Abstract
Introduction Heat shock response (HSR) pathway is a highly conserved cellular process. HSF1 is a master transcriptional regulator responsible for the expression of several important heat shock proteins (HSP), which can effectively protect critical client proteins from misfolding and degradation, thus maintaining intracellular integrity under stressed conditions. Recent studies have demonstrated the direct connections between HSR players and tumor cell survival, validating HSR players as novel molecular targets in anticancer treatment. Small molecule screening has produced some promising HSR inhibitors for anticancer treatment. In this article, we aim to summarize the main findings from HSR inhibitors on recent clinical and preclinical studies.
Methods The authors reviewed all the relevant papers of HSR inhibitors with an emphasis on human and animal studies.
Results More than 18 unique chemical identities have been discovered with confirmed inhibition of HSR pathway. Among them, two natural products and their derivatives are currently in various phases of clinical studies. Detailed works are required to define the exact mechanisms of actions (MOA) for these compounds.
Conclusion Many hurdles in clinical application still need to be effectively addressed, such as undesirable drug toxicity and off-target effects; narrow therapeutic window; poor PK/PD profiles, etc. Recent reports on synergistic drug combination, advanced prodrug design, smart nanoparticle packaging, and RNA aptamer selection offer promising solutions to overcome these challenges. Future advancements in this fast-growing area can potentially lead to the next-generation cancer therapeutics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cancer is a malignant disease characterized by uncontrolled cell growth. Genetic and epigenetic alterations can activate oncogenes whose activities are necessary for tumor initiation and maintenance, a phenomenon called “oncogene addiction.” [75]. As a result of this oncogenic transformation, cancer cells are known to exhibit “stress phenotype”, such as high levels of DNA damage, aneuploidy, and reactive oxygen species [21]. These cancer cells constantly express mutated oncogenes with misfolded protein structures. Comparing to their normal counterparts, the transformed malignant cells demand a much higher expression level of molecular chaperone proteins, including HSP70, HSP90 and HSP27, etc., to preserve protein homeostasis for tumor cell survival [6]. The tumor dependency of molecular chaperone machinery creates an attractive model of cancer therapeutics by selective targeting key HSR players [17].
Heat shock factor 1 (HSF1) is the master transcription factor responsible for controlling the heat shock response [15]. HSF1 is normally maintained in an inactive monomeric state through binding to a complex containing heat shock protein (HSP) 70, HSP90 and HSP40 (Fig. 1). When cellular stress occurs by the multiple known causes of protein misfolding (such as cancer cell transformation, DNA damage, etc.), HSF1 dissociates from the chaperone complex, translocates into the nucleus, and forms active phosphorylated trimers. This activated HSF1 trimer thus binds to the heat shock element (HSE) in the Promoter regions of many heat shock proteins. As a result, activation of HSF1 leads to the mRNA induction and translation of HSP70 and other chaperone family proteins. In addition, HSF1 has a large number of target genes encoding proteins with versatile cytoprotective functions [2]. This highly conserved protective machinery is now believed to be adopted by the majority of cancer cells, through which the so-called “protein folding pressure” can be mostly relieved to facilitate the survival of malignant cells [33].
Illustration of HSF1 trimerization and activation inside the cell with four types of inhibitory mechanisms of small molecules. (1) Inhibition of HSF1 phosphorylation or HSF1 dephosphorization; (2) Interference of HSF1 trimer binding to HSE; (3) Blocking HSF1 mediated transcription activation; (4) hampering mRNA translation of HSF1 and HSP. For a total of 18 compounds in this review (including two prodrugs), 14 compounds show some evidence in one type of the inhibitory mechanisms of HSF1 (listed above), although more detailed studies are essential. The MOAs for the remaining four compounds need to be determined
Since HSF1 essentially controls the expression of all cellular HSP in tumor cells, it has been proposed to be a novel anticancer target with “non-oncogene addiction” features [68]. Dai and colleagues demonstrated that genetic HSF1 deficiency in transgenic mice inhibits tumorigenesis in a mouse skin cancer model. Similar results were also observed in genetic models containing oncogenic mutations of the RAS oncogene or inactivating mutations in the tumor suppressor p53 [14]. Other target validation results from different groups also confirmed that tumor cells have a much greater dependence on HSF1 function compared to normal cells, suggesting that cancer cells are becoming “HSF1 addicted” [43]. Thus, small molecule intervention pf tumor HSR pathway, as monotherapy or combined with other drugs, has been suggested to be a novel avenue for cancer therapeutics [15].
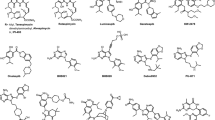
In recent years, efforts have been made to screen and identify small molecule HSR inhibitors [16]. Through various compound screening platforms, such as reporter based high throughput screening, image-based phenotypic screening and chemical SAR approach, more than 18 unique chemical identities or structure derivatives have been discovered with confirmed inhibition of HSR pathway (Fig. 1; [65]). According to Clinincaltrial.gov, two of the natural products, Quercetin [59] and triptolide [49] and their respective prodrugs (QC12 and Minnelide), are in various stages of clinical trial studies (Table 1 and Fig. 2). Seven of these compounds were reported to have positive antiproliferative effects in the mouse xenograft models carrying different tumor cell lines, including breast, skin, colon, hepatoma, and pancreatic cancer lines (Table 2 and Fig. 3). The remaining seven compounds and one RNA aptamer are in the early stage of preclinical studies, with only cell line data available (Table 3 and Fig. 4). More detailed works are required to define the exact mechanisms of actions (MOA) for these compounds. As shown in Fig. 1, the inhibitory mechanisms can be primarily categorized into four types: (1) Inhibition of HSF1 phosphorylation or HSF1 dephosphorization; (2) Interference of HSF1 trimer binding to HSE; (3) Blocking HSF1 mediated transcription activation; (4) hampering mRNA translation of HSF1 and HSP. The undefined MOAs and diverse chemotypes make the clinical study of these drug candidates less predictable. In this chapter, the authors reviewed the recent progress and lessons learned from human and animal model studies. Several excellent reviews are also available on the current status of MOAs, chemical structures and screening of HSR inhibitors [16, 28, 65].
