Abstract
Germ cells are a sexual reproductive cell type at any stage from primordial germ cells (PGCs) to mature gametes. Germ line stem cells are important for genetic transmission to future generations. In this review, we focus on female germ line stem cells (FGSCs), spermatogonial stem cells (SSCs), and PGCs. In addition, we summarize current research progress concerning PGC specification, migration, and development, SSC properties, their niche, and fate decisions, as well as the history and current research of FGSCs and their applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 The Origin and Fate of Germ Cells in Mammals
In many organisms, a primary event during development is the segregation of germ cells from somatic cells. Germ cell development ensures the perpetuation of genetic information across the generations. In mammals, primordial germ cells (PGCs) are the first cell type established during embryogenesis and are the common precursors of both oocytes and spermatozoa.
1.1 PGC Specification in Mammals
In many invertebrates, PGCs are determined by the inheritance of maternal factors deposited in the egg, and only blastomeres containing germ cell determinants develop into germ cells. However, in mammals, pluripotent epiblast cells acquire a germ cell fate in response to extrinsic and intrinsic signaling molecules. Prior to gastrulation, the mouse embryo consists of three distinct cell lineages: the epiblast, extraembryonic endoderm, and trophectoderm. PGCs are derived from the proximal fraction of the population of epiblast cells that will mainly give rise to the extraembryonic mesoderm. Transplantation experiments have demonstrated that signals from extraembryonic tissues are critical for PGC fate specification (Tam and Zhou 1996). In the mouse, PGCs are identified as an alkaline phosphatase-positive cell population in the developing allantois [embryonic day (E) 6.5–7.5].
Bone morphogenetic protein (BMP) signaling from extraembryonic tissues is essential for PGC specification in mouse embryos. Bmp4, which is expressed in the extraembryonic ectoderm prior to gastrulation and subsequently in the extraembryonic mesoderm, is required for the generation of PGCs. A previous study of Bmp4 mutant embryos revealed a significant reduction in the number of PGCs in heterozygous mutant embryos, and no PGCs were detected in homozygous mutant embryos (Lawson et al. 1999). Bmp8b, which is expressed in the extraembryonic ectoderm in pregastrula and gastrula stage mouse embryos, is also required for PGC generation (Ying et al. 2000). Bmp4 and Bmp8b may form heterodimers to induce the formation of PGCs (Ying et al. 2001). Targeted inactivation of the Bmp2 gene, which is primarily expressed in the endoderm of pregastrula and gastrula stage mouse embryos, significantly reduces the number of PGCs (Ying and Zhao 2001). Moreover, WNT signaling in the epiblast plays a role in PGC formation. Wnt3 in the epiblast ensures its responsiveness to BMP4 for PGC differentiation (Ohinata et al. 2009). Dullard (also known as C-terminal domain nuclear envelope phosphatase 1; Ctdnep1) is a member of the serine/threonine phosphatase family of the C-terminal domain of eukaryotic RNA polymerase II. A recent study revealed that Dullard is essential for the formation of PGCs in the mouse embryo as a positive regulator of WNT signaling (Tanaka et al. 2013).
After induction by BMP and WNT signals, epiblast cells are regulated by PR domain proteins PRDM1 (also known as B lymphocyte induced maturation protein 1, Blimp1) and PRDM14. PRDM1, a potential transcriptional repressor of a histone methyltransferase subfamily, has a critical role in the foundation of the mouse germ cell lineage. PRDM1 promotes the expression of Stella (also known as Dppa3), a definitive PGC marker, and represses the expression of somatic cell genes, particularly members of the Hox gene family. In Prdm1 knockout mouse embryos, PGC-like cells fail to repress the expression of somatic cell genes, Hoxa1 and Hoxb1, and PGCs lacking PRDM1 do not properly migrate or proliferate (Ohinata et al. 2005). Prdm14, a PR domain-containing transcriptional regulator, has been found to be important for PGC specification in mice. Similar to Prdm1-knockout mice, PGCs are almost completely lost by E12.5 in Prdm14 mutant embryos (Yamaji et al. 2008). Another study has demonstrated that a conserved mesodermal factor, T, which is induced by WNT3, is essential for the activation of Prdm1 and Prdm14 via binding to distinct regulatory elements in these genes for direct upregulation, thereby delineating the downstream PGC program (Aramaki et al. 2013). Recently, an in vitro study revealed that simultaneous overexpression of Prdm1, Prdm14, and Tfap2c (also known as AP2γ) rapidly and efficiently directs epiblastlike cells derived from embryonic stem cells (ESCs) or induced pluripotent stem cells or (iPSCs) into a PGC state (Nakaki et al. 2013). Another study demonstrated that, in principle, PRDM1, AP2γ, and PRDM14 are sufficient for PGC specification and the unprecedented resetting of the epigenome toward a basal state (Magnusdottir et al. 2013).
1.2 PGC Migration in Mammals
In the mouse, PGCs begin to migrate from the primitive streak to the endoderm (the future hindgut) at E7.5. They continue to migrate through the hindgut endoderm at E8.0 and then migrate bilaterally toward the dorsal body wall at E9.5 to finally colonize the gonadal ridges at E10.5.
There are several factors that play important roles during PGC migration in mammals. PGCs lacking β1 integrins fail to migrate normally to the gonads (Anderson et al. 1999). IFITM (interferon-induced transmembrane) proteins are cell surface proteins implicated in diverse cellular processes including cell adhesion. Knockdown of Ifitm1 by RNA interference in the primitive streak leads to failure of PGC migration into the endoderm (Tanaka et al. 2005). During the later stage of migration, the interaction of stromal cell-derived factor 1 (expressed by the body wall mesenchyme and genital ridges) and its G-protein-coupled receptor, chemokine (CXC motif) receptor 4 (expressed by the migrating germ cells), is required for the colonization of the gonads by PGCs (Molyneaux et al. 2003). Foxc1 encodes a forkhead/winged-helix transcription factor expressed in many embryonic tissues. Many PGCs fail to migrate normally to the gonadal ridge in Foxc1 null mouse embryos, remaining trapped in the hindgut, although the germ cells are specified correctly (Mattiske et al. 2006).
1.3 PGC Development in Mammals
Following gonadal sex determination, germ cells in the testis initially proliferate and then undergo mitotic cell cycle arrest at G0/G1. The germ cells that differentiate from PGCs to type A spermatogonia, including spermatogonial stem cells (SSCs), are termed gonocytes. Gene expression patterns change dynamically during the transition from PGCs to gonocytes and SSCs (Culty 2009). After arriving at the genital ridge at approximately 10.5 days post-coitus (dpc), female germ cells are called oogonia and develop into clusters of cells called germ line cysts or oocyte nests. Subsequently, the oogonia enter meiosis and become oocytes. During fetal and neonatal development, germ line cysts break apart into single oocytes, which are intruded by pregranulosa cells to form primordial follicles (Pepling 2006, 2012; Pepling and Spradling 2001).
