Abstract
The molecular chaperone HSP90 (heat shock protein 90) has become a crucial target in cancer therapeutics as its function has been implicated with various types of malignant transformation. Numerous HSP90 inhibitors have been identified so far and many of them have also been clinically tested. Although most of these are natural or their derived inhibitors including geldanamycin and its derivatives 17-AAG and 17-DMAG have shown efficacy, their easy success has been hindered in various stages of the clinical studies due to poor solubility and cytotoxicity. However, recently substantial published documents reported that the systemic targeting of the HSP90 inhibitors using nano-based drug delivery system could provide a possible clinical solution to overcome their limitation. In this chapter, we review the initial development of various HSP90 inhibitors from natural to synthetically derived one and their clinical studies. We also review their limitations and future perspectives as a possible potential agent in the cancer therapeutics by their systemic and control delivery to the target site using the nano-drug delivery system. Also, the application of combined therapy has also been discussed in the current chapter using HSP90 inhibitors and nanocarrier. In addition, we also discuss the therapeutic approaches like photothermal where nano carrier is not only used as a carrier for the systemic delivery of HSP90 inhibitors but also as a therapeutic agent.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
According to WHO, cancer is the second most leading cause of death which counts nearly one out of six globally and about ten million new cases are reported every year (Siegel et al. 2017). Among the major therapeutic techniques practiced for the treatment of cancer, chemotherapy and radiation are most common. The genetic vulnerability or instability of cancer cells along with the presence of cellular machinery such as molecular chaperones (heat shock proteins) are responsible to protect them against the initial treatment (Hölzel et al. 2013; Young et al. 2004). For example, when the cancer cells are treated with chemotherapeutic drugs that causes some initial damage but due to the genetic plasticity nature of cancer cells, cancer cells protect themselves by the mutation of the targeted receptor against the chemotherapeutic drugs (Meacham and Morrison 2013) and thus accruing multi-drug resistance (MDR) (Markman et al. 2013; Parveen and Sahoo 2008). Similarly, molecular chaperones, known as heat shock proteins, also help cancer cells from environmental stress due to various therapies such as elevated temperature, oxidative damage caused by photothermal therapy, and hypoxia (caused by low oxygen due to rapid metabolism) (Young et al. 2004). When cancer cells are exposed to environmental stress like elevated temperature and hypoxia, cells induce the synthesis of high amount of heat shock proteins (HSP) that play an important role in regulating and guiding of proteins to their correct structural conformation, which in turn protect cancer cells from various modes of therapy such as photothermal and chemotherapy (Meacham and Morrison 2013). Since most of the client proteins of HSP90 are oncoproteins, such phenomena plays an important role in cancer transformation and progression.
Further, the advancement in the molecular genetics and genomics, more specialized and personalized methods for cancer treatment such as gene therapy, hormone therapy, and immunotherapy have been developed with enormous specificity with reduced systemic toxicity. However, they also have suffered a similar fate due to the genetic instability and vulnerability nature of cancer cells that may cause mutation of the targeted gene or receptor and becoming hormone-independent. So, the two important key factors which play an important role in such adoption are genetic vulnerability nature of cancer cells and molecular chaperones (HSP). Hence, this two factors can be targeted for efficient therapeutic strategy (Audisio et al. 2011; Bagatell and Whitesell 2004). Therefore, one of the recommended way for the treatment of cancer is to target HSP90 that helps cancer cells to adapt such applied environmental stress derived from various therapies like photothermal therapy (Neckers 2002). Further, HSP also function as biochemical buffers to endure numerous genetic alterations present within tumor cells that would otherwise be fatal and also allows mutant proteins to retain or even gain function under such imbalanced signaling condition (Bagatell and Whitesell 2004). Therefore, HSP can be used as therapeutic target for efficient cancer treatment as its expression accounts for the cancer cell’s ability to maintain its protein homeostasis even under the hostile conditions such as hypoxia and acidic microenvironment (Pearl and Prodromou 2006; Solit and Chiosis 2008). In mammalian cells, HSP90 is predominant and widespread among other molecular chaperones and also play key role in folding, stability, transportation of various oncoproteins (Neckers 2007; Pearl and Prodromou 2006). Therefore, over the decades, HSP90 has become a main molecular target for the efficient cancer treatment by developing small molecule HSP90 inhibitors, thereby triggering the proteasomal degradation of their oncogenic ‘client’ proteins for the cancer treatment (Mahalingam et al. 2009).
1.1 HSP 90 as Target Molecule for Cancer Treatment
Mainly four different types of mammalian HSP have been classified, primarily based on their molecular weight: HSP90, HSP70, HSP60 and small HSP (15–30 kDa). Among them, HSP90 is the most abundant molecular chaperones found in the eukaryotes complexes with huge number of proteins regarded as ‘clients’ whose folding, stability, transportation is regulated by HSP90. Interestingly, many of its clients are oncoproteins which is why HSP90 plays an important role in the cancer development and has been regarded as a target molecule for the therapy in cancer (Trepel et al. 2010). The Schematic representation of HSP90 cycle and the effects of HSP90 inhibition for its client oncoprotein degradation for cancer therapy were shown in the Fig. 8.1. The synthesis of new client proteins begins with the association of HSP40 and HSP70 through forming HSP70–HSP40 chaperone complex. Then the client proteins are be delivered to the ADP-bound “open” form of HSP90 via the TPR-containing co-chaperone HSP70-HSP90 organizing protein (HOP). The role of HOP in the HSP70–HSP40 chaperone complex provides the reversible link between HSP70 and HSP90 through the MEEVD peptide which allows the transfer of client proteins to HSP90. The binding of client proteins leads to conformational changes conforming the ATP-bound “closed” state of HSP90 with the association of the co-chaperone p23. Then kinases are delivered to HSP90 by an alternative method using a complex with the co-chaperone cell-division cycle 37 homolog (Cdc37). This interaction between HSP90 and other co-chaperone induces conformational changes in client proteins that allow their maturation and activation. The binding of the co-chaperone Activator of HSP90 ATPase 1 (Aha1) to the HSP90 causes hydrolysis of ATP that further leads to the release of mature and active client protein. However, inhibition of HSP90 by small-molecule inhibitors acting at the N-terminal nucleotide site blocks ATP binding and subsequent hydrolysis. The binding of the small-molecule inhibitors at the N-terminal of HSP90 leads to the blocking of ATP binding to HSP90 that causes the termination of the chaperone cycle and degradation of the client proteins by ubiquitin-dependent proteasomal degradation. Thus, there is a gaining interest in the clinical development of HSP90 inhibitors from identification of new HSP90 inhibitors and their modification for the optimization of the pharmaceutical properties including pharmacokinetic, pharmacodynamic and toxicological. Over a decade, considerable progress has happened on many fronts such as introduction of chemically distinct HSP90 inhibitors with improved pharmaceutical properties and the preparation of improved formulations making them potentially a the first-in-class agent for cancer treatment. The development of HSP90 inhibitors from natural to synthetic one and the new nanostructure-based formulations are also discussed further.
