Abstract
The present research study investigates bioremediation potential of biostimulated microbial culture isolated from heavy metals waste disposal contaminated site located at Bhayander (east), Mumbai, India. The physicochemical and microbial characterization including heavy metal contaminants have been studied at waste disposal site. The microorganisms adapted at heavy metal-contaminated environment were isolated, cultured, and biostimulated in minimal salt medium under aerobic conditions in a designed and developed laboratory bioreactor. Heavy metals such as Fe, Cu, and Cd at a selected concentration of 25, 50, and 100 μg/ml were taken in bioreactor wherein biostimulated microbial culture was added for bioremediation of heavy metals under aerobic conditions. The remediation of heavy metals was studied at an interval of 24 h for a period of 21 days. The biostimulated microbial consortium has been found effective for remediation of Cd, Cu, and Fe at higher concentration, i.e., 100 mg/l up to 98.5%, 99.6%, and 100%, respectively. Fe being a micronutrient was remediated completely compared to Cu and Cd. During the bioaccumulation of heavy metals by microorganisms, environmental parameters such as pH, total alkalinity, electronic conductivity, biological oxygen demand, chemical oxygen demand, etc. were monitored and assessed. The pilot scale study would be applicable to remediate heavy metals from waste disposal contaminated site to clean up the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid industrialization and urbanization have dramatically increased the generation of wastes and new types of pollutants. Widespread pollution by heavy metals generated from various industrial and agricultural activities has adverse effects on human health and ecosystems (Nriagu and Pacyna 1988). Heavy metal toxicity can result in damaged or reduced mental and central nervous function; lower energy levels; and damage to blood composition, lungs, kidneys, liver, and other vital organs. Long-term exposure may result in slowly progressing physical, muscular, and neurological degenerative processes that mimic Alzheimer’s disease, Parkinson’s disease, muscular dystrophy, and multiple sclerosis. Allergies are not uncommon, and repeated long-term contact with some metals or their compounds may even cause cancer (International Occupational Safety and Health Information Centre 1999). Metals are released into the environment from natural weathering processes of the Earth’s crust, soil erosion, mining, industrial discharge, urban runoff, sewage effluents, air pollution, and pest or disease control agents applied to plants, among other sources (Fargasova 1994; Alloway 1995; Raskin and Ensley 2000). Heavy metals are of serious concern as they are nonbiodegradable, highly toxic, and are present in a variety of waste streams that contaminate the environment. Thus, treatment technologies for contaminated water, sediments, and soils with metals should be developed. Biological, physical, and chemical methods have been developed for water purification and metal recovery operations from wastewater (Schwartz et al. 2001; Mercier et al. 2001; Thaveemaitree et al. 2003).
Bioremediation processes are very attractive in comparison to physicochemical methods for heavy metal removal because they are less expensive and highly efficient even at low heavy metal concentrations (Gadd and White 1993). The use of microorganisms in the removal of metals from contaminated environment is generally considered promising (Green-Ruiz et al. 2008). Many soils contaminated with heavy metals contain heavy metal degrading microorganisms. Due to limited environmental factors, they are often limited to degradation. The biostimulation method stimulates their activity by providing nutrients, oxygen, and other amendments. Studies conducted by Straube et al. (2003) have also shown the effectiveness of the biostimulation method. Bacteria show remarkable resistance to essentially all toxic metal ions of environmental concern. Such resistance to toxic metals may be possible either due to exclusion of ions, biotransformation, accumulation, and production of low molecular weight binding proteins (Summers 1978; Silver and Misra 1988). To survive under metal-stressed conditions, bacteria have evolved several types of mechanisms in order to tolerate the uptake of heavy metal ions. These mechanisms include the efflux of metal ions outside the cell, accumulation and complexation of the metal ions inside the cell, and reduction of the heavy metal ions to a less toxic state (Nies 1999).
