Abstract
Primordial Germ Cells (PGC) are the progenitors of the germ-line and differentiate into spermatozoa or oocytes. They are responsible for the transmission of genetic and epigenetic information from one generation to the other. Since the nineteenth century, PGCs have been investigated using different techniques. Regardless of their mode of specification, inheritance or induction, PGCs arise early in development and migrate by a combination of passive and active movements towards the gonadic ridges. The migration of PGCs is very similar to the path taken by metastasis and germ cells are very often proposed as a model for the study of cell migration. Their pathway of migration is regulated by different signals that interact with Hsp90, an ATP-dependent chaperone associated with numerous tumors and used to grade the malignancy. Furthermore, some of the signals regulating PGCs have been proved to have a role in different cancers. This underlines the idea that PGCs could be an interesting tool to study long-distance cell migration and perhaps cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Primordial Germ Cells (PGCs) strongly express Heat Shock Protein 90 (Hsp90) during their entire life cycle. Understanding the role of Hsp90 during migration and differentiation of PGCs constitutes a major challenge, as well as establishing their putative function during evolution of species. Some relationships between PGCs migration and cancer dissemination could suggest common roles for Hsp90 in both processes. In this contribution, we will discuss the state of the art in the implications of Hsp90 in germs cells migration, and its potential correlation with the metastatic processes.
1.1 Identification of the Primordial Germ Cells
Primordial Germ Cells (PGCs) are present in all sexually reproducing animals. They differentiate into spermatozoa or oocytes. In this way, they represent the origin of any new organisms and are responsible for the transmission of genetic and epigenetic information across generations. PGCs are easily recognized thanks to their histological characteristics (Niewkoop and Sutasurya 1979). They are described as large cells (10–20 μm) with a round, oval or pear- shape. The cell nucleus is as well of a substantial size (6–10 μm) with a sparse content rending the nuclear membrane more evident. Depending on the stage of development and the species, the cytoplasm is filled with a various amount of lipid droplets that displace the nucleus in an eccentric position. Glycogen is also identified present and is of primary interest in the PAS-positive identification of these cells. The cytoplasm has a granular appearance with numerous ribosomes and contains a structure called the center sphere, cytoplasmic crescent or attraction sphere. It is made of one or two centrioles, mitochondrias and yolk granules. Eosinophilic granules have been considered as a usefull histological criteria to identify germ cells in fish (Nagai et al. 2001; Gamo 1961; Timmermans 1996; Kazama-Wakabayashi et al. 1999). PGCs also exhibit lamellopodias and fillopodias. This ability is related to their capacity to migrate and will be discussed in greater detail in the description of the migration pathways. Since Weishmann first identified the difference between germinal and somatic cells in the nineteenth century, CGPs were studied by several authors using their morphological characteristics (Heys 1931; Everett 1945; Niewkoop and Sutasurya 1979). Different stainings among which we can mention the Periodic acid-Schiff, the Best’s Carmine or the Hematoxylin and eosin were used. At this time, the demonstration of alkaline phosphatase activity seemed to be an appropriate way to identify and locate these cells (Fig. 4.1).