Chemical structures of seven inhibitors of HSR pathway with data available only in various cancer cell lines. These inhibitors include three natural products (2,4-Bis(4-hydroxybenzyl) phenol, cardenolide CL-43, stresgenin B), four synthetic compounds (α−acyl amino carboxamides, IHSF115, NZ-28, 4,6-disubstituted pyrimidine) and one RNA aptamer (iaRNAHSF1)
2 HSR Inhibitors Currently in Human Clinical Studies (Table 1 and Fig. 2)
2.1 Quercetin
Quercetin is one of the bioactive flavonoids found ubiquitously in fruits, vegetables and beverages [59]. Previous publications established its role as a natural antiproliferative agent against various cancer cell lines from diverse lineages and mouse xenograft models [44]. Using a gel shift assay, Nagai et al. reported that quercetin down-regulates HSF1 by the decrease of HSF1-HSE binding, rather than inhibition of HSF1 trimerization [47]. Yang and colleges used quercetin-loaded liposomes to treat rats with R3230 breast adenocarcinoma. An increase of the tumor destruction/endpoint survival in vivo was observed as compared to the control treatment group [83]. Importantly, this study confirmed that HSF1 is required for quercetin-induced cancer cell death and quercetin can directly down-regulate HSF1. Moreover, quercetin was also tested in other xenograft mouse models, including hepatoma [92], ovarian cancer [39, 40], breast cancer [25, 34, 64], colon cancer [25, 54, 81]. In addition to its inhibitory role in the heat shock response pathway, these animal studies showed that quercetin can also perform its anti-cancer effects through modulating cyclins, pro-apoptotic, autophagy, PI3K/Akt and mitogen-activated protein kinase (MAPK) molecular pathways, etc.
Quercetin has been proposed as a sensitizer and protects non-cancer cells from the side effects of conventional cancer therapies [59]. The safety and potential usefulness of quercetin for cancer treatment have been documented in both animal experiments and a phase I clinical trial. The first clinical trial phase I study was done in the UK [20]. The authors investigated the pharmacokinetics of quercetin with short i.v. infusion and found 1,400 mg/m2 as the safe bolus dose. The maximum tolerated dose was determined as 1,700 mg/m2 three weekly. The anti-tumor effects were recorded with confirmed inhibition of lymphocyte tyrosine kinase activity. However, dimethyl sulfoxide was used as a solubilized vehicle and was unsuitable for further clinical development of quercetin.
According to clinicaltrial.gov, several clinical trials of quercetin are currently registered for the treatment of COPD, Hepatitis C and Type II diabetes, etc. Some of these trials are designed to study Quercetin as a dietary supplement for cancer prevention. For monotherapy of Quercetin, a phase II clinical trial is currently active to investigate its effects on chemoprevention for squamous cell carcinoma in patients with Fanconi anemia. Quercetin was designed to be orally administered for a maximum total daily dose of 4000 mg/day for 24 months (ClinicalTrials.gov Identifier: NCT03476330). The clinical data is expected to be available after 2023.
2.2 QC12 (Analog Prodrug of Quercetin)
In order to overcome the solubility issue of quercetin, a synthetic chemistry effort was undertaken to produce water-soluble derivatives of quercetin [46]. The most promising candidates of these synthetic compounds, called QC12, was selected. QC12 can be hydrolyzed in vivo and releases quercetin and glycine. Unfortunately, QC12 or quercetin was not detected in plasma following oral administration of QC12, confirming it was not orally bioavailable. As a result, QC12 entered the clinical phase I study with i.v. administration. The authors can detect peak QC12 concentration at >100 μM in plasma. Although quercetin was found in all patients following i.v. infusion. The relative bioavailability of quercetin is estimated to be only 20–25% released from QC12. This unsatisfactory Phase I results prevented QC12 for further clinical application [61].
2.3 Triptolide
Triptolide has been reported as one of the most potent inhibitors of heat shock response pathway [10, 49]. Westerheide et al. identified triptolide as an inhibitor of the HSF1 pathway through small molecule screening [76]. Although triptolide does not inhibit the earlier steps in the HSF1 multistep activation process, including trimer formation, hyperphosphorylation, or translocation and binding HSP70 promoter, the inhibitory effects of triptolide on HSP70 expression was reported to be at the level of transcription by interfering with the proper activity of the C-terminal transactivation domain of HSF1. Significantly reduced tumor cell viability was reported after triptolide was incubated with pancreatic cell lines, including PANC-1 and MiaPaCa-2. Triptolide also induces pancreatic cancer apoptosis via inhibition of heat shock protein 70 at mRNA level [52]
A number of mouse xenograft models were adopted to evaluate the anti-cancer effects of triptolide. Some representative examples including breast cancer [35, 36, 79], Prostate cancer [26], lung cancer [57, 58], pancreatic cancer [52], gastric cancer and melanoma [78, 82], etc. Comparing to all known HSR pathway inhibitors, triptolide exhibits a broader spectrum of anticancer activity against the common types of human cancer.
Using a semi-synthetic derivative of triptolide F60008, a phase I and PK/PD study were performed in patients with advanced solid tumors [31]. Twenty patients were enrolled, but hematological side effects were reported such as mild grade anemia. Other mild grade toxicities included constipation, fatigue, vomiting, diarrhea and nausea. Importantly, two lethal events were documented with increased caspase-3 activity and overt apoptosis in neutrophils and monocytes. PK data showed high variability between tested patients. The narrow therapeutic window and undesired water solubility largely limited the clinical application of triptolide [73, 74]. However, the prodrug of triptolide, namely Minnelide, has become an excellent candidate in the clinical study (next). In addition, a recent report on the smart drug delivery system can be an alternative solution to address toxicity issues of triptolide [78].