In mammals, meiotic initiation occurs at different time points in male and female germ cells. Female germ cells enter meiosis at around 13.5 dpc and arrest at the diplotene stage beginning at 17.5 dpc (Speed 1982), whereas male germ cells start meiosis at puberty. Retinoic acid (RA) is produced in the mesonephros of both sexes, which is postulated to diffuse or flow into the adjacent gonad. Stra8 (stimulated by retinoic acid gene 8), which is induced by RA, is a premeiotic gene required for meiotic initiation. In the fetal ovary, high levels of RA induce germ cells to enter meiosis (Baltus et al. 2006; Bowles et al. 2006; Koubova et al. 2006; Vernet et al. 2006; Lin et al. 2008). However, meiosis is not triggered in the fetal testis because RA is degraded by the retinoid enzyme CYP26B1. In Cyp26b1-knockout male fetal gonads, germ cells enter meiosis (Bowles et al. 2006). A study of Cyp26b1-, Fgf9 (fibroblast growth factor 9)-, and double-knockout embryos demonstrated that fibroblast growth factor (FGF) 9 produced in the fetal testis acts directly on germ cells to inhibit meiosis, making them less responsive to RA (Bowles et al. 2010). A recent study showed that PRC1 (polycomb repressive complex 1) has gene dosage effects on PGC development and coordinating the timing of sex differentiation of female PGCs by antagonizing extrinsic RA signaling to ensure proper timing of meiotic induction (Yokobayashi et al. 2013). In addition, Dazl (Lin et al. 2008), Msx1/2 (Le Bouffant et al. 2011), Dmrt1 (Matson et al. 2010; Krentz et al. 2011), Nodal (Souquet et al. 2012), and Notch pathways (Feng et al. 2014) regulate the initiation of meiosis. However, a study of Raldh2 (retinaldehyde dehydrogenase-2)-knockout mice lacking RA synthesis and signaling in the mesonephros and adjacent gonad revealed that STRA8 expression in the fetal ovary does not require RA signaling (Kumar et al. 2011).
The conventional theory is that all germ cells in the fetal ovary enter meiosis, thereby committing to oogenesis. The number of germ cells is determined after birth. However, this view has been challenged. There are reports that female germ line stem cells (FGSCs) with the ability to produce functional oocytes still exist in neonatal and adult mouse ovaries (Zou et al. 2009). Subsequently, FGSCs have been discovered in the ovaries of reproductive-age woman (White et al. 2012) and rats (Zhou et al. 2014).
2 Female Germ Line Stem Cells
2.1 Introduction
FGSCs are a new class of germ cells in mammals. The recent identification and isolation of FGSCs from mouse and human ovaries have opened a new research area in stem cell biology, developmental biology, and reproductive biology as well as reproductive medicine. Although we know little about FGSCs and significant research needs to be performed at present, we believe that FGSCs might shed light on the preservation of fertility in reproductive-age women under the conditions of premature ovarian failure or chemotherapy. Recently, FGSCs were isolated and cultured from postnatal mammals, which allows us to study their biological characteristics and applications. In this section, we will discuss the progress of FGSC research.
2.2 History of FGSC Research
In the early 1950s, it was thought that postnatal germ line stem cells (GSCs) only existed in the male testis. However, in females, a fixed number of primordial follicles exist in the ovaries, and the defined follicle pool serves as the source of oogenesis over the life span of mammals (Zuckerman 1951; Rudkin and Griech 1962; Borum 1967; Peters and Crone 1967). From then on, although there have been different views from some researchers (Ying and Zhao 2001; Ohinata et al. 2005, 2009; Tanaka et al. 2013; Yamaji et al. 2008), the existence of a non-renewing follicle pool after birth in mammals has become a central dogma in classical reproductive biology.
Recently, Johnson et al. (2004) suggested that the female ovary may have regenerative activity in juvenile and adult mice in vivo by examining changes in follicle numbers from birth to adulthood. Subsequently, they showed that peripheral blood (PB) or bone marrow (BM) transplantation restores oocyte production in wild-type mice sterilized by chemotherapy and in ataxia telangiectasia-mutated gene-deficient mice (Johnson et al. 2004). Therefore, they concluded that BM and PB may be potential sources of female germ cells that can generate oocytes in adulthood. Unfortunately, this view spawned a wave of skepticism and controversy, as well as reports with contradictory findings, claiming there is no evidence for the formation of oocytes from BM cells in mice (Eggan et al. 2006; Gosden 2004).
In 2009, our laboratory successfully isolated FGSCs from neonatal and adult mouse ovaries by two enzymatic digestion steps and mouse vasa homolog (MVH)-magnetic bead sorting. Furthermore, a neonatal mouse FGSC line was established and cultured for more than 1 year, whereas the adult mouse FGSCs was cultured for more than 6 months. These long-term cultured FGSCs maintained a normal karyotype, high telomerase activity, and their capacity to differentiate into functional oocytes, and offspring were generated after transplantation into ovaries (Zou et al. 2009). Considering the low purification efficiency based on MVH-magnetic bead sorting, we screened other germ cell-specific markers and found that the germ line-specific protein Fragilis as a selection maker can remarkably improve the purification efficiency (Zou et al. 2011). Moreover, transgenic or gene knockdown mice were prepared by FGSC transplantation. The gene transfer efficiency was up to 29–37 % (Zhang et al. 2011). In addition, we isolated and cultured rat FGSCs with the abilities to produce fat-1 transgenic rats after transplantation in vivo and differentiate into oocytes in vitro (Zhou et al. 2014).
In 2012, White et al. extended our previously described protocol and culture system by isolating FGSCs from adult mice and reproductive-age (20–30-year-old) women using MVH as the selection maker and fluorescence-activated cell sorting (FACS) (White et al. 2012; Woods and Tilly 2013).
In fact, FGSCs are found not only in rodents (mice and rats) and primates (humans), but also in other animals including vertebrate species such as fish including zebra fish (Wong et al. 2013) and teleost medaka (Nakamura et al. 2010). More importantly, using a retrospective phylogenetic-based method, a study showed preservation of the female germ line in both young and old mice (Reizel et al. 2012). Therefore, the existence of FGSCs has been demonstrated through cell biology and genetic analysis.
2.3 Current FGSC Research Progress
2.3.1 FGSC Origin and Their Location
In the mouse, PGCs arise within the proximal epiblast, begin to migrate along the hindgut at E8.5, and then arrive at the genital ridge at around E10.5. PGCs proliferate during their migration, thereby increasing their population. In the gonadal ridge, PGCs are considered as oogonia (Durcova-Hills et al. 2003). The oogonia divide mitotically in a short period. Subsequently, oogonia cease mitosis and enter meiosis I and arrest at this phase. Based on the current research of FGSCs, not all oogonia enter into meiosis, and a small number of GSCs exist during reproductive life (Bukovsky et al. 2008). However, the exact biological processes of differentiation from PGCs to FGSCs are unknown. Single-cell analysis and real-time, high-resolution imaging systems might facilitate future studies of these processes.
To investigate the location of FGSCs, bromodeoxyuridine (BrdU) has been injected into female mice followed by dual immunofluorescence staining of BrdU and MVH. The results indicated that FGSCs are located in the cortical surface of ovaries (Zou et al. 2009).
2.3.2 FGSC Isolation and Culture
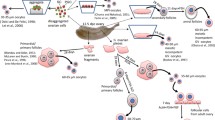
Separation of FGSCs from ovaries requires knowledge of both the ovarian tissue structure and cell morphology. A schematic diagram of the major steps for FGSC isolation is shown in Fig. 1.1. Generally, there are two main methods to obtain pure FGSCs from a single cell suspension after two enzymatic (collagenase IV and trypsin) digestion steps, namely the differential plating method and the immunotargeted purification method [magnetic-activated cell sorting (MACS) and FACS]. Immunotargeting is largely based on a specific antibody targeting the surface markers on GSCs.
