Abstract
Cannabinoids influence cardiovascular variables in health and disease via multiple mechanisms. The chapter covers the impact of cannabinoids on cardiovascular function in physiology and pathology and presents a critical analysis of the proposed signalling pathways governing regulation of cardiovascular function by endogenously produced and exogenous cannabinoids. We know that endocannabinoid system is overactivated under pathological conditions and plays both a protective compensatory role, such as in some forms of hypertension, atherosclerosis and other inflammatory conditions, and a pathophysiological role, such as in disease states associated with excessive hypotension. This chapter focuses on the mechanisms affecting hemodynamics and vasomotor effects of cannabinoids in health and disease states, highlighting mismatches between some studies. The chapter will first review the effects of marijuana smoking on cardiovascular system and then describe the impact of exogenous cannabinoids on cardiovascular parameters in humans and experimental animals. This will be followed by analysis of the impact of cannabinoids on reactivity of isolated vessels. The article critically reviews current knowledge on cannabinoid induction of vascular relaxation by cannabinoid receptor-dependent and –independent mechanisms and dysregulation of vascular endocannabinoid signaling in disease states.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
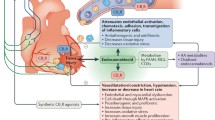
Cannabinoids influence the function of many organs and systems and apart from the well-known neurobehavioral and analgesic effects, cannabinoids exert a profound effect on cardiovascular, immune, digestive, reproductive function, and influence cell fate, body temperature, bone formation and other aspects of human physiology. Key cardiovascular parameters such as blood pressure, vasomotor control, cardiac contractility, vascular inflammation, preconditioning and angiogenesis are controlled by cannabinoids. Changes in the levels of circulatory cannabinoids and cannabinoid receptor expression in the vasculature as well as the associated perturbations in cannabinoid signalling have been detected under a number of pathophysiological conditions including obesity, diabetes, advanced liver cirrhosis, cardiotoxicity, circulatory shock, atherosclerosis and hypertension [1,2,3,4,5,6,7,8]. Consequently, the endocannabinoid system is widely accepted to represent an attractive therapeutic target to tackle a range of abnormalities including cardiovascular disorders [9,10,11].
Because of psychotopic and pain-relieving effects, the members of the plant family Cannabaceae have a long history of cultivation and human use both for recreational and medical purposes, rooting through several thousand years [12]. Recent boosted recreational marijuana abuse and accessibility of a growing number of synthetic psychoactive cannabinoids with greatly increased potencies as compared to that of Δ9-tetrahydrocannabinol (THC) , the main psychoactive constituent of marijuana, are coincided with the reported serious cardiovascular events such as myocardial infarction, cardiomyopathy, arrhythmias and stroke with documented fatalities even among young and relatively healthy men [13,14,15]. While the mechanisms for these events are still not entirely clear, these observations emphasize a casual association between recreational cannabis abuse and cardiovascular abnormalities, calling for a need to advance our understanding of the fundamental mechanisms so severely affecting cardiovascular function by cannabis. Besides the principal phytocannabinoid THC, which binds to cannabinoid receptors and determines psychoactive properties of the plant, cannabis contains an extensive number of non-psychoactive phytocannabinoids with low affinity binding at cannabinoid receptors type 1, CB1, and type 2, CB2, such as cannabidiol , cannabinol, cannabidivarin and cannabigerol and others. Because of beneficial effects in a range of disorders and a lack of psychoactivity, therapeutic effects of these compounds and their mechanisms of action is a subject of intense research [10].
The discovery of THC, the main psychoactive phytocannabinoid contained in Cannabis plant [16], was the first major step in the recognition of the role of the endocannabinoid system in health and disease. Cannabinoid research received a strong impetus following the identification and characterization of CB1 [17] and CB2 receptors [18, 19]. The latter was initially identified as peripherally restricted receptor expressed by immune cells. Subsequent studies have shown that the CB2 receptors are also distributed in vascular cells [20, 21] and central and peripheral nervous system [22,23,24,25].
The endogenous ligands for cannabinoid receptors, anandamide and 2-arachidonoyl glycerol (2-AG) were detected initially in the brain [26, 27] and gut [28]. Subsequent studies showed that endocannabinoids are synthetized at the plasma membranes of virtually all cell types including vascular [29,30,31,32], cardiac cells [33], monocytes and platelets [34]. Cardiovascular pathologies, such as coronary circulatory dysfunction, myocardial infarction, hypertension, atherosclerosis and diseases accompanied by vascular dysfunction, such as diabetes, obesity and cirrhosis, are associated with alterations in cannabinoid signaling and increased plasma levels of 2-AG and anandamide [1, 5, 34,35,36,37,38].
Under normal conditions, CB1 receptors have been detected in different vascular beds, including endothelial cells from rat mesentery [37, 39, 40], rat [2, 32] and human aorta [41], human hepatic artery endothelial cells [42], rat aortic smooth muscle cells [43], pointing at engagement of the endocannabnoid system in regulation of vascular function. Indeed, endocannabinoids and their synthetic analogues exert hypotensive and cardiodepressant effects, control cardiac contractile reactions [1, 11, 44]. The chapter will review the impact of cannabinoids on cardiovascular function.
5.2 Effects of Cannabinoids on Cardiovascular Parameters
Highly diverse actions of cannabinoids are mediated via surprisingly wide number of targets, spanning from classical G protein-coupled cannabinoid receptors, non-CB1/CB2 targets, including G-protein coupled receptors GPR18, GPR35, GPR55 and GPR119 [45,46,47,48,49,50], and a broad number of ion transport systems [51,52,53,54]. The reactions of cardiovascular variables in response to cannabinoids depend on several factors and besides the type of predominantly stimulated cannabinoid receptors, there are many other determinants of the reaction, such as direct targeting of ion channels and transporters located in plasmalemma, engagement in the response of non-CB1/CB2 receptors, intracellular ion channels [55, 56] or intracellularly located receptors for cannabinoids [57,58,59]. Via stimulation of specific cannabinoid receptors, cannabinoids may attenuate or intensify cardiovascular pathological states and, accordingly, play a protective or pathophysiological role.
The molecular mechanisms underlying diverse effect of cannabinoids and their synthetic analogues on vascular function , although progressively unveiled in the last two decades, are not yet entirely clear. In vivo effects of cannabinoids involve sites of action in the central [60, 61] and the peripheral nervous system [62, 63] as well as both cannabinoid receptor-dependent and –independent targets located on cardiac myocytes [64,65,66], vascular smooth muscle [40, 67,68,69,70,71] and endothelial cells [6, 40, 52, 53, 72,73,74,75].
Apart from regulation of vasoactivity, cannabinoids influence cardiac performance and modulate ischemia-reperfusion injury [8, 76,77,78], endothelial [79], and smooth muscle cell migration [71], angiogenesis [80, 81], vascular wall inflammation and atherogenesis [3, 82]. We will briefly review the literature describing the impact of smoked cannabis and intravenous THC, as well as central and peripheral regulation of cardiovascular parameters by cannabinoids.
5.2.1 Effects of Marijuana Smoking on Cardiovascular Parameters
The cardiovascular effects elicited by marijuana smoking largely depend on chemical composition of the plant, specifically, the THC content, the dose inhaled and the smoking method. The impact of cannabinoids on cardiovascular parameters has been studies since early 1970s. The studies were mostly directed on examining the effect of THC as the principle active ingredient of Cannabis sativa [83, 84]. From the very beginning of investigation of the effects of constituents of cannabis on cardiovascular parameters, the differences in the effects of THC and cannabidiol both on heart rate and some psychological reactions were noticed [85]. Early reports on the effects of cannabis on humans were focused mainly on psychotropic effects and pointed at pharmacological difference between oral injestion and inhalation of smoke of cannabis products [86]. Smoking cannabis was shown to lead to a potent bronchodilation of human airways [87] and an immediate increase in heart rate up to 90 beats per minute that may last more than 1 h and an increase in limb blood flow [88, 89]. These responses were not observed after administration of propranolol, a beta-adrenergic blocker, pointing for beta-adrenergic stimulation [89]. However, repeated users within several days or weeks develop a tolerance to the initial effects and experience bradycardia and hypotension. Most of these studies conclude that THC alters autonomic control of the cardiovascular system resulting in parasympathetic dominance. Early [90, 91] and more recent [92] studies have shown that marijuana smoking is associated with increased cerebral blood flow. Systematic reviews of the reported cases indicate that marijuana smoking is linked to increased likelihood of development of severe cardiovascular events including atrial fibrillation, enhanced left ventricular systolic function, transient loss of consciousness and a fall, ventricular arrhythmias, coronary artery disease, severe stroke development, peripheral arteritis [15, 93,94,95].
5.2.2 Effects of Cannabinoids on Blood Pressure and Heart Rate
5.2.2.1 Human Studies
In healthy men volunteers, 30 mg of THC received orally increased the heart rate and blood pressure [85, 96]), while cannabidiol (15–60 mg) produced no effect when administered alone and blocked the effect of THC when the drugs were administered together [85]. Acute administration of cannabidiol at higher dose (600 mg) was reported to reduce resting blood pressure and the blood pressure increase elicited by exercise and mental stress [97]. The uncovered differences in the impacts of THC and cannabidiol on heart rate and blood pressure in early 1970s [85, 96, 98] initiated an extensive research in this field aiming to identify the mechanisms underlying the impact of cannabinoids on cardiovascular system. While acute administration of THC generally results in an increase in blood pressure and heart rate, repeated administration of THC decreases blood pressure and heart rate. In conscious humans, an acute intravenous administration of THC in the dose of 25 μg/kg elicits tachycardia without significant alterations in systolic and diastolic blood pressures [99]. Prior beta adrenergic blockade partially inhibited this response. Acute oral or intravenous administration of THC at higher dose (0.2–0.3 mg/kg) resulted in an increase in both heart rate and blood pressure in healthy volunteers [96, 100]. The reactions, however, switched to the opposite when THC was injested for a prolonged time [101]. These data point at complex mechanisms involved in bidirectional regulation of cardiovascular parameters by cannabinoids.