2.4 Minnelide (Analog Prodrug of Triptolide)
Most human clinical studies of triptolide were performed with its analog prodrug Minnelide. Minnelide is a phosphonooxymethyl prodrug with three times more water solubility than its parent molecule triptolide. Minnelide can be converted into triptolide by phosphatase in vivo [51]. Chugh et al. reported that Minnelide is as effective as triptolide in inhibition of pancreatic tumor cells both in vitro and in vivo [12]. In two mouse xenograft models of human colon adenocarcinoma and ovarian cancer, Minnelide was effective in reducing or eliminating tumors with a well-tolerated safety profile [51]. Banerjee and colleagues reported that Minnelide induces cell death in a number of pancreatic cancer cell lines and reduces tumor volume in multiple xenograft mouse models, including pancreatic cancer and hepatoma [5]. Similar results were also reported on several pancreatic and melanoma xenograft models [18, 63]. Minnelide also exhibits inhibitory effects of HSP70 on the human gastric tumor xenograft mouse model, both as a single agent and in combination with chemotherapy agent CPT-11 [3]. Using an in vivo imaging system, Giri and colleagues reported Minnelide significantly decreased leukemic burden in multiple xenograft models of acute myeloid leukemia at doses easily achievable in patients [23]
The first open-label, phase I, safety clinical trial of Minnelide was completed in patients with advanced gastrointestinal tumors in 2016 (ID: NCT01927965). The primary objective of this study was to determine the maximum tolerated dose and the dose-limiting toxicities of Minnelide. Although no phase I data is currently available, a continued Minnelide phase II study is expected to begin for treating gastrointestinal malignancy [63]. According to Clinicaltrial.gov, there are three active clinical trials of Minnelide in human cancer treatment. A phase 1, open label, pilot study was initiated in 2018 on the pharmacokinetic and pharmacodynamic property of Minnelide with adult patients of AML (ID: NCT03347994). Minnelide capsule was also tested alone or in combination with protein-bound paclitaxel in patients with advanced solid tumor (various cancer types). This open label, phase 1 trial started in 2017 and is expected to be finished in 2021 (ID: NCT03129139). Promising response data from early stage trials has led to a phase II, international open-label trial of Minnelide in patients with refractory pancreatic cancer (ID: NCT03117920, [55]).
Interestingly, all current inhibitors of heat shock response in the clinical trial study are of natural origin or prodrug of natural compounds. Expectations are particularly high for positive results of Minnelide as monotherapy or combined treatment with other cancer drugs. Meantime, more data on animal PK/PD, toxicity, SAR analysis are becoming available for synthetic HSR inhibitors. Human clinical studies of this category of synthetic compounds are likely to be increased in the near future.
3 HSR Inhibitors Currently in Preclinical Animal Studies (Table 2 and Fig. 3)
3.1 PW3405
PW3405 was discovered as a potent heat shock response pathway inhibitor via a large-scale, unbiased, high content image-based screening in our group [89]. This synthetic compound demonstrated a nanomolar potency against HSF1 granulation after heat stress. Our study showed that the decrease of heat shock response is achieved through inhibition of HSF1 phosphorylation at the Ser326 activating site. Thus, a potential intracellular kinase inhibitory mechanism was proposed [90]. The results from in vivo pancreatic cancer PC-3 xenograft models showed encouraging results with reduced tumor volume at a well-tolerated dose schedule (manuscript in preparation). Currently, PW3405 alone, or in combination with chemotherapy agents, are being investigated with several relevant mouse xenograft models. The results will be reported in due course.
3.2 Cantharidin
Cantharidin is a type of terpenoid secreted by the blister beetle Mylabris phalerata. A cell-based screening led to the discovery of this natural compound with potent activity against HSF1 [29]. Li and colleagues reported that cantharidin inhibits the growth of triple-negative breast cancer cells in vitro [37]. After the treatment of cantharidin, the tumor growth in MDA-MB-231 and MDA-MB-468 xenografts mice was reduced through inducing apoptosis of tumor cells. Moreover, the combination of cantharidin and antiangiogenic therapeutics presents additive antitumor effects against pancreatic cancer xenografts in vivo [80], although an unfavorable proangiogenic side effect was recorded. Anticancer effects of cantharidin were also reported in mouse skin cancer xenografts [38]. Using liposomal encapsulated cantharidin, increased anticancer effects were reported in a HepG2-bearing hepatocellular carcinoma xenograft model [91]. Although cantharidin has been a traditional Chinese remedy, there is no human clinical trial data recorded in clinicaltrial.gov.
3.3 KNK437
KNK437 was first reported as an inhibitor of HSF1 and HSP induction in human colon carcinoma cell [85]. The compound inhibits HSP expression at the mRNA level while it does not increase thermos-sensitivity in nontolerant cells. Since then, several animal tumor studies have been reported. In a mouse transplantable tumor model, Koishi et al. reported that KBK437 can inhibit thermotolerance via the inhibition of HSP72, thus improving the efficacy of clinical fractioned hyperthermia [32]. Another recent study showed KNK437 can inhibit colorectal cancer in vivo, with a significant reduction of HSP40 family member A1 expression [84]. Similar to quercetin, KNK437 may not be sufficiently potent for clinical use as high concentrations of compound are required to demonstrate inhibitory activity. The use of such high concentrations of low potency compounds increases the likelihood of off-target effects [53].
3.4 KRIBB11
Yoon and colleagues reported a synthetic compound named KRIBB11 that can directly bind to HSF1 in a western blot analysis [86]. It was proposed that the association of HSF1 and KRIBB11 can further inhibit the transcription process of heat shock proteins. In the same study, KRIBB11 can inhibit the growth of colon cancer cells in BALB/c nude mouse xenograft regression model. These results, particularly the evidence of direct HSF1 binding, promoted in vivo animal study of this compound. KRIBB11 was intraperitoneally administrated in a myeloma xenograft model, a significant decrease in tumor volume was observed [22]. At the molecular level, a significant reduction of HSP27 protein expression was documented in the KRIBB11 treated tumor group comparing to the control group. Using an orthotopic xenograft mouse model, KRIBB11 and AKT inhibitor MK-2206 in combination can result in the synergistic killing of breast cancer cells and inhibit tumor growth [7]. Recently, Parekh reported that KRIBB11 exhibits primary myeloma cell killing and cytotoxicity in stromal coculture, thus eliminating tumor cell protection inside the bone microenvironment in myeloma [50]. Although KRIBB11 exhibits modest in vivo efficacy in xenograft models as a single agent, its efficacy of HSF1 inhibition may be further improved by rational combination therapy.
3.5 Fisetin
Kim and colleagues reported their results of fisetin, a dietary flavonoid, on its inhibitory activity of HSF1 [30]. The downregulation of HSP70, HSP27 and BAG3 by fisetin significantly reduces the cellular levels of Bcl-2, Bcl-xL and Mcl-1 proteins, followed by apoptotic tumor cell death. Further analysis indicated that fisetin inhibits HSF1 by blocking the binding of HSf1 to HSP70 promoter. Treatment of colon cancer xenograft mice model with fisetin caused inhibition of HCT-116 cell growth in vivo. Another example is the results from a mouse liver cancer model. The orthotopically implanted tumors were inhibited by fisetin with a prolonged survival rate [39, 40]. Recently, a mouse xenograft model with luciferase expression in human pancreatic PANC-1 tumor cells was reported [27]. Bioluminescence can be emitted after the injection of luciferin intraperitoneally in a living mouse. Using this noninvasive method, tumor volume was recorded kinetically with significant size reduction in the fisetin treated group as compared to the control group. In an effort to improve the solubility and therapeutic index of fisetin, poly (lactic acid) nanoparticles loaded with fisetin were also developed. The data showed that drug-loaded nanoparticles were superior to that of free drug solution when tested against HCT116 colon cancer cells in vitro and antitumor test in a xenograft 4 T1 breast cancer model in vivo [19].
