Abstract
Chimeric antigen receptor (CAR) T-cells are a form of adoptive immunotherapy constituted of autologous T-cells engineered with a receptors that is able to target tumor antigens. Treatment with CAR19 cells leads to rapid response in a significant proportion of patients with relapsed or refractory aggressive B-cell lymphomas. However, relapses post CAR-T cell therapy are common. In this chapter, we will discuss what is currently known about mechanisms of resistance to CAR-T cell therapy in B-cell lymphomas or leukemias.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
CD19-directed genetically modified autologous T-cell immunotherapy is comprised of autologous T-cells collected from a patient and genetically engineered to encode an anti-CD19 chimeric antigen receptor (CAR) (Fig. 9.1) [1]. The structure of the anti-CD19 CAR T cell products (CAR19) recently evaluated in B-cell lymphoma trials are displayed in Fig. 9.2 [2]. Treatment with CAR19 cells leads to rapid response in the majority of patients with relapsed or refractory (r/r) aggressive B-cell lymphomas, including complete responses (CR). Notwithstanding the rapid initial response, a significant proportion of patients will eventually face disease relapses or progression, making crucial to understand mechanisms of treatment failure to CAR T cell therapy. Currently, CAR T cells with novel target antigens, such as CD22, CD20, κ-light chain for B-cell lymphomas, and CD30 for Hodgkin lymphoma and T-cell lymphomas are being investigated in several clinical trials (Fig. 9.1). While the era of CAR T cell therapy is in its infancy and there are large gaps in our understanding of the reasons cellular immunotherapy fails, a few mechanisms have become evident and will be the focus of our discussion in this chapter.
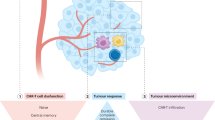
CAR T cell therapy – principle and clinical trial overview. (A) The CAR T cell therapy process. T-cells are isolated from blood of the patient or a donor, activated, and then genetically engineered to express the CAR construct (an example shown in gray above the vector particle in violet). After ex vivo expansion of the CAR T cells, they are formulated into the final product. The patient undergoes either a conditional chemotherapy or the CAR T cell product is directly infused. (B) Schematic representation of a T-cell receptor (TCR) and four types of CARs being displayed on the surface of a T-cell while contacting their antigen (red) on the tumor cells. The single-chain variable fragment (scFv) as ligand-binding domain mediating tumor cell recognition in CARs is shown in light blue with the VH and VL domains being connected via a along flexible linker and transmembrane domain to intracellular signaling domains. Pro-inflammatory cytokines or co-stimulatory ligands expressed by the CAR T cells are depicted for the 4th generation. (C) Overview of so-called smart CAR T cell products. Pooled CAR T cell products consist of two or more single-targeting CAR T cell types with distinct antigen specificities. Multi-CAR T cells harbor several CAR molecules with different antigen specificities. A tandem CAR T ell expresses a CAR construct harboring two ligand-binding domains with different antigen specificities. In a conditional CAR T cell activation and co-stimulation are separated on two CAR constructs recognizing different target antigens. In the split CAR construct the ligand-binding or signaling domain is physically separated allowing controlled CAR T cell activation. iCAR T cells additionally express a receptor engineered to recognize an antigen expressed on normal tissue to provide an inhibitory signal in turn. In addition CAR T cells can be equipped with suicide genes or switches (e.g. iCasp9) allowing ablation of CAR T cells. (D) Left, status of published CAR T cell gene therapy trials or trials registered at clinicaltrials.gov including long-term follow-up studies. The status of one trial is unknown and not listed. The total number of clinical trials (dark blue bars) is compared to published clinical trials (light blue bars). The asterisk indicate zero trials. Right, phases of CAR T cell gene therapy trials. Long-term follow-up studies are not included. For nine trials, the phase classification is unknown. The asterisk indicate zero trials [1]