5.2.2.2 Animal Studies
The hemodynamic effects of cannabinoids in conscious rats are quite different from those observed in anesthetized rats [102, 103]. In anaesthetized rats, the most prominent response to anandamide and THC infusion is a long-lasting hypotension and bradycardia, generally ascribed as phase 3 of the triphasic response [104,105,106]. The long-lasting hypotension and bradycardia evoked by anandamide infusion to anesthetized rats is preceded by an immediate brief drop in blood pressure and heart rate ascribed as phase 1, which was followed by a brief (30–60 s) pressor response and tachycardia, ascribed as phase 2. The long lasting depressor effect of anandamide, but not the two initial transient phases, is inhibited by rimonabant [105, 107] and after transaction of the cervical spinal cord or blockade of alfa-adrenergic receptors [103, 104, 107], suggesting that the depressor response is due to CB1 receptor-mediated inhibition of norepinephrine release from pepripheral sympathetic nerve terminals in the heart and vasculature and subsequent inhibition of catecholamine release [108]. It was shown also that in anaesthetized rats, cannabidiol and its synthetic analogue O-1918, which have low affinity to CB1 and CB2 receptors, elicit a prolonged decrease in blood pressure, heart rate and mesenteric and renal blood flow, masking/reducing the similar cardiovascular effects of anandamide that are normally observed in the absence of cannabidiol and O-1918 [109]. The authors attributed the hypotensive effects of anandamide in anesthetized rats to stimulation of the third type cannabinoid receptor sensitive to O-1918 [109]. In the earlier study of Malinowska et al [110], the anandamide-evoked decrease in heart rate and blood pressure in anaesthetized rats (phase 3) has been attributed to stimulation of the CB1 and TRPV1 receptors.
The phases 1 and 2 are absent in TRPV1-defficient mice [106, 107, 111], pointing for the involvement of TRPV1 receptor. A TRPV1 agonist capsaicin was shown to be more potent than methanandamide and anandamide at eliciting an immediate short-lasting decrease in heart rate and blood pressure that is inhibited by a selective TRPV1 antagonist capsazepine [110], suggesting that a short-lived depressor effect of anandamide is evoked by the Bezold-Jarisch reflex [112]. A brief pressor response (phase 2) is enhanced after alfa-receptor blockade or cervical cord transaction [104].
Unlike the responses elicited by THC and anandamide, the hypotension and bradycardia elicited by 2-AG is insensitive to rimonabant and is preserved in CB1 knock- out mice [113]. However, cardiovascular effects of 2-AG were found to be masked by a rapid degradation of the endocannabinoid by a monoacylglycerol lipase with generation of arachidonic acid. A metabolically stable 2-AG analogue 2-AG ether was found to elicit hypotension that is sensitive to rimonabant and absent in CB1 knock-out mice [113], suggesting the CB1 receptor-mediated signalling, possibly through sympathetic nerves innervating the resistance vessels.
In contrast to anaesthetized rats, in conscious rats, intravenous administrations of anandamide , its stable analogue methanandamide, THC and WIN55212-2 fail to produce a prolonged hypotension, but result in a brief pressor response, that was potentiated by rimonabant [105], indicating that the CB1 receptor-dependent signaling attenuates the pressor response. Consistent with the idea that stimulation of CB1 receptor results in vasodilation, intravenous administration of a synthetic CB1 and CB2 receptor agonist WIN55512-2 to pithed, conscious rabbits in which the sympathetic outflow was continuously stimulated electrically was shown to decrease blood pressure and heart rate [114] and the effect was antagonized by rimonabant.
In other study performed on conscious rats, anand amide (75–1250 μg/kg) elicited a short-lived increase in arterial blood pressure associated with vasoconstriction in renal, mesenteric and hindquarters vascular beds [115]. When anandamide was administered at the higher dose (2.5 mg/kg) to conscious rats, a pressor response was preceded by a transient fall in arterial blood pressure. After high dose of anandamide, the hindquarters vasoconstriction was followed by vasodilation. Intriguingly, in conscious rats, none of the hemodynamic responses to anandamide were found to be influenced by antagonism of CB1 receptors with AM251 [115], suggesting that the anandamide-evoked responses may not involve CB1 receptors. In contrast, in the presence of beta2 adrenoceptor antagonist ICI 118551 the hindquarters vasodilation was inhibited and the pressor response prolonged. Similar to anandamide, WIN55212-2 and HU-210 evoked a pressor response associated with renal and mesenteric vasoconstriction and hindquarters vasodilation that was antagonized by AM251 and the beta2 receptor antagonist ICI 118551 [116], a finding consistent with the involvement of beta2 adrenoreceptors in the CB1 receptor-mediated hindquarters vasodilation. Notably, AM251 [116] has no noticeable effects on resting hemodynamics and blood pressure, suggesting negligible role of CB1 receptor-dependent signaling in cardiohemodynamics under normal conditions.
Conclusively, studies on anesthetized and conscious rats demonstrate complexity of hemodynamic effects of cannabinoids. While in anesthetiized rats, cannabinoids evoke a triphasic response, the most prominent of which is a sustained vasodilation, in conscious rats, cannabinoids evoke dose-dependent brief pressor response. In the absence of anesthetics, the only vascular bed that shows vasodilation is the hindquarters.
5.3 Effects of Cannabinoids on Reactivity of Isolated Vessels
The mechanisms of action of cannabinoids and cannabinoid-like substances on vascular cells have been widely studied in isolated vessel preparations with the use of wire myography. In a great number of isolated pre-contracted vascular preparations, cannabinoids produce vasodilation of varying degree, however, constriction responses have also been reported [117], emphasizing complex vascular cannabinoid pharmacology. While it is established that both CB1 and CB2 are distributed in both endothelial and vascular smooth muscle cells [21, 32, 40, 41], a link between stimulation of vascular cannabinoid receptors and vasodilation remains controversial, with the prevailing conclusions that the relaxation of healthy arteries may not require stimulation of vascular cannabinoid receptors. In fact, the mechanisms affecting vasomotor activity elicited by topically applied cannabinoids independently of CB1 and CB2 receptor stimulation are extremely versatile and seem to be predominantly responsible for both endothelium-dependent and -independent relaxation in a vast number of vascular beds.
Numerous studies indicate that the mechanisms of cannabinoid-induced vasodilation vary between species, vessel type and have regional differences [70, 118,119,120]. Conclusions of different research groups on the involvement of CB1 receptor in the responses of isolated vessels to anandamide and participation of endothelium-dependent mechanisms in these responses sometimes are controversial even with regard to the same vascular bed, adding some confusion into the topic [119, 121]. Thus, in isolated rat mesenteric artery, the relaxation to anandamide has been identified as endothelium- and CB1 receptor-independent [121, 122], whereas other groups showed endothelium-dependency of the response in the same artery with [119] or without [123] CB1 receptor involvement. It is possible that the choice of different constricting agents, unspecific CB1 antagonists and the method of de-endothelization influenced the results obtained.
Anandamide at 30 μM was shown to relax U-46619-pre-contracted rat aortic rings by 22% in endothelium-independent manner [124]. The relaxation was unaffected by rimonabant, AM251 and capsaicin, but was reduced to 13% in pertussis toxin-pre-treated preparations, allowing the authors to suggest the involvement of yet unidentified non-CB1/CB2 cannabinoid receptor located on smooth muscle cells [124]. In other study [125], anandamide (100 μM) gradually relaxed rat aortic rings pre-contracracted with phenylephrine by 51% within 7–10 min before reaching a plateau and the relaxation was reduced to 20% following removal of the endothelium. The relaxation was insensitive to pre-treatment with rimonabant and the CB2 antagonist SR144528, but was inhibited by O-1918, allowing the authors to suggest the involvement of unidentified non-CB1/CB2 cannabinoid receptor located on endothelial cells [125]. In rat aortic rings pre-contracted with the combined presence of U-46619 and methoxamine, the endothelium-dependent relaxation to anandamide was shown to be insensitive to CB1 and CB2 receptor antagonists, but inhibited by peroxisome proliferator-activated receptor (PPAR) gamma antagonist GW9662 [59], suggesting that the relaxation is mediated by stimulation of nuclear PPAR gamma receptor. In phenylephrine-pre-contracted aortic rings isolated from normotensive sham-operated rats subjected to excision of the left renal artery without clipping, the maximal relaxation to 30 μM anandamide amounted 4% only [20]. As could be seen from these studies, the proposed mechanisms governing the anandamide-evoked relaxation of the same vascular preparations principally differ between different research groups. The reasons for the discrepancies are unclear but might be related to variations in wire myography protocols or the constricting agent used (a thromboxane mimetic U-46619 vs. phenylephrine).