3.6 Racoglamide A
Santagata et al. adopted a well-designed reporter-based assay to screen for inhibitors of HSF1 activation. With a diversified library of 300,000 compounds from the NIH Molecular Libraries Probe Center Network, racoglamide A was identified as an inhibitor of HSF1 activation with nanomolar potency against multiple cancer cell lines [62]. Importantly, racoglamide A can significantly suppress tumor growth in an M0–91 acute myeloid leukemia xenograft mouse model with no evidence of systemic toxicity. In another study, the combination of racoglamide A and a human circularly permuted TRAIL (CPT) exhibits an efficient treatment towards mice xenografted with the CPT-resistant human acute T-cell leukemia cell line Molt-4 [77]. Furthermore, racoglamide A was reported to reduce the tumor size in a patient-derived pancreatic cancer xenograft mouse model without noticeable toxicity in vivo [73, 74]. Since racoglamide A is a natural Chinese herb compound with a relatively safe profile, this inhibitor or its derivatives may possibly advance to human study pending more efficacy data in animal models.
3.7 CCT251236
Using an image-based phenotypic screen, Cheeseman et al. reported the discovery of a new chemical probe, bisamide (CCT251236) as a potent inhibitor of the HSF1 stress pathway [9]. Efforts have been made to make analogs to improve solubility and bioavailability of this bisamide compound series with satisfactory mouse pharmacokinetics data. Importantly, CCT251236 displays efficacy in a human ovarian carcinoma xenograft model. In addition. CCT251236 demonstrates relatively low toxicity and was well tolerated in a mouse multidose tolerability study. The Pirin protein was proposed to have a possible role in the bisamide phenotype and the cellular effects of modulating the HSF1 pathway [9, 11]. In addition, significant anti-myeloma efficacy was observed in a myeloma xenograft mouse model after the treatment of CCT251236 through oral administration [50]. Although this group of HSR inhibitors has shown promising results in different xenograft mouse models, more animal model data, including PK/PD, MOA and biomarker studies, are essential for their advancements to the human clinical trial. Recent progress on nanoparticles and prodrugs can accelerate this translational process.
4 HSR Inhibitors in Early Preclinical Studies (Cancer Cell Lines, Table 3 and Fig. 4)
Seven compounds and one RNA aptamer were reported with antiproliferative effects in various cancer cell lines (Table 3), although publicly available data are limited for many compounds in this group. 2,4-Bis(4-hydroxybenzyl) phenol was discovered to induce the dephosphorylation of HSF1 at Ser326 [87], a similar HSF1 inhibitory mechanism as PW3405 [89]. This compound can induce growth arrest and apoptosis of NCI-H460 human lung cancer cells. Using a smart synthetic library, Bach et al. found another HSR inhibitor, namely alpha-acyl aminocarboxamides, which can induce apoptosis in multiple myeloma cells [4]. Cardenolide CL-43, a natural compound, was identified through a heat-shock element-luciferase reporter system [48]. CL-43 can effectively inhibit the levels of all major HSP in the HCT-116 colon cancer line with no cytotoxicity observed in human fibroblasts.
An in-silico screening of lead-like library along with a cell-based assay led to the discovery of compound IHSF115 [70]. This compound can bind to an isolated HSF1 DNA binding-domain fragment in vitro and inhibit its transcriptional activity. IHSF115 exhibits a broad anticancer capacity in a panel of 33 cancer lines, with high sensitivity observed in multiple myeloma lines. In another study, Zaarur et al. identified NZ28 after screening of 20,000 compounds from several diversity compound libraries [88]. This compound potently inhibits the induction of HSP by heat shock, proteasome, and Hsp90 inhibitors in a variety of cell lines. An NZ28 analog, called emunin, strongly sensitizes myeloma cells to proteasome and HSP90 inhibitors as well as prostate carcinoma cells to proteasome inhibitors. Importantly, both NZ28 and emunin cause potent inhibition of HPS72 induction after heat shock in all tested cell lines.
Another phenotypic screen of 200,000 small molecules identified 4,6-disubstituted pyrimidines as a potent inhibitor of the HSF1 stress pathway [60]. Efforts on SAR and analog analysis led to the improvement of the HSF1 pathway inhibition to 14 nM potency with a U2OS human osteosarcoma tumor cell line. Interestingly, biochemical data showed high binding affinity (sub-micromolar to the single-digit micromolar range) of selected 4,6-pyrimidines to CDK9, suggesting possible roles of CDK9 in the inhibitory function of HSR. Unfortunately, this chemical series showed high clearance in mouse pharmacokinetic experiments, which were unsuitable for progression into the animal model study. Lastly, Stresgenin B was isolated as an inhibitor of the HSR pathway from a culture broth of Streptomyces sp. AS-9. This natural product showed inhibition of heat-induced reporter gene expression, including HSP70, HSP 90 and HSP110. Significant cytotoxicity against a panel of 6 cancer lines was documented with single to double-digit micromolar potency [1]
In summary, inhibitors in this category are either in their early stage of the preclinical study or have chemical liability (such as PK issues) that prevent them from entry into animal and human testing. It is also possible that the relevant animal data have not become publicly available at this point.
5 Lessons Learned from Preclinical and Clinical Studies
5.1 Improvement of Therapeutic Window by Prodrug Design and Nanoparticle Packaging
Many inhibitors in the HSR pathway show unfavorable chemical properties (low solubility and bioavailability) with a narrow therapeutic window. Treatments with increased dosages usually lead to undesirable cytotoxicity. Recent advancements of prodrug design research make it possible to intentionally design current HSR inhibitors as prodrugs for improved therapeutic effects [56]. FDA has already approved at least 30 prodrugs, providing a promising direction for the prodrug development of HSF1 pathway inhibitors. On the other hand, some of the inhibitors are of natural origin with significant challenges in the medicinal chemistry approach and prodrug design. Nanoparticles or liposome encapsulation can be introduced for the packaging of these natural compounds with improved efficacy [72]. With the advance of cancer biomarker research and imaging technology, this type of nanomedicines can be better targeted to cancerous tissues with precision.