To establish FGSC lines, FGSC isolation and purification protocols have been developed based on techniques for isolation and in vitro expansion of SSCs. Such a method described in our online protocol is able to isolate FGSCs from ovarian tissue (Wang et al. 2013). It is important to note that the homogeneity of the starting materials and standardization of the isolation protocol are key factors for obtaining desired cells. In present, there is no unique surface marker for GSCs (SSCs and FGSCs) purification. Therefore, the markers should be carefully selected for GSC isolation. Using germ line surface markers may obtain GSCs, whereas other pluripotency-related makers such as stage-specific embryonic antigen (SSEA)-1 may not be appropriate for GSC selection (Nakaki et al. 2013). In our opinion, regardless of the surface marker, probing the biological identity of the obtained cells is an issue of urgent priority.
In addition, a stable culture system is crucial to maintain the propagation and features of FGSCs in vitro. From our experience in stem cell culture, we believe that basic medium, a feeder layer, and growth factors play a major role in FGSC culture, although there is still some discrepancy between optimal culture conditions and the microenvironment of FGSCs in vivo. Growth factors, such as glial cell line-derived neurotrophic factor (GDNF), FGF2, epidermal growth factor (EGF), and leukemia inhibitory factor (LIF), are important for SSC and FGSC propagation (Wu et al. 2008; Xiong et al. 2011; Yuan et al. 2009). Among these factors for in vitro culture, GDNF is critical for GSC proliferation.
2.3.3 FGSC Characterization
FGSCs can be characterized based on SSC and other stem cell-related research by their morphology and gene expression profiles, as well as functional assays. Interestingly, isolated FGSCs have a morphology common with freshly isolated SSCs, including a large cell body with little cytoplasm, helical nuclei with slight staining, a large nucleus/cytoplasm ratio, and a nuclear diameter of 12–20 µm. The growth pattern of FGSCs and SSCs is also similar. For example, most FGSCs grow with a typical grapelike morphology in primary culture. Both FGSCs and SSCs express germ cell-specific markers (MVH, Fragilis, Blimp-1, Dazl, and Stella) but not pluripotency-related proteins (Nanog, SSEA-1, and Sox2). Moreover, long-term cultured FGSCs maintain a normal karyotype (40, XX), alkaline phosphatase activity, and a female imprinting pattern (Zou et al. 2009). In addition, the most important functional analysis of FGSCs is through oogenesis in vitro or in vivo.
2.3.4 FGSC Transplantation
In the mouse, transplantation has been used as a functional assay to study the biological characteristics of GSCs. Although SSC transplantation is considered as a quite mature technology, FGSC transplantation research is still lacking. In fact, transplantation can be divided into two categories: direct in situ injection (Zou et al. 2009) and tissue grafting (White et al. 2012). Although the grafting has advantage that GSCs still remain in their microenvironment and interact with their neighboring or supporting cells, the direct injection strategy can meet the need of gametogenesis requirement (Zou et al. 2009). Recent studies have shown that the combination of organ culture and transplantation provides a new strategy for functional sperm preparation in vitro (Gohbara et al. 2010; Yokonishi et al. 2013; Sato et al. 2011a, b, 2012, 2013). However, whether functional oocytes can be generated from FGSCs using this platform is still unknown.
To eliminate effects from endogenous germ cells, recipient females of transplantation can be sterilized with cyclophosphamide and busulphan. Furthermore, non-endogenous germ cells generated by genetic modification would be more convenient for transplantation. To ensure a good outcome after transplantation, some critical points need consideration, such as carefully moving the intestines away from the inside of the abdominal cavity and not damaging the connective tissue or underlying structures of the ovaries. The details of transplantation have been described previously (Wang et al. 2013).
2.4 Applications of FGSCs
Stem cells have a great potential for use in regenerative medicine because of their self-renewal and multi-lineage differentiation abilities. From a clinical perspective, as a new type of adult stem cell, FGSCs may be applicable from the preservation of endangered species to ovarian aging therapy, as well as treating infertility caused by radiation and chemotherapy, even though embryo and oocyte cryopreservation are currently available to restore fertility. Moreover, FGSCs are an alternative source of mitochondria for ooplasmic transfer (Harvey et al. 2007; Barritt et al. 2001). From a basic research perspective, as a female germ cell precursor, FGSCs can be studied to understand the molecular mechanisms of oogenesis and folliculogenesis. Although numerous reports have shown that pluripotent stem cells including ESCs and iPSCs are able to differentiate into oocytes (Hubner et al. 2003; Hayashi et al. 2012), their direct differentiation is currently limited by low efficiency.
Since the discovery of pluripotent stem cells, they have created a new research area. Recent studies have reported that mouse SSCs can be converted into pluripotent stem cells under certain culture conditions (Ko et al. 2010; Conrad et al. 2008; Guan et al. 2006; Kanatsu-Shinohara et al. 2004, 2008; Seandel et al. 2007; Golestaneh et al. 2009; Kossack et al. 2009). Based on our previous research, we have found that FGSCs share common characters with SSCs, including their shape, growth pattern, and functions during gametogenesis (Wu et al. 2013). Therefore, we attempted FGSC conversion to pluripotent stem cells. As a result, similar to SSC conversion, we found that FGSCs can be converted into pluripotent stem cells under certain culture conditions (Wang et al. 2014). Consequently, the generation of patient-specific FGSC-derived pluripotent stem cells is feasible and provides a foundation for personalized regenerative applications. Moreover, SSCs can transdifferentiate into reproductive and non-reproductive cells and tissues in certain microenvironments (Zhang et al. 2013; Simon et al. 2009). Whether FGSCs can transdifferentiate into other types of cells is still unknown. If FGSC transdifferentiation occurs, FGSCs will become more widely applicable.
Although FGSCs have a wide range of applications, which is similar to that of SSCs (shown in Fig. 1.2), we must have a clear understanding of these cells. To reveal more aspects of FGSC biology, studies of SSCs in mice and FGSCs in Caenorhabditis elegans and Drosophila can offer us new insights for further exploration. More importantly, new technologies and equipment used by scientists with different backgrounds will be helpful to further FGSC research.
3 Spermatogonial Stem Cells
3.1 Introduction
Continual spermatogenesis lays the foundation for male fertility, which is highly dependent on SSCs, a very small population accounting for only about 0.02–0.03 % of the germ cell population (Tegelenbosch and de Rooij 1993). The existence of SSCs has been proposed since the 1950s, but the related research progress has been difficult and little has been clarified in this field (De Rooij and Russell 2000). Traditional studies of SSCs highly relied on morphology and not considered the deeper aspects of their molecular mechanisms. In 1994, Brinster and colleagues developed a transplantation technique to investigate SSC functionally (Brinster and Avarbock 1994). Briefly, donor testicular cells are dissociated and transplanted into the efferent duct of infertile recipient mice. After 6 weeks to 2 months, offspring are produced with the donor haplotype. This technique is of great importance because it allows relatively easy identification of SSCs and counting of SSC numbers by considering that each colony in the seminiferous tubules arises from a single SSC (Kanatsu-Shinohara et al. 2006). Another important milestone was the development of an in vitro SSC culture system in 2003 by Kanatsu-Shinohara et al. (2003). In the presence of GDNF, FGF2, LIF, EGF, and other cytokines, germ cells from neonatal mice are able to proliferate and form clusters of spermatogonia in long-term culture in vitro. Transplantation experiments have confirmed that SSC numbers are greatly increased in this system. In vitro culture systems are of great importance because they allow in vitro studies and the generation of large numbers of SSCs for molecular and biochemical studies (Kanatsu-Shinohara and Shinohara 2013). Owing to these two techniques and other traditional methods, SSC studies have advanced further to molecular mechanisms and signal transduction.