Obviously, varying outcomes of the studies as for the requirement for the given cannabinoid receptor in the vasodilation to cannabinoids are unlikely to be solely explained by intrinsic variations in the cannabinoid receptor expression between the vascular beds. A part of the problem is that the pharmacology and molecular modes of action of cannabinoid receptor agonists and antagonists developed and widely used in wire myography studies as selective have not been clearly defined. Mounting number of studies points for additional, cannabinoid-receptor-independent effects and targets, such as PPAR, TRPV4 and large conductance calcium-activated potassium (BKCa) channels. Employment of highly selective cannabinoid receptor agonists and antagonists with precise molecular mechanisms of action is essential to delineate possible role of cannabinoid receptors in vascular effects of cannabinoids in health and disease. Another possible contributor into variable outcomes of the studies is the choice of technique to capture the mechanisms of vascular cannabinoid signalling and clarify whether the response requires specific receptor. Electrophysiological studies on isolated endothelial and smooth muscle cells and intact vascular preparations allowed to identify a large number ion-transporting systems targeted by cannabinoids and cannabinoid-like substances, including BKCa channels [72, 126, 127], intermediate conductance calcium-activated potassium (IKCa ) channels [53], voltage-gated Ca2+ channels of L and T type [128,129,130], TRPV4 [74], TRPA1 [131, 132], and the TASK subfamily of two pore domain K+ channels responsible for background K+ currents [133,134,135,136], Na+-Ca2+ exchanger (NCX) [65, 127, 137, 138] and Na+-K+- ATPase [52, 139, 140]. All these players are present in the vasculature, determining the membrane potential of endothelial and smooth muscle cells, the release of variety of endothelium-derived vasoactive substances and contractile responses to cannabinoids.
5.3.1 Endothelium-Independent Relaxation to Cannabinoids
The earliest study describing the effect of locally applied endocannabinoids on vascular reactivity showed that in anesthetized rabbits, topically applied anandamide and THC dilate cerebral arterioles [141]. The dilation was suggested to be mediated by the release of endogenous arachidonic acid. The study initiated an intense research into the effects and the mechanisms of action of cannabinoids on blood vessels. In experiments performed on pre-contracted rat superior mesenteric arterial bed, anandamide was found to induce endothelium-independent relaxation that was suppressed by the CB1 receptor inhibitor rimonabant [39]. Relaxations to carbachol and Ca2+ ionophore A23187 were also sensitive to rimonabant, suggesting that carbachol and anandamide share the mechanisms of action and that anandamide is an endothelium-derived hyperpolarizing factor (EDHF), an entity responsible for endothelium-dependent relaxation under conditions of inhibition of nitric oxide (NO) synthesis. This mechanism was also proposed to govern the relaxation in rat coronary artery [142]. Since these observations, much attention has been given to investigation of the mechanisms of vasoactive effects of anandamide and other cannabinoids. However, in the rat isolated perfused mesenteric vascular bed, HU-210, WIN55212-2 and THC failed to cause vasodilation and even produced constriction [143]. The possibility that anandamide acts as a mediator of NO-independent vasodilation to endothelium-dependent vasodilators was extensively evaluated in late 1990s in different vascular beds, including isolated rat mesenteric arteries [70, 118, 144, 145], rat hepatic [146] and rat coronary arteries [147], guinea-pig basilar artery [148] and in anaesthetized rabbits [149] with general conclusion that anandamide is not EDHF in these vascular beds.
5.3.1.1 Potential Role of Cannabinoid Receptors in Vasodilation
The involvement of cannabinoid receptors in the vasodilation to anandamide and other cannabinoids in different vascular beds has been most extensively investigated using wire myography approach with the help of pharmacological modulators of cannabinoid receptors. Less often, pressure myography is used. In a typical wire myography protocol, the excised vessels are cut into approximately 2 mm-long segments and the rings are attached to hooks mounted in a Mulvany-Halpern wire myograph [150] and then properly stretched to achieve a largest contractile response to a submaximal dose of one of the contractile agents such as phenylephrine, methoxamine or U-46619, a stable thromboxane A2 receptor agonist. Following stabilization of the tone, the substances of interest are applied and the vasorelaxation is computed as the ratio to the imposed contraction. In wire myography, tension is measured under isometric conditions. The limitation of the method is that optimum resting tension is not the same as physiological and the attachment of vessel ring leads to nonphysiological geometry and loading [151, 152]. It should be noted, that in the absence of imposed contraction, i.e. in the absence of stimulated Ca2+ entry into smooth muscle cells, most of the cannabinoids fail to cause a significant changes in the baseline tension and THC elicits a significant contraction [124].
While the majority of studies concluded that neither CB1, nor CB2 receptors are involved in endothelium-dependent or –independent vasodilation to cannabinoids in healthy arteries [69, 70, 122, 123, 144, 153,154,155], some limited number of reports point for engagement of CB1 receptors [37, 43, 67, 119, 156,157,158] or CB2 receptors [37, 40] in the vasodilation. In many early and recent vascular myography studies, rimonabant and structurally very close compound AM251 have been the most widely employed CB1 receptor antagonists. Rimonabant was first described by Sanofi Aventis as a selective and orally active inverse CB1 receptor agonist with CB1 affinity in low nanomolar range [159]. It should be noted that although the low nM concentrations of rimonabant and AM251 are required to block CB1 receptors, these compounds are frequently used in low micromolar (1–3 μM) concentrations in assessing the role of CB1 receptor in the vasodilation [124, 125, 158, 160, 161]. At these concentrations, these CB1 antagonists display a number of CB1 receptor-independent effects. Thus, at 1 μM rimonabant inhibits cannabinoid-induced hypotension and mesenteric vasodilation via a target distinct from CB1 receptor, as the effect is observed in CB1 and CB1/CB2 knock-out mice [123]. The unspecific effects of rimonabant include inhibition of myo-endothelial gap junctions [162], endothelium-dependent relaxation to carbachol, acetylcholine, bradykinin, Ca2+ ionophor A23187 and ionomycin [39, 142, 143, 157, 162], direct suppressive action on the BKCa channel function [163] and Ca2+ entry mechanism [164]. In the isolated quinea pig carotid artery, rimonabant at concentrations 0.1–10 μM hyperpolarizes vascular smooth muscle cells by up to 10 mV and significantly inhibits the smooth muscle cell hyperpolarization evoked by acetylcholine [70, 118]. Rimonabant at 10 μM was shown to strongly attenuate the relaxation of cannulated pre-contracted rat mesenteric artery induced by levcromacalim, an opener of ATP-sensitive K+ (KATP) channel [122]. Similar to rimonabant, AM251 was found to have a number of non-specific effects [54, 131, 163, 165,166,167]. Interestingly, the activation of TRPV1 channels by anandamide was reported to be antagonized by rimonabant and AM251, although at concentrations higher than those required for CB1 antagonism. Collectively, the wealth of data points for non-specific effects of widely used CB1 receptor antagonists rimonabant and AM251, warranting considerations in interpretation of the relevant data. Cannabinoid receptor-independent targets for CB1 antagonists rimonabant and AM251 and for the cannabidiol analogue O-1918 widely used as a “selective” antagonist of “endothelial cannabinoid receptor” are listed in Table 5.1.
Vasoactive properties of anandamide are most frequently studied among other cannabinoids. There is a general consensus that this endocannabinoid elicits a relaxation in a vast number of isolated pre-contracted vascular preparations with both endothelium-independent and –dependent mechanisms. The results published are somewhat controversial even when the studies from the same vascular bed are compared.
Anandamide was shown to elicit endothelium-independent relaxation of pre-contracted rat small mesenteric artery [39, 121, 143, 145, 157, 168, 169], rat coronary artery [168], rat aorta [124]. In another study performed on isolated rat mesentery pre-contracted with phenylephrine, de-endothelization slightly but significantly reduced the relaxation to anandamide [143]. Removal of the endothelium fails to inhibit the relaxation of pre-contracted rat gastric arteries in response to the stable anandamide analoque methanandamide [69] and the anandamide-evoked smooth muscle cell hyperpolarization in the rat small mesenteric artery [118]. In other study performed on isolated rat small mesenteric artery, the hyperpolarization to anandamide was endothelium-dependent and sensitive to a selective inhibitor of ATP-sensitive K+ (KATP) channel glibenclamide, but not rimonabant [70].
In a number of isolated pre-contracted vascular preparations, including those from human [170] and rat pulmonary arteries [171], rabbit [162] and human mesenteric arteries [172], bovine [153] and sheep coronary artery [173], anandamide produces endothelium-dependent relaxation. In contrast, in isolated porcine coronary artery with or without endothelium, anandamide (30 μM) did not modify the tension and the membrane potential of smooth muscle cells [70].
Reports on the effect of 2-AG on reactivity of isolated vessels point for engagement of both endothelium-dependent [174] and –independent mechanisms [117, 174, 175]. 2-AG can dually modulate the contractile reactions, as both the relaxing and constricting responses have been described. Consistent with the described hypotensive effect [113, 176], 2-AG was shown to cause relaxation of isolated vascular preparations [30, 67, 174, 175, 177]. In isolated pre-contracted bovine coronary arteries, the relaxation to 2-AG depends on the intact endothelium and is blocked by inhibition of phospholipase C, FAAH, cyclooxygenase (COX) and cytoxhrome C450 [30]. In contrast, in rabbit pre-contracted mesenteric arteries, the relaxation to 2-AG is endothelium-independent but sensitive to rimonabant [175]. In mesenteric arteries isolated from patients undergoing surgical treatment of bowel carcinoma and inflammatory bowel disorders, the relaxation to 2-AG is endothelium-independent and is insensitive to the antagonists of CB1 and CB2 receptors AM251 (100 nM) and AM-630 (100 nM) [177]. De-sensitization of TRPV1 channels and FAAH inhibition failed to affect the dilation that was reduced by the COX-1 inhibitors [177].