The present research study deals with the bioremediation of heavy metals in bioreactor by biostimulation of microorganisms isolated from heavy metal-contaminated waste disposal site. The small-scale industries located at Bhayander (east) which is situated between the north latitude 19.305740° (10 mode) = 19°18′20″ (60 mode) and east longitude 72.862411° (10 mode) = 72°51′44″ (60 mode) carry out various processes/operations which include grinding, mixing, plating, washing, milling, cutting, rubbing, etc. During these processes, the metal-contaminated solid waste is collected and dumped in a waste disposal ground. Domestic waste is also directly disposed on the ground by these small-scale industries. Also, rainwater comes in contact with the contaminant, leaches the soluble part of the metals into the surrounding environment, and causes heavy metal pollution. This study involves physicochemical and biological characterization of the waste disposal site. Microbial culture isolated was further biostimulated by providing nutrients and aerobic conditions. Although microorganisms were present in contaminated soil, they were not necessarily present in numbers for bioremediation. Therefore, it was necessary to stimulate the growth and activity of microorganisms. The adapted microbial culture was further used as a biomass for bioremediation of selected heavy metals from waste disposal site, i.e., Fe, Cu, and Cd at an appropriate concentration (25, 50, and 100 μg/ml) in a bioreactor under laboratory conditions. The bioremediation was carried out in a glass vessel wherein aerobic conditions were maintained, and a mechanical stirrer was provided for the close contact of microorganisms with contaminant. After 3 weeks, metals were remediated completely. The biostimulated microbial culture was found to be very effective for bioremediation of heavy metals, which can be used as a culture for environmental cleanup of metal-contaminated sites.
Materials and methods
Sampling site
The metal generated through various small-scale industries engaged in operations such as metal plating, grinding, mixing, rubbing, milling, cutting, manufacturing of machinery tools, etc. is disposed of at a waste disposal site located at Bhayander (east), Mumbai, India. The soil/effluent/sludge samples were collected from five different places of waste disposal site in precleaned polythene bottles/bags for physicochemical and microbial characterization assays.
Physicochemical characterization of sample
Soil was air-dried, ground, and passed through a 2-mm pore size sieve, and stored in sealed containers at room temperature. Microbial characterization was done immediately after sampling. The samples were analyzed for various physicochemical parameters like pH, temperature, electrical conductivity (EC), total solids (TS), total hardness (TH), total suspended solids (TSS), total alkalinity (TA), phosphorus (P), total dissolved solids (TDS), including biological characterization like biological oxygen demand (BOD), chemical oxygen demand (COD), and dissolved oxygen (DO). The parameters were analyzed as per the “APHA, Standard Methods for Water and Waste Water Analysis” volume 2, 1989 (APHA 1979, 1989).
Bioremediation of heavy metals
Microbial biomass
Microorganisms, used as biomass for bioremediation of heavy metals, were isolated from soil samples collected from various locations of metal-contaminated waste disposal site at Bhayander, east. Nutrient agar was used for their isolation and enumeration. Serial dilution method was adopted for soil, sludge, and effluent, using sterile distilled water as diluent. Each dilution (0.1 ml) was spread on nutrient agar plates in triplicates, incubated for 24 h at room temperature, and the colonies were counted on a colony counter. Colony-forming units (CFU) per milliliter were calculated according to the following formula:
Preparation and activation of metal-contaminated sample
The sample collected from metal-contaminated sites was diluted with water in 1:1 ratio. The prepared slurry was aerated and activated in a glass vessel for a week at room temperature (30–35°C). The sample was treated by “biostimulation method,” wherein the required amount of nutrients and electron acceptors, such as phosphorus, nitrogen, oxygen, or carbon source (here glucose was used as carbon source) and consortium of pre-existing microorganisms, were added. The sample slurry was supplemented with basal salt solutions which serve as a source for inorganic nutrients (C:N:P). This helps in the growth and proliferation of biomass. The salt solution comprises (in grams per liter): Na2HPO4, 2.67; NH4Cl, 5.35; citric acid, 4; glucose, 10; and blended with 6 ml of trace elements solution. Trace elements solution contains (in grams per liter): CaCl2·2H2O, 0.05; MgSO4, 5; MnSO4·7H2O, 0.035; and FeSO4·7H2O, 0.2. After the addition of nutrients and consortium, the sample was subjected to aeration (by air pump) and agitation (by stirring, with the help of magnetic stirrer). Samples were collected in glass vials on daily and weekly basis.