3.2 SSC Properties
3.2.1 SSC Classification
SSCs residue on the basement compartment of seminiferous tubules and are surrounded by a highly complex microenvironment called the niche that is responsible for sophisticated and orchestrated regulation of the balance between SSC self-renewal and differentiation (Kostereva and Hofmann 2008). In mice, SSCs are undifferentiated spermatogonial cells of As (A-single), Apr (A-paired), and Aal (A-aligned chains of 4, 8, and 16 cells, and 32 in rare cases) configurations based on their topographical arrangement (Ohinata et al. 2005; Yamaji et al. 2008). Undifferentiated as spermatogonial cells are thought to be the most primitive type of spermatogonial cell, which will divide into two Apr cells interconnected by intercellular bridges, further division produces intercellular interconnected Aal4, Aal8, and Aal16 cells. Aal spermatogonia convert to differentiating type A spermatogonia (A1–4) that further progress to In (intermediate) and B spermatogonia. Finally, type B spermatogonia divide into primary spermatocytes, and mitosis converts to meiosis to produce haploid spermatozoa (Phillips et al. 2010). A single SSC undergoes 11–12 divisions on average to eventually produce 2048 or 4096 spermatozoa (De Rooij and Russell 2000) (Fig. 1.3). Classically, Apr and Aal cells are thought to be the progenitors committed to differentiate. However, increasing evidence shows that some Apr and Aal cells are potential SSCs. Through lineage tracing in a transplantation assay, Nakagawa et al. (2007) found that transit-amplifying spermatogonia are also able to form colonies in a germ cell-depleted testis, indicating their stem cell ability. Moreover, during tissue regeneration after testis injury, a significantly greater number of cells contribute to the stem cell pool than that under normal conditions, further confirming the progenitor cell potential.
Compared with rodents, the true identity of SSCs remains largely unknown in adult humans. Unlike propose model in mice previously mentioned, in a widely accepted model, there are two kinds of type A spermatogonia in human: a dark type A spermatogonia (Adark) and pale type A spermatogonia (Apale) according to their staining pattern and nuclear morphology. Both types of A spermatogonia are stem cells. Apale spermatogonia are the active stem cells responsible for normal self-renewal and generating type B spermatogonia, which further divide into spermatozoa, while Adark spermatogonia are the reserve stem cells with rare mitotic activity but are active under injury and disease states (Clermont 1963, 1966, 1972). However, this model has been challenged by Ehmcke and colleagues who proposed a revised model in which Apale spermatogonia are self-renewing progenitors and Adark spermatogonia are the true stem cells. In a non-human study, they showed that Apale spermatogonia undergo higher mitosis than previously thought and their increase in number affects the total number of germ cells (Ehmcke and Schlatt 2006; Ehmcke et al. 2006). Nevertheless, a lack of evidence has limited our understanding of human SSCs and more studies are required.
3.2.2 SSC Characteristics
The lack of specific SSC markers has greatly hindered our understanding of SSCs. However, expression profiles are slowly being revealed spermatogonia stem (progenitor) cells (SPC), indicating exclusive expression of many genes. The strong adherence of SSCs to laminin, the main component of the extracellular matrix of basement membranes, led to the clarification of β1 and α6 integrins as surface markers of SSCs (Shinohara et al. 1999). Subsequently, more surface markers have been identified, such as thymus cell antigen-1 (Thy-1), Ep-CAM, CD9, GDNF receptors GFRα1 and c-Ret, and GPR125, some of which allow the enrichment of SSCs by FACS and MACS (Buageaw et al. 2005; Kanatsu-Shinohara et al. 2004; Tokuda et al. 2007; Kubota et al. 2003; Anderson et al. 1999; Naughton et al. 2006). GFRα1, a co-receptor of GNDF with c-Ret, tends to be expressed in As and Aal cells and appears to represent a relatively primitive proportion of spermatogonia. Combined with gravity sedimentation on a bovine serum albumin gradient, Hofmann et al. were able to isolate SSCs to 98 % purity using GFRα1 for antibody selection. However, purification with GFRα1 is only possible from pubescent mice but not adults, and the cell survival in culture is low (Hofmann et al. 2005; Ebata et al. 2005). Many studies have successfully enriched SSCs with an antibody against Thy-1 and realized their long-term cultivation. However, the cells are a mixture of spermatogonia at various stages, which is sufficient for most researchers (Kubota et al. 2004). c-kit is the receptor for stem cell factor (SCF), which was previously thought to be expressed by SSCs but later identified as a marker of differentiation (Shinohara et al. 1999). In multi-parameter cell sorting, negative selection with c-kit and positive selection with another surface marker will result in a higher percentage of SSCs. c-kit is expressed in late Aal spermatogonia to early spermatocytes, and its expression is often used to identify Aal cell conversion to differentiating spermatogonia A1 (Shinohara et al. 2000; Lennartsson and Rönnstrand 2012; Zhang et al. 2013).
Many transcription factors that promote self-renewal have been proposed as SSC markers. According to their response to GDNF, a key extracellular factor that promotes self-renewal, transcription factors can be divided into GDNF-dependent or GDNF-independent factors. GDNF is the most important extrinsic factor that regulates SSC self-renewal in a dose-dependent manner, and it is essential to culture SSCs in vitro (Meng et al. 2000). To define downstream effectors of GDNF signaling, Oatley and Brinster conducted microarray analysis of cultured germ cells. In their study, GDNF was removed and re-added to the cultured cells, and then, microarray analysis was performed at various time points to determine GDNF-inducible factors. Six genes responded most dramatically to GDNF, which were proposed to be the downstream effectors of GDNF signals, including Bcl6b, Etv5, Lhx1, Egr2, Egr3, and Tspan8 (Oatley et al. 2006). In vitro disruption of Bcl6b with siRNA significantly affects the proliferation of SSCs. Moreover, Bcl6b-null mice exhibit the same progressive defect as GDNF-null mice, further confirming that Bcl6b is a downstream effector of GDNF (Oatley et al. 2006). Subsequently, Etv5-knockout mice were generated and showed a similar phenotype (Chen et al. 2005). Inhibitor of DNA binding protein 4 (ID4) is another GDNF-inducible factor. However, ID4 is unique because it is exclusively expressed in As cells but not in Apr or Aal cells (Oatley et al. 2011). Recently, NONOS2, an RNA-binding protein that is preferentially expressed in As and Apr cells, was found to be a downstream effector of GDNF signaling. A lack of NANOS2 results in the same phenotype as that of GDNF-null mice, whereas NANOS2 overexpression will compensate for GFRα1 depletion in mice (Sada et al. 2012). GDNF signals through three pathways to downstream effectors for the promotion of SSC self-renewal, including PI3 K-AKT, SFK, and Ras/ERK1/2, which also cross talk with each other (Lee et al. 2007; Braydich-Stolle et al. 2007; He et al. 2008). Promyelocytic leukemia zink factor (PLZF), also known as ZFP145 and ZBTB16, is the first identified intrinsic factor that is exclusively expressed in undifferentiated spermatogonia in the testis. Disruption of PLZF leads to progressive germ cell loss, indicating the essential role of PLZF in SSC maintenance (Buaas et al. 2004; Costoya et al. 2004). The exact mechanism of PLZF has not been fully clarified in the maintenance of SSCs, although some details have been revealed, which will be discussed below. Oct4, another GDNF-independent maintenance factor, is also exclusively expressed in undifferentiated spermatogonia in the adult testis. Oct4 disruption in cultured GSCs notably reduces both their proliferation and survival rates, suggesting its indispensable role in SSC self-renewal (Dann et al. 2008). However, the downstream and upstream molecules of OCT4 signaling are almost unknown in SPCs, which require further study.