5.2 Combined Application with Chemotherapy Drugs or Drugs against a Different Cancer Target
Shevtsov et al. recently published an excellent review on combination therapy of current anticancer drugs with molecular chaperone inhibitors [66]. An optimal drug combination has been proposed to simultaneously target cytoprotective mechanisms (i.e., heat shock response pathway) and malignant proliferation drivers (oncogenes, signal transduction players, cell cycle players, etc.) Such a combination strategy can allow these drugs to act synergistically while reducing doses of individual drugs and related unfavorable side effects [42]. Several HSR inhibitors have been applied to combinational therapy with synergistic anticancer effects. For example, Xiong et al. reported that triptolide can significantly enhance the antiproliferative effects of doxorubicin in human breast cancer line MCF-7 and MDA-MB-468 [79]. In combination with curcumin, triptolide also significantly reduces tumor cell proliferation in the ovarian cancer SKOV-3 line [41]. KBIBB11 and CL-43 also exhibit similar synergistic effects when combined with AKT inhibitor [7] and conventional chemotherapy agents [48]. Thus, screening for the best combination of these drugs may be the quickest way to create a novel cancer therapy with HSR pathway inhibitors.
5.3 Target Validation and Use of RNA Aptamer
Due to the complex nature of HSR pathways, the underlying molecular mechanisms for the majority of HSR pathway inhibitors are not clearly identified. Only two inhibitors, namely KRIBBII and IHSF115, demonstrate direct biochemical binding evidence to HSF1 in vitro. More detailed target identification studies are essential for the success of future drug development. Alternatively, recent publications on the RNA aptamer approach can open a different avenue to address this critical issue [61]. The complex tertiary folded structure of RNA aptamer makes it possible to achieve superior binding affinity and selectivity for a presumed cellular target. Currently, one aptamer drug has already been approved by the FDA [69], so more advanced projects can be initiated in this promising area [45].
5.4 HSF1 as a Potential Biomarker for Clinical Trial Design and Prognosis Prediction
It has been reported that elevated levels of HSF1 are generally associated with poor prognosis [8]. After analysis of over 3000 cancer patient samples, Wan and colleagues reported HSF1 overexpression as an unfavorable prognostic biomarker for a number of solid tumors, including breast cancer, hepatocellular carcinoma, non-small-cell lung cancer and pancreatic cancer, but not in osteosarcoma [71]. The recent advances in high-resolution imaging at single cell level, in combination with new molecular probes of cell types and metabolic states, will allow sensitive detection of HSF1/HSP expression of tumor samples in real time [13]. Thus, HSF1 pathway biomarkers can enable the development of tools for early cancer diagnosis, drug response prediction and therapeutic monitoring. Future clinical trial designs will particularly benefit from the better translation of basic science insights at the single cell level towards precision cancer treatment [24].
6 Conclusions
Significant progress have been made in the drug discovery and development of small molecule inhibitors against HSR pathways. Along with RNA aptamers, prodrugs, nanoparticles and combined therapy approaches, novel HSF1pathways-based cancer therapeutics will be created with tremendous clinical values.
Abbreviations
- HCS:
-
high content screening
- HSE:
-
heat shock element
- HSF1:
-
heat shock factor 1
- HSP:
-
heat shock protein
- HSR:
-
heat shock response
- HTS:
-
high throughput screening
- MOA:
-
mechanism of action
- NCT:
-
ClinicalTrials.gov identifier number
- PD:
-
pharmacodynamics
- PK:
-
pharmacokinetics
- SAR:
-
structure-activity relationship
- target ID:
-
target identification
References
Akagawa H, Takano Y, Ishii A, Mizuno S, Izui R, Sameshima T, Kawamura N, Dobashi K, Yoshioka T (1999) Stresgenin B, an inhibitor of heat-induced heat shock protein gene expression, produced by Streptomyces sp. AS-9. J Antibiot (Tokyo) 52(11):960–970
Anckar J, Sistonen L (2011) Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 80:1089–1115
Arora N, Alsaied O, Dauer P, Majumder K, Modi S, Giri B, Dudeja V, Banerjee S, Von Hoff D, Saluja A (2017) Downregulation of Sp1 by Minnelide leads to decrease in HSP70 and decrease in tumor burden of gastric cancer. PLoS One 12(2):e0171827
Bach M, Lehmann A, Brünnert D, Vanselow JT, Hartung A, Bargou RC, Holzgrabe U, Schlosser A, Chatterjee M (2017) Ugi Reaction-Derived α-Acyl Aminocarboxamides Bind to Phosphatidylinositol 3-Kinase-Related Kinases, Inhibit HSF1-Dependent Heat Shock Response, and Induce Apoptosis in Multiple Myeloma Cells. J Med Chem 60(10):4147–4160. https://doi.org/10.1021/acs.jmedchem.6b01613
Banerjee S, Saluja A (2015) Minnelide, a novel drug for pancreatic and liver cancer. Pancreatology 15(4 Suppl):S39–S43
Calderwood SK, Gong J (2016) Heat shock proteins promote Cancer: It’s a protection racket. Trends Biochem Sci 41(4):311–323
Carpenter RL, Sirkisoon S, Zhu D, Rimkus T, Harrison A, Anderson A, Paw I, Qasem S, Xing F, Liu Y, Chan M, Metheny-Barlow L, Pasche BC, Debinski W, Watabe K, Lo HW (2017) Combined inhibition of AKT and HSF1 suppresses breast cancer stem cells and tumor growth. Oncotarget 8(43):73947–73963
Carpenter RL, Gökmen-Polar Y (2019) HSF1 as a Cancer biomarker and therapeutic target. Curr Cancer Drug Targets 19(7):515–524