Using this technique, Chiquoine (1954) was the first to give a complete description of the origin and migration of the PGCs in the mouse embryo. Nevertheless, this method has been declared unreliable by some because the morphological criteria used to recognize the germ cells could be observed in the entirety of the embryo’s tissues (Hargitt 1925; Simkins 1923, 1928). The fact that the selective staining of PGCs with this technique was based on a short incubation in the buffer can explain the raise of criticism. Furthermore, it already underlined the need of a more specific method for the identification of the PGCs. Immunohistochemistry is largely used for the identification of PGCs. Stage-specific embryonic antigens (SSEA) is a glycoprotein found on the PGC’s surface and is thus considered as a reliable marker for the study of these cells (Yon and Akbulut 2015; Fox et al. 1981; Shevinsky et al. 1982). Nevertheless, SSEA positivity has been observed in non-germinal tissue depending on the stage of the embryo. Fox et al. (1981) studied the localization of SSEA-1 in mouse embryo and reported positivity on the surface of ectodermal cells, visceral endoderm, primordium of the heart and somites, in the neural crest, the visceral yolk sac and gut endoderm just to mention a few. D’Costa and Petitte (1999) confirmed the observations made by Fox et al. in turkey embryos indicating that SSEA-1 is not germline-specific. Another cell-surface carbohydrate used to recognize PGCs is the embryonic mouse antigen 1 (EMA-1) (Hahnel and Eddy 1986). However, a study made by Urven et al. (1988) reported that only one third to one fifth of the PAS-positive PGCs were labeled by antiEMA-1 suggesting that EMA-1 is not the best marker to identify and follow the migration of the germ cells. The vasa gene was first identified in Drosophila by Lasko and Ashburner (1988). This member of the posterior group genes is required in germline development. Furthermore, the absence of the vasa transcript from somatic cells suggested that this transcript is germline specific. The vasa protein is a member of the DEAD (Asp-Glu-Ala-Asp) family of ATP-dependent RNA helicases which expression is restricted to the germline. Vasa homologues have been identified in Caenorhabditis, mouse, zebrafish, rats, xenopous and humans (Deborah and Bennett 1993; Fujiwara et al. 1994; Olsen et al. 1997; Castrillon et al. 2000; Noce et al. 2001; Tsunekawa et al. 2000). Other markers have been used in various studies on PGCs. Among those, we can quote the work of Vanmuylder et al. (2002). This study described the expression of the two cytosolic isoform of Hsp90 in mice, Hsp86 and Hsp84, respectively corresponding to the human Hsp90α and Hsp90β. PGCs strongly expressed Hsp86 from their appearance on the yolk sac until they reaches the genital ridges. Hsp86-positive PGCs were successively observed at the base of the allantois (Fig. 4.2), in the epithelium lining the hindgut, migrating into the dorsal mesentery (Fig. 4.3) and finally into the genital ridges. Gonadic expression of Hsp86 is limited to the germline. They confirmed the idea of the protective role of the members of the Hsp90 family and that Hsp86 could be the protein that preserve mammals from phenotypic mutations.
1.2 Migration Pathways of the Primordial Germ Cells
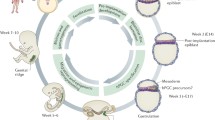
Primordial germ cells arise early in development and migrate by a combination of passive and active movements towards the gonadic ridges. There are two different mechanisms described for PGCs formation: Inheritance of a germ plasma or induction by signals. The first mode is the one observed in Invertebrates such as Drosophila or Caenorhabidis and in nonmammalian vertebrates such as frogs and fishes (Kazama-Wakabayashi et al. 1999). The germ plasm, also called pole plasm in flies, consists of maternally derived RNAs, RNA-binding-protein and organelles and is transmitted to the future germ cells during the first divisions of the embryo. On the other side, in mammals, PGCs are specified in the epiblast shortly before or during gastrulation and are under the influence of specific signals secreted by surrounding cells (Ginsburg et al. 1990; McLaren 2003; Molyneaux and Wylie 2004; Saitou and Yamaji 2012; Tam and Zhou 1996). In the mouse, primordial germ cells appear at the base of the allantois at day 7,5. They migrate through the intestinal endoderm between day 8 and day 9 before colonizing the genital ridge through migration in the dorsal mesentery. Fuss and Felix were the first to study the migration of the germ cells in human embryos (Fuss 1911; Felix 1911). The pathway of this migration is very similar to the one observed in mice. Human PGCs arise in the endoderm of the yolk sac at the end of the 3rd week. They migrate through the gut epithelium during the 5th week to reach the dorsal mesentery and finally colonize the gonadic ridges at the beginning of the 6th week (De Felici 2013; McKay et al. 1953). It is of interest to indicate that this pathway of migration is different in other species. In birds, PGCs are first localized in the central zone of the epiblast. They are then moved anteriorly to form the germinal crescent with the apparition of the primitive streak. They enter the forming vascular network at stage Hamburger Hamilton (HH) 10. They are localized in the extra-embryonic vessels at stage 12 and in the embryonic vessels at stage 13. They leave the blood circulation coming out the visceral branches of the aorta and reach the genital ridges (Swift 1914; Ginsburg 1997). In reptiles, the pathway depends on the group of classification. In Anapsids, the migration is interstitial while in Diapsids, the migration will be a combination of the interstitial and circulatory modes for the primitive ones and only circulatory for the superior ones (Dustin 1910). The migration of PGCs is, especially in birds, very similar to the path taken by metastasis and this is why germ cells are often proposed as a model for the study of cell migration. Lejong et al. (2018) reported that geldanamycin, a well-known Hsp90 inhibitor with potent anti-tumor activity, decreases the number of germ cells in the mouse embryo. In 2009, preliminary results of Vanmuylder et al. demonstrated that Geldanamycin administration reduces the number of HSP90-positive germ cells in the mouse embryo. They also found cells with morphological features similar to PGCs in the pelvis as shown in teratoma (Fig. 4.4). In their study, Lejong et al. (2018) compared the immune reactivity of the PGCs for both Hsp90 and Vasa (Fig. 4.5). Those were identical and the authors therefore expressed the idea that Hsp90 could be considered as a reliable marker for PGCs investigation. Furthermore, the cells found in the pelvis suggested that some cells at least got lost. These results suggest that an inhibition of the Hsp90 could decrease the capacity of the germ cells to migrate.