3.2.3 Reversibility and Heterogeneity
Recent studies have proposed that undifferentiated spermatogonia are not uniform as previously thought and that morphologically classified SPCs exhibit different molecular and biology characters among themselves. At all stages of undifferentiated SSCs (As, Apr, and Aal cells), NGN3 expression can be detected, but there are undifferentiated SSCs that are negative for NGN3, suggesting molecular heterogeneity among undifferentiated SSCs (Yoshida et al. 2007). Lineage tracing under the control of NGN3 expression revealed that most labeled cells are committed to differentiation, while a very small population are stem cells that account for only about 10 % of total SSCs (Nakagawa et al. 2007). Quantitative analysis revealed that about 10 % of As spermatogonia are GFRα1 negative, but transplantation has demonstrated their clonogenic ability. In contrast, cells selected using GFRα1 show almost no clonogenic ability. In addition, GFRα1 expression is not the same among Apr spermatogonia (Grisanti et al. 2009). Taking these evidences together, heterogeneity does exist among undifferentiated SSCs. Such heterogeneity raises the possibility that there may be more stages among the undifferentiated types of SSCs. Morphological classification may be not truly reflect the actual status of undifferentiated spermatogonia, and a better classification system combined with molecular characters should be developed.
Under normal conditions, the vast majority of NGN3-expressing spermatogonial cells are transit-amplifying cells committed to differentiate. However, in transplantation assays, a significantly larger number of NGN3-expressing spermatogonial cells are clonogenic and contribute to regeneration in lineage tracing experiments. This finding demonstrates that in addition to true stem cells, transit-amplifying cells can revert to SSCs (Nakagawa et al. 2007; Yoshida et al. 2007). Nakagawa et al. (2007) referred to this subpopulation as potential stem cells. Such a functionally distinct population of undifferentiated spermatogonia possesses the potential for self-renewal but do not show this ability in undisrupted testis. Similar results have been obtained in two studies showing that c-kit-positive spermatogonia both in vivo and in vitro, which are usually thought to be the differentiating subpopulation, are also able to regenerate recipient testis, although with a significantly lower ability compared with that of the c-kit-negative fraction (Barroca et al. 2009; Morimoto et al. 2009). Considering the existence of potential stem cells, transplantation assays may overestimate the number of true SSCs. However, estimations of total SSCs at <2000 per testis according to transplantation assays are similar to those in a study by Nakagawa et al. (2007) based on the boundary of one SSC territory, although this strategy is somehow confusing (Nakaki et al. 2013; Reizel et al. 2012). It is still unclear whether only some SPCs are reversible or all SPCs are able to convert to SSCs under certain conditions. The former possibility may indicate the complexity or heterogeneity of the undifferentiated spermatogonia population, while the latter may represent the phenotypic plasticity. If heterogeneity is important, a certain phase may mark the undirected differentiation. If there is a certain phase, it might be possible to characterize a molecular phenotype that marks the specific point, but no such marker has been revealed thus far. It is also possible that undifferentiated spermatogonia show plasticity and can be reversed under certain conditions, such as transplantation and tissue regeneration, which is important for the robustness of the spermatogenesis system.
3.3 SSC Niche
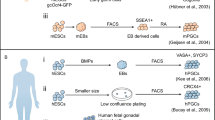
In sexually reproductive animals, the SSC niche is the specialized microenvironment that harbors the stem cells and precisely regulates their self-renewal and differentiation. In Drosophila and C. elegans, whose gonads are polarized, its localization is identified by a specialized somatic compartment that supports the stem cells. However, in mammals, this specialized microenvironment has not been proposed (Spradling et al. 2001; Yamashita and Fuller 2005). Seminiferous tubules are comprised of basal and adluminal compartments separated by tight junctions among Sertoli cells, and all spermatogonia lie in the basal compartment. Together with morphological research showing that SSCs are situated on the basal membrane, we can speculate that the SSC niche must be located somewhere adjoining the basal membrane in the basal compartment. Sertoli cells and the basal membrane composed of peritubular and extracellular matrix provide the structural basis for the SSC niche. However, no report has shown the functional difference of sertoli cells and the basal membrane, suggesting that these two components are not the main factors that dictate the location of the SSC niche (Wang et al. 2013). Another factor must maintain the SSC niche, which is probably derived from interstitial cells. Oatley et al. (2009) reported that cultured thy1+ germ cells are enriched for colony-stimulating factor 1 receptor (Csf1r), and the addition of colony-stimulating factor 1 (Csf1) greatly enhances the self-renewal of SSCs, but not the total germ cells, as confirmed by transplantation assays (Oatley et al. 2009). Csfr1 is expressed by Leydig cells that are not evenly to distributed in the interstitium of seminiferous tubules, suggesting that interstitial cells, such as Leydig cells, may contribute to the formation of the SSC niche. Yoshida et al. (2007) showed that undifferentiated spermatogonia are more likely to reside within the area that the vasculature goes through in the seminiferous interval using time-lapse imaging of green fluorescent protein. This result suggests that the SSC niche is located in this area because SSCs are a subpopulation of undifferentiated spermatogonia. Moreover, an alternate pattern of the vasculature system leads to rearrangement of the undifferentiated spermatogonia, which further confirms the vasculature-associated niche location (Yoshida et al. 2007). Despite the association of the vasculature and Leydig cells, the true location of the niche is still under debate owing to the lack of specific SSC markers (Fig. 1.4).
It is thought that the number of SSC niches decides the potential SSC number. Therefore, it is important to reveal which factors influence the number of niches. Ectopically expressed human GDNF in mouse sertoli cells results in a dramatic increase of SSCs in the testis, indicating that high GDNF levels may increase the number of SSC niches (Yomogida et al. 2003). Oatley et al. (2011) found that the number of SSCs is possibly dictated by the number of Sertoli cells that secrete GDNF. A threefold increase in the number of colony-forming cells in seminiferous tubules of recipient mice occurs after transplantation of SSCs from PTU-treated mice with an increased number of sertoli cells at puberty compared with that in normal mice. Furthermore, PTU-treated mice exhibit threefold more accessible niches than that in normal mice with normal sertoli cells. In addition, they found that expansion of the niche number is influenced by neither the vasculature nor the interstitial cell populations (Oatley et al. 2011). SSC numbers are strictly regulated by GDNF in a dose-dependent manner. Overexpression of GDNF results in accumulation of SSCs, and heterozygous mutants show depletion of germ cells including SSCs (Meng et al. 2000). Follicle-stimulating hormone (FSH) upregulates GDNF and during testis damage. GDNF expression is increased possibly through FSH to maintain a normal number of SSC niches (Tadokoro et al. 2002). Other factors, such as Sin3A and RA, may also affect the niche number. Germ cells transplanted into RA-deficient mice show less colony formation, whereas mice with sertoli cells lacking Sin3A show germ cell depletion (Payne et al. 2010; McLean et al. 2002).
The study of the niche has been difficult owing to the complicated three-dimensional structure of seminiferous tubules in vivo. Developing a three-dimensional culture system may provide a possible strategy to solve this problem. Using the testicular cells of infertile mice as feeder cells, Kanatsu-Shinohara et al. (2012) reconstructed the SSC niche to some extent in a culture system, and the cultured SSCs migrated beneath the sertoli cells and formed cobblestone colonies. In this system, they found that the chemokine CXCL12 contributes to the SSC homing efficiency. Another study from Yokonishi and colleagues demonstrated that dissociated testicular cells are able to aggregate in suspension culture and form seminiferous tubules after transfer and culture on the surface of an agarose gel. When cultured GSCs were added to this autoreconstructed system, the GSCs differentiate before the meiotic phase (Yokonishi et al. 2013). This autoreconstruction of testicular cells is extraordinary and may be of great value for in vitro study of the niche.