Physicochemical characterization of the sample was determined according to soil chemical analysis (Jackson 1973). The collected samples were characterized for the assessment of its microbial diversity. The slurry was grown on nutrient agar plate, and different microbial colonies were isolated based on morpho-colonial characteristics. The samples taken were digested with nitric acid for heavy metal analysis by atomic absorption spectroscopy (AAS).
Experimental setup for bioremediation of heavy metals
In this process, contaminated solid materials (such as soil and degraded sediments), microorganisms, and water were made into slurry within a bioreactor. It was a triphasic system containing air, water, and solids. Water serves as suspension medium for nutrients, trace elements, pH adjustment, chemicals, and desorbed contaminants. Suspended particulate matter (solid) forms the biologically inert substratum containing contaminants. Air provides oxygen for bacterial growth. Bioremediation of heavy metals (Fe, Cu, and Cd) was carried out in a bioreactor, having dimensions 20 × 20 × 25 cm, with provision for air (12 mg DO/l), to maintain the aerobic conditions (Fig. 1, Singh and Fulekar 2007). Five hundred milliliters of 1:1 diluted sample was taken in the bioreactor wherein mechanical stirrer was provided for agitation of biomass and maintaining close contact of microorganism with contaminant. The activated microbial culture was taken as microbial biomass for biodegradation of heavy metals. The concentration of heavy metals, i.e., Fe, Cu, and Cd were 25, 50, and 100 mg/l, respectively. Sampling was done daily for 7 days, further on the 14th day and 21st day to study the complete biodegradation of Fe, Cu, and Cd. The physicochemical parameters such as pH, temperature, EC, TA, BOD, COD, and DO have been determined daily and then, after an interval of 1 week for two consecutive weeks.
The number of CFU per milliliter was counted every 24 h. The CFU was determined by aseptically plating serially diluted samples on sterile minimal agar plates. The agar plates were incubated for 24 h at 37°C.
Sample preparation for metal analysis
For analysis of metal ion concentration in the effluent samples, the method of spectrophotometry was adopted. Filtrate of effluent samples (20 ml) was taken in a glass beaker, and 1 ml of concentrated HNO3 was added and kept on a hot plate for slow boiling. Exposing the sample to high temperature removes all organic matters. When the volume was added and again heated till the volume was reduced to 10 ml, clear solution (without any suspended particles) was obtained. After obtaining a clear solution, 5 N NaOH was added, which was then passed through a Whatman No.41 filter paper, into a volumetric flask and the volume of the filtrate was made up to the mark, by adding deionized distilled water. Digested samples were store in clean vials at −20°C till analysis. Metal analysis was carried out using AAS (APHA, AWWA WPCF 1998).
Results and discussion
The environmental contaminated site located at Bhayander (east), Mumbai was selected for sampling the water, sludge, and soil for their physicochemical and microbial characterization.