In isolated rat aorta, 2-AG is ineffective at influencing the basal tone, but in pre-contracted rings, the endocannabinoid induces a biphasic response consisting of a transient relaxation followed by a sustained constriction [117], the responses being unaffected by endothelial denudation and inhibition of both types of cannabinoid receptors. Unlike in human mesenteric artery, in rat aorta, pretreatment with COX-1 inhibitor indomethacin failed to inhibit the weak relaxation to 2-AG , however, abolished the contraction phase both in endothelium-intact and denuded rings.
Experimental data derived from the study of the effect of THC, a CB1 agonist, on vascular contractility, also fails to support the engagement of CB1 receptor in the dilator response. In pre-contracted rat superior mesenteric artery, 3 μM THC elicited a marginal relaxation amounting 4% only. The relaxation was enhanced to 16% in the presence of the COX inhibitor indomethacin [120], pointing that the stimulated release of vasoconstrictor prostanoids masks the vasodilation. De-endothelization and 100 nM rimonabant had no effect on the relaxation. Higher concentrations of THC (10–100 μM) elicit a rimonabant-sensitive vasoconstriction that was converted to a weak relaxation following de-endothelization [120]. In third order branches of mesenteric artery, however, THC was shown to elicit endothelium-independent vasorelaxation, sensitive to charybdotoxin, a dual inhibitor of BKCa and IKCa channels, and apamin, a selective blocker of small conductance Ca2+ -activated K+ channel, but insensitive to antagonists of CB1 and TRPV1 receptors. In these arteries, THC was shown to inhibit the contractile response elicited by Ca2+ re-addition, indicating for inhibition of Ca2+ influx into smooth muscle cells [120].
5.3.1.2 The Role of TRPV1 Channels
Vasorelaxation to anandamide may involve stimulation of TRPV1 receptor as first demonstrated in isolated rat hepatic and mesenteric arteries and guinea pig basilar arteries [155]. The relaxation was shown to be abolished after treatment with capsazepin, a TRPV1 antagonist, and involves calcitonin gene-related peptide released from perivascular sensory nerves [155]. In that study, rimonabant (0.3 μM) failed to inhibit the vasodilator effect of anandamide. Neither 2-AG , nor synthetic CB1 and CB2 agonists were able to mimic the effect of anandamide [155], strongly indicating that neither of the known cannabinoid receptors mediate the relaxation of these arterial beds. However, the anandamide-induced vasodilation was abolished by capsazepine, a selective TRPV1 antagonist. The vasodilation was not reproduced following TRPV1 desensitization by pretreatment with capsaicin, a selective TRPV1 agonist. Following this original observation, several other studies confirmed, at least partial engagement of this mechanism in the dilation to anandamide [119, 121, 122]. However, no evidences for TRPV1 role in vasodilation to anandamide were obtained in the rat pulmonary [171] and rat coronary [168] arteries. The role of TRPV1 in relaxation to anandamide was further confirmed in pre-contracted small mesenteric artery, where anandamide was shown to produce endothelium-independent relaxation that was reduced following capsaicin pre-treatment [119, 121]. In the study of Ho and Hiley [121], the relaxation to anandamide, although was sensitive to 3 μM rimonabant, was unaffected by AM251 (3 μM) or CB2 receptor inhibitor SR144528, suggesting that perivascular TRPV1, but not CB1, plays a major role in the relaxation. In contrast, in the study of O’Sullivan et al. [119] the relaxation of small mesenteric arteries to anandamide was partially endothelium-dependent and reduced by both rimonabant (100 nM) and AM251 (100 nM), implicating activation of CB1 receptors. The only difference between the studies is that in the study of Ho and Hiley [121] the arteries were pre-contracted with α1-adrenergic receptor agonist methoxamine, while in the study of O’Sullivan [119], U-46619, a thromboxane A2 receptor agonist, was used as a constrictor. Similarly, in isolated rat gastric arteries, methanandamide, a metabolically stable analogue of anandamide , induces endothelium-independent relaxation [69] insensitive to AM251 and the CB2 receptor antagonists AM-630 and SR144528 when applied either alone or in combination. The authors found that the addition of exogenous CGRP relaxed pre-contracted arteries and capsaicin- and capsazepine pre-treatment only slightly inhibited the relaxation to methanandamide. Ca2+ -induced vasodilation was found to be inhibited in the presence of methanandamide, suggesting that the endocannabinoid induces smooth muscle relaxation by CB1 and CB2 receptor-independent inhibition of Ca2+ entry [69]. In rat mesenteric and gastric arteries, methanandamide and anandamide induce a slowly developing smooth muscle cell hyperpolarization that is reproduced by exogenous CGRP and abolished by capsazepine.
The expression of the mRNA coding for the TRPV1 receptor was detected also in endothelial cells from rat mesenteric artery, where anandamide at nanomolar concentrations was shown to elicit an acute release of NO secondary to stimulation of endothelial TRPV1 [178].
5.3.1.3 The Role of KCa Channels in Endothelium-Independent Vasodilation
Several lines of evidences point for involvement of Ca2+ -dependent K+ (KCa) channels in endothelium-independent relaxation to cannabinoids occurred independently on cannabinoid receptors. In isolated pre-contracted segments of rat superior mesenteric artery, the relaxation to anandamide is associated with the smooth muscle cell hyperpolarization due to activation of BKCa and, likely, IKCa channels [144]. The relaxation was found to be endothelium- and CB1 receptor- independent, as rimonabant even at 5 μM failed to modify the relaxation, and HU-210, a selective CB1 receptor agonist, and WIN55212-2, a non-selective cannabinoid receptor agonist , failed to reproduce the effect of anandamide [144]. This observation is in line with electrophysiological studies performed on isolated rat mesenteric artery, where anandamide-evoked smooth muscle cell hyperpolarization is unaffected by 1 μM rimonabant [70, 118]. In addition, neither the synthetic CB1 receptor agonists HU-210 and WIN55212-2, nor palmitoylethanolamide, a CB2 receptor agonist, affected the membrane potential of the smooth muscle cells [70, 118]. In smooth muscle cells of the main mesenteric artery [118], guinea pig carotid artery and porcine coronary artery [70], anandamide at up to 30 μM failed to produce shifts in the membrane potential.
In isolated pre-contracted rat aortic rings, arachidonylcyclopropylamide (ACPA), a selective CB1 receptor agonist, elicits a weak relaxation (~20%) occurred independently of the presence of endothelium [43]. The relaxation was reduced to 9% in the presence of iberiotoxin, a selective BKCa inhibitor. In rat superior mesenteric artery, the relaxation to 30 μM ACPA was reduced by endothelial denudation from 18% to 8% [40]. In rings with intact endothelium, iberiotoxin diminished the relaxation to 9%, while in endothelium-free rings, the BKCa inhibitor fully inhibited the relaxation [40].
5.3.1.4 Inhibition of Ca2+ Entry as a Mechanism of Relaxation to Cannabinoids
In order to observe the relaxation to cannabinoids, they are administered to pre-contracted arterial segments, i.e under conditions of prestimulated Ca2+ entry into smooth muscle cells. Clearly, any interference with any of the Ca2+ entry pathways would decrease the tone. In a number of vascular myography studies an inhibition of Ca2+-induced relaxation by cannabinoids and their analogues was observed, indicating that one of the mechanisms of endothelium-independent vasorelaxation to these compounds is an inhibition of Ca2+ entry into smooth muscle cells [69, 121, 157, 179]. These observations are supported by electrophysiological studies that identified anandamide as a direct inhibitor of NCX [65, 127], and voltage gated Ca2+ channel of L- and T- type [180, 181]. Noteworthy, both NCX and L- type Ca2 channels are crucial determinant of the contraction imposed by norepinephrine and phenylephrine [182, 183] and targeting these systems by cannabinoids should be considered in assessing the mechanisms of vasodilation elicited by cannabinoids.
5.3.2 Endothelium-Dependent Relaxation to Cannabinoids
Endothelium-dependent component of relaxation to anandamide and some other endogenous lipid signaling molecules belonging to N-acyl amino acids, such as N-arachidonoyl glycine, NAGly, [184, 185] and N-arachidonoyl L-serine [186], has been reported in a number of vascular beds with the dominated but yet unproven hypothesis that a novel endothelial G-protein coupled cannabinoid receptor, the so called endothelial cannabinoid receptor (CBe) also referred in literature as abnormal cannabidiol or endothelial atypical cannabinoid receptor, underlies the dilation [109, 123, 143, 154, 161, 172, 187]. Despite some inconsistencies in the proposed signalling mechanisms reviewed earlier [188], the observed sensitivity of cannabinoid-evoked vasodilation to high (micromolar) concentration of CB1 receptor blockers rimonabant and AM251, and the cannabidiol analogue O-1918 was explained by a possibility that these compounds selectively target CBe receptor that remained to be identified [73, 109, 119, 123, 143, 160, 170, 172, 184, 189,190,191]. Stimulation of CBe was postulated to be coupled to EDHF [119] and NO [184] release, leading to a delayed hypotension in anesthetized rats [109].