1.3 Molecular Control of PGC Migration
PGCs specification and correct migration are under the influence of different signals. In 1996, Tam and Zhou transplanted distal epiblast close to the extraembryonic ectoderm before E6,5 and observed the formation of PGCs even though cells from the distal epiblast are not supposed to generate this cellular type. On the other hand, transplantation of proximal epiblast into the distal region, far away from the extraembryonic ectoderm, never gave rise to PGCs. They concluded that, in the gastrulating mouse embryo, PGCs specification depends on signals coming from the extraembryonic ectoderm. Bone morphogenetic proteins (Bmps) are members of the transforming growth factor β superfamily of intercellular signaling proteins, TGFβ. At least three Bmps have been demonstrated to be involved in the generation and migration of PGCs: Bmp4, Bmp2 and Bmp8 (Ying et al. 2000, Ying and Zhao 2001; Machev et al. 2004; Lawson et al. 1999; de Sousa Lopes et al. 2004; Dudley et al. 2007). BMP4 is strongly expressed before gastrulation in the extraembryonic ectoderm. Bmp4 homozygous null embryos are depleted of PGCs and lack allantois. Heterozygous embryos are normal regarding the morphological features but contain less PGCs compared to the wild-type embryos. Lawson et al. proposed a model in which two signals are required to generate PGCs and allantois. The first signal consists of a high concentration of Bmp4 which specifies a group of cells that will become either PGCs or allantoic cells depending on a second signal. Bmp2 and Bmp4 have an additive effect in PGC specification. Indeed, Bmp2+/− Bmp4+/− embryos have even less PGCs than the Bmp4 heterozygous mutant embryo. Bmp signaling also regulates PGCs motility. Dudley et al. (2007) studied the role of Bmps on cultured PGCs in migration. They found that Bmp4 has a bi-phasic effect on PGCs number. Treatment with low doses of Bmp4 increases this number whereas high doses decreases it. The BMP-inhibitor Noggin has the opposite effect. Furthermore, they observed that treatment of the slices with Noggin greatly affects PGCs motility. Hsp90 inhibition has been proved to destabilizes TGFβ signaling by decreasing the levels of Bmps receptors such as Alk2, Bmpr1a, Bmpr2 and Actr2 (Haupt et al. 2012). De Sousa Lopes et al. demonstrated that Bmp4 signals through ALK2 for PGCs formation. Furthermore, phosphorylation of the Bmps receptors results in the phosphorylation of Smad-1, Smad-5 and Smad-8. Smad-1 and Smad-5 mutant mouse embryos display a great decrease even a complete lack in PGCs.
Cell migration including the one of the PGCs implies several intracellular modifications including cytoskeleton reorganization. As described earlier, filopodias are one of the morphological features of PGCs in migration. Terayama et al. (2013) made a study on Xenopus primordial germ cells. They results showed that germ cells motility requires actin polymerization and myosin activity and that PGCs migration depends on ROCK/RhoA signaling. Hsp90 binds actin in vitro (Koyasu et al. 1986). Taiyab and Mohan Rao (2011) demonstrated that geldanamycin decreased cell motility and invasion. They showed that Hsp90 inhibition decreases the number of membrane protrusions in F111 cells. They observed a significant decrease in the levels of RhoA and mDia2, members of the Rho family known to be involved in the generation of contractile force and in the formation of lamellipodias.