3.4 Fate Decisions of SSCs
SSC self-renewal and differentiation must be sophistically balanced to maintain normal spermatogenesis and avoid tumorigenesis. When the balance shifts to self-renewal, there is accumulation of stem cells and decreases in the number of developed germ cells. In contrast, when the balance shifts to differentiation, maintenance defects will occur, eventually leading to infertility. This effect is best illustrated by paracrine regulation of GDNF secreted by sertoli cells. Meng et al. (2000) developed two transgenic mouse strains: one with overexpression of GDNF and the other with heterozygous ablation of GDNF. All transgenic mice showed decreased germ cell development and reduced fertility, and some even showed infertility. Mice overexpressing GDNF under the control of the promoter of testis-specific, human translation elongation factor showed larger clusters of spermatogonia, indicating accumulation of undifferentiated spermatogonia. Moreover, as the mice aged, these clusters grew larger and began to invade into the interstitium, and most of the mice generated non-metastatic tumors after 1 year of age. Through BrdU incorporation and apoptosis staining, they found no marked enhancement in the total proliferation rate. Thus, it was inhibition of differentiation rather than hyperproliferation that was responsible for the SSC accumulation. In GDNF+/− mice, although most were fertile, the depletion of germ cells increased with age and eventually resulted in only Sertoli cells in seminiferous tubules, indicating a maintenance defect of SSCs (Meng et al. 2000). This dose-dependent effect highlights the importance of precise regulation of GDNF and the role of GDNF in SSC fate decisions. GDNF is the ligand for co-receptors GFRα1/Ret, and its binding is able to activate several intrinsic signaling pathways such as PI3K/AKT/MEK and Src (He et al. 2008; Braydich-Stolle et al. 2007; Oatley et al. 2007). Many downstream transcription factors have been revealed, such as Bcl6b, Etv5, NANOS2, and ID4 (Oatley et al. 2006, 2011; Sada et al. 2009, 2012). Knockout or overexpression of these transcription factors have been performed in mice, confirming their indispensable roles in SSC maintenance. In addition to the most important extrinsic factor (GDNF), FGF2 and CSF1 play a role in SSC self-renewal, but not in the balance between self-renewal and differentiation (Oatley et al. 2009; Ishii et al. 2012).
An important intrinsic factor that regulates SSC fate is the transcriptional repressor Plzf. Ablation of Plzf leads to progressive germ cell loss due to exhaustion of the SSCs. One possible mechanism has been proposed by Filipponi et al. (2007). They revealed that Plzf directly binds to the promoter region of the kit gene, a character of spermatogonia differentiation, thus repressing the expression of kit, the SCF receptor (Filipponi et al. 2007). By inducing Redd1 expression, Plzf can oppose with mTORC1 whose hyperactivity leads to stem cell exhaustion. Through activation of PI3 K/AKT signaling, mTORC1 activates as a downstream effector. However, activation of mTORC1 suppresses the expression of the GDNF co-receptor GFRα1/Ret, which in turn inhibits GDNF signaling for SPC self-renewal. In the absence of Plzf, excessive amounts of GDNF are able to promote SPC self-renewal. Thus, the fate of SPCs is controlled by cross talk between Plzf-Redd1-mTORC1 and AKT/PI3K-mTORC1 signaling pathways in which mTORC1 plays the central role. The addition of rapamycin, a specific inhibitor of mTORC1, to Plzf−/− cultured GSCs restores expression of the receptor and partly rescues the GDNF signal (Hobbs et al. 2010). Another study from the same group revealed that Plzf–Sall4 antagonism decides the fate of SPCs. Sall4, a zing-finger transcription factor, restricts Plzf to non-cognate chromatin domains and induces the expression of the differentiation factor kit. In turn, Plzf opposes Sall4 functions and induces Sall1 expression. In vitro treatment of GSCs with RA, an indispensable extrinsic factor for initiation of differentiation, downregulates Plzf expression and increases the number of kit-positive spermatogonia. Though Sall4 transient upregulation in vivo, this change is accompanied by increasing numbers of kit+ spermatogonia (Hobbs et al. 2012). Considering the importance of Plzf in SSC fate decision, it is crucial to determine the regulatory mechanisms of Plzf expression. Identifying such factors will greatly enhance our understanding of the mechanisms of SSC fate decisions.
4 Perspectives
The expression profiles of undifferentiated spermatogonia involved in self-renewal and differentiation have been gradually revealed during the last few decades. However, there are no specific molecular markers that are unique to the subpopulation with both self-renewal and commitment abilities. Id4 may be a potential marker because it is the only identified molecule that is exclusively expressed by some As cells (Oatley et al. 2011). Identifying such molecules will greatly improve our understanding of fate decisions. It is still unclear whether there is a stage that marks the irreversibility of spermatogonia or whether it is the biological plasticity that leads to the heterogeneity among undifferentiated spermatogonia. Clarification of these aspects may change the current model of SSC development.
Development of a three-dimensional culture system for SSCs is another future challenge. In vivo studies have been difficult owing to the complex microenvironment. However, a three-dimensional SSC culture system will greatly facilitate niche studies. An in vitro culture system that supports the entire differentiation process and is able to produce haploid germ cells would be a useful tool. In vitro culture of SSCs has resolved the problem of rarity, but only establishment of an in vitro differentiation system will realize clinical applications. Dann et al. (2008) reported that expansion of cultured GSC with RA increases the number of germ cells positive for kit. However, they also noted premature meiosis and incomplete differentiation (Dann et al. 2008). Sato and Katagiri (2013) developed an organ culture system that supports all differentiation stages. Mature sperm can be obtained using this system, and in vitro fertilization has generated offspring (Sato et al. 2013). Nevertheless, this system still depends on testis organ fragments, thereby limiting its application to clinical use. Transplantation of rat or hamster SSCs into mouse testes results in exogenous spermatogenesis, which indicates conservation among species (Ogawa et al. 1999). Combining organ culture with xenogeneic testis culture is possible to fully support spermatogenesis, which needs further validation.
The signaling network that promotes SSC self-renewal and differentiation remains largely unknown. Microarray and transgenic mouse analyses have provided many potential genes that are important for SSC maintenance and differentiation, such as PHF13, SALL4, CDH1, and OCT4, but their exact roles have not been clarified (Tokuda et al. 2007; Gassei and Orwig 2013; Bordlein et al. 2011). As previously mentioned, the upstream molecules of Plzf remain unknown. Clarification of such networks will provide a better understanding of SSC self-renewal and differentiation to easily manipulate SSCs. Rodent SSC culture is efficient, but the culture of SSCs from other species is difficult (Kanatsu-Shinohara and Shinohara 2013). Understanding the molecular networks may improve the culture efficiency in other species.
References
Anderson R, Fassler R, Georges-Labouesse E, Hynes RO, Bader BL, Kreidberg JA, Schaible K, Heasman J, Wylie C. Mouse primordial germ cells lacking beta1 integrins enter the germline but fail to migrate normally to the gonads. Development. 1999a;126:1655–64.
Anderson R, Schaible K, Heasman J, Wylie C. Expression of the homophilic adhesion molecule, Ep-CAM, in the mammalian germ line. J Reprod Fertil. 1999b;116:379–84.
Aramaki S, Hayashi K, Kurimoto K, Ohta H, Yabuta Y, Iwanari H, Mochizuki Y, Hamakubo T, Kato Y, Shirahige K, Saitou M. A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Dev Cell. 2013;27:516–29.
Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–4.
Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16:513–6.
Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, Allemand I, Riou L, Fouchet P. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–6.
Bordlein A, Scherthan H, Nelkenbrecher C, Molter T, Bosl MR, Dippold C, Birke K, Kinkley S, Staege H, Will H, Winterpacht A. SPOC1 (PHF13) is required for spermatogonial stem cell differentiation and sustained spermatogenesis. J Cell Sci. 2011;124:3137–48.
Borum K. Oogenesis in the mouse. A study of the origin of the mature ova. Exp Cell Res. 1967;45:39–47.
Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600.
Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, Koopman P. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell. 2010;19:440–9.
Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304:34–45.
Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci. 1994;91:11303–7.
Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–52.
Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011–6.
Bukovsky A, Gupta SK, Virant-Klun I, Upadhyaya NB, Copas P, Van Meter SE, Svetlikova M, Ayala ME, Dominguez R. Study origin of germ cells and formation of new primary follicles in adult human and rat ovaries. Methods Mol Biol. 2008;450:233–65.
Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM, Hassell JA, Chandrashekar V, Hofmann MC, Hess RA, Murphy KM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–4.
Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35–51.
Clermont Y. Renewal of spermatogonia in man. Am J Anat. 1966;118:509–24.
Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236.
Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–9.
Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–9.
Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C. 2009;87:1–26.
Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–37.
De Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–98.
Durcova-Hills G, Wianny F, Merriman J, Zernicka-Goetz M, McLaren A. Developmental fate of embryonic germ cells (EGCs), in vivo and in vitro. Differentiation. 2003;71:135–41.
Ebata KT, Zhang X, Nagano MC. Expression patterns of cell-surface molecules on male germ line stem cells during postnatal mouse development. Mol Reprod Dev. 2005;72:171–81.
Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–14.
Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction. 2006;132:673–80.
Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update. 2006;12:275–82.
Feng YM, Liang GJ, Pan B, Qin XS, Zhang XF, Chen CL, Li L, Cheng SF, De Felici M, Shen W. Notch pathway regulates female germ cell meiosis progression and early oogenesis events in fetal mouse. Cell Cycle. 2014;13:782–91.
Filipponi D, Hobbs RM, Ottolenghi S, Rossi P, Jannini EA, Pandolfi PP, Dolci S. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–81.
Gassei K, Orwig KE. SALL4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PLoS ONE. 2013;8:e53976.
Gohbara A, Katagiri K, Sato T, Kubota Y, Kagechika H, Araki Y, Araki Y, Ogawa T. In vitro murine spermatogenesis in an organ culture system. Biol Reprod. 2010;83:261–7.
Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115–26.
Gosden RG. Germline stem cells in the postnatal ovary: is the ovary more like a testis? Hum Reprod Update. 2004;10:193–5.
Grisanti L, Falciatori I, Grasso M, Dovere L, Fera S, Muciaccia B, Fuso A, Berno V, Boitani C, Stefanini M, Vicini E. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27:3043–52.
Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–203.
Harvey AJ, Gibson TC, Quebedeaux TM, Brenner CA. Impact of assisted reproductive technologies: a mitochondrial perspective of cytoplasmic transplantation. Curr Top Dev Biol. 2007;77:229–49.
Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–5.
He ZP, Jiang JJ, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M. GDNF upregulates c-fos transcription via the Ras/ERK1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells. 2008;26:266–78.
Hobbs RM, Seandel M, Falciatori I, Rafii S, Pandolfi PP. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell. 2010;142:468–79.
Hobbs RM, Fagoonee S, Papa A, Webster K, Altruda F, Nishinakamura R, Chai L, Pandolfi PP. Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell. 2012;10:284–98.
Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–24.
Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF 3rd, Boiani M, Scholer HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–6.
Ishii K, Kanatsu-Shinohara M, Toyokuni S, Shinohara T. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development. 2012;139:1734–43.
Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–50.
Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–87.
Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6.
Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004a;119:1001–12.
Kanatsu-Shinohara M, Toyokuni S, Shinohara T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol Reprod. 2004b;70:70–5.
Kanatsu-Shinohara M, Inoue K, Miki H, Ogonuki N, Takehashi M, Morimoto T, Ogura A, Shinohara T. Clonal origin of germ cell colonies after spermatogonial transplantation in mice. Biol Reprod. 2006;75:68–74.
Kanatsu-Shinohara M, Lee J, Inoue K, Ogonuki N, Miki H, Toyokuni S, Ikawa M, Nakamura T, Ogura A, Shinohara T. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78:681–7.
Kanatsu-Shinohara M, Inoue K, Takashima S, Takehashi M, Ogonuki N, Morimoto H, Nagasawa T, Ogura A, Shinohara T. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell. 2012;11:567–78.
Ko K, Arauzo-Bravo MJ, Kim J, Stehling M, Scholer HR. Conversion of adult mouse unipotent germline stem cells into pluripotent stem cells. Nat Protoc. 2010;5:921–8.
Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–49.
Kostereva N, Hofmann MC. Regulation of the spermatogonial stem cell niche. Reprod Domest Anim. 2008;43(Suppl 2):386–92.
Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–9.
Krentz AD, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol. 2011;356:63–70.
Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci. 2003;100:6487–92.
Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71:722–31.
Kumar S, Chatzi C, Brade T, Cunningham TJ, Zhao XL, Duester G. Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat Commun 2011;2.
Lawson KA, Dunn NR, Roelen BAJ, Zeinstra LM, Davis AM, Wright CVE, Korving JPWFM, Hogan BLM. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–36.
Le Bouffant R, Souquet B, Duval N, Duquenne C, Herve R, Frydman N, Robert B, Habert R, Livera G. Msx1 and Msx2 promote meiosis initiation. Development. 2011;138:5393–402.
Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–9.
Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92:1619–49.
Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–7.
Magnusdottir E, Dietmann S, Murakami K, Gunesdogan U, Tang FC, Bao SQ, Diamanti E, Lao KQ, Gottgens B, Surani MA. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat Cell Biol. 2013;15:905–U322.
Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19:612–24.
Mattiske D, Kume T, Hogan BLM. The mouse forkhead gene Foxc1 is required for primordial germ cell migration and antral follicle development. Dev Biol. 2006;290:447–58.
McLean DJ, Russell LD, Griswold MD. Biological activity and enrichment of spermatogonial stem cells in vitamin A-deficient and hyperthermia-exposed testes from mice based on colonization following germ cell transplantation. Biol Reprod. 2002;66:1374–9.
Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–93.
Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O’Brien W, Raz E, Littman D, Wylie C, Lehmann R. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130:4279–86.
Morimoto H, Kanatsu-Shinohara M, Takashima S, Chuma S, Nakatsuji N, Takehashi M, Shinohara T. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS ONE. 2009;4:e7909.
Nakagawa T, Nabeshima Y-i, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206.
Nakaki F, Hayashi K, Ohta H, Kurimoto K, Yabuta Y, Saitou M. Induction of mouse germ-cell fate by transcription factors in vitro. Nature 2013;501:222–+.
Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328:1561–3.
Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–21.
Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci. 2006;103:9524–9.
Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842–51.
Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–9.
Oatley MJ, Kaucher AV, Racicot KE, Oatley JM. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod. 2011a;85:347–56.
Oatley MJ, Racicot KE, Oatley JM. Sertoli cells dictate spermatogonial stem cell niches in the mouse testis. Biol Reprod. 2011b;84:639–45.
Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol Reprod. 1999;60:515–21.
Ohinata Y, Payer B, O’Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, Saitou M, Surani MA. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–13.
Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell. 2009;137:571–84.