Schematic representation of the role of HSP90 in client protein synthesis and its inhibition by small HSP 90 inhibitors molecules (Butler et al. 2015)
1.2 First Generation HSP90 Inhibitor: Natural Inhibitor
1.2.1 N-Terminal HSP90 Natural Inhibitor
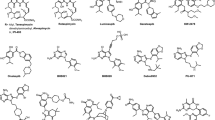
The discovery of geldanamycin (GM) as an HSP90 inhibitor has sparked an interest in both industry and academic sectors for developing small molecule-based HSP90 inhibitors with clinical viability as an effective strategy for the cancer treatment (Patel et al. 2011). More often, natural product/compounds play a vital role during drug discovery. In the case of HSP90, natural products geldanamycin (GA) and radicicol (RD) (Fig. 8.2), mimics the structure which is adopted by ATP in the N-terminal nucleotide-binding pocket of HSP90 (Sidera and Patsavoudi 2014). Thus, the selective binding of both GA and RD to the N-terminal nucleotide-binding pocket of HSP90 eventually leads to inhibition of the intrinsic activities of HSP90 by preventing the ATP binding. This fact increases the recruitment of ubiquitin ligase to HSP90 chaperone complex and induces the depletion of oncogenic HSP90 client proteins via the proteasome degradation of these client proteins (Mimnaugh et al. 1996; Roe et al. 1999; Soga et al. 2013).
Chemical structures of the natural HSP90 inhibitors (Li et al. 2009)
1.2.2 C-Terminal HSP90 Inhibitor
Novobiocin and coumermycin A are two important naturally occurring C-terminal HSP90 inhibitor (Fig. 8.2). Novobiocin is an amino-coumarin containing antibiotic which was isolated from Staphylococcus saprophyticus. It binds to the ATP-binding site present in the C-terminal domain of HSP90 (Byrd et al. 2016). Thus, the binding of novobiocin to HSP90 lead to the disturbance of the interaction of between HSP90 complex and it’s both co-chaperones namely p23 and HSP70, thereby the destabilization of HSP90−client protein interactions. Another related coumarin antibiotics as the C-terminal HSP-90 inhibitor, Coumermycin A1 have shown to disrupt C-terminal dimerization and halt the conformational cycle (Allan et al. 2006). Like Novobiocin, Coumermycin A1 also have been shown to disrupt interactions of partner co-chaperones that bind HSP90 at both the C- and N-terminus, i.e., Cdc37, p23, Hsc70, FKBP52, and PP5, thus destabilization of HSP90−client protein complex (Allan et al. 2006; Marcu et al. 2000).
Recently, natural inhibitors like isoflavone derrubone and epigallocatechin 3-gallate (EGCG) have also been isolated and tested (Fig. 8.2). Isoflavone derrubone was isolated from the Indian tree Derris robusta and which exhibits its ability to inhibit the interaction of HSP90 and Cdc37 with heme-regulated eIF2α kinase (HRI), an HSP90 client kinase, and also showed anticancer potential in human breast cancer cell lines (Hadden et al. 2007). Similarly, catechin, epigallocatechin 3-gallate (EGCG) was isolated from a green tea polyphenol that also shows its ability to inhibit the transcriptional activity of aryl hydrocarbon receptor (AhR) through binding to the C-terminus of HSP90 (Palermo et al. 2005). The discovery of EGCG has lead to the development of novel HSP90 inhibitors by providing scaffolds for the design of new molecules.
1.3 Second Generation HSP90 Inhibitor: Derivatives or Analog of Natural HSP90 Inhibitor
Despite the potential anti-tumor activity of natural HSP90 inhibitors GA and RD, the poor “drug-like properties, limited in vivo stability, poor solubility and significant hepatotoxicity limits their clinical application (Ge et al. 2006; Haque et al. 2016; Wang et al. 2015). Although both GA and RD proved to have poor solubility, they are too toxic and unstable for clinical applications, each of which have a profound effect on the development of new HSP90 inhibitors using them as a lead compound.
1.3.1 Analogues of GA
1.3.1.1 17-Allylamino-17-Demethoxygeldanamycin (17-AAG)
In order to overcome these limitations of GA, new analogues or derivatives were synthesized using the structure of GA by chemical modifications of position 17, which are known as 17-allylamino-17-demethoxygeldanamycin (17-AAG) and 17-(2 dimethyl aminoethyl) amino-17-demethoxygeldanamycin (17-DMAG) (Fig. 8.3) (Hollingshead et al. 2005; Nimmanapalli et al. 2001). 17-AAG is the first HSP90 inhibitor tested for clinical trials as an antineoplastic drug due to its significant anti-cancer potential. However, the approval of 17-AAG was also hindered due to its poor solubility and bioavailability as well as hepatotoxicity and nephrotoxicity effects (Jhaveri et al. 2014). Further, the development of 17-AAG faces several problems like production/formulation issues as well as patent expiry concerns (http://www.myelomabeacon.com/news/2010/07/22/ tanespimycin-development-haulted/).
Chemical structures of the N terminal GA analogs HSP90 inhibitors (Sidera and Patsavoudi 2014)
1.3.1.2 17-(2-Dimethylaminoethyl)amino-17-Demethoxygeldanamycin (17-DMAG)
Another GA analogue entered into clinical trials was 17-(2-dimethylaminoethyl)amino-17-demethoxygeldanamycin (17-DMAG) in 2005(http://clinicaltrials.gov/ct2/show/NCT00088868). However, 17-DMAG showed very interesting results such as better water solubility, oral bioavailability and greater anticancer activity compared to 17-AAG due to the presence of an ionizable amino group as a result of the substitution of the C-17 methoxy group of GM with N, N-dimethylethylamine (Messaoudi et al. 2008). However, Kosan discontinued 17-DMAG development due to based on several factors, including the strength of intellectual property protection and risk, clinical experience to date, and time to commercialization compared to 17-AAG.