The “endothelial cannabinoid receptor” hypothesis has been challenged by recent electrophysiological demonstrations that cannabinoids, cannabinoid-like substances and synthetic analogues at concentrations that are commonly considered to be CBe specific, bidirectionally efficiently affect the BKCa activity in cell-free patches excised from native cells and cells heterologously expressing BKCa α, α+β1 or α+β4 subunits [72, 126, 163, 192,193,194,195], the effect being dependent on the plasma membrane cholesterol content [126, 194]. The topic of lipid- and cannabinoid-induced regulation of BKCa channel function is covered in a review of Bukiya and Dopico [196]. Cholesterol, an essential component of eucariotic plasma membranes, plays an important role in regulation of the activity of a number of membrane proteins [197,198,199], including TRP [200, 201], BKCa channels [202, 203] and cannabinoid signaling [204]. The latter is achieved via direct interaction between cholesterol and anandamide molecules by the establishment of a hydrogen bond and via regulation of interaction between anandamide and CB1 receptor [205, 206]. Consistent with view that direct cannabinoid-BKCa targeting is responsible for the relaxation, the hyperpolarizing effect of NAGly, a proposed ligand for GPR18 and a candidate for CBe [189, 207], was shown to be fully intact in endothelial cells following intracellular dialysis with GPR18 antibody [163]. A number of studies have provided evidences against the GPR18 involvement in the action of NAGly [127, 208,209,210], prompting a reconsideration of the concept of existence of a third type cannabinoid receptor required for endothelium-dependent vasodilation to cannabinoids.
Apart of direct targeting BKCa channels, NCX, TRPV1, V4, PPAR gamma and voltage-dependent Ca2+ channels, vasodilation to cannabinoids may also require cannabinoid interaction with vascular CB1 and CB2 receptors. In human mesenteric arteries collected during surgical operations from patients suffering from bowel carcinoma and inflammatory bowel disease, the CB1 receptor was concluded to mediate endothelium-dependent vasodilation to anandamide , with no evidence for engagement of CB2 receptor [172]. Cannabidiol , a phytocannabinoid wih low affinity to CB1 and CB2 receptors that exhibits high potency as an antagonist of both CB1 and CB2 receptors (Thomas et al. [244]), was shown to induce endothelium-dependent vasodilation of human mesenteric arteries sensitive to blockers of CB1 receptor and TRPV1 channels [172]. A synthetic CB1 receptor agonist CP55,940 produced a greater relaxation compared to that evoked by anandamide. In endothelium-intact rings obtained from rat superior mesenteric arteries, a highly potent CB1 receptor agonist ACPA (EC50 = 2.2 nM) used at extremely high concentration 30 μM was shown to relax pre-contracted arteries by 18% in NO-dependent manner [40] and the relaxation was partially BKCa-dependent. Endothelial denudation reduced the vasodilator effect of ACPA to 7.7%. In this study, a CB2 receptor agonist JWH-133 (30 μM) relaxed pre-contracted mesenteric arteries by 14% in NO-dependent manner. Similar results were obtained in another study performed in isolated endothelium-intact rat mesenteric arteries [37], where anandamide, ACPA and the CB2 agonist JWH-015 elicited concentration-dependent vasodilation. Blockade of TRPV1 channels partially decreased the relaxation, while the blockade of CGRP receptors completely inhibited the relaxation to anandamide. Pre-treatment of vascular preparations with AM251 and the CB2 antagonist AM630, only slightly decreased the relaxation responses to anandamide allowing to conclude that CB1, CB2 and TRPV1 mediate the relaxation to anandamide [37]. The conclusion of the two aforementioned studies on involvement of both CB1 and CB2 receptors in the vasorelaxation of rat mesenteric artery to cannabinoids contrasts with that derived by others [70, 118, 125, 143, 144]. Electrophysiological studies addressing the effect of CB2 receptor agonists on electrical responses of vascular cells are quite limited. In healthy arteries, CB2 receptor agonists failed to elicit measurable shifts in membrane potential either in smooth muscle [70, 118] or endothelial cells [6]. The action potential-driven endocannabinoid release in hyppocampal pyramidal cells is accompanied by a long-lasting CB2-dependent hyperpolarization [25]. Table 5.2 summarizes the key findings on the cannabinoid receptor-dependent and -independent targets governing relaxation to cannabinoids.
5.4 Dysregulation of Vascular Endocannabinoid Signaling in Disease States
Circulating levels of endocannabinoids and cannabinoid receptor expression are altered in disease states . Under various pathophysiological conditions accompanied by vascular abnormalities, the vasodilator potencies of cannabinoid receptor agonists differ from those observed in healthy arteries. Cannabinoids may exert both the beneficial and deleterious effects on cardiovascular system [38, 82]. The majority of beneficial effects of cannabinoids have been ascribed to CB2 receptor stimulation. Signalling via CB1 and CB2 receptors differentially affects vascular inflammation. Stimulation of vascular and cardiac CB1 receptors contributes to pathophysiology of various cardiovascular diseases via promotion of oxidative and nitrosative stress, activation of mitogen-activated protein kinase and cell demise [66, 211,212,213,214]. In contrast, a wealth of experimental data indicate that stimulation of CB2 receptors displays cardioprotective effect [215,216,217,218], reduces cerebral ischemic injury [219, 220], limits inflammation, oxidative/nitrosative stress, cell demise [221], progression of atherosclerosis [3, 214, 222,223,224], prevents nephrotoxicity [225]. An increased expression level of CB2 receptors in the cardiovascular system under pathophysiological conditions, such as inflammation and tissue injury, is considered to represent a compensatory protective mechanism [216, 226,227,228]. In the vasculature, CB2 receptor protein expression was shown to be up-regulated under some pathophysiological conditions, including atherosclerosis, inflammatory insults and DOCA-salt hypertension model [21, 226, 229,230,231].
In mesenteric arteries excised from young obese rats, anandamide produced an attenuated endothelium-dependent relaxation [37]. The reduction was accompanied by a decreased CB1, CB2, but not TRPV1, protein expression level. In this model, the relaxation responses to acetylcholine and CB1 and CB2 receptor agonists ACPA and JWH-015 were also decreased. The responses were, however, restored following pre-incubation of the arteries with FAAH inhibitor URB597, indicating that an increased anandamide degradation is responsible for attenuation of the relaxant responses.
Overactivation of cannabinoid system contributes to an increased vasodilation and hypotension. Thus, in patients with cirrhosis, the plasma anandamide and the CB1 receptor expression levels in vascular endothelial cells are elevated [42, 232]. The vasodilator state in chronic liver cirrhosis and hypotension in advanced cirrhosis is determined by activation of vascular endothelial cells cannabinoid CB1 receptors by endogenous cannabinoids and is reversed by the rimonabant treatment [42]. In cirrhotic mesenteric vessels, an increased relaxation to anandamide is mediated by an enhanced signalling via CB1 receptors, perivascular TRPV1 channels and vascular KCa channels [233, 234]. However, while in cirrhotic liver tonic CB1 stimulation plays a pathophysiological role determining chronic vasodilator state, in spontaneously hypertensive rats, chronic CB1 activation seems to represent a part of protective mechanism directed to reduction of blood pressure. In normotensive rats, CB1 receptor antagonism had no effect on blood pressure and other hemodynamic parameters. However, in spontaneously hypertensive rats (SHR), the expression of CB1 receptor is increased in heart and aortic endothelium as compared with Wistar-Kyoto rats, and rimonabant elicited a further increase in blood pressure and myocardial contraction with no change in heart rate [2], pointing at protective effect of tonic CB1 receptor activation. As intracerebroventricular microinjection of rimonabant did not influence blood pressure, it is highly expected that the effect of intravenous rimonabant is mediated by peripheral mechanisms [2]. Similar hypertensive effects were observed with AM251 when administered to either hypertensive salt-sensitive Dahl rats maintained on high salt diet, or to rats with angiotensin II–induced hypertension. Elevation of the endogenous anandamide level by FAAH inhibitor URB597 had no detectable hemodynamic effect in control rats, however, decreased arterial blood pressure in hypertensive rats [2, 235]. The hypotensive effects of WIN55212-2 are described in conscious rats with several forms of experimental hypertension, including SHR rats and Wistar rats made acutely hypertensive by infusion of angiotensin II and arginine vasopressin [235, 236], but not in transgenic hypertensive rats [102] Consistent with the upregulated role of endocannabinoid system in blood pressure regulation in hypertension, anandamide dose-dependently decrease the mean arterial blood pressure in conscious hypertensive, but not normotensive, rats [235]. While in normotensive rats WIN55212-2 elevates blood pressure, in acutely hypertensive rats WIN55212-2 produces hypotensive effect that was attenuated by AM251 [235]. In conscious hypertensive transgenic rats, however, the pressure and vasoconstrictor effect of WIN55212-2 are little affected [102].
In hypertension, alterations in cannabinoid signalling are model-specific. Thus, while in SHR both cardiac and plasma levels of anandamide and 2-AG are decreased, in DOCA-salt model, the endocannabinoid levels are elevated [231]. In both models, the CB1 receptor expression is higher in the heart and aortic endothelium [2, 33, 231]. However, higher CB2 receptor expression was detected only in DOCA-salt model [231]. Injection of THC (1.5 mg/kg) to rats with experimental renal hypertension elicited a significant decrease in blood pressure and heart rate [237]. The reaction developed within 15 min and in 24 h the parameters returned to the initial levels. However, daily THC injections for 3–5 weeks did not produce any difference in the heart rate and the systolic blood pressure between the control and hypertensive groups.