Cheeseman MD, Chessum NE, Rye CS, Pasqua AE, Tucker MJ, Wilding B, Evans LE, Lepri S, Richards M, Sharp SY, Ali S, Rowlands M, O’Fee L, Miah A, Hayes A, Henley AT, Powers M, Te Poele R, De Billy E, Pellegrino L, Raynaud F, Burke R, van Montfort RL, Eccles SA, Workman P, Jones K (2017) Discovery of a chemical probe Bisamide (CCT251236): an orally bioavailable efficacious Pirin ligand from a heat shock transcription factor 1 (HSF1) phenotypic screen. J Med Chem 60(1):180–201
Chen SR, Dai Y, Zhao J, Lin L, Wang Y, Wang Y (2018) A mechanistic overview of Triptolide and Celastrol, Natural Products from Tripterygium wilfordii Hook F. Front Pharmacol 9:104
Chessum NEA, Sharp SY, Caldwell JJ, Pasqua AE, Wilding B, Colombano G, Collins I, Ozer B, Richards M, Rowlands M, Stubbs M, Burke R, McAndrew PC, Clarke PA, Workman P, Cheeseman MD, Jones K (2018) Demonstrating in-cell target engagement using a Pirin protein degradation probe (CCT367766). J Med Chem 61(3):918–933
Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK (2012) A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 4(156):156ra139
Condeelis J, Weissleder R (2010) In vivo imaging in cancer. Cold Spring Harb Perspect Biol 2(12):a003848
Dai C, Whitesell L, Rogers AB, Lindquist S (2007) Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130(6):1005–1018
Dai C, Sampson SB (2016) HSF1: Guardian of Proteostasis in Cancer. Trends Cell Biol 26(1):17–28
Dayalan Naidu S, Dinkova-Kostova AT (2017) Regulation of the mammalian heat shock factor 1. FEBS J 284(11):1606–1627
Dong B, Jaeger AM, Thiele DJ (2019) Inhibiting heat shock factor 1 in Cancer: a unique therapeutic opportunity. Trends Pharmacol Sci:pii: S0165-6147(19) 30234-2
Dudeja V, Sharma N, Modi S, Merchant NB, Vickers SM, Banerjee S, Saluja A (2017) Evaluation of efficacy of oral administration of pro-drug of triptolide against pancreatic cancer. J Clin Onco 35(4 suppl):296
Feng C, Yuan X, Chu K, Zhang H, Ji W, Rui M (2019) Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int J Biol Macromol 125:700–710
Ferry DR, Smith A, Malkhandi J, Fyfe DW, de Takats PG, Anderson D, Baker J, Kerr DJ (1996) Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res 2(4):659–668
Fitzgerald DM, Hastings PJ, Rosenberg SM (2017) Stress-induced mutagenesis: implications in Cancer and drug resistance. Annu Rev Cancer Biol 1:119–140
Fok JHL, Hedayat S, Zhang L, Aronson LI, Mirabella F, Pawlyn C, Bright MD, Wardell CP, Keats JJ, De Billy E, Rye CS, Chessum NEA, Jones K, Morgan GJ, Eccles SA, Workman P, Davies FE (2018) HSF1 is essential for myeloma cell survival and a promising therapeutic target. Clin Cancer Res 24(10):2395–2407
Giri B, Gupta VK, Yaffe B, Modi S, Roy P, Sethi V, Lavania SP, Vickers SM, Dudeja V, Banerjee S, Watts J, Saluja A (2019) Pre-clinical evaluation of Minnelide as a therapy for acute myeloid leukemia. J Transl Med 17(1):163
Garralda E, Dienstmann R, Piris-Giménez A, Braña I, Rodon J, Tabernero J (2019) New clinical trial designs in the era of precision medicine. Mol Oncol 13(3):549–557
Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K, Kouretas D, Tzanakakis G, Nikitovic D, Anisimov NY, Spandidos DA, Tsatsakis AM, Rezaee R (2017) Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol Rep 38(2):819–828
Huang W, He T, Chai C, Yang Y, Zheng Y, Zhou P, Qiao X, Zhang B, Liu Z, Wang J, Shi C, Lei L, Gao K, Li H, Zhong S, Yao L, Huang ME, Lei M (2012) Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS One 7(5):e37693
Jia S, Xu X, Zhou S, Chen Y, Ding G, Cao L (2019) Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis 10(2):142
Kijima T, Prince T, Neckers L, Koga F, Fujii Y (2019) Heat shock factor 1 (HSF1)-targeted anticancer therapeutics: overview of current preclinical progress. Expert Opin Ther Targets 23(5):369–377
Kim JA, Kim Y, Kwon BM, Han DC (2013) The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J Biol Chem 288(40):28713–28726
Kim JA, Lee S, Kim DE, Kim M, Kwon BM, Han DC (2015) Fisetin, a dietary flavonoid, induces apoptosis of cancer cells by inhibiting HSF1 activity through blocking its binding to the hsp70 promoter. Carcinogenesis 36(6):696–706
Kitzen JJ, de Jonge MJ, Lamers CH, Eskens FA, van der Biessen D, van Doorn L, Ter Steeg J, Brandely M, Puozzo C, Verweij J (2009) Phase I dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumors. Eur J Cancer 45(10):1764–1772
Koishi M, Yokota S, Mae T, Nishimura Y, Kanamori S, Horii N, Shibuya K, Sasai K, Hiraoka M (2001) The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clin Cancer Res 7(1):215–219
Lang BJ, Guerrero-Giménez ME, Prince TL, Ackerman A, Bonorino C, Calderwood SK (2019) Heat shock proteins are essential components in transformation and tumor progression: Cancer cell intrinsic pathways and beyond. Int J Mol Sci 20(18):pii: E4507
Lee YK, Shin JI, Lee WS, Lee HG, Hwang JT, Park OJ (2009) Inhibitory properties of tumor growth by Quercetin in Xenograft models. Cancer Prev Res 14(1):54–59
Li J, Liu R, Yang Y, Huang Y, Li X, Liu R, Shen X (2014) Triptolide-induced in vitro and in vivo cytotoxicity in human breast cancer stem cells and primary breast cancer cells. Oncol Rep 31(5):2181–2186
Li H, Pan GF, Jiang ZZ, Yang J, Sun LX, Zhang LY (2015) Triptolide inhibits human breast cancer MCF-7 cell growth via downregulation of the ERα-mediated signaling pathway. Acta Pharmacol Sin 36(5):606–613
Li HC, Xia ZH, Chen YF, Yang F, Feng W, Cai H, Mei Y, Jiang YM, Xu K, Feng DX (2017a) Cantharidin inhibits the growth of triple-negative breast cancer cells by suppressing autophagy and inducing apoptosis in vitro and in vivo. Cell Physiol Biochem 43(5):1829–1840. https://doi.org/10.1159/000484069