Payne CJ, Gallagher SJ, Foreman O, Dannenberg JH, Depinho RA, Braun RE. Sin3a is required by sertoli cells to establish a niche for undifferentiated spermatogonia, germ cell tumors, and spermatid elongation. Stem Cells. 2010;28:1424–34.
Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–32.
Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143:139–49.
Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–51.
Peters H, Crone M. DNA synthesis in oocytes of mammals. Arch Anat Microsc Morphol Exp. 1967;56:160–70.
Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–78.
Reizel Y, Itzkovitz S, Adar R, Elbaz J, Jinich A, Chapal-Ilani N, Maruvka YE, Nevo N, Marx Z, Horovitz I, Wasserstrom A, Mayo A, Shur I, Benayahu D, Skorecki K, Segal E, Dekel N, Shapiro E. Cell lineage analysis of the mammalian female germline. PLoS Genet. 2012;8:e1002477.
Rudkin GT, Griech HA. On the persistence of oocyte nuclei from fetus to maturity in the laboratory mouse. J Cell Biol. 1962;12:169–75.
Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–8.
Sada A, Hasegawa K, Pin PH, Saga Y. NANOS2 acts downstream of glial cell line-derived neurotrophic factor signaling to suppress differentiation of spermatogonial stem cells. Stem Cells. 2012;30:280–91.
Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, Matoba S, Ogura A, Ogawa T. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011a;2:472.
Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011b;471:504–7.
Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nature Protocols 2013;8:2098–2104
Sato T, Yokonishi T, Komeya M, Katagiri K, Kubota Y, Matoba S, Ogonuki N, Ogura A, Yoshida S, Ogawa T. Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc Natl Acad Sci U S A. 2012;109:16934–8.
Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protoc. 2013;8:2098–104.
Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–50.
Shinohara T, Avarbock MR, Brinster RL. β1-and α6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci. 1999;96:5504–9.
Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97:8346–51.
Simon L, Ekman GC, Kostereva N, Zhang Z, Hess RA, Hofmann MC, Cooke PS. Direct transdifferentiation of stem/progenitor spermatogonia into reproductive and nonreproductive tissues of all germ layers. Stem Cells. 2009;27:1666–75.
Souquet B, Tourpin S, Messiaen S, Moison D, Habert R, Livera G. Nodal signaling regulates the entry into meiosis in fetal germ cells. Endocrinology. 2012;153:2466–73.
Speed RM. Meiosis in the foetal mouse ovary. I. An analysis at the light microscope level using surface-spreading. Chromosoma. 1982;85:427–37.
Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104.
Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113:29–39.
Tam PP, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol. 1996;178:124–32.
Tanaka SS, Nakane A, Yamaguchi YL, Terabayashi T, Abe T, Nakao K, Asashima M, Steiner KA, Tam PPL, Nishinakamura R. Dullard/Ctdnep1 modulates WNT signalling activity for the formation of primordial germ cells in the mouse embryo. PLoS ONE. 2013;8.
Tanaka SS, Yamaguchi YL, Tsoi B, Lickert H, Tam PP. IFITM/Mil/fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev Cell. 2005;9:745–56.
Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200.
Tokuda M, Kadokawa Y, Kurahashi H, Marunouchi T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod. 2007;76:130–41.
Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110.
Wang H, Shi L, Xiang J, Ding X, Luo H, Wang S, Wu J. Isolation, culture and transplantation of female germline stem cells from neonatal and prepubertal mice. Protoc Exch. 2013;. doi:10.1038/protex.2013.004.
Wang H, Jiang M, Bi H, Chen X, He L, Li X, Wu J. Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol. 2014;6:164–71.
White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–U176.
Wong TT, Tesfamichael A, Collodi P. Production of zebrafish offspring from cultured female germline stem cells. PLoS ONE. 2013;8:e62660.
Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc. 2013;8:966–88.
Wu J, Zhang Y, Tian GG, Zou K, Lee CM, Yu Q, Yuan Z. Short-type PB-cadherin promotes self-renewal of spermatogonial stem cells via multiple signaling pathways. Cell Signal. 2008;20:1052–60.
Wu J, Luo H, Wang H. Germline stem cells. Curr Top Dev Biol. 2013;102:97–126.
Xiong J, Wang H, Guo G, Wang S, He L, Chen H, Wu J. Male germ cell apoptosis and epigenetic histone modification induced by Tripterygium wilfordii Hook F. PLoS ONE. 2011;6:e20751.
Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet. 2008;40:1016–22.
Yamashita YM, Fuller MT. Asymmetric stem cell division and function of the niche in the Drosophila male germ line. Int J Hematol. 2005;82:377–80.
Ying Y, Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol. 2001;232:484–92.
Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol. 2000;14:1053–63.
Ying Y, Qi XX, Zhao GQ. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc Natl Acad Sci U S A. 2001;98:7858–62.
Yokobayashi S, Liang CY, Kohler H, Nestorov P, Liu Z, Vidal M, van Lohuizen M, Roloff TC, Peters AH. PRC1 coordinates timing of sexual differentiation of female primordial germ cells. Nature. 2013;495:236–40.
Yokonishi T, Sato T, Katagiri K, Komeya M, Kubota Y, Ogawa T. In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol Reprod. 2013;89:1–6.
Yokonishi T, Sato T, Katagiri K, Ogawa T. In vitro spermatogenesis using an organ culture technique. Methods Mol Biol. 2013;927:479–88.
Yomogida K, Yagura Y, Tadokoro Y, Nishimune Y. Dramatic expansion of germinal stem cells by ectopically expressed human glial cell line-derived neurotrophic factor in mouse Sertoli cells. Biol Reprod. 2003;69:1303–7.
Yoshida S, Nabeshima Y, Nakagawa T. Stem cell heterogeneity: actual and potential stem cell compartments in mouse spermatogenesis. Ann N Y Acad Sci. 2007a;1120:47–58.
Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007b;317:1722–6.
Yuan Z, Hou R, Wu J. Generation of mice by transplantation of an adult spermatogonial cell line after cryopreservation. Cell Prolif. 2009;42:123–31.
Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, Sun K, Zou K, Wang L, Xiong J, Xiang J, Wu J. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3:132–41.
Zhang Z, Gong Y, Guo Y, Hai Y, Yang H, Yang S, Liu Y, Ma M, Liu L, Li Z, Gao WQ, He Z. Direct transdifferentiation of spermatogonial stem cells to morphological, phenotypic and functional hepatocyte-like cells via the ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin E. Cell Commun Signal. 2013a;11:67.
Zhang L, Tang J, Haines CJ, Feng H, Lai L, Teng X, Han Y. c-kit expression profile and regulatory factors during spermatogonial stem cell differentiation. BMC Dev Biol. 2013b;13:38.
Zhou L, Wang L, Kang JX, Xie WH, Li XY, Wu CQ, Xu B, Wu J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod. 2014;20:271–81.
Zou K, Yuan Z, Yang ZJ, Luo HC, Sun KJ, Zhou L, Xiang J, Shi LJ, Yu QS, Zhang Y, Hou RY, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–U424.
Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20:2197–204.
Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–109.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Shanghai Jiao Tong University Press, Shanghai and Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Wu, J., Zheng, Z., Wang, H., Mei, X., Ding, X., Li, X. (2015). Primordial Germ Cells and Germ Line Stem Cells. In: Zhao, R. (eds) Stem Cells: Basics and Clinical Translation. Translational Medicine Research, vol 1. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7273-0_1
Download citation
DOI: https://doi.org/10.1007/978-94-017-7273-0_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7272-3
Online ISBN: 978-94-017-7273-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)