Physicochemical characterization
The samples collected from five different places of the contaminated site located in Bhayander (east), Mumbai. The samples were analyzed for different physicochemical parameters. The physicochemical parameters studied show pH (7.4–8.5), temperature (27–29°C), acidity (11.1–62.5 mg/l), TA (98–700 mg/l), total organic carbon (TOC, 0.00144–0.0168%), BOD (240.83–1,374.42 mg/l), COD (13,600–29,066.7 mg/l), EC (0.16–1.65 mS/cm), TH (70–83 mg/l, Table 1). Clemente et al. (2007) have also demonstrated similar values after physicochemical characterization of heavy metal-contaminated site. Basu et al. (1997) have also reported high levels of BOD and COD in heavy metal-contaminated area which supported growth of chromate-tolerant bacteria (Basu et al. 1997). The concentrations of sulfate (32,007.33 mg/l), phosphorus (476.55 mg/l), and nitrogen (0.78 mg/l) show that the microbial consortium has sufficient supply of nutrients to survive and grow in the metal-contaminated environment. The microbial consortium adapted to the conditions at the contaminated site was assessed, which shows the presence of bacteria, fungi, and Actinomycetes, etc. (Table 2). Microbial analysis of contaminated samples shows the presence of predominant organisms such as Pseudomonas, Bacillus, and Escherichia coli. Other microorganisms found to be present have been shown in Table 1. Microbial diversity in the metal-contaminated samples was observed in terms of CFU per milliliter.
Soil, sludge, and effluent samples were assessed for metal ion concentrations (Cu, Cd, Fe, Zn, and Pb). It was found that the effluent sample contained Cu, Fe, and Zn, while the sludge sample showed the presence of Cd, Cu, Zn, Pb, and Fe, whereas in soil Cd and Pb was not detected (Table 3). Heavy metals like Cu, Fe, and Zn were present above the recommended US EPA (Environmental Protection Agency) standards, only Cd and Pb were the ones were present in very low concentration in all the samples.
Adaptation of microbial consortium by biostimulation
Effluent samples contain a large number of microorganisms, thus demonstrating rich microbial diversity. Microbial consortium isolated from contaminated samples was adapted using Biostimulation method for a period of 14 days. During experiments, environmental parameters such as pH, temperature, EC, TA, DO, BOD, and COD were maintained and monitored (Table 4).
After treatment, significant changes were observed in the physiochemical characteristics and metal ion concentrations (as shown in Table 4). When the sample was tested for the physiochemical parameters, it was observed that the pH decreased till day 7, indicating that the native microorganisms are uptaking the metal ions for their growth. But by day 14, the pH reached to 7 indicating that the organism may have reached saturation and are precipitating out the metal ions in less toxic form. Metal precipitation caused by some bacteria producing ammonia, organic bases, or hydrogen sulfide that precipitates metals as hydroxides or sulfides. In a similar study, chromate-reducing bacteria, Enterobacter cloacae, resistant to chromate, reduced Cr(VI) to Cr(III) which is precipitated at pH 7 and thus detoxified (Wang et al. 1989).
EC estimates the amount of TDS or the total amount of dissolved ions in the minimal medium which was found to vary from 7.28 to 11.26 mS/cm (Fig. 2). BOD of the treated sample decreased to a level wherein it can be estimated that the native microorganisms were able to degrade the organic materials present in the effluent sample, and this also indicates the presence of aerobic microorganisms. COD of the sample decreased, but did not come to the acceptable limit. Since the COD is slightly above the acceptable limit, the effluent needs to be processed even further before being discharged in various kinds of water.
After the treatment of the effluent sample for 2 weeks, it was observed that the metal ions were completely removed from the sample, except Cu and Zn which still remained but in a very low amount which was in the acceptable limits. Thus, decrease in the metal ion concentration shows that the native organisms were capable of removing the metal ions from the sample, may be utilizing it as a growth factor.
Bioremediation of heavy metals in bioreactor
After spiking the effluent sample with different concentrations (100, 50, and 25 ppm) of metal ions Cu, Cd, and Fe for 3 weeks, it was observed that the pH of the sample was slightly alkaline and that the pH of the sample containing 25 ppm showed more reduction compared to the one with 100 and 50 ppm. Such reduction in pH indicates metal uptake by the grown native microorganisms. The increase in pH results in favoring the dissolution of metals and makes it more available for microorganisms. The temperature (in degrees Celsius) of all the three concentrations (Fig. 3) was in the range of 29 ± 0.7°C to 28 ± 0.8°C. The viable count (CFU per milliliter) of microorganisms during bioremediation confirms the growth and proliferation of microorganisms. The viable counts (in CFU per milliliter × 1011) of microorganisms were increased from 0.913 to 2.93, 0.11 to 2.10, and 0.45 to 3.89, respectively (Fig. 4) in 25, 50, 100 mg/l of heavy metals (Fe, Cu, and Cd).