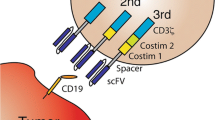
Anti-CD19 CAR T cell products evaluated in pivotal trials in B-cell lymphomas. The intracellular domain of axicabtagene ciloleucel (ZUMA-1 trial) is composed of two signaling domains, CDɜζ and a co-stimulatory domain, CD28. Tisagenlecleucel (JULIET trial) and lisocabtagene maraleucel (TRANSCENT trial) use CD137 (4-1BB) as co-stimulatory domain. The co-stimulatory domain promotes the T-cell activation and persistence of CAR T cells [2]
Anti-CD19 CAR T Cell Therapy in Lymphoma
Axicabtagene ciloleucel (axi-cel) was granted the US Food and Drug Administration (FDA) regular approval in 2017 for the treatment of patients with r/r large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphomas, and DLBCL arising from follicular lymphoma (tFL). The approval was based on the results of a seminal Phase 2 study published by Neelapu et al. [3]. In this multicenter study (ZUMA-1 trial), 111 patients with r/r DLBCL, primary mediastinal B-cell lymphoma (PMBCL), or tFL were included. Patients received a target dose of 2 × 106 CAR19 cells per kilogram of body weight after conditioning regimen of low-dose cyclophosphamide and fludarabine. Axi-cel was administered to 101 patients, with objective response rate (RR) of 82%, and CR rate of 54%. Most CRs were durable, the overall rate of survival at 18 months was 52% [3]. At 27.1 months, the median overall survival (OS) was not reached, and the median progression-free survival (PFS) was 5.9 months [4]. Interestingly, CAR T cell levels during the first month of therapy seem to be associated with efficacy of the product [3, 5].
Tisagenlecleucel (tisa-cel) was approved by the US FDA in 2018 for adult patients with r/r DLBCL and tFL after at least two prior lines of therapy, including anthracycline and rituximab, or relapsing after an autologous stem cell transplant (SCT). Tisa-cel was tested in a Phase 2 multicenter study (JULIET trial) involving adult patients with r/r DLBCL [6]. A total of 93 patients received tisa-cel infusions and were included in the efficacy analysis of JULIET trial. The best overall response rate was 52%, including 40% of patients achieving CR and 12% achieving partial response (PR). At 12 months after the initial response, the rate of relapse-free survival was estimated to be 65% (79% among patients in CR) [6].
In addition to axi-cell and tisa-cel, lisocabtagene maraleucel (liso-cel) has also been studied in a Phase 1 multicenter study (TRANSCEND trial) [7]. The difference between liso-cell and tisa-cell or axi-cel is that liso-cel is a CAR T cell product administered in defined composition at a precise dose of CD8 and CD4 CAR T cells (Fig. 9.1). Adult patients with r/r DLBCL, PMBCL, tFL, or mantle cell lymphoma (MCL) were included and an interim analysis of the Phase 1 of the trial. Results showed that, with a median follow-up of 8 months, 80% of 73 patients treated achieved an objective response, and duration of response was not reached. The frequency of objective response at 6 months was 47% [7]. Main toxicity associated to CAR-T cell therapy includes development of cytokine release syndrome (CRS), neurotoxicity and B-cell aplasia (Table 9.1). For patients experiencing a relapse after an autologous or allogeneic stem cell transplant (SCT), administration of CAR T cell therapy seems to be safe and efficacious [8,9,10].
There is very limited experience with the use of CAR T therapy to treat lymphoma in pediatric and adolescent patients. Recently, Rivers et al. reported 5 pediatric patients (range 12–18 years) with r/r CD19+ NHL (DLBCL, PMBCL, or gray zone B-cell) treated in an ongoing Phase 2 trial [11]. Patients received 1 × 106/Kg CAR19 cells as a 1:1 ratio of CD4 and CD8 cells, following lymphodepletion with fludarabine and cytarabine. One patients had history of auto/allo-SCT (PMBCL), 3 had had received immunotherapy (nivolumab or brentuximab vedotin). Similar to adult patients, the most common side effects were (mild) CRS (n = 4) and (mild) neurotoxicity (N = 2). At 3 weeks, anti-tumor response was observed in 4/5 patients, and 2/3 evaluable subjects were in CR at week 9. One subject had a CD19− progression at week 9, after initial response. One subject obtained CR, but eventually recurred with CD19+ disease despite ongoing CAR-T cell persistence [11].