Closely related to the cytoskeleton is the cadherin superfamily. These glycoproteins are expressed on the surface membrane and play a role in cell adhesion. During their migration, PGCs form a network through cytoplasmic processes or filopodias. Bendel-Stenze et al. (2000) investigated the expression of cadherins during PGCs migration. At E 8,5, PGCs were negative for E-Cadherin. From E9,5, the expression of E-cadherin was in function of the location of the PGCs. The cells still nested in the hindgut remained negative whereas the ones migrating towards the genital ridges started being positive. E-cadherin expression was strongly expressed at the junctions between germ cells and in the genital ridges. The culture of slices of E10,5 embryos with E-cadherin blocking antibodies prevented the condensation of PGCs into the gonadal ridge (GR) and ectopic germ cells were observed. Epithelial mesenchymal transition (EMT) is a process in which cells gain the capacity to migrate. E-cadherin plays a key role in this process. Hsp90 secreted by colorectal cancer cells has been proved to facilitate EMT by decreasing E-cadherin expression (Chen et al. 2013).
Steel factor, is a short-chain helical cytokine that binds to the c-kit receptor, expressed by PGCs throughout their migration. The Steel-c-kit interaction is mandatory for PGCs survival. A mechanism called midline death is set up after E9,5 to get rid of the ectopic cells, mostly in the midline (Gu et al. 2006). PGCs that remain in the midline after E10,5 die by apoptosis. Steel −/− mutant embryos display an important loss of PGCs before E9. Gu et al. (2006) showed that the culture of these embryos in a medium supplemented in Steel factor restored the protection against apoptosis whereas blocking the c-kit receptor removed this protective effect. Hsp90 inhibition by STA-9090 has been proved to downregulate Kit phosphorylation and tumor growth in malignant mast cell tumor (Lin et al. 2008).
The chemokine stromal cell-derived factor 1 (SDF-1) provides directional clues for the migration f PGCs (Ara et al. 2003; Doitsidou et al. 2002; Stebler et al. 2004; Takeuchi et al. 2010). SDF-1 is a chemokine which acts via a G protein-coupled receptor, CXCR4. SDF-1 is involved in lymphocyte and blood vessels development, cardiac septum formation and embryonic viability. Toshiaki et al. demonstrated that PGCs migration in SDF-1−/− mice was unaffected until E9,5. However, only 14% of PGCs were found in the genital ridges of the mutants. The amount of PGCs remaining in the hindgut and the mesentery was also higher. SDF-1 has a key role in post- natal vasculogenesis after ischemic injuries. Ischemia induces an increase in SDF-1 expression which in turn generates the recruitment of endothelium progenitor cells. Wan et al. (2010) demonstrated that Hsp90 inhibition with geldanamycin decreases SDF-1 expression and the neovascularization.
Pfeiffer et al. (2018) also investigated the role of Hsp90 in the migration of PGCS in zebrafish embryos. To do so, they compared the RNA expression profile of motile PGCs with that of PGCS in the gonads and with that of somatic cells. Hsp90 is strongly expressed in PGCs all along their migration. Mutant embryos showed impaired colonization of the gonads due to a significant decrease in the displacement and straightness of the migration path of the PGCs. They observed the presence of two Microtubule Organizing Center (MOTC) in embryos knocked down for Hsp90. This interferes with a correct polarization of the cell and so, prevents a correct migration pathway. In mutant embryos, about twice more PGCs located in ectopic positions were observed compared to the control embryos. Pfeiffer et al. (2018) also noted defects in cell cycle progression with a prolonged time spent in the S/G2/M phases in embryos with decreased or no Hsp90 activity. This again interferes with the polarization of the cell and prevents a correct response to the guidance clues.
1.4 Hsp90: An Interesting Tool in Epigenetic Evolution
The first evidence that HSP90 could play a role on biological evolution was brought by Rutherford and Lindquist (1998), in Drosophila melanogaster. Heterozygotous HSP83 mutant flies exhibited some homeotic abnormalities. Outcrossing mutant flies with normal strains displayed same kinds of abnormalities. Little changes in temperature increases the amount of abnormalities in heterozygotous, as well as exposition to a HSP90 inhibitor, the geldanamycin. The authors concluded that, with HSP90 buffering inhibition, cryptic variants due to normal variation can phenotypically express. These results were confirmed by Sollars et al. (2003), who observed in drosophila exposed to geldanamycin and trichostatin an abnormal expression of wingless in the eye imaginal discs, associated with abnormal eye phenotypes. Mutation of the trithorax genes were also observed, and the abnormalities were heritable in the offspring.