Numerous beneficial cardiovascular effects have been reported for in vivo cannabidiol treatment in a number of disorders. It was shown that cannabidiol treatment improves endothelium-dependent relaxation in mesentery of diabetic fatty rats and leads to improvement of serum biomarkers [238], prevents cerebral infarction [239], is cerebroprotective via cannabinoid receptor-independent pathway [240, 241], attenuates cardiac dysfunction and vascular inflammation, reduces infarct size, oxidative stress and inflammatory pathway in diabetic cardiomyopathy [242, 243].
5.5 Concluding Remarks
Cannabinoids, through central and local mechanisms, affect key cardiovascular parameters in health and disease, such as heart rate, blood pressure, vascular and cardiac contractility and inflammation. Studies over the last decades demonstrated that endocannabinoid system is overactivated under pathological conditions and plays both a pathophysiological role, such as in disease states associated with excessive hypotension, and a protective compensatory role, such as in some forms of hypertension and inflammatory conditions. Mechanisms of local regulation of vascular reactivity by cannabinoids include modulation of a number of ion transporting systems. This modulation may be accomplished either in cannabinoid receptor-dependent or –independent mechanisms. A main emphasis on the local regulation of cardiovascular function by endocannabinoids has been devoted to examining the role of specific type of cannabinoid receptors in vasodilation. Ironically, cannabinoid receptor antagonists of first generation designed and widely used in functional myography assays as selective were later found to be of low selectivity, displaying off-target effects on a number of ion transporting systems. Consequently, the conclusions yielded are at times speculative and controversial, often overlooking receptor-independent effects of cannabinoids and cannabinoid receptor blockers. To advance our understanding of cannabinoid actions on the vasculature, more information is essentially needed on the impact of selective cannabinoid receptor stimulation in disease states and the mechanisms of direct action of cannabinoids on the function of endothelial and smooth muscle cell ion channels and how these effects are translated into mechanotransduction, regulation of inflammation, angiogenesi, etc. Similar to steroids, general anesthetics and alcohols, cannabinoids modulate the function of a number of ion channels. An important still unanswered question is whether the given effect of cannabinoids requires direct interactions between cannabinoid molecules with specific channel protein subunit or the effect is indirect, due to change in lipid composition of the plasma membrane and changing the physical parameters of plasmalemma. Taking into account the vital role of potassium and TRP channels in physiology and pathophysiology of cardiovascular system, better insights into the intrinsic mechanisms of modulation of the channel function by cannabinoids, would not only advance our basic knowledge of local modulation of vascular function by cannabinoids, but pave the way for development of new selective cannabinoid receptor and ion channel modulators and their therapeutic application.
Abbreviations
- 2-AG:
-
2-Arachidonoylglycerol
- ACPA:
-
arachidonylcyclopropylamide
- BKCa :
-
large conductance calcium-activated potassium channel, KCa1.1
- CB1:
-
cannabinoid receptor type 1
- CB2:
-
cannabinoid receptor type 2
- CBe:
-
endothelial cannabinoid receptor
- CGRP:
-
calcitonin gene-related peptide
- COX:
-
cyclooxygenase, prostaglandin-endoperoxide synthase
- DOC salt hypertension:
-
deoxycorticosterone acetate-induced hypertension
- EDHF:
-
endothelium-derived hyperpolarizing factor
- FAAH:
-
fatty acid amide hydrolase
- IKCa :
-
intermediate conductance calcium-activated potassium channel, KCa3.1
- KATP:
-
ATP-sensitive potassium channel
- NAGly:
-
N-arachidonoyl glycine
- NCX:
-
Na+-Ca2+ exchanger
- NO:
-
nitric oxide
- PPAR:
-
peroxisome proliferator-activated receptor
- SHR:
-
spontaneously hypertensive rats
- TASK:
-
TWIK-related acid-sensitive potassium channel
- THC:
-
Δ9-tetrahydrocannabinol
- TRPA:
-
transient receptor potential cation channel subfamily A (ankyrin)
- TRPV:
-
transient receptor potential cation channel subfamily V (vanniloid)
References
Pacher P, Steffens S (2009) The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol 31(1):63–77
Batkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J et al (2004) Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 110(14):1996–2002
Carbone F, Mach F, Vuilleumier N, Montecucco F (2014) Cannabinoid receptor type 2 activation in atherosclerosis and acute cardiovascular diseases. Curr Med Chem 21(35):4046–4058
Godlewski G, Alapafuja SO, Batkai S, Nikas SP, Cinar R, Offertaler L et al (2010) Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol 17(11):1256–1266
Hopps JJ, Dunn WR, Randall MD (2012) Enhanced vasorelaxant effects of the endocannabinoid-like mediator, oleamide, in hypertension. Eur J Pharmacol 684(1-3):102–107
Bondarenko AI, Panasiuk O, Okhai I, Montecucco F, Brandt KJ, Mach F (2018) Ca2+-dependent potassium channels and cannabinoid signaling in the endothelium of apolipoprotein E knockout mice before plaque formation. J Mol Cell Cardiol 115:54–63
Capettini LS, Savergnini SQ, da Silva RF, Stergiopulos N, Santos RA, Mach F et al (2012) Update on the role of cannabinoid receptors after ischemic stroke. Mediat Inflamm 2012:824093
Montecucco F, Di Marzo V (2012) At the heart of the matter: the endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol Sci 33(6):331–340
Pertwee RG (2012) Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond Ser B Biol Sci 367(1607):3353–3363
Martin Gimenez VM, Noriega SE, Kassuha DE, Fuentes LB, Manucha W (2018) Anandamide and endocannabinoid system: an attractive therapeutic approach for cardiovascular disease. Ther Adv Cardiovasc Dis 12(7):177–190
Sierra S, Luquin N, Navarro-Otano J (2017) The endocannabinoid system in cardiovascular function: novel insights and clinical implications. Clin Auton Res 8
Baron EP (2015) Comprehensive review of medicinal marijuana, cannabinoids, and therapeutic implications in medicine and headache: what a long strange trip it’s been. Headache 55(6):885–916
Wolff V, Jouanjus E (2017) Strokes are possible complications of cannabinoids use. Epilepsy Behav 70(Pt B):355–363
Pacher P, Steffens S, Hasko G, Schindler TH, Kunos G (2017) Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 15(3):151–166
Singh A, Saluja S, Kumar A, Agrawal S, Thind M, Nanda S et al (2018) Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther 7(1):45–59
Lerner M (1963) Marihuana: tetrahydrocannabinol and related compounds. Science 140(3563):175–176
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346(6284):561–564
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365(6441):61–65
Howlett AC (1995) Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol 35:607–634
Guo Z, Liu YX, Yuan F, Ma HJ, Maslov L, Zhang Y (2015) Enhanced vasorelaxation effect of endogenous anandamide on thoracic aorta in renal vascular hypertension rats. Clin Exp Pharmacol Physiol 42(9):950–955
Schley M, Stander S, Kerner J, Vajkoczy P, Schupfer G, Dusch M et al (2009) Predominant CB2 receptor expression in endothelial cells of glioblastoma in humans. Brain Res Bull 79(5):333–337
Brusco A, Tagliaferro PA, Saez T, Onaivi ES (2008) Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann N Y Acad Sci 1139:450–457
Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR et al (2011) Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci 14(9):1160–1166
Ishiguro H, Horiuchi Y, Ishikawa M, Koga M, Imai K, Suzuki Y et al (2010) Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry 67(10):974–982
Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK et al (2016) Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 90(4):795–809
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K et al (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215(1):89–97
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G et al (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946–1949
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50(1):83–90
Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK et al (1997) Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest 100(6):1538–1546
Gauthier KM, Baewer DV, Hittner S, Hillard CJ, Nithipatikom K, Reddy DS et al (2005) Endothelium-derived 2-arachidonylglycerol: an intermediate in vasodilatory eicosanoid release in bovine coronary arteries. Am J Physiol Heart Circ Physiol 288(3):H1344–H1351
Sugiura T, Kodaka T, Nakane S, Kishimoto S, Kondo S, Waku K (1998) Detection of an endogenous cannabimimetic molecule, 2-arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonoylglycerol a possible vasomodulator? Biochem Biophys Res Commun 243(3):838–843
Szekeres M, Nadasy GL, Turu G, Soltesz-Katona E, Benyo Z, Offermanns S et al (2015) Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol Cell Endocrinol 403:46–56
Pacher P, Batkai S, Kunos G (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58(3):389–462
Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK et al (2001) Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J Am Coll Cardiol 38(7):2048–2054