1.3.1.3 IPI-504 [17-Allylamino-17-Demethoxygeldanamycin Hydroquinone Hydrochloride]
Infinity Pharmaceuticals developed a water-soluble HSP90 inhibitor, IPI-504 (retaspimycin) which is a reduced and hydroquinone hydrochloride salt derivative of 17-AAG (Fig. 8.3) (Sydor et al. 2006). The 17-AAG was reduced using sodium dithionite, followed by conversion to the preparation of IPI-504. Actually, both 17-AAG and IPI-504 co-exist in redox equilibrium in vivo as the hydroquinone forms for IPI-504 and quinine forms for 17-AAG prepared through the oxidoreductases action. Since, the hepatotoxicity of GM is primarily due to the presence of the quinone ring, as in IPI-504, the hydroquinone group is in the reduced form might results in reduced toxicity. The intravenous administration of IPI-504 has reached to Phase II and III clinical trials (http://clinicaltrials.gov/ct2/show/NCT00817362, and http://clinicaltrials.gov/ct2/show/ NCT00688766). However, since IPI-504 and 17-AAG are interconvertible in vivo condition due to the action of the oxidoreductases action, its phase III trial of 17-AAG was terminated (Sydor et al. 2006).
1.3.1.4 IPI-493 (17-Desmethoxy-17-Amino Geldanamycin)
The Infinity Pharmaceuticals was also developed an orally administrated HSP90 inhibitor17-AG (17-amino-17-demethoxygeldanamycin; IP-493) which is the primary active and long-lived metabolite of IPI-504 and 17-AAG (Fig. 8.3). Although IPI-493 reached Phase I studies, its clinical study was suffered due to its poor pharmaceutical properties such as low solubility and the difficulty of establishing pharmacologically relevant doses of administration (http://clinicaltrials.gov/ct2/show /NCT00724425?term=IPI-493&rank=1). Moreover, due to better clinical relevant properties of IPI-504, the development of IPI-493 agent was stopped and focus was turned exclusively on retaspimycin. Till date, IPI-504 is the most active and clinically feasible GA-derived-HSP90 inhibitor. The analog of ansamycin with devoid of quinone/hydroquinone component was obtained by ‘muta-synthesis’ and was developed by Kosan Biosciences and displayed comparable activities to the IPI-504 in vitro condition but better efficacy in a mouse xenograft model (Menzella et al. 2009). A genetically engineered microorganism ‘Actinosynemma pretiosum Biotica technology was able to secrete the natural HSP90 inhibitor ‘macbecin’ (Fig. 8.3) (Martin et al. 2008; Tanida et al. 1980). Further, non-quinone analogue of macbecin was developed (Fig. 8.3) with high binding affinity with Kd of 3 nM while the Kd of macbecin was 240 nM) and it was also found to be less toxic than 17- AAG, as it lacks the toxic benzoquinone pharmacophore group with a maximum tolerated dose (MTD) greater than 250 mg/kg.
1.3.2 Analogues of RD
The resorcinol core of RD is the critical element for its binding to HSP90. However, In addition to a resorcinol moiety (Fig. 8.4), RD also contains unstable and reactive epoxide and α,β,γ,δ-unsaturated carbonyl groups which makes its unstable in serum and devoid under in vivo condition. However, based on the resorcinol core of RD, a number of molecules have been developed and entered into clinical trials like NVP-AUY922, KW-2478, and AT13387 as well as STA-9090.
Chemical structures of the N terminal RD analogs HSP90 inhibitors (Jhaveri et al. 2014)
1.3.2.1 NVP-AUY922/VER52296
Workman and colleagues from the Cancer Research UK Center for Cancer Therapeutics, first identified the resorcinol containing pyrazole CCT018159 from an HTS of a library of 56,000 compounds capable of inhibiting the ATPase activity of yeast HSP90 using the malachite green detection of inorganic phosphate (Cheung et al. 2005; Rowlands et al. 2004). They used a structure-based approach and optimized the isoxazole NVP-AUY922/VER52296 as a potent HSP90 inhibitor (Fig. 8.4) (Gaspar et al. 2010; Jensen et al. 2008). Subsequently, this led a candidate, now is being developed by Novartis and, is currently in phase I/II clinical trials for patients with refractory multiple myeloma and HER2 + and ER + metastatic breast cancer as a single agent as well as in combination therapy. From the Phase I trial results in advanced solid tumors, it showed that the maximum tolerated dose (MTD) of weekly was 70 mg/m2 (Samuel et al. 2010). Further, Dose-limiting toxicities (DLTs) of NVP-AUY922/VER52296 included darkening of vision, atrial flutter, diarrhea, and fatigue. However, around 20% of the patients tested developed night blindness with a grade 3 or higher eye disorders at the MTD. Also, 16 patients have been found to develope SD and 9 had a partial metabolic response fluorodeoxyglucose positron emission tomography (FDG-PET) scans (Samuel et al. 2010). A Phase II expansion trial of NVP-AUY922/VER52296 in HER2-positive and ER + metastatic breast cancer also have been reported two PD responses on FDG-PET (Schroder et al. 2011).
1.3.2.2 AT13387
AT13387 was discovered by Astex Pharmaceuticals through a fragment-based drug discovery approach for the optimization of a resorcinol-containing group having a high HSP90 binding affinity (Fig. 8.4) (Murray et al. 2010; Woodhead et al. 2010). This fragment screening consisted of a combination of nuclear magnetic resonance and high-throughput X-ray crystallography. One of the fragments have been identified with a phenolic chemotype and selected, which was further optimized via structure-guided design to obtain AT-13387 (Murray et al. 2010; Woodhead et al. 2010).
AT13387 was evaluated in Phase I trials in patients with advanced solid tumors. The various dosing schedules were studied which includes weekly or bi-weekly intravenous infusions for a period of 3 weeks in a 28-day cycle (Do et al. 2012; Jhaveri et al. 2012; Mahadevan et al. 2012). A dosage of 260 mg/m2 has been identified MTD for the weekly study (Mahadevan et al. 2012). It is currently in three Phase I/II trials under evaluation in either alone or in combination with: (i) abiraterone in the treatment of CRPC which is no longer responding to abiraterone (NCT01685268); (ii) crizotinib in the treatment of NSCLC (NCT01712217); and (iii) imatinib in patients with unresectable and/or metastatic GIST whose tumor has progressed treatment with a maximum of three TKIs (NCT01294202).