Li CC, Yu FS, Fan MJ, Chen YY, Lien JC, Chou YC, Lu HF, Tang NY, Peng SF, Huang WW, Chung JG (2017b) Anticancer effects of cantharidin in A431 human skin cancer (Epidermoid carcinoma) cells in vitro and in vivo. Environ Toxicol 32(3):723–738. https://doi.org/10.1002/tox.22273
Liu Y, Gong W, Yang ZY, Zhou XS, Gong C, Zhang TR, Wei X, Ma D, Ye F, Gao QL (2017a) Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 22(4):544–557
Liu XF, Long HJ, Miao XY, Liu GL, Yao HL (2017b) Fisetin inhibits liver cancer growth in a mouse model: relation to dopamine receptor. Oncol Rep 38(1):53–62
Liu L, Xiong X, Shen M, Ru D, Gao P, Zhang X, Huang C, Sun Y, Li H, Duan Y (2018) Co-delivery of Triptolide and Curcumin for ovarian Cancer targeting therapy via mPEG-DPPE/CaP nanoparticle. J Biomed Nanotechnol 14(10):1761–1772
Lopez JS, Banerji U (2017) Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol 14(1):57–66
Luo J, Solimini NL, Elledge SJ (2009) Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136(5):823–837
Miles SL, McFarland M, Niles RM (2014) Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease. Nutr Rev 72(11):720–734
Morita Y, Leslie M, Kameyama H, Volk DE, Tanaka T (2018) Aptamer therapeutics in Cancer: current and future. Cancers (Basel) 10(3):pii: E80
Mulholland PJ, Ferry DR, Anderson D, Hussain SA, Young AM, Cook JE, Hodgkin E, Nagai N, Nakai A, Nagata K (1995) Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun 208(3):1099–1105
Nagai N, Nakai A, Nagata K (1995) Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun 208(3):1099–1105. https://doi.org/10.1006/bbrc.1995.1447
Nikotina AD, Koludarova L, Komarova EY, Mikhaylova ER, Aksenov ND, Suezov R, Kartzev VG, Margulis BA, Guzhova IV (2018) Discovery and optimization of cardenolides inhibiting HSF1 activation in human colon HCT-116 cancer cells. Oncotarget 9(43):27268–27279
Noel P, Von Hoff DD, Saluja AK, Velagapudi M, Borazanci E, Han H (2019) Triptolide and its derivatives as Cancer therapies. Trends Pharmacol Sci 40(5):327–341
Parekh S (2018) Targeting HSF1: a prime integrator of Proteotoxic stress response in myeloma. Clin Cancer Res 24(10):2237–2238
Patil S, Lis LG, Schumacher RJ, Norris BJ, Morgan ML, Cuellar RA, Blazar BR, Suryanarayanan R, Gurvich VJ, Georg GI (2015) Phosphonooxymethyl prodrug of Triptolide: synthesis, physicochemical characterization, and efficacy in human Colon adenocarcinoma and ovarian Cancer Xenografts. J Med Chem 58(23):9334–9344
Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK (2007) Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res 67(19):9407–9416
Powers MV, Workman P (2007) Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett 581(19):3758–3769
Priego S, Feddi F, Ferrer P, Mena S, Benlloch M, Ortega A, Carretero J, Obrador E, Asensi M, Estrela JM (2008) Natural polyphenols facilitate elimination of HT-29 colorectal cancer xenografts by chemoradiotherapy: a Bcl-2- and superoxide dismutase 2-dependent mechanism. Mol Cancer Ther 7(10):3330–3342
Propper D, Han H, Hoff DV, Borazanci E, Reya T, Ghergurovich J, Pshenichnaya I, Antal C, Condjella R, Sharma S, O’Dwyer P, Littlewood T, Patel H, Saluja A, Velagapudi M, Yang L, Downes M, Evans R, Evan G (2019) Abstract CT165: phase II open label trial of minnelideTM in patients with chemotherapy refractory metastatic pancreatic cancer. Cancer Res 79(13 Supplement)
Rautio J, Meanwell NA, Di L, Hageman MJ (2018) The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov 17(8):559–587
Reno TA, Kim JY, Raz DJ (2015) Triptolide inhibits lung Cancer cell migration, invasion, and metastasis. Ann Thorac Surg 100(5):1817–1824
Reno TA, Tong SW, Wu J, Fidler JM, Nelson R, Kim JY, Raz DJ (2016) The triptolide derivative MRx102 inhibits Wnt pathway activation and has potent anti-tumor effects in lung cancer. BMC Cancer 11(16):439
Reyes-Farias M, Carrasco-Pozo C (2019) The anti-Cancer effect of Quercetin: molecular implications in Cancer metabolism. Int J Mol Sci 28:20(13)
Rye CS, Chessum NE, Lamont S, Pike KG, Faulder P, Demeritt J, Kemmitt P, Tucker J, Zani L, Cheeseman MD, Isaac R, Goodwin L, Boros J, Raynaud F, Hayes A, Henley AT, de Billy E, Lynch CJ, Sharp SY, Te Poele R, Fee LO, Foote KM, Green S, Workman P, Jones K (2016) Discovery of 4,6-disubstituted pyrimidines as potent inhibitors of the heat shock factor 1 (HSF1) stress pathway and CDK9. Medchemcomm 7(8):1580–1586
Salamanca HH, Antonyak MA, Cerione RA, Shi H, Lis JT (2014) Inhibiting heat shock factor 1 in human cancer cells with a potent RNA aptamer. PLoS One 9(5):e96330
Santagata S, Mendillo ML, Tang YC, Subramanian A, Perley CC, Roche SP, Wong B, Narayan R, Kwon H, Koeva M, Amon A, Golub TR, Porco JA Jr, Whitesell L, Lindquist S (2013) Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science 341(6143):1238303
Sethi V, Giri B, Garg B, Modi S, Banerjee S, Ramakrishnan S, Saluja A, Dudeja V (2017) A pre-clinical evaluation of minnelide in treating melanoma. J Am Coll Surg 225(4):E46. https://doi.org/10.1016/j.jamcollsurg.2017.07.641
Seymour LW, Kerr DJ (2001) Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann Oncol 12(2):245–248
Sharma C, Seo YH (2018) Small molecule inhibitors of HSF1-activated pathways as potential next-generation anticancer therapeutics. Molecules 23(11):pii: E2757
Shevtsov M, Multhoff G, Mikhaylova E, Shibata A, Guzhova I, Margulis B (2019) Combination of anti-Cancer drugs with molecular chaperone inhibitors. Int J Mol Sci 20(21):pii: E5284
Shin YC, Ko SG (2016) Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in HER2-overexpressing BT-474 breast cancer cells. Oncol Rep 36(1):31–42
Solimini NL, Luo J, Elledge SJ (2007) Non-oncogene addiction and the stress phenotype of cancer cells. Cell 130(6):986–988