In the plant Arabidopsis thaliana, exposition to two Hsp90 inhibitors (geldanamycin and radicicol) leads to an increase of phenotypical variation (Queitsch et al. 2002). The conclusions were that HSP90 buffers the expression of genetic variations, affects developmental plasticity and increases stability against stochastic process. The cavefish astyanax mexicanum was treated by radicicol (Rohner et al. 2013). The surface-dwelling strain (which possess eyes) exhibited morphological variations of both eye and eye-socket, whereas cave-dwelling strains leaded to a reduction of the eyeless orbital cavity. Peuβ et al. (2015) demonstrated in insects that downregulation of Hsp90 can be reduced in cases of genetic variation and that it can be beneficial. In the nematodes Caenorhabidis elegans and c. briggsae, specific knockdown of Hsp90 demonstrated that HSP90 regulates the mobility of transposable elements, considered as drivers of genomic evolutionary changes (Ryan et al. 2016).
It is also classically considered that the primary role of HSP90 is to prevent aggregation of denatured proteins (Stankiewicz and Mayer 2012). Several examples of refolding of denatured or muted proteins by HSP90 were described (Miyata and Yahara 1992; Buchner 1999; Yahara 1999). Some proteins act as transcriptional regulators. Therefore, failure in refolding of defective proteins by HSP90 can uncover silent mutations. HSP90 can thus be considered by a potential regulator of evolvability (Wagner et al. 1999). The fact that Hsp90 is strongly expressed in the germ-line without any interruption from the first stages of embryonic development to the adult in mammals, including the humans (Figs. 4.6, 4.7, and 4.8) (Vanmuylder et al. 2002; Louryan et al. 2002, 2003) suggest that its protective function could be particularly needful in this cell-line, in order to stabilize the offspring against phenotypical changes due to cryptic genetic variations. Furthermore, the fact that HSP90 function can be reduced by temperature changes suggest a possible role in biological evolution associated with dramatic climatic changes.
1.5 Hsp90, Germ Cell and Cancer
The importance of the regulation of cell cycle and migration mechanisms by Hsp90 is well known and characterized as much in germ cells than in cancer cells (Pfeiffer et al. 2018). Hsp90 has a key role in folding/unfolding of proteins but its function extends far beyond. Hsp90 is expressed in the cytosol under normal conditions and is involved in various steps of development, differentiation, apoptosis and oncogenesis (Neckers and Workman 2012). Hsp90 is used as a marker of malignancy for several tumors. The human orthologue HSP90 has been specially studied in the context of cancer spread (reviewed in Wu et al. 2017). For example, Hsp86, corresponding to the human Hsp90α, is overexpressed in pleomorphic adenoma (mixed tumor (Fig. 4.9)) and in some Warthin tumors (cystadenolymphoma) especially in germinative centers (Fig. 4.10) probably due to malignant transformation rates in mixed tumor compared to other adenoma or higher recurrence rate for benign tumors (Vanmuylder et al. 2000; Wang et al. 2013). HSP86 expression in embryonic cells since their appearance at the surface of yolk sac until gonadic mature area is also maintained in yolk sac tumor (Fig. 4.11) (Vanmuylder et al. 2004). Hsp86 inhibition by geldanamycin reduces germ cells migration and increases ectopic germ cell populations, similar to teratomas (Fig. 4.7) (Vanmuylder et al. 2009). Primordial germ cells arise early in development and migrate through different tissues to reach the genital ridges. This mechanism is under the control of several signal pathways some of which have been highlighted in cancer spread. Migration of PGCs seems therefore an interesting model to study long distance cell migration and perhaps cancer.
2 Conclusions
Hsp90 is clearly involved in PGCs migration and stabilization, and could play a significant role in the protection of germ lineage against phenotypic variations. The study of Hsp90 in germ cells migration could constitute a now interesting way to establish similarities between embryonic and neoplastic proliferation and migration.
