adsorp Sci Technol 21:643–650
Sen M, Dastidar MG (2011) Biosorption of Cr (VI) by resting cells of Fusarium solani. Iran J Environ Health Sci Eng 8:153–158
Seshikala D, Charya MS (2012) Effect of pH on chromium biosorption. Int J Pharma Bio Sci 2:298–302
Shouaib A, Badar T, Aslam N (2011) Removal of Pb (II), Cu (II) and Cd (II) from aqueous solution by some fungi and natural adsorbents in single and multiple metal systems. Pak J Bot 43:2997–3000
Singh A, Gauba P (2014) Mycoremediation: a treatment for heavy metal pollution of soil. J Civ Eng Environ Technol 1:59–61
Singh S, Kumar M (2006) Heavy metal load of soil, water and vegetables in peri-urban Delhi. Environ Monit Assess 120:79–91
Singh J, Lingamdinne LP, More NS, Shankar S, Koduru JR (2017) Toxic metals contamination in the environment: toxicological effects and bioremediation approaches for environmental cleanup. In: Environmental pollutants and their bioremediation approaches. CRC Press, Boca Raton, pp 209–240
Singh J, Rawat K S and Kumar A (2013) Mobility of Cadmium in sewage sludge applied soil and its uptake by Radish (Raphanus sativus L.) and Spinach (Spinacia oleracea L.), Int J Agric Food Sci Technol 4(4):291–296
Singh J, Rawat KS, Kumar A, Singh A (2013a) Effect of sewage sludge and bio-fertilizers on physicochemical properties of alluvial soil Biochem Cell. Arch 13(2):191–202
Sharma A, Kumar A, Dhaka TS (2012a) Impact on sugar factory effluent on chlorophyll and protein contents of Cicer arietinum and Tigonella foenum-gracecum. Curr Adv Agric Sci 4(1):62–63
Sharma A, Kumar A, Dhaka TS (2012b) Impact of sugar factory effluent on leghaemoglobin and soluble proteins of Cicer arietinum and Trigonella foenum-graecum. Vegetose 25(1):287–289
Smith ML, Bruhn JN, Anderson JB (1992) The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature 356(2):428–431
Srivastava P, Hasan SH (2011) Biomass of Mucor heimalis for the biosorption of cadmium from aqueous solutions: equilibrium and kinetic studies. Bioresources 6:3656–3675
Srivastava V, Sarkar A, Singh S, Singh P, de Araujo ASF, Singh RP (2017) Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front Environ Sci 5:1–19. https://doi.org/10.3389/fenvs.2017.00064
Suresh PV, Chandrasekaran M (1998) Utilization of prawn waste for chitinase production by the marine fungus Beauveria bassiana by solid state fermentation. World J Microbiol Biotechnol 14:655–660
Tahir A (2012) Resistant fungal biodiversity of electroplating effluent and their metal tolerance index. In: Sebayang D (ed) Electroplating. InTech, Rijeka, pp 137–144. ISBN: 978-953-51-0471-1
Tan T, Cheng P (2003) Biosorption of metal ions with Penicillium chrysogenum. Appl Biochem Biotechnol 104:119–128
Taştan BE, Ertuğrul S, Dönmez G (2010) Effective bioremoval of reactive dye and heavy metals by Aspergillus versicolor. Bioresour Technol 101:870–876
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Molecular, clinical and environmental toxicology. Springer, Basel, pp 133–164
Thenmozhi R, Arumugam K, Nagasathya A, Thajuddin N, Paneerselvam A (2013) Studies on mycoremediation of used engine oil contaminated soil samples. Adv Appl Sci Res 4:110–118
Thippeswamy B, Shivakumar C, Krishnappa M (2012a) Accumulation potency of heavy metals by Saccharomyces sp. indigenous to paper mill effluent. J Environ Res Dev 6:439–445
Thippeswamy B, Shivakumar C, Krishnappa M (2012b) Bioaccumulation potential of Aspergillus niger and Aspergillus flavus for removal of heavy metals from paper mill effluent. J Environ Biol 33:1063
Tu X, Huang G-H (2005) A novel biosorbent: characterization of the spent mushroom compost and its application for removal of heavy metals. J Environ Sci 17:756–760
United States Geological Survey. (2012) Minerals Resources Program. https://www.usgs.gov/energy-and-minerals/mineral-resources-program
Venugopal B, Luckey T (1978) Metal toxicity in mammals. In: Chemical toxicology of metals and metalloids. Academic Press, New York, pp 32–36