1.3.2.3 Ganetespib (STA-9090)
STA-9090 (Fig. 8.3) is a novel resorcinol HSP90 inhibitor containing triazole developed by Synta Pharmaceuticals (Ying et al. 2011). Goldman et al. have evaluated phase I trial of STA-9090 in 35 patients having advanced solid tumors with an intravenously received the STA-9090 weekly for 3 weeks in a 28-day cycle. It was determined that STA-9090 of MTD was 216 mg/m2. Dose-limiting toxicity (DLT) of STA-9090 was found as fatigue, diarrhea, and elevated amylase. The other common adverse side effects were anemia, abdominal pain, dyspnea, constipation, and nausea. It was also noticed one partial response (PR) in a patient with rectal cancer and several stable diseases (SD) suffering from gastrointestinal non-small cell lung cancer, stromal tumor, and renal cell carcinoma (Goldman et al. 2010). Another phase I clinical trials were evaluated for ganetespib in advanced solid malignancies using different dosing schedules intravenously such as weekly for 3 weeks or twice-weekly dosing for 3 weeks in a 28-day cycle. DLT of STA-9090 included grade 3 diarrhea, grade 3 amylase elevations as well as grade 3 and 4 asthenia. PR was noticed in one patient with metastatic colorectal cancer whereas SD was observed in 23 patients. Disease control rate was 24.5% of the patients (Goldman et al. 2010). For the twice-weekly dosing trial of STA-9090, DLT of elevated liver enzymes was observed. Ganetespib (STA-9090) was tolerated up to the dosage of 120 mg/m2; the dose cohort has been expanded to 144 mg/m2 and dose escalation is ongoing (Cho et al. 2011).
A safety and efficacy of phase I/II study for STA-9090 was conducted in patients suffering from acute myeloid leukemia and other hematological malignancies and evaluated with a dosage schedule of weekly infusions for four consecutive weeks per cycle at three dose levels (120–200 mg/m2). DLT of elevated liver enzymes was noticed in one patient. There were no adverse responses observed, but one patient with refractory acute myeloid leukemia had developed SD. The recommended phase II dose has not been identified and study is ongoing (Lancet et al. 2010). Moreover, The combination of ganetespib with chemotherapeutic agents such as doxorubicin and taxanes have shown enhanced cytotoxic effects (Proia et al. 2013). The evaluation of the combination of ganetespib and weekly paclitaxel was planned in the ENCHANT-1 trial. Another trial of ganetespib and docetaxel in Triple-negative breast cancer (TNBC) was planned by Synta Pharmaceuticals. Phase I clinical trial for a combination of ganetespib, trastuzumab and paclitaxel is ongoing in HER2-positive trastuzumab-refractory metastatic breast cancer (MBC) (NCT02060253). Further, Patients with Gastrointestinal Stromal Tumors (GIST) who had failed previous treatment with imatinib and sunitinib, were also treated with single-agent ganetespib with a dosing schedule of weekly for 3 weeks in a 28-day cycle (Demetri et al. 2011). Correlatives included HSP90 client proteins and positron emission tomography (PET) imaging using biopsies both pre- and post-treatment. The primary endpoint was the Clinical Benefit Rate (CBR) (CR + PR + SD >16 weeks). In this study, 12 SD was noticed in 23 patients in this Simons 2-stage model, while 7 out of the 123 patients reported with a >20% decrease in the standardized uptake value (SUV) which measured using PET imaging. These data, therefore, suggested that once-weekly treatment with ganetespib might not be optimal in patients with Gastrointestinal Stromal Tumors (GIST) and therefore accumulation is limited to patients with platelet-derived growth factor receptor alpha (PDGFRA) mutations to allow evaluation of other dosage schedules and combinations.
1.3.2.4 KW-2478
KW-2478 is one of the important analogs of resorcinol that was discovered by Kyowa Hakko Kirin Pharma using a binding assay where HSP90 molecules were fixed onto a plate and the compounds were added to identify competitive inhibitors of a labeled RD (Fig. 8.4). The endeavors resulted in the identification of competitive inhibitors of RD which was eventually given the clinically fiscal candidature through evaluation of X-ray crystallography, cell-based screening, and in vivo models (Cavenagh et al. 2008; Nakashima et al. 2010). A phase I study of KW-2478, in patients was completed in patients with relapsed/refractory multiple myeloma, chronic lymphocytic leukemia or B-cell non-Hodgkin’s lymphoma. KW-2478 (14–99 mg/m2) was administered with an intravenously dosing schedule of daily on days 1–5 of a 14-day cycle. There were no DLTs observed in the patients up to a dosage of 99 mg/m2. Drug-related toxicities included grade 1/2 hypertension in one patient and grade 3 QTc prolongation in another. A combination phase I/II trial for KW-2478 and bortezomib was evaluated with administered on days 1, 4, 8, 11 of a 21-day cycle in patients with relapsed and refractory multiple myeloma (Baylon et al. 2013; Jhaveri et al. 2012). In the Phase I trial, a total of 95 patients were involved, while there were 80 patients in the Phase II trial. The common side effects included diarrhea, nausea, vomiting, fatigue and peripheral neuropathy. Also, grade 4 thrombocytopenia was noticed in five patients and grade 4 neutropenia was observed in three patients. The induction of HSP70 was also observed in the all peripheral blood mononuclear cells (PBMC) patients during Phase I trial. Overall response rate (ORR) was found to be 39% and progression-free survival (PFS) was 26.4 weeks. ORR was 48% in the bortezomib-naive patients (n = 50) (Baylon et al. 2013).
1.3.3 Purine and Purine-Like Analogs
The rational design of HSP90 inhibitors was made possible with the availability of the X-ray crystal structures of ATP/ADP as well as GM and RD bound N-terminal nucleotide-binding domain of HSP90. The GHKL ATPase protein family like HSP90 has a unique and distinguishing Bergerat fold among most other ATPases which results in a distinctive conformation adaptation by a bound nucleotide that enables the selective inhibition of HSP90 by small-molecules over other ATP/ADP binding proteins (Chène 2002). Based on the unique fold of HSP90 as well as a structure-based approach, Chiosis et al., have rationally designed the first reported synthetic HSP90 inhibitor, PU3 (Fig. 8.5, 1 entry), using the co-crystal structures of HSP90 with its ligands such as GM, RD and ATP/ADP (Chiosis et al. 2001). The purine-linker-aryl is an essential motif found in PU3 which was used as a prototype, (Fig. 8.4). By incorporating various functional groups, potent and selective HSP 90 inhibitors candidates with favorable pharmaceutical properties were designed. Among various derivatives of PU3, purines like CNF2024/BIIB021, EC144, MPC-3100, MPC-0767, and PU-H71 as well as the purine-like Debio 0932 (CUDC-305) have advanced to clinical level.
Chemical structures of the purine-scaffold analogs HSP90 inhibitors (Sidera and Patsavoudi 2014)
1.3.3.1 CNF 2024/BIIB021
CNF 2024/BIIB021 was discovered and first developed by Conforma Therapeutics and later by Biogen Idec (Fig. 8.5) (Zhang et al. 2010). CNF2024/BIIB021 is unique amongst other members in this class in that the aryl moiety is attached to the 9-position of the purine (Kasibhatla et al. 2007). Although, CNF 2024/BIIB021 agent was evaluated in phase I and II clinical trials. But the clinical development of CNF 2024/BIIB021 was halted thereafter (Mitchell 2011).