Abbreviations
- actr2:
-
Actin related protein 2 homolog
- alk2:
-
Activin receptor-like kinase-2
- Bmp:
-
Bone morphogenetic proteins
- Bmpr:
-
Bone morphogenetic protein receptor
- EMT:
-
Epithelial-mesenchymal transition
- GR:
-
Gonadal ridge
- Hsp:
-
Heat shock protein
- MOTC:
-
Microtubule organizer center
- PGC:
-
Primordial germ cells
- ROCK/RhoA:
-
Rho-associated protein kinase
- TGFβ:
-
Transforming growth factor β
References
Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T (2003) Impaired colonization of the gonads by primordial gem cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc Natl Acad Sci 100:5319–5323
Bendel-Stenze MR, Gomperts M, Anderson R, Heasman J, Wylie C (2000) The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech Dev 91:143–152
Buchner J (1999) HSP90 & Co.-a holding for folding. Trends Biochem Sci 24:136–141
Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP (2000) The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci U S A 97:9585–9590
Chen WS, Chen CC, Chen LL, Lee CC, Huang TS (2013) Secreted heat shock protein 90α (HSP90α) induces nuclear factor-κB-mediated TCF12 protein expression to down-regulate E-cadherin and to enhance colorectal cancer cell migration and invasion. J Biol Chem 288:9001–9010
Chiquoine DA (1954) The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat Rec 118:135–146
D’Costa S, Petitte JN (1999) Characterization of stage-specific embryonic antigen-1 (SSEA-1) expression during early development of the turkey embryo. Int J Dev Biol 43:349–356
De Felici M (2013) Origin, migration, and proliferation of human primordial germ cells. In: Coticchio B, De Santis L (eds) Oogenesis. Springer, London, pp 19–37
Deborah LR, Bennett LK (1993) glh-1, a germ-line putative RNA helicase from Caenorhabditis, has four zinc fingers. Proc Natl Acad Sci U S A 90:9300–9304
Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E (2002) Guidance of primordial germ cell migration by the chemokine SDF-1. Cell 111:647–659
Dudley BM, Runyan C, Takeuchi Y, Schaible K, Molyneaux K (2007) BMP signaling regulates PGC numbers and motility in organ culture. Mech Dev 124:68–77
Dustin AP (1910) L’origine et l’évolution des gonocytes chez les reptiles. Arch Biol 25:495–534
Everett NB (1945) The present status of the germ cell problem in vertebrates. Biol Rev Camb Philos Soc 20:45–55
Felix W (1911) Die Entwicklung der Harm- und Geschlechtsorgane. In: Keibel-Mall Handbuch der Entwicklungageschichte des Menschen, vol 2. Hirzel, Leipzig, pp 732–955
Fox N, Damjanov I, Martinez-Hernandez A, Knowel BB, Solter D (1981) Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissue. Dev Biol 83:391–398
Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T (1994) Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci U S A 91:12258–12262
Fuss A (1911) Uber extraregionare Geschlechtszellen bei einem menschlichen Embryo von 4 Wochen. Anat Am 39:407–409
Gamo H (1961) On the origin of germ cells and formation of gonad primordia in the medaka, Olyzias latipes. J Zool 13:101–115
Ginsburg M (1997) Primordial germ cell development in avians. Poult Sci 76:91–95
Ginsburg M, Snow MHL, Mc Laren A (1990) Primordial germ cells in the mouse embryo during gastrulation. Development 110:521–528
Gu Y, Runyan C, Shoemaker A, Surani A, Wylie C (2006) Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development 136:1295–1303
Hahnel AC, Eddy EM (1986) Cell surface markers of mouse primordial germ cells defined by two monoclonal antibodies. Gamete Res 15:1235–1244
Hargitt GT (1925) The formation of the sex glands and germ cells of mammals. J Morph Physiol 40:517–557
Haupt A, Joberty G, Bantscheff M, Frohlich H, Stehr H, Schweiger MR, Fischer A, Kerick M, Boerno ST, Dahl A, Lappe M, Lehrach H, Gonzalez C, Drewes G, Lange BMH (2012) Hsp90 inhibition differentially destabilizes MAP linase and TGF-beta signaling components in cancer cells revealed by kinase-targeted chemoproteomics. BMC Cancer 12:38–50