Quercioli A, Pataky Z, Vincenti G, Makoundou V, Di Marzo V, Montecucco F et al (2011) Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur Heart J 32(11):1369–1378
Montecucco F, Matias I, Lenglet S, Petrosino S, Burger F, Pelli G et al (2009) Regulation and possible role of endocannabinoids and related mediators in hypercholesterolemic mice with atherosclerosis. Atherosclerosis 205(2):433–441
Lobato NS, Filgueira FP, Prakash R, Giachini FR, Ergul A, Carvalho MH et al (2013) Reduced endothelium-dependent relaxation to anandamide in mesenteric arteries from young obese Zucker rats. PLoS One 8(5):e63449
Pires PW (2018) Cannabinoids during ischemic strokes: friends or foes? Am J Physiol Heart Circ Physiol 314(6):H1155–H11H6
Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM et al (1996) An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun 229(1):114–120
Lopez-Dyck E, Andrade-Urzua F, Elizalde A, Ferrer-Villada T, Dagnino-Acosta A, Huerta M et al (2017) ACPA and JWH-133 modulate the vascular tone of superior mesenteric arteries through cannabinoid receptors, BKCa channels, and nitric oxide dependent mechanisms. Pharmacol Rep 69(6):1131–1139
Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A et al (2000) Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J 346(Pt 3):835–840
Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J et al (2001) Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med 7(7):827–832
Sanchez-Pastor E, Andrade F, Sanchez-Pastor JM, Elizalde A, Huerta M, Virgen-Ortiz A et al (2014) Cannabinoid receptor type 1 activation by arachidonylcyclopropylamide in rat aortic rings causes vasorelaxation involving calcium-activated potassium channel subunit alpha-1 and calcium channel, voltage-dependent, L type, alpha 1C subunit. Eur J Pharmacol 729:100–106
Batkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, Kunos G (2007) Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol 293(3):H1689–H1695
Bradshaw HB, Lee SH, McHugh D (2009) Orphan endogenous lipids and orphan GPCRs: a good match. Prostaglandins Other Lipid Mediat 89(3–4):131–134
Burstein S, McQuain C, Ross A, Salmonsen R, Zurier RE (2011) Resolution of inflammation by N-arachidonoylglycine. J Cell Biochem 112(11):3227–3233
Brown AJ (2007) Novel cannabinoid receptors. Br J Pharmacol 152(5):567–575
Irving A, Abdulrazzaq G, Chan SLF, Penman J, Harvey J, Alexander SPH (2017) Cannabinoid receptor-related orphan G protein-coupled receptors. Adv Pharmacol 80:223–247
Zhao P, Abood ME (2013) GPR55 and GPR35 and their relationship to cannabinoid and lysophospholipid receptors. Life Sci 92(8-9):453–457
Montecucco F, Bondarenko AI, Lenglet S, Burger F, Piscitelli F, Carbone F et al (2016) Treatment with the GPR55 antagonist CID16020046 increases neutrophil activation in mouse atherogenesis. Thromb Haemost 116(5):987–997
Oz M (2006) Receptor-independent effects of endocannabinoids on ion channels. Curr Pharm Des 12(2):227–239
Bondarenko A, Waldeck-Weiermair M, Naghdi S, Poteser M, Malli R, Graier WF (2010) GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells. Br J Pharmacol 161(2):308–320
Bondarenko AI, Malli R, Graier WF (2011) The GPR55 agonist lysophosphatidylinositol directly activates intermediate-conductance Ca2+-activated K+ channels. Pflugers Arch 462(2):245–255
Pertwee RG (2010) Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem 17(14):1360–1381
Bednarczyk P, Koziel A, Jarmuszkiewicz W, Szewczyk A (2013) Large-conductance Ca2+-activated potassium channel in mitochondria of endothelial EA.hy926 cells. Am J Physiol Heart Circ Physiol 304(11):H1415–H1427
Bondarenko AI, Jean-Quartier C, Malli R, Graier WF (2013) Characterization of distinct single-channel properties of Ca2+ inward currents in mitochondria. Pflugers Arch 465(7):997–1010
Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B (2009) Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci 29(7):2053–2063
Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E et al (2012) Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat Neurosci 15(4):558–564
O’Sullivan SE, Kendall DA, Randall MD (2009) Time-dependent vascular effects of Endocannabinoids mediated by peroxisome proliferator-activated receptor gamma (PPARgamma). PPAR Res 2009:425289
Niederhoffer N, Szabo B (2000) Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J Pharmacol Exp Ther 294(2):707–713
Grzeda E, Schlicker E, Luczaj W, Harasim E, Baranowska-Kuczko M, Malinowska B (2015) Bi-directional CB1 receptor-mediated cardiovascular effects of cannabinoids in anaesthetized rats: role of the paraventricular nucleus. J Physiol Pharmacol 66(3):343–353
Niederhoffer N, Schmid K, Szabo B (2003) The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedeberg’s Arch Pharmacol 367(5):434–443
Malinowska B, Godlewski G, Bucher B, Schlicker E (1997) Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn Schmiedeberg’s Arch Pharmacol 356(2):197–202
Li Q, Ma HJ, Song SL, Shi M, Li DP, Zhang Y (2012) Effects of anandamide on potassium channels in rat ventricular myocytes: a suppression of I(to) and augmentation of K(ATP) channels. Am J Phys Cell Physiol 302(6):C924–C930
Al Kury LT, Yang KH, Thayyullathil FT, Rajesh M, Ali RM, Shuba YM et al (2014) Effects of endogenous cannabinoid anandamide on cardiac Na/Ca exchanger. Cell Calcium 171:3485–3498
Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L et al (2010) CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res 85(4):773–784
Su JY, Vo AC (2007) 2-Arachidonylglyceryl ether and abnormal cannabidiol-induced vascular smooth muscle relaxation in rabbit pulmonary arteries via receptor-pertussis toxin sensitive G proteins-ERK1/2 signaling. Eur J Pharmacol 559(2-3):189–195
Van den Bossche I, Vanheel B (2000) Influence of cannabinoids on the delayed rectifier in freshly dissociated smooth muscle cells of the rat aorta. Br J Pharmacol 131(1):85–93
Breyne J, Van de Voorde J, Vanheel B (2006) Characterization of the vasorelaxation to methanandamide in rat gastric arteries. Can J Physiol Pharmacol 84(11):1121–1132
Chataigneau T, Feletou M, Thollon C, Villeneuve N, Vilaine JP, Duhault J et al (1998) Cannabinoid CB1 receptor and endothelium-dependent hyperpolarization in guinea-pig carotid, rat mesenteric and porcine coronary arteries. Br J Pharmacol 123(5):968–974
Rajesh M, Mukhopadhyay P, Hasko G, Huffman JW, Mackie K, Pacher P (2008) CB2 cannabinoid receptor agonists attenuate TNF-alpha-induced human vascular smooth muscle cell proliferation and migration. Br J Pharmacol 153(2):347–357
Bondarenko AI, Malli R, Graier WF (2011) The GPR55 agonist lysophosphatidylinositol acts as an intracellular messenger and bidirectionally modulates Ca2+-activated large-conductance K+ channels in endothelial cells. Pflugers Arch 461(1):177–189
Stanley CP, Hind WH, Tufarelli C, O’Sullivan SE (2015) Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc Res 19
Ho WS, Zheng X, Zhang DX (2015) Role of endothelial TRPV4 channels in vascular actions of the endocannabinoid, 2-arachidonoylglycerol. Br J Pharmacol 172(22):5251–5264
Suleimani YMA, Hiley CR (2010) Lysophosphatidylinositol (LPI) mediates vasorelaxation of the rat mesenteric resistance artery and induces calcium release in rat mesenteric artery endothelial cells. In: Proceedings of the British Pharmacological Society Winter Meeting 2010, London, 81(1)
Lepicier P, Bouchard JF, Lagneux C, Lamontagne D (2003) Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol 139(4):805–815
Lamontagne D, Lepicier P, Lagneux C, Bouchard JF (2006) The endogenous cardiac cannabinoid system: a new protective mechanism against myocardial ischemia. Arch Mal Coeur Vaiss 99(3):242–246
Mukhopadhyay P, Horvath B, Rajesh M, Matsumoto S, Saito K, Batkai S et al (2011) Fatty acid amide hydrolase is a key regulator of the endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med 50(1):179–195
Mo FM, Offertaler L, Kunos G (2004) Atypical cannabinoid stimulates endothelial cell migration via a Gi/Go-coupled receptor distinct from CB1, CB2 or EDG. Eur J Pharmacol 489(1-2):21–27
Zhang X, Maor Y, Wang JF, Kunos G, Groopman JE (2010) Endocannabinoid-like N-arachidonoyl serine is a novel pro-angiogenic mediator. Br J Pharmacol 160(7):1583–1594
Pisanti S, Picardi P, Prota L, Proto MC, Laezza C, McGuire PG et al (2011) Genetic and pharmacologic inactivation of cannabinoid CB1 receptor inhibits angiogenesis. Blood 117(20):5541–5550
Mach F, Montecucco F, Steffens S (2008) Cannabinoid receptors in acute and chronic complications of atherosclerosis. Br J Pharmacol 153(2):290–298
Weil AT, Zinberg NE, Nelsen JM (1968) Clinical and psychological effects of marihuana in man. Science 162(3859):1234–1242
Hollister LE (1971) Actions of various marihuana derivatives in man. Pharmacol Rev 23(4):349–357
Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA (1974) Cannabidiol interferes with the effects of delta 9 – tetrahydrocannabinol in man. Eur J Pharmacol 28(1):172–177
Kiplinger GF, Manno JE (1971) Dose-response relationships to cannabis in human subjects. Pharmacol Rev 23(4):339–347
Van Hoozen BE, Cross CE (1997) Marijuana. Respiratory tract effects. Clin Rev Allergy Immunol 15(3):243–269
Gash A, Karliner JS, Janowsky D, Lake CR (1978) Effects of smoking marihuana on left ventricular performance and plasma norepinephrine: studies in normal men. Ann Intern Med 89(4):448–452