1.3.3.2 5-(2-Amino-4-Chloro-7-((4-Methoxy-3,5-Dimethylpyridine-2-yl)Methyl)-7H-Pyrrolo[2,3-d]Pyrimidin-5-yl)-2-Methylpent-4-yn-2-ol) [EC 144]
The same company, Biogen Idec also disclosed a series of pyrrolo [2,3-d]pyrimidine (deazapurine) analogs among them, EC144 (Fig. 8.5) proved to have the best combination of pharmacokinetic properties and efficacy in murine cancer models (Shi et al. 2012; Sidera and Patsavoudi 2014). Daiichi Sankyo Inc. also prepared another compound pyrazolo [3,4- d] pyrimidine-based on the tricyclic structure as a potential HSP90 inhibitor (i.e., Fig. 8.5, entry 4) for different cancer cell lines with an IC50 value around the nanomolar range (Ohsuki et al. 2012).
1.3.3.3 MPC-3100 and MPC-0767
MPC-3100 (Fig. 8.5) developed for finding HSP90 inhibitor using the purine-scaffold by Myrexis Inc. (Samlowski et al. 2011). MPC-3100 was evaluated in a Phase I trial for 21 days with a week off in a 28-day cycle in 26 patients with recurrent or refractory cancer. Patients received either dosage of 50,100, 165, 245 or 340 mg/m2 daily for 21 days with a week off in a 28-day cycle or with daily continuously dosage for 28 days of 240 or 320 mg every 12 h. The most common adverse effects developed were nausea, grade 1 or 2 diarrhea, vomiting and fatigue (http://clinicaltrials.gov/show/ NCT00920205). The DLT was supraventricular tachycardia at a dose of 245 mg/m2. Whereas, patients treated total daily doses >600 mg/day developed Grade 1–3 gastrointestinal and grade 1/2 visual adverse events (Samlowski et al. 2011). Moreover, due to the poor solubility and bioavailability of MPC-3100, Myrexis also developed novel L-alanine ester pro-drug of MPC-3100, MPC-0767 with the planned introduction of a pro-drug, MPC-0767 with improved aqueous solubility compared to MPC-3100 and equivalent tumor regressions efficacy. The Company submitted an IND on MPC-0767 in the first quarter of 2012 (http://www.myriadpharma.com/component/content/article/2-product-pipeline/23-HSP90-inhibitorprogram).
1.3.3.4 Debio 0932 (CUDC-305)
Scientists at Curis designed the Debio 0932 (CUDC-305) (Fig. 8.5) by replacing the N3 of the purine with a carbon and the iodine by dimethylamine (Bao et al. 2009; Isambert et al. 2012; Taldone et al. 2013). In 2010, Debiopharm initiated the Phase I trial of Debio 0932 in patients with solid tumors or lymphoma (Isambert et al. 2012) (http://clinicaltrials.gov/ct2/show/NCT01168752). Debio 0932 was administered orally with a dosage of 5–100 mg in both daily and every other day regimens with a total of 80 patients. The drug was well tolerated at doses up to 1600 mg every 2 days and 1000 mg/day. The most common adverse side effects of the study were diarrhea, asthenia and decreased appetite with no ocular or cardiotoxicity being reported. One DLT of febrile neutropenia was reported in the every-other-day dosing study where second DLTs of asthenia and diarrhea were observed with the daily dosing study. However, Debio 0932 showed promising activity with partial response (PR) study in two patients, one patient with KRAS-mutant lung cancer and the other patient with breast cancer (Isambert et al. 2012). Further, the daily dosing cohort study has been expanded to a Phase I/II trial of Debio-0932 in combination with standard-of-care in the first- and second-line treatment of non-small cell lung cancer (NCT01714037).
1.3.3.5 PU-H71
PU-H71 is a purine-based compound discovered at Memorial Sloan-Kettering cancer center, developed by Samus therapeutics and NCI (Fig. 8.5) (Marubayashi et al. 2010; Mitchell 2011; Taldone and Chiosis 2009). A unique feature of PU-H71 is the presence of endogenous iodine atom (127I) that has been replaced with the PET radionuclide 124I which results as an imaging agent 124I-PUH71 (He et al. 2006). Currently, PU-H71 has been evaluated in phase I clinical trial in patients with advanced malignancies and under the license to Samus Therapeutics (http://clinicaltrials.gov/ct2/show/NCT01393509). Recently, Memorial Sloan-Kettering cancer centre has also announced to conduct a pilot study in cancer patients for PET imaging using I-PUH71 (http://clinicaltrials.gov/ct2/show/NCT01269593). We have summerized few important HSP90 inhibitors and their clinical study details in Table 8.1.