Heys F (1931) The problem of the origin of germ cells. Q Rev Biol 6:1–45
Kazama-Wakabayashi M, Yamaha E, Yamazaki F (1999) The elimination and duplication of lower part of blastoderm effects on the number of primordial germ cells in goldfish. Fish Sci 65:577–582
Koyasu S, Nishida E, Kodawaki T, Matsuzaki F, Lida K, Harada F, Kasuga M, Sakai H, YaharaTwo I (1986) Mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc Natl Acad Sci U S A 83:8054–8058
Lasko PF, Ashburner M (1988) The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335:611–617
Lawson KA, Ray Dunn N, Roelen BAJ, Zeinstra LM, Davis AM, Wright CVE, Korving JPWFM, Hogan BLM (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13:424–436
Lejong M, Choa-Duterre M, Vanmuylder N, Louryan S (2018) Geldanamycin administration reduces the amount of primordial germ cells in the mouse embryo. Morphologie 102: 219-224. https://doi.org/10.1016/JMorpho.2018.05.001
Lin TY, Bear M, Du Z, Foley KP, Ying W, Barsoum J, London C (2008) The novel HSP90 inhibitor STA-9090 exhibits activity against Kit-dependent and -independent malignant mast cell tumors. Exp Hematol 36:1266–1277
Louryan S, Evrard L, Glineur R, Vanmuylder N (2002) Protéines de choc thermique, embryogenèse et évolution. Bull Mem Acad R Med Belg 157:293–299
Louryan S, Vanmuylder N, Lambot MA, Rooze M (2003) HSP86: un rôle dans l’évolution humaine ? Anthropol Praehist 114:1–5
Machev N, Fuhrmann G, Viville S (2004) Ontogénèse des cellules germinales primordiales. Med Sci 20:1091–1095
McKay D, Hertig AT, Adams EC, Danziger S (1953) Histochemical observations on the germ cells of human embryos. Anat Rec 17:201–219
McLaren A (2003) Primordial germ cells in the mouse. Dev Biol 262:1–15
Miyata A, Yahara I (1992) The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem 7:7042–7047
Molyneaux K, Wylie C (2004) Primordial germ cell migration. Int J Dev Biol 48:537–544
Nagai T, Yamaha E, Arai K (2001) Histological differentiation of primordial germ cells in zebrafish. Zool Sci 18:215–223
Neckers L, Workman P (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 18:64–76
Niewkoop D, Sutasurya LA (1979) Primordial germ cells in the chordates. Cambridge University Press, Cambridge
Noce T, Okamoto-Ito S, Tsunekawa N (2001) Vasa homolog genes in mammalian germ cell development. Cell Struct Funct 26:131–136
Olsen LC, Aasland R, Fjose A (1997) A vasa-like gene in zebrafish identifies putative primordial germ cells. Mech Dev 66:95–105
Peuβ R, Eggert H, Armitage SAO, Kurtz J (2015) Downregulation of the evolutionary capacitor Hsp90 is mediated by social cues. Proc R Soc B 282:20152041. https://doi.org/10.1098/rspb.2015.2041
Pfeiffer J, Tarbashevich K, Bandemer J, Palm T, Raz E (2018) Rapid progression through the cell cycle ensures efficient migration of primordial germ cells-the role of Hsp90. Dev Biol 436:84–93
Queitsch C, Sangster TA, Lindquist S (2002) Hsp9O as a capacitor of phenotypic variation. Nature 417:618–624
Rohner N, Jarosz DF, Kowalko JE, Yoshizawa M, Jeffery WR, Borowsky RL, Lindquist S, Tabin CJ (2013) Cryptic variations on morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 342:1372–1375
Rutherford SL, Lindquist S (1998) Hsp90 as a capacitor for morphological evolution. Nature 396:336–342
Ryan CP, Brownlie JC, Whyard S (2016) Hsp90 and physiological stress are linked to antonomous transposon mobility and heritable genetic change in nematodes. Genome Biol Evol 8:3794–3805
Saitou M, Yamaji M (2012) Primordial germ cells in mice. Cold Spring Harb Perspect Biol 4:1–19
Shevinsky LH, Knowles BB, Damjanov I, Solter D (1982) Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell 30:697–705
Simkins CS (1923) Origin and migration of the so-called primordial germ cells in the mouse and rats. Acta Zool 4:241–278
Simkins CS (1928) Origin of sex cells in man. Am J Anat 41:249–272
Sollars V, Lu X, Wang S, Garfinkel MD, Ruden DM (2003) Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nat Genet 33:70–74