Mechanisms of CAR T Cell Resistance
Immune Scape from Antigen Loss
A significant proportion of relapses post CAR T cell therapy seem to be associated with immune scape from antigen loss of CD19, but the exact mechanisms of antigen loss in lymphoma therapy have yet to be understood. However, insights into possible mechanisms of antigen loss are being revealed by several studies done in pediatric and adult patients with B-acute lymphoblastic leukemia (ALL) suffering CD19 negative (CD19−) B-ALL relapse after treatment with CAR19 cell therapy. Lack of CD19 expression has been shown to occur due to either mutations, alternative splicing in CD19, or by mutations in the B-cell receptor protein CD81. Those mechanisms are further discussed below.
CD19 Mutation and CD19 Alternative Splicing (exon 2 skipping)
The most known mechanism of CAR19 resistance is the emerging dominance of leukemic cells harboring isoforms of CD19 lacking the transmembrane domain or the targeted exon, under the selective pressure of CAR T cells. Sotillo et al. detected hemizygous deletions within chromosome 16 spanning the CD19 locus and de novo frameshift and missense mutations in exon 2 of CD19 in some relapse samples [12]. The investigators also described alternatively spliced CD19 mRNA species, including one lacking exon 2, and demonstrated that exon 2 skipping bypasses exon 2 mutations in B-ALL cells and allows expression of the N-terminally truncated CD19 variant, which fails to trigger killing by CAR19 [12]. More recently, Fisher et al. analyzed the expression of CD19 isoforms in a cohort of subjects with CD19+ B-ALL [13]. They demonstrated that an alternatively spliced CD19 mRNA isoform lacking exon 2, and therefore the CAR19 epitope, but not isoforms lacking the transmembrane and cytosolic domains were expressed in the leukemia blasts at diagnosis and in the bone marrow of nonleukemia donors, suggesting that some of the CD19 isoforms contributing to CAR19 escape already preexist at diagnosis and could evolve as a dominant clone during CAR19 therapy [13].
Another mechanism of CD19 loss can be due to mutations in other genes that express other proteins of the B-cell receptor complex. To signal with the B-cell receptor, CD19 complexes with CD21, CD81, and CD225. Homozygous mutations in the CD81 gene have been demonstrated to cause congenital immunodeficiency in humans [14]. Braig et al. demonstrated resistance to anti-CD19/CD3 BiTE therapy (blinatumomab) in patients with B-ALL via disrupted CD19 membrane trafficking [15]. At relapse post blinatumomab, patient’s CD19− blasts were surface CD81−, which led to non-CD19 processing and maturation in the Golgi complex [15]. Although not yet demonstrated, it is highly plausible that the similar mechanism play a role in antigen scaping in B-cell lymphomas.
Myeloid Switch
MLL-rearranged CD19+ B-cell ALL are responsive to CAR19 therapy as demonstrated in a cohort of 7 patients who achieved CR after CAR T-cell therapy [16]. However, 2 patients relapsed, both with a myeloid phenotype leukemia approximately 1 months after CAR T cell infusion. One patient had no evidence of disease in the bone marrow after therapy on day 22 by flow cytometry, but karyotyping and FISH studies revealed persistent MLL rearrangement. On day 35, circulating blasts were present and expressed myeloperoxidase, CD4, and CD64 without CD19 or other B-cell lineage antigens, consistent with acute myeloid leukemia (AML). FISH for MLL rearrangement and IGH deep sequencing demonstrated that both B-ALL and AML were clonally related. Second patient was a young child with MLL-rearranged CD19+ B-ALL who relapsed after 30 days of receiving CAR19 therapy with an abnormal myeloid population without B-lineage antigens but persistent presence of MLL rearrangement [16]. CAR19 was detected in blood, and there was B-cell aplasia at the time of AML diagnosis. Deep sequencing of the IGH gene was negative for the rearrangement previously noted in the lymphoid blasts, suggesting myeloid relapse occurred form an immature stem cell clone [16]. Those two cases illustrate that myeloid switch is a mechanism of CAR T-cell resistance, likely due to presence of rearranged MLL, reprogramming or de-differentiation of previously committed B-cell lymphoid blasts (case 1) or myeloid differentiation of a noncommitted precursor or selection of a preexisting myeloid clone after CAR19 therapy (case 2).