2 Conclusions
Although numerous natural HSP90 inhibitors, as well as their analogues, have been identified with significant anti-tumor activity (as an antineoplastic drug), they failed in clinical trials due to their poor water solubility and poor bioavailability as well as cytotoxic effects such as hepatotoxicity and nephrotoxicity (Petersen et al. 2018). These problems are the main hindrance for the potential application of HSP90 inhibitors as an anti-tumor drug development in cancer. Recently, to overcome such problems, nano-delivery system has also been introduced to allow better drug delivery to the target site by eliminating unwanted toxic effects (Lamotte et al. 2017). In the area of nanomedicine, nano-sized drug carriers can offer several pharmacokinetic advantages compared to conventional methods such as controlled release of drugs leading to an improved bioavailability, biodistribution, therapeutic index, apparent solubility of drugs, prolonged activity and significantly less adverse effects (Lamotte et al. 2017). Recently, the self-assembled biodegradable nanoparticles of recombinant human gelatin (GHG) modified with alpha-tocopheryl succinate (rHG-TOS) have efficiently been encapsulated and delivered 17-AAG, the HSP90 inhibitor in cancer which indeed enhanced the anticancer effect in the tumor model with better biocompatibility (Won et al. 2011). In another study, a lipophilic GA prodrug was prepared and incorporated into methoxy-capped poly(ethylene glycol)-block-poly(ε-caprolactone) (mPEG-b-PCL) micelles to reduce the toxicity of GA. This formulation showed very promising results as the distribution volume of lipophilic GA prodrug in mice was decreased significantly and also the area under curve (AUC) was enhanced significantly, compared to the free drug. Moreover, lipophilic GA prodrug- mPEG-b-PCL conjugate systems also exhibited a sustained release of the drug and reduced systemic toxicity with high efficacy against MCF-7 breast cancer cells (Xiong et al. 2008). Sauvage et al. (2016) have successfully applied a strategy for a C-terminal inhibitor of HSP90, 6BrCaQ for its systemic delivery to the target site (Sauvage et al. 2016). As 6BrCaQ is a potential inhibition of HSP90 functions via degradation of its client proteins but its application is limited due to low solubility under in vivo conditions (Audisio et al. 2011). Hence, the liposomal nano-encapsulation of 6BrCaQ Sauvage et al. (2016) increased its solubility and induced apoptosis (caspase 3 cleavage) and cell cycle arrest (G2/M) in prostate cancer PC-3 cells. Further, the preliminary in vivo studies of this formulation have shown encouraging anti-cancer potential in the breast cancer nude mice model (unpublished data). The result demonstrates that the encapsulation of insoluble HSP90 inhibitors like 6BrCaQ in suitable nanocarriers like liposome could allow overcoming the limitation associated with insoluble HSP90 inhibitors and their clinical evaluation. In another report, Zhang et al. (2015) prepared mesoporous carbon nanospheres stabilized by phospholipid for systemic delivery of amphiphobic SNX-2112 and demonstrated to enhance its antitumor effect (Zhang et al. 2015). Since SNX-2112 is amphiphobic drug, it is insoluble in both water and oil and its drug delivery was indeed a formidable challenge. The problem was overcome by the systemic delivery of SNX-2112 using carbon nanospheres. Bruni et al. (2017) also formulated poorly water-soluble drugs such as 17-AAG into nano-vectors to enhance its leishmanicidal activity with reduced cytotoxicity (Bruni et al. 2017). There are few reports, where HSP90 inhibitors are used for combinational therapy by loading to a potential nano-carrier having therapeutic properties like photodynamic and photothermal ability. Lin et al. (2016) have recently prepared a nanoformulation of organic and biocompatible nanoporphyrin-based drug delivery for the HSP90 inhibitors like 17AAG or 17DMAG for combination therapy, where the nanocarriers act a carrier as well as a therapeutic agent due to its photodynamic and photothermal potential. The formulation was very effective with an advantage of minimal skin phototoxicity (Lin et al. 2016). In another study, Rochani et al. (2016) have prepared formulation of poly (lactic-co-glycolic acid) (PLGA) coated, 17AAG and Fe3O4 loaded magnetic nanoparticle, where magnetic hyperthermia of magnetic nanoparticle in combination with the HSP 90 inhibitory effect of 17AAG was used to treat pancreatic cancer treatment and results were promising (Rochani et al. 2016). Although the research of using nanocarrier for HSP90 inhibitors as well as the combination therapy with other drugs is still not sufficient, there are studies which have already entered clinical trials. So we have summarized the literature about nanoparticulate systems carrying an HSP90 inhibitor alone or with other cytotoxic drugs in Table 8.2. Thus, we can conclude that the future perspective of HSP90 inhibitor as a potential anti-cancer agent should focus on its systematic delivery to the target site by proper formulation using nano-delivery systems including vesicles, hydrogels. Such approaches might provide the clinical solution by overcoming the above-mentioned limitations such as poor water solubility and cytotoxic effects. Therefore, more work should be accomplished in the line of HSP90 inhibitors using nano-based delivery for higher drug distribution with a lower dose.
Abbreviations
- 17-AAG:
-
17-allylamino-17demethoxygeldanamycin
- 17-DMAG:
-
17-(2-dimethylaminoethyl)amino-17-demethoxygeldanamycin
- ADP:
-
adenosine diphosphate
- AhR:
-
aryl hydrocarbon receptor
- ATP:
-
adenosine triphosphate
- CBR:
-
clinical benefit rate
- DLT:
-
dose-limiting toxicities
- EGCG:
-
Epigallocatechin 3-gallate
- FDG-PET:
-
fluorodeoxyglucose positron emission tomography
- GIST:
-
gastrointestinal stromal tumors
- GM:
-
Geldanamycin
- HOP:
-
HSP90 organizing protein
- HSP:
-
heat shock protein
- IPI-493:
-
17-desmethoxy-17-amino Geldanamycin
- IPI-504:
-
17-allylamino-17-demethoxygeldanamycin Hydroquinone Hydro-chloride
- MDR:
-
multi-drug resistance
- MTD:
-
maximum tolerated dose
- NSCLC:
-
non-small-cell lung carcinoma
- ORR:
-
overall response rate
- PBMC:
-
peripheral blood mononuclear cells
- PCL:
-
poly(ε-caprolactone)
- PDGFRA:
-
platelet-derived growth factor receptor alpha
- PEG:
-
poly(ethylene glycol)
- PET:
-
positron emission tomography
- PLGA:
-
poly (lactic-co-glycolic acid)
- PR:
-
partial response
- RD:
-
radicicol
- SD:
-
stable diseases
- SUV:
-
standardized uptake value
- TNBC:
-
triple-negative breast cancer
References
Allan RK, Mok D, Ward BK, Ratajczak T (2006) Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90 evidence that coumarin antibiotics disrupt Hsp90 dimerization. J Biol Chem 281:7161–7171