de Sousa Lopes SM, Roelen BA, Monteiro RM, Emmens R, Lin HY, Li E, Lawson KA, Mummery CL (2004) BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev 18:1838–1849
Stankiewicz M, Mayer MP (2012) The universe of Hsp90. Biol Mol Concepts 3:79–97
Stebler J, Spieler D, Slanchev K, Molyneaux K, Richter U, Cojocaru V, Tarabykin V, Wylie C, Kessel M, Raz E (2004) Primordial germ cell migration in the chick and mouse embryo: the role of the chemokine SDF-1/CXCL12. Dev Biol 272:351–361
Swift CH (1914) Origin and early history of the primordial germ-cells in the chick. Am J Anat 15:483–516
Taiyab A, Rao CM (2011) Hsp90 modulates actin dynamics: inhibition of Hsp90 leads to decreased cell motility and impairs invasion. Biochim Biophys Acta 1813:213–221
Takeuchi T, Tanigawa Y, Minamide R, Ikenishi K, Komiya T (2010) Analysis of SDF-1/CXCR4 signaling in primordial germ cell migration and survival or differentiation in Xenopus laevis. Mech Dev 27:146–158
Tam PP, Zhou SX (1996) The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol 178:124–132
Terayama K, Kataoka K, Morichika K, Orii H, Watanabe K, Mochii M (2013) Developmental regulation of locomotive activity in Xenopus primordial germ cells. Develop Growth Differ 55:217–228
Timmermans LPM (1996) Origin and differentiation of primordial germ cells in vertebrates, especially fishes. Neth J Zool 46:147–162
Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T (2000) Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 127:2741–2750
Urven LE, Erickson CA, Abbott UK, McCarrey JR (1988) Analysis of germ line development in the chick embryo using an anti-mouse EC cell antibody. Development 103:299–304
Vanmuylder N, Evrard L, Daelemans P, Dourov N (2000) Chaperones in the parotid gland: localization of heat shock proteins in human salivary glands. Cells Tissues Organs 167:199–205
Vanmuylder N, Werry-Huet A, Rooze M, Louryan S (2002) Heat shock protein HSP86 expression during mouse embryo development, especially in the germ-line. Anat Embryol 205:301–306
Vanmuylder N, Lambot M-A, Rooze M, Noël J-C, Louryan S (2004) HSP86, cellules germinales at yolk sac tumor. Ann Pathol 24:473–475
Vanmuylder N, Larbi H, Choa-Duterre M, Salvia P, Rooze M, Louryan S (2009) Geldanamycin administration reduces the number of HSP86-positive germ cells in the mouse embryo: preliminary results. Rev Med Brux 30:23–27
Wagner GP, Chiu CH, Hansen TF (1999) Is Hsp90 a regulator of evolvability? J Exp Zool 285:116–118
Wan YQ, Zhang XM, Wang XD, Wang BJ, Wang W (2010) 17-AAG, a Hsp90 inhibitor attenuates the hypoxia induced expression of SDF-1α and ILK in mouse RPE cells. Mol Biol Rep 37:1203–1209
Wang G, Gu X, Chen L, Wang Y, Cao B, E Q (2013) Comparison of the expression of 5 heat shock proteins in benign and malignant salivary gland tumor tissues. Oncol Lett 5:1363–1369
Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S (2017) Heat shock proteins and cancer. Trends Pharmacol Sci 38:226–256
Yahara I (1999) The role of HSP90 in evolution. Gene Cells 4:375–379
Ying Y, Zhao GQ (2001) Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol 232:484–492
Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ (2000) Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol 14:1053–1063
Yon N, Akbulut C (2015) Identification of primordial germ cells: cytological, histological and immunohistochemical aspects. Braz Arch Biol Technol 58:222–228
Acknowledgements
The authors thank Mrs. M. Choa-Duterre for histological sections.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lejong, M., Vanmuylder, N., Louryan, S. (2019). Hsp90 in the Migration of Primordial Germ Cells: A Model to Study Long-Distance Cell Migration and Perhaps Cancer?. In: Asea, A., Kaur, P. (eds) Heat Shock Protein 90 in Human Diseases and Disorders. Heat Shock Proteins, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-030-23158-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-23158-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23157-6
Online ISBN: 978-3-030-23158-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)