Senescence and Exhaustion of CAR-T Cell Population
Yang et al. demonstrated that the presence of T-cell receptor (TCR) antigen can provoke loss in CD8+ CAR T cell efficacy associated with T-cell exhaustion and apoptosis [17]. Using an immunocompetent, syngeneic murine model of CD19-targeted CAR T cell therapy for B-ALL in which the CAR is introduced into T-cells with known TCR specificity, they demonstrated that loss of CD8 CAR T-cell efficacy associated with T-cell exhaustion and apoptosis when TCR antigen is present [17]. Long et at. also demonstrated that tonic CAR CD3-ζ phosphorylation, triggered by antigen-independent clustering of CAR single-chain variable fragments can induce early exhaustion of CAR T-cells that limit antitumor efficacy [18]. Interestingly, CD28 co-stimulation augments, whereas 4-1BB co-stimulation reduces, exhaustion induced by persistent CAR signaling [18].
Accidental Transfection of Tumor Cells and CD19 “Masking”
The manufacturing process of CAR-T cell requires collection of mononuclear cells from peripheral blood by apheresis and several steps to T-cell purification, expansion, and transfection of viral vector carrying the CAR. Absence of circulating tumor cells has not been considered a critical requirement for such therapy given that occasional malignant B-cells collected during apheresis would be selected out, not expanded and/or not transduced during the manufacturing process.
However, Ruella et al. recently described a B-ALL patient treated with CAR19 cell therapy who experienced a CD10+CD19− ALL relapse caused by accidental transfection of tumor cells with CD19 “masking” [19]. Evaluation of the leukemia cells revealed that the B-leukemia cells were CAR-transduced B-cell blasts (CARB) by immunophenotyping, suggesting that malignant B-cells can survive the manufacturing process and be transfected with the lentivirus containing the CAR [19]. Such transfection was not inconsequential and the lack of CD19 expression was not caused by any known mechanism of antigen loss. In fact, CD19 mRNA transcripts were identified at the baseline and at relapse, and CD19 protein expression was also detected by immunohistochemistry. Confocal microscopy demonstrated that colocalization of CAR19 and CD19 on the cell surface of the relapsed leukemia, leading to the hypothesis that the lack of detection of CD19 by flow cytometry was due to CAR10 binding in cis to CD19 on the cell surface and “masking” the epitope detection by flow cytometry. Antigen masking by transduction of B-ALL with CAR was demonstrated in vitro to be also possible during the manufacturing process of anti-CD22 CAR-T cell products [19]. CARB would initially be a minute fraction of the disease burden and not interfere with response at first. Over time, however, it gives rise to a resistant subclone manifesting clinically as leukemia relapse. So far, this mechanism of resistance has only been described in B-ALL and it seems to be a rare phenomenon, but it remains at least hypothetically possible in aggressive lymphomas as well.
Future Directions
One possible approach to overcome immune scape from antigen loss is the simultaneous or sequential targeting of more than one B-cell specific antigen. In fact, CD22-targeting CAR T cells are effective in r/r B-ALL and have recently been proven active in patients with relapse after anti-CD19 CAR T cell therapy [20]. Failure to anti-CD22 CAR T appears linked to the decrease in antigen density, rather than modification of the CD22 molecule [20]. The concomitant targeting of both CD19 and CD22 can potentially reduce the risk of relapse given that it would be unlikely that a single cell would develop simultaneous mechanisms of scape for both targets. Clinical trials are currently being performed with CAR T cell products targeting both CD19 and CD22.