Audisio D, Messaoudi S, Cegielkowski L et al (2011) Discovery and biological activity of 6BrCaQ as an inhibitor of the Hsp90 protein folding machinery. ChemMedChem 6:804–815
Bagatell R, Whitesell L (2004) Altered Hsp90 function in cancer: a unique therapeutic opportunity. Mol Cancer Ther 3:1021–1030
Bao R, Lai C-J, Wang D-G et al (2009) Targeting heat shock protein 90 with CUDC-305 overcomes erlotinib resistance in non–small cell lung cancer. Mol Cancer Ther 8(12): 3296–3306
Baylon HG, Caguioa PB, Davies FE et al (2013) A phase 1/2 study of KW-2478, an Hsp 90 inhibitor, in combination with bortezomib (BTZ) in patients (Pts) with relapsed/refractory (R/R) multiple myeloma (MM). Am Soc Hematol 122: 1967
Bruni N, Stella B, Giraudo L, Della Pepa C, Gastaldi D, Dosio F (2017) Nanostructured delivery systems with improved leishmanicidal activity: a critical review. Int J Nanomedicine 12:5289
Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM, Workman P (2015) Maximizing the therapeutic potential of HSP90 inhibitors. Mol Cancer Res 13:1445–1451: molcanres. 0234.2015
Byrd KM, Subramanian C, Sanchez J et al (2016) Synthesis and biological evaluation of novobiocin core analogues as Hsp90 inhibitors. Chem Eur J 22:6921–6931
Cavenagh J, Yong K, Byrne J et al (2008) The safety, pharmacokinetics and pharmacodynamics of KW-2478, a novel HSP90 antagonist, in patients with B-cell malignancies: a first-in-man, phase I, multicentre, open-label, dose escalation study. Am Soc Hematol 112(11): 2777
Chène P (2002) ATPases as drug targets: learning from their structure. Nat Rev Drug Discov 1:665
Cheung K-MJ, Matthews TP, James K et al (2005) The identification, synthesis, protein crystal structure and in vitro biochemical evaluation of a new 3, 4-diarylpyrazole class of Hsp90 inhibitors. Bioorg Med Chem Lett 15:3338–3343
Chiosis G, Timaul MN, Lucas B et al (2001) A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem Biol 8:289–299
Cho D, Heath E, Cleary J et al (2011) A phase I dose-escalation study of the Hsp90 inhibitor ganetespib (STA-9090) administered twice weekly in patients with solid tumors: updated report. J Clin Oncol 29:3051–3051
Demetri G, Heinrich M, Chmielowski B et al (2011) An open-label phase II study of the Hsp90 inhibitor ganetespib (STA-9090) in patients (pts) with metastatic and/or unresectable GIST. J Clin Oncol 29:10011–10011
Do KT, Speranza G, Chen AP et al. (2012) Phase l study assessing a two-consecutive-day (QD× 2) dosing schedule of the HSP90 inhibitor, AT13387, in patients with advanced solid tumors. Paper presented at the Proc Am Soc Clin Oncol
Gaspar N, Sharp SY, Eccles SA et al (2010) Mechanistic evaluation of the novel HSP90 inhibitor NVP-AUY922 in adult and pediatric glioblastoma. Mol Cancer Ther 9:1219–1233
Ge J, Normant E, Porter JR et al (2006) Design, synthesis, and biological evaluation of hydroquinone derivatives of 17-amino-17-demethoxygeldanamycin as potent, water-soluble inhibitors of Hsp90. J Med Chem 49:4606–4615
Goldman J, Raju R, Gordon G, Vukovic V, Bradley R, Rosen L (2010) A phase I dose-escalation study of the Hsp90 inhibitor STA-9090 administered once weekly in patients with solid tumors. J Clin Oncol 28:2529–2529
Hadden MK, Galam L, Gestwicki JE, Matts RL, Blagg BS (2007) Derrubone, an inhibitor of the Hsp90 protein folding machinery. J Nat Prod 70:2014–2018
Haque A, Alam Q, Zubair Alam M et al (2016) Current understanding of HSP90 as a novel therapeutic target: an emerging approach for the treatment of cancer. Curr Pharm Des 22:2947–2959
He H, Zatorska D, Kim J et al (2006) Identification of potent water soluble purine-scaffold inhibitors of the heat shock protein 90. J Med Chem 49:381–390
Hollingshead M, Alley M, Burger AM et al (2005) In vivo antitumor efficacy of 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride), a water-soluble geldanamycin derivative. Cancer Chemother Pharmacol 56:115–125
Hölzel M, Bovier A, Tüting T (2013) Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer 13:365
Isambert N, Hollebecque A, Berge Y et al (2012) A phase I study of Debio 0932, an oral HSP90 inhibitor, in patients with solid tumors. Am Soc Clin Oncol 8(12): 3296–3306
Jensen MR, Schoepfer J, Radimerski T et al (2008) NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res 10:R33
Jhaveri K, Taldone T, Modi S, Chiosis G (2012) Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta (BBA)-Mol Cell Res 1823:742–755
Jhaveri K, Ochiana SO, Dunphy MP et al (2014) Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin Investig Drugs 23:611–628
Kasibhatla SR, Hong K, Biamonte MA et al (2007) Rationally designed high-affinity 2-amino-6-halopurine heat shock protein 90 inhibitors that exhibit potent antitumor activity. J Med Chem 50:2767–2778
Lamotte S, Späth GF, Rachidi N, Prina E (2017) The enemy within: targeting host–parasite interaction for antileishmanial drug discovery. PLoS Negl Trop Dis 11:e0005480
Lancet JE, Smith BD, Bradley R, Komrokji RS, Teofilovici F, Rizzieri DA (2010) A phase I/II trial of the potent Hsp90 inhibitor STA-9090 administered once weekly in patients with advanced hematologic malignancies. Am Soc Hematol 116(21):3294
Li Y, Zhang T, Schwartz SJ, Sun D (2009) New developments in Hsp90 inhibitors as anti-cancer therapeutics: mechanisms, clinical perspective and more potential. Drug Resist Updat 12:17–27
Lin T-y, Guo W, Long Q et al (2016) HSP90 inhibitor encapsulated photo-theranostic nanoparticles for synergistic combination cancer therapy. Theranostics 6:1324
Mahadevan D, Rensvold DM, Kurtin SE et al (2012) First-in-human phase I study: results of a second-generation non-ansamycin heat shock protein 90 (HSP90) inhibitor AT13387 in refractory solid tumors. Am Soc Clin Oncol 3028
Mahalingam D, Swords R, Carew JS, Nawrocki S, Bhalla K, Giles F (2009) Targeting HSP90 for cancer therapy. Br J Cancer 100:1523
Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM (2000) The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem 275:37181–37186
Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY (2013) Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev 65:1866–1879
Martin CJ, Gaisser S, Challis IR et al (2008) Molecular characterization of macbecin as an Hsp90 inhibitor. J Med Chem 51:2853–2857
Marubayashi S, Koppikar P, Taldone T et al (2010) HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest 120:3578–3593
Meacham CE, Morrison SJ (2013) Tumour heterogeneity and cancer cell plasticity. Nature 501:328
Menzella HG, Tran T-T, Carney JR et al (2009) Potent non-benzoquinone ansamycin heat shock protein 90 inhibitors from genetic engineering of Streptomyces hygroscopicus. J Med Chem 52:1518–1521