Dissolved oxygen was found to decrease as growth of microorganism increased (Fig. 5). The increase in BOD indicates growth of microorganisms at various heavy metal concentrations (Fig. 6). Transition metal ions in general are toxic to the extent that they lead to immobilization of microbes through complex formation with the enzymes and amino acids found in the microbial cells. Alternatively, the microbes may also not be able to survive in the presence of metal ions together due to impaired metabolic activities (Mittal and Goel 2010). It was observed that after biostimulation, microorganisms became more adapted to heavy metal toxicity. Therefore, increase in growth of microorganisms results in increase BOD values. The reduction in COD values indicates low concentration of organic compounds. The sample after treatment for 21 days can be discharged in various water bodies; this is due to the efficiency of the treatment used for remediation (Fig. 7).
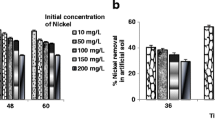
The results obtained from bioremediation of heavy metals in bioreactor are presented in Fig. 8. Data show effective reduction in heavy metal ion concentrations. Biodegradation started within 48 h, i.e., lag phase was observed whereby the microorganisms adapted/acclimatized to the respective metal concentration and continued up to 14 days. After 21 days, all metals were found completely remediated. The percentage degradation of heavy metals at varying concentrations (25 to 100 mg/l) ranged from 16.6% to 100%, whereas at the end of the experiment, lower concentrations (25, 50 mg/l) were totally degraded and percentage degradations at higher concentration, i.e., 100 mg/l were found to be 98.5%, 99.6%, and 100% for Cd, Cu, and Fe, respectively. Being a nutrient, iron was completely utilized in comparison to copper and cadmium. After 14 days, biostimulated microbial culture could degrade most toxic heavy metal cadmium. Thus, our research findings show that biostimulation has enhanced microbial activity for effective degradation of heavy metals.
Conclusion
The small-scale industries located in Bhayander (east), Mumbai carried out various operations wherein heavy metal wastes generated was disposed of at a dumping ground near the industrial belt. The physicochemical and microbial characterization has been studied at different sites in the dumping ground. The heavy metals like Cd, Cu, Zn, Pb, and Fe were present in the dumping ground. The microbial consortium isolated was biostimulated by aeration and mechanical stirring and used for bioreator purpose under controlled environment. Heavy metals such as Cd, Cu, and Fe were spiked at different concentrations for biostimulation and bioremediated using microbial consortium from the heavy metal dumping ground. The adapted microbial consortium used as a culture was found to be effective for remediation of heavy metal by way of precipitation, adsorption, and chemical transformation during the process of bioremediation. The biostimulated process used for activation of microbial consortium has been found effective for remediation of heavy metals from the contaminted sites.
The laboratory bioreactor developed for remediation of heavy metals using biostimulated microbial consortium under controlled environmental conditons was found to be effective and efficient to decontaminate heavy metals from waste disposal site. The microorganisms adapted at solid waste disposal site have been used as a source for bioaccumualtion/bioadsorption/bioabsorption of heavy metals. The designed and developed laboratory bioreactor can be modified in length and size as per the requirement in the field wherein the biostimulated microbial culture would be added for enhancement of remediation of heavy metals. This would have direct application in the present study for bioremediation of heavy metals to clean up the environment.
References
Alloway, B.J. (1995). Soil processes and the behavior of heavy metals. In: B.J. Alloway (Ed.), Heavy metals in soils. London: Blackie Academic and Professional, 11–37.
APHA. (1979). Standard methods for examination of water and waste water. Washington: American Public Health Association.
APHA. (1989). Standard methods for examination of water and waste water. Washington: American Public Health Association.