Another possible way to overcome resistance to CAR T cell therapy would be trying to revert tumor-induced immunosuppression and immune exhaustion using immune checkpoint inhibitors. Chong et al. recently reported a case of a patient with DLBCL treated with PD-1 blocking antibody after progression post CAR19 therapy [21]. Overexpression of PD-L1 has been demonstrated in relapsed DLBCL samples post CAR19. Following PD-1 blockade with pembrolizumab, the patient had a clinically significant response and expansion of CART19 cells. Currently the use of pembrolizumab is being tested in a clinical trial setting in patients with CD19+ lymphomas who failed post CAR19 therapy.
Abbreviations
- ALL:
-
Acute Lymphoblastic Leukemia
- AML:
-
Acute Myeloid Leukemia
- Axi-cel:
-
Axicabtagene Ciloleucel
- CAN:
-
Copy-number Alteration
- CAR:
-
Chimeric Antigen Receptor
- CARB:
-
CAR-transduced B-cell leukemia
- CR:
-
Complete Response
- CRS:
-
Cytokine Release Syndrome
- DLBCL:
-
Diffuse Large B-Cell Lymphoma
- Liso-cel:
-
Lisocabtagene Maraleucel
- LOH:
-
Loss Of Heterozygosity
- MCL:
-
Mantle Cell Lymphoma
- OS:
-
Overall Survival
- PFS:
-
Progression-Free Survival
- PMBCL:
-
Primary Mediastinal B-Cell Lymphoma
- PR:
-
Partial Response
- r/r:
-
Relapsed/refractory
- RR:
-
Response Rate
- SCT:
-
Stem Cell Transplant
- TCR:
-
T-Cell Receptor
- tFL:
-
DLBCL arising from Follicular Lymphoma
- Tisa-cel:
-
Tisagenlecleucel
- WES:
-
Whole-genome sequencing
References
Hartmann J, Schussler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9(9):1183–97.
Strati P, Neelapu SS. Chimeric antigen receptor-engineered T cell therapy in lymphoma. Curr Oncol Rep. 2019;21(5):38.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42.
Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
Abramson JS, Gordon LI, Palomba ML, Lunning MA, Arnason JE, Forero-Torres A, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol. 2018;36(15_suppl):7505.
Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016;127(24):2980–90.
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–39.
Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23(2):242–9.
Rivers J, Annesley C, Summers C, Finney O, Pulsipher M, Wayne A, et al. Early response data for pediatric patients wiht non-Hodgkin lymphoma treated with CD19 chimeric antigen receptor (CAR) T-cells. Br J Haematol. 2018;182(Suppl. 1). (23):Abstract 29
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–95.
Fischer J, Paret C, El Malki K, Alt F, Wingerter A, Neu MA, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187–95.
van Zelm MC, Smet J, Adams B, Mascart F, Schandene L, Janssen F, et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest. 2010;120(4):1265–74.
Braig F, Brandt A, Goebeler M, Tony HP, Kurze AK, Nollau P, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129(1):100–4.
Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406–10.
Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. 2017;9(417):eaag1209.
Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–90.
Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24(10):1499–503.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8.
Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039–41.
Acknowledgements
The authors would like to thank Erin Morris, RN in her assistance in the preparation of this manuscript.
Disclosure of Conflict of Interest
L.J.M has received research support from Celgene, Janssen, Amgen, and GlaxoSmithKline; honorarium from Celgene, Amgen, Karyopharm, GlaxoSmithKline, and Kite; and speaker fees from Amgen and Sanofi. A.C.X has no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Xavier, A.C., Costa, L.J. (2019). Resistance to Chimeric Antigen Receptor T-Cell Therapy. In: Xavier, A., Cairo, M. (eds) Resistance to Targeted Therapies in Lymphomas . Resistance to Targeted Anti-Cancer Therapeutics, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-030-24424-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-24424-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-24423-1
Online ISBN: 978-3-030-24424-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)