Messaoudi S, Peyrat J, Brion J, Alami M (2008) Recent advances in Hsp90 inhibitors as antitumor agents. Anti-Cancer Agents Med Chem (Formerly Curr Med Chem-Anti-Cancer Agents) 8:761–782
Mimnaugh EG, Chavany C, Neckers L (1996) Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem 271:22796–22801
Mitchell P (2011) Biogen Idec restructures, sharpens neurology focus. Nature Biotechnology 29: 7–8
Murray CW, Carr MG, Callaghan O et al (2010) Fragment-based drug discovery applied to Hsp90. Discovery of two lead series with high ligand efficiency. J Med Chem 53:5942–5955
Nakashima T, Ishii T, Tagaya H et al (2010) New molecular and biological mechanism of anti-tumor activities of KW-2478, a novel non-ansamycin Hsp90 inhibitor, in multiple myeloma cells. Clin Cancer Res 16:2792–2802: clincanres. 3112. subyr
Neckers L (2002) Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med 8:S55–S61
Neckers L (2007) Heat shock protein 90: the cancer chaperone. In: Heat shock protein cancer. Springer, Dordrecht, pp 231–52
Nimmanapalli R, O’Bryan E, Bhalla K (2001) Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res 61:1799–1804
Ohsuki S, Tengeiji A, Ikeda M, Shibata Y, Nagata C, Shimada T (2012) Pyrazolopyrimidine derivative: Google Patents
Palermo CM, Westlake CA, Gasiewicz TA (2005) Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry 44:5041–5052
Parveen S, Sahoo SK (2008) Polymeric nanoparticles for cancer therapy. J Drug Target 16:108–123
Patel HJ, Modi S, Chiosis G, Taldone T (2011) Advances in the discovery and development of heat-shock protein 90 inhibitors for cancer treatment. Expert Opin Drug Discovery 6:559–587
Pearl LH, Prodromou C (2006) Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem 75:271–294
Petersen ALOA, Campos TA, Dantas DAS et al (2018) An in vitro assessment on the efficacy of a novel liposomal formulation containing the Hsp-90 inhibitor, 17-AAG. Front Cell Infect Microbiol 8:303
Proia DA, Zhang C, Sequeira M et al (2013) Preclinical activity profile and therapeutic efficacy of the HSP90 inhibitor ganetespib in triple-negative breast cancer. Clin Cancer Res: clincan res 20(2):413–424
Rochani AK, Balasubramanian S, Girija AR et al (2016) Dual mode of cancer cell destruction for pancreatic cancer therapy using Hsp90 inhibitor loaded polymeric nano magnetic formulation. Int J Pharm 511:648–658
Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 42:260–266
Rowlands MG, Newbatt YM, Prodromou C, Pearl LH, Workman P, Aherne W (2004) High-throughput screening assay for inhibitors of heat-shock protein 90 ATPase activity. Anal Biochem 327:176–183
Samlowski W, Papadopoulos K, Olszanski A et al (2011) Phase 1 study of HSP90 inhibitor MPC-3100 in subjects with refractory or recurrent cancer. Mol Targets Cancer Ther 10(11): A96-A96
Samuel T, Sessa C, Britten C et al (2010) AUY922, a novel HSP90 inhibitor: final results of a first-in-human study in patients with advanced solid malignancies. J Clin Oncol 28:2528–2528
Sauvage F, Franzè S, Bruneau A et al (2016) Formulation and in vitro efficacy of liposomes containing the Hsp90 inhibitor 6BrCaQ in prostate cancer cells. Int J Pharm 499:101–109
Schroder C, Pedersen J, Chua S et al (2011) Use of biomarkers and imaging to evaluate the treatment effect of AUY922, an HSP90 inhibitor, in patients with HER2+ or ER+ metastatic breast cancer. J Clin Oncol 29:e11024–e11e24
Shi J, Van de Water R, Hong K et al (2012) EC144 is a potent inhibitor of the heat shock protein 90. J Med Chem 55:7786–7795
Sidera K, Patsavoudi E (2014) HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov 9:1–20
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30
Soga S, Akinaga S, Shiotsu Y (2013) Hsp90 inhibitors as anti-cancer agents, from basic discoveries to clinical development. Curr Pharm Des 19:366–376
Solit DB, Chiosis G (2008) Development and application of Hsp90 inhibitors. Drug Discov Today 13:38–43
Sydor JR, Normant E, Pien CS et al (2006) Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci 103:17408–17413
Taldone T, Chiosis G (2009) Purine-scaffold Hsp90 inhibitors. Curr Top Med Chem 9:1436–1446
Taldone T, Patel PD, Patel M et al (2013) Experimental and structural testing module to analyze paralogue-specificity and affinity in the Hsp90 inhibitors series. J Med Chem 56:6803–6818
Tanida S, Hasegawa T, Higashide E (1980) Macbecins I and II, new antitumor antibiotics. J Antibiot 33:199–204
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10:537
Wang J, Li Z, Lin Z et al (2015) 17-DMCHAG, a new geldanamycin derivative, inhibits prostate cancer cells through Hsp90 inhibition and survivin downregulation. Cancer Lett 362:83–96
Won Y-W, Yoon S-M, Sonn CH, Lee K-M, Kim Y-H (2011) Nano self-assembly of recombinant human gelatin conjugated with α-tocopheryl succinate for Hsp90 inhibitor, 17-AAG, delivery. ACS Nano 5:3839–3848
Woodhead AJ, Angove H, Carr MG et al (2010) Discovery of (2, 4-Dihydroxy-5-isopropylphenyl)-[5-(4-methylpiperazin-1-ylmethyl)-1, 3-dihydroisoindol-2-yl] methanone (AT13387), a novel inhibitor of the molecular chaperone Hsp90 by fragment based drug design. J Med Chem 53:5956–5969
Xiong MP, Yáñez JA, Remsberg CM et al (2008) Formulation of a geldanamycin prodrug in mPEG-b-PCL micelles greatly enhances tolerability and pharmacokinetics in rats. J Control Release 129:33–40
Ying W, Du Z, Sun L et al (2011) Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther 11(2):475-484
Young JC, Agashe VR, Siegers K, Hartl FU (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5:781
Zhang H, Neely L, Lundgren K et al (2010) BIIB021, a synthetic Hsp90 inhibitor, has broad application against tumors with acquired multidrug resistance. Int J Cancer 126:1226–1234
Zhang X, Zhang T, Ye Y et al (2015) Phospholipid-stabilized mesoporous carbon nanospheres as versatile carriers for systemic delivery of amphiphobic SNX-2112 (a Hsp90 inhibitor) with enhanced antitumor effect. Eur J Pharm Biopharm 94:30–41
Acknowledgements
The authors sincerely thank the Science and Engineering Research Board, Government of India, for financial support (Grant No. SERB/F/4290/2016-17) and National Institute of Technology Rourkela, Government of India, for providing the infrastructural facility for the preparation of the chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Somu, P., Paul, S. (2019). HSP90 and Its Inhibitors for Cancer Therapy: Use of Nano-delivery System to Improve Its Clinical Application. In: Asea, A., Kaur, P. (eds) Heat Shock Protein 90 in Human Diseases and Disorders. Heat Shock Proteins, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-030-23158-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-23158-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23157-6
Online ISBN: 978-3-030-23158-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)