Stein CA, Castanotto D (2017) FDA-approved oligonucleotide therapies in 2017. Mol Ther 25(5):1069–1075
Vilaboa N, Boré A, Martin-Saavedra F, Bayford M, Winfield N, Firth-Clark S, Kirton SB, Voellmy R (2017) New inhibitor targeting human transcription factor HSF1: effects on the heat shock response and tumor cell survival. Nucleic Acids Res 45(10):5797–5817
Wan T, Shao J, Hu B, Liu G, Luo P, Zhou Y (2018) Prognostic role of HSF1 overexpression in solid tumors: a pooled analysis of 3,159 patients. Onco Targets Ther 11:383–393
Wang AZ, Langer R, Farokhzad OC (2012) Nanoparticle delivery of cancer drugs. Annu Rev Med 63:185–198
Wang B, Li Y, Tan F, Xiao Z (2016a) Chinese herb derived-Rocaglamide a is a potent inhibitor of pancreatic cancer cells. Am J Transl Res 8(2):1047–1054
Wang X, Zhao F, Lv ZM, Shi WQ, Zhang LY, Yan M (2016b) Triptolide disrupts the actin-based Sertoli-germ cells adherens junctions by inhibiting rho GTPasesexpression. Toxicol Appl Pharmacol 310:32–40
Weinstein IB (2002) Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science 297(5578):63–64
Westerheide SD, Kawahara TL, Orton K, Morimoto RI (2006) (2006) Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem 281(14):9616–9622
Wu Y, Giaisi M, Köhler R, Chen WM, Krammer PH, Li-Weber M (2017) Rocaglamide breaks TRAIL-resistance in human multiple myeloma and acute T-cell leukemia in vivo in a mouse xenogtraft model. Cancer Lett 389:70–77
Xie M, Wu J, Ji L, Jiang X, Zhang J, Ge M, Cai X (2019) Development of Triptolide self-microemulsifying drug delivery system and its anti-tumor effect on gastric Cancer Xenografts. Front Oncol 9:978
Xiong J, Su T, Qu Z, Yang Q, Wang Y, Li J, Zhou S (2016) Triptolide has anticancer and chemosensitization effects by down-regulating Akt activation through the MDM2/REST pathway in human breast cancer. Oncotarget 7(17):23933–23946
Xu MD, Liu L, Wu MY, Jiang M, Shou LM, Wang WJ, Wu J, Zhang Y, Gong FR, Chen K, Tao M, Zhi Q, Li W (2018) The combination of cantharidin and antiangiogenic therapeutics presents additive antitumor effects against pancreatic cancer. Oncogenesis 7(11):94
Yang K, Lamprecht SA, Liu Y, Shinozaki H, Fan K, Leung D, Newmark H, Steele VE, Kelloff GJ, Lipkin M (2000) Chemoprevention studies of the flavonoids quercetin and rutin in normal and azoxymethane-treated mouse colon. Carcinogenesis 21(9):1655–1660
Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L (2003) Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther 2(1):65–72
Yang W, Cui M, Lee J, Gong W, Wang S, Fu J, Wu G, Yan K (2016) Heat shock protein inhibitor, quercetin, as a novel adjuvant agent to improve radiofrequency ablation-induced tumor destruction and its molecular mechanism. Chin J Cancer Res 28(1):19–28
Yang S, Ren X, Liang Y, Yan Y, Zhou Y, Hu J, Wang Z, Song F, Wang F, Liao W, Liao W, Ding Y, Liang L (2019) KNK437 restricts the growth and metastasis of colorectal cancer via targeting DNAJA1/CDC45 axis. Oncogene. https://doi.org/10.1038/s41388-019-0978-0. [Epub ahead of print]
Yokota S, Kitahara M, Nagata K (2000) Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res 60(11):2942–2948
Yoon YJ, Kim JA, Shin KD, Shin DS, Han YM, Lee YJ, Lee JS, Kwon BM, Han DC (2011) KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J Biol Chem 286(3):1737–1747
Yoon T, Kang GY, Han AR, Seo EK, Lee YS (2014) 2,4-Bis(4-hydroxybenzyl)phenol inhibits heat shock transcription factor 1 and sensitizes lung cancer cells to conventional anticancer modalities. J Nat Prod 77(5):1123–1129
Zaarur N, Gabai VL, Porco JA Jr, Calderwood S, Sherman MY (2006) Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res 66(3):1783–1791
Zhang D, Zhang B (2016) Selective killing of cancer cells by small molecules targeting heat shock stress response. Biochem Biophys Res Commun 478(4):1509–1514
Zhang D, Zhang B (2018) High content screening of small molecule modulators targeting heat shock response pathway. In: Alexzander AA, Asea PK (eds) Heat shock proteins and stress, vol 15, pp 141–165
Zhang X, Lin CC, Chan WK, Liu KL, Yang ZJ, Zhang HQ (2017) Augmented anticancer effects of Cantharidin with liposomal encapsulation: In Vitro and In Vivo evaluation. Molecules 22(7):pii: E1052
Zhou J, Fang L, Liao J, Li L, Yao W, Xiong Z, Zhou X (2017) Investigation of the anti-cancer effect of quercetin on HepG2 cells in vivo. PLoS One 12(3):e0172838
Acknowledgements
The authors would like to thank all the supports received from everyone at Alpine Therapeutics. Special acknowledgements are given to Sydney Zhang for his generous help during the preparation of this manuscript. This study was funded by private research funding through Alpine Therapeutics, Inc.
Disclosure of Interests
All authors declare they have no conflict of interest.
Ethical Approval for Studies Involving in Humans
This article does not contain any studies with human participants performed by any of the authors.
Ethical Approval for Studies Involving in Animals
This article does not contain any studies with animals performed by any of the author.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zhang, D., Wang, D., Zhang, B. (2020). Small Molecule Inhibitors Targeting Heat Shock Response Pathways: Lessons from Clinical and Preclinical Studies in Cancer Therapeutics. In: Asea, A.A.A., Kaur, P. (eds) Heat Shock Proteins in Human Diseases. Heat Shock Proteins, vol 21. Springer, Cham. https://doi.org/10.1007/7515_2020_2
Download citation
DOI: https://doi.org/10.1007/7515_2020_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62288-6
Online ISBN: 978-3-030-62289-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)