Vinciguerra V, D’Annibale A, Delle Monache G, Sermanni GG (1995) Correlated effects during the bioconversion of waste olive waters by Lentinus edodes. Bioresour Technol 51:221–226
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36:169–182
Yadav AN (2018) Biodiversity and biotechnological applications of host-specific endophytic fungi for sustainable agriculture and allied sectors. Acta Sci Microbiol 1:01–05
Yadav AN, Sachan SG, Verma P, Saxena AK (2015a) Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J Biosci Bioeng 119:683–693
Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, Saxena AK (2015b) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31:95–108
Yadav AN, Verma P, Kumar M, Pal KK, Dey R, Gupta A, Padaria JC, Gujar GT, Kumar S, Suman A, Prasanna R, Saxena AK (2015c) Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann Microbiol 65:611–629
Yadav AN, Sachan SG, Verma P, Saxena AK (2016) Bioprospecting of plant growth promoting psychrotrophic Bacilli from cold desert of north western Indian Himalayas. Indian J Exp Biol 54:142–150
Yadav KK, Gupta N, Kumar V, Singh JK (2017) Bioremediation of heavy metals from contaminated sites using potential species: A review. Indian J Environ Prot 37:65–84
Yadav A, Verma P, Kumar R, Kumar V, Kumar K (2017a) Current applications and future prospects of eco-friendly microbes. EU Voice 3:21–22
Yadav AN, Kumar R, Kumar S, Kumar V, Sugitha T, Singh B, Chauhan VS, Dhaliwal HS, Saxena AK (2017b) Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. J Appl Biol Biotechnol 5:1–13
Yadav AN, Verma P, Kumar V, Sangwan P, Mishra S, Panjiar N, Gupta VK, Saxena AK (2018a) Biodiversity of the genus Penicillium in different habitats. In: Gupta VK, Rodriguez-Couto S (eds) New and future developments in microbial biotechnology and bioengineering, Penicillium system properties and applications. Elsevier, Amsterdam, pp 3–18. https://doi.org/10.1016/B978-0-444-63501-3.00001-6
Yadav KK, Gupta N, Kumar A, Reece LM, Singh N, Rezania S, Khan SA (2018b) Mechanistic understanding and holistic approach of phytoremediation: a review on application and future prospects. Ecol Eng 120:274–298
Yadav KK, Gupta N, Kumar V, Khan SA, Kumar A (2018c) A review of emerging adsorbents and current demand for defluoridation of water: bright future in water sustainability. Environ Int 111:80–108
Yadav AN, Mishra S, Singh S, Gupta A (2019a) Recent advancement in white biotechnology through fungi Volume 1: Diversity and enzymes perspectives. Springer International Publishing, Cham
Yadav AN, Mishra S, Singh S, Gupta A (2019b) Recent advancement in white biotechnology through fungi. Volume 2: Perspective for value-added products and environments. Springer International Publishing, Cham
Yang Y, Liang Y, Han X, Chiu T-Y, Ghosh A, Chen H, Tang M (2016) The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci Rep 6:20469
Zhu Y, Yu H, Wang J, Fang W, Yuan J, Yang Z (2007) Heavy metal accumulations of 24 asparagus bean cultivars grown in soil contaminated with Cd alone and with multiple metals (Cd, Pb, and Zn). J Agric Food Chem 55:1045–1052
Acknowledgments
The authors are grateful to Prof. Harcharan Singh Dhaliwal, Vice Chancellor, Eternal University, Baru Sahib, Himachal Pradesh, India, for providing infrastructural facilities and constant encouragement.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kumar, A. et al. (2019). Fungal Phytoremediation of Heavy Metal-Contaminated Resources: Current Scenario and Future Prospects. In: Yadav, A., Singh, S., Mishra, S., Gupta, A. (eds) Recent Advancement in White Biotechnology Through Fungi. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-25506-0_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-25506-0_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25505-3
Online ISBN: 978-3-030-25506-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)