APHA, AWWA WPCF. (1998). Standard methods for the examination of water and wastewater. American Public Health Association/American Waterworks Association/Water Environmental Federation, Washington DC.
Basu, M., Bhattacharya, S., & Paul, A. K. (1997). Isolation and characterization of chromium-resistant bacteria from tannery effluents. Bulletin of Environmental Contamination and Toxicology, 58, 535–542.
Clemente, R., de la Fuente, C., Moral, R., & Bernal, M. P. (2007). Changes in microbial biomass parameters of a heavy metal-contaminated calcareous soil during a field remediation experiment. Journal of Environmental Quality, 36, 1137–1144.
Fargasova, A. (1994). Effect of Pb, Cd, Hg, As and Cr on germination and root growth of Sinapis alba seeds. Bulletin of Environmental Contamination and Toxicology, 52, 452.
Gadd, G. M., & White, C. (1993). Microbial treatment of metal pollution—A working biotechnology? Trends Biotechnology, 11(8), 353–359.
Green-Ruiz, C., Rodriguez-Tirado, V., & Gomez-Gil, B. (2008). Cadmium and zinc removal from aqueous solutions by Bacillus jeotgali: pH, salinity and temperature effects. Bioresource Technology, 99, 3864–3870.
International Occupational Safety and Health Information Centre. (1999). Metals. In Basics of chemical safety (Chapter 7). Geneva: International Labour Organization.
Jackson, M. L. (1973). Soil chemical analysis. New Delhi: Prentice-Hall of India.
Mercier, G., Duchesne, J., & Blackbum, D. (2001). Prediction of metal efficiency from contaminated soils by physical methods. Journal of Environmental Engineering, 127, 348–358.
Mittal, K. S., & Goel, S. (2010). BOD exertion and OD600 measurements in presence of heavy metal ions using microbes from dairy wastewater as a seed. Journal Water Resource and Protection, 2, 478–488.
Nies, D. H. (1999). Microbial heavy metal resistance. Applied Microbiology and Biotechnology, 51, 730–750.
Nriagu, J. O., & Pacyna, J. M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature, 333, 134–139.
Raskin, I., & Ensley, B. D. (2000). Phytoremediation of heavy metals: Using plants to clean the environment. New York: Wiley.
Schwartz, C., Gerard, E., Perronnet, K., & Morel, J. L. (2001). Measurement of in situ phytoextraction of zinc by spontaneous metallophytes growing on a former smelter site. Science of the Total Environment, 279, 215–221.
Silver, S., & Misra, T. K. (1988). Plasmid mediated heavy metal resistances. Annual Review of Microbiology, 42, 717–743.
Singh, D., & Fulekar, M. H. (2007). Bioremediation of phenol using microbial consortium in bioreactor. IRFB, 1, 32–38.
Straube, W. L., Nestler, C. C., Hansen, L. D., Ringleberg, D., Pritchard, P. H., & Jones-Meehan, J. (2003). Remediation of polyaromatic hydrocarbons (PAHs) through landfarming with biostimulation and bioaugmentation. Acta Biotechnologica, 23(2–3), 179–196.
Summers, A. C. (1978). Microbial transformations of metals. Annual Review of Microbiology, 32, 637–672.
Thaveemaitree, Y., Polprasert, C., & Seung-Hwan, L. (2003). Application of electrochemical process for landfill leachate treatment with emphasis on heavy metal and organic removal. Environmental Technology, 24, 1135–1145.
Wang, P., Mori, T., Komoril, K., Ssatsu, M., Toda, K., & Ohtake, H. (1989). Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Applied and Environmental Microbiology, 55(7), 1665–1669.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fulekar, M.H., Sharma, J. & Tendulkar, A. Bioremediation of heavy metals using biostimulation in laboratory bioreactor. Environ Monit Assess 184, 7299–7307 (2012). https://doi.org/10.1007/s10661-011-2499-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2499-3