Beaconsfield P, Ginsburg J, Rainsbury R (1972) Marihuana smoking. Cardiovascular effects in man and possible mechanisms. N Engl J Med 287(5):209–212
Mathew RJ, Wilson WH, Tant SR (1989) Acute changes in cerebral blood flow associated with marijuana smoking. Acta Psychiatr Scand 79(2):118–128
Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE (1992) Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab 12(5):750–758
O’Leary DS, Block RI, Koeppel JA, Schultz SK, Magnotta VA, Ponto LB et al (2007) Effects of smoking marijuana on focal attention and brain blood flow. Hum Psychopharmacol 22(3):135–148
Rezkalla S, Kloner RA (2018) Cardiovascular effects of marijuana. Trends Cardiovasc Med
Korantzopoulos P, Liu T, Papaioannides D, Li G, Goudevenos JA (2008) Atrial fibrillation and marijuana smoking. Int J Clin Pract 62(2):308–313
Pacher P, Steffens S, Hasko G, Schindler TH, Kunos G (2018) Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 15(3):151–166
Weiss JL, Watanabe AM, Lemberger L, Tamarkin NR, Cardon PV (1972) Cardiovascular effects of delta-9-tetrahydrocannabinol in man. Clin Pharmacol Ther 13(5):671–684
Jadoon KA, Tan GD, O’Sullivan SE (2017) A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 15:2(12)
Vollmer RR, Cavero I, Ertel RJ, Solomon TA, Buckley JP (1974) Role of the central autonomic nervous system in the hypotension and bradycardia induced by (-)-delta 9-trans-tetrahydrocannabinol. J Pharm Pharmacol 26(3):186–192
Kanakis C Jr, Pouget JM, Rosen KM (1976) The effects of delta-9-tetrahydrocannabinol (cannabis) on cardiac performance with and without beta blockade. Circulation 53(4):703–707
Malit LA, Johnstone RE, Bourke DI, Kulp RA, Klein V, Smith TC (1975) Intravenous delta9-Tetrahydrocannabinol: effects of ventilatory control and cardiovascular dynamics. Anesthesiology 42(6):666–673
Benowitz NL, Jones RT (1975) Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin Pharmacol Ther 18(3):287–297
Gardiner SM, March JE, Kemp PA, Bennett T (2001) Regional haemodynamic responses to the cannabinoid agonist, WIN 55212-2, in conscious, normotensive rats, and in hypertensive, transgenic rats. Br J Pharmacol 133(3):445–453
Lake KD, Compton DR, Varga K, Martin BR, Kunos G (1997) Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther 281(3):1030–1037
Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G (1996) Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension 28(4):682–686
Lake KD, Martin BR, Kunos G, Varga K (1997) Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension 29(5):1204–1210
Malinowska B, Baranowska-Kuczko M, Schlicker E (2012) Triphasic blood pressure responses to cannabinoids: do we understand the mechanism? Br J Pharmacol 165(7):2073–2088
Varga K, Lake K, Martin BR, Kunos G (1995) Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol 278(3):279–283
Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G (1996) Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol 118(8):2023–2028
Zakrzeska A, Schlicker E, Baranowska M, Kozlowska H, Kwolek G, Malinowska B (2010) A cannabinoid receptor, sensitive to O-1918, is involved in the delayed hypotension induced by anandamide in anaesthetized rats. Br J Pharmacol 160(3):574–584
Malinowska B, Kwolek G, Gothert M (2001) Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedeberg’s Arch Pharmacol 364(6):562–569
Pacher P, Batkai S, Kunos G (2004) Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol 558(Pt 2):647–657
Krayer O (1961) The history of the Bezold-Jarisch effect. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 240:361–368
Jarai Z, Wagner JA, Goparaju SK, Wang L, Razdan RK, Sugiura T et al (2000) Cardiovascular effects of 2-arachidonoyl glycerol in anesthetized mice. Hypertension 35(2):679–684
Niederhoffer N, Szabo B (1999) Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br J Pharmacol 126(2):457–466
Gardiner SM, March JE, Kemp PA, Bennett T (2002) Complex regional haemodynamic effects of anandamide in conscious rats. Br J Pharmacol 135(8):1889–1896
Gardiner SM, March JE, Kemp PA, Bennett T (2002) Influence of the CB(1) receptor antagonist, AM 251, on the regional haemodynamic effects of WIN-55212-2 or HU 210 in conscious rats. Br J Pharmacol 136(4):581–587
Stanke-Labesque F, Mallaret M, Lefebvre B, Hardy G, Caron F, Bessard G (2004) 2-Arachidonoyl glycerol induces contraction of isolated rat aorta: role of cyclooxygenase-derived products. Cardiovasc Res 63(1):155–160
Vanheel B, Van de Voorde J (2001) Regional differences in anandamide- and methanandamide-induced membrane potential changes in rat mesenteric arteries. J Pharmacol Exp Ther 296(2):322–328
O’Sullivan SE, Kendall DA, Randall MD (2004) Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br J Pharmacol 142(3):435–442
O’Sullivan SE, Kendall DA, Randall MD (2005) The effects of Delta9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br J Pharmacol 145(4):514–526
Ho WS, Hiley CR (2003) Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx. Br J Pharmacol 139(3):585–597
Harris D, McCulloch AI, Kendall DA, Randall MD (2002) Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J Physiol 539(Pt 3):893–902
Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR et al (1999) Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A 96(24):14136–14141
O’Sullivan SE, Kendall DA, Randall MD (2005) Vascular effects of delta 9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. Eur J Pharmacol 507(1-3):211–221
Herradon E, Martin MI, Lopez-Miranda V (2007) Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. Br J Pharmacol 152(5):699–708
Godlewski G, Offertaler L, Osei-Hyiaman D, Mo FM, Harvey-White J, Liu J et al (2009) The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J Pharmacol Exp Ther 328(1):351–361
Bondarenko AI, Drachuk K, Panasiuk O, Sagach V, Deak AT, Malli R et al (2013) N-arachidonoyl glycine suppresses Na+/Ca2+ exchanger-mediated Ca2+ entry into endothelial cells and activates BK channels independently of G-protein coupled receptors. Br J Pharmacol 169(4):933–948
Chemin J, Cazade M, Lory P (2014) Modulation of T-type calcium channels by bioactive lipids. Pflugers Arch 466(4):689–700
Barbara G, Alloui A, Nargeot J, Lory P, Eschalier A, Bourinet E et al (2009) T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids. J Neurosci 29(42):13106–13114
Yang W, Li Q, Wang SY, Gao F, Qian WJ, Li F et al (2016) Cannabinoid receptor agonists modulate calcium channels in rat retinal Muller cells. Neuroscience 313:213–224
Patil M, Patwardhan A, Salas MM, Hargreaves KM, Akopian AN (2011) Cannabinoid receptor antagonists AM251 and AM630 activate TRPA1 in sensory neurons. Neuropharmacology 61(4):778–788
Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM (2008) Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci 28(5):1064–1075
Maingret F, Patel AJ, Lazdunski M, Honore E (2001) The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J 20(1-2):47–54
Veale EL, Buswell R, Clarke CE, Mathie A (2007) Identification of a region in the TASK3 two pore domain potassium channel that is critical for its blockade by methanandamide. Br J Pharmacol 152(5):778–786
Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM et al (2006) Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res 98(8):1072–1080
Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, Weston AH (2004) Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol 142(1):192–202
Bondarenko AI, Montecucco F, Panasiuk O, Sagach V, Sidoryak N, Brandt KJ et al (2017) GPR55 agonist lysophosphatidylinositol and lysophosphatidylcholine inhibit endothelial cell hyperpolarization via GPR-independent suppression of Na+-Ca2+ exchanger and endoplasmic reticulum Ca2+ refilling. Vasc Pharmacol 89:39–48
Al Suleimani YM, Al Mahruqi AS (2017) The endogenous lipid N-arachidonoyl glycine is hypotensive and nitric oxide-cGMP-dependent vasorelaxant. Eur J Pharmacol 794:209–215
Bondarenko A, Sagach V (2006) Na+-K+-ATPase is involved in the sustained ACh-induced hyperpolarization of endothelial cells from rat aorta. Br J Pharmacol 149(7):958–965
Steffens M, Feuerstein TJ (2004) Receptor-independent depression of DA and 5-HT uptake by cannabinoids in rat neocortex--involvement of Na(+)/K(+)-ATPase. Neurochem Int 44(7):529–538
Ellis EF, Moore SF, Willoughby KA (1995) Anandamide and delta 9-THC dilation of cerebral arterioles is blocked by indomethacin. Am J Phys 269(6 Pt 2):H1859–H1864
Randall MD, Kendall DA (1997) Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. Eur J Pharmacol 335(2-3):205–209
Wagner JA, Varga K, Jarai Z, Kunos G (1999) Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension 33(1 Pt 2):429–434
Plane F, Holland M, Waldron GJ, Garland CJ, Boyle JP (1997) Evidence that anandamide and EDHF act via different mechanisms in rat isolated mesenteric arteries. Br J Pharmacol 121(8):1509–1511
White R, Hiley CR (1997) A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol 122(8):1573–1584
Zygmunt PM, Hogestatt ED, Waldeck K, Edwards G, Kirkup AJ, Weston AH (1997) Studies on the effects of anandamide in rat hepatic artery. Br J Pharmacol 122(8):1679–1686
Fulton D, Quilley J (1998) Evidence against anandamide as the hyperpolarizing factor mediating the nitric oxide-independent coronary vasodilator effect of bradykinin in the rat. J Pharmacol Exp Ther 286(3):1146–1151
Zygmunt PM, Sorgard M, Petersson J, Johansson R, Hogestatt ED (2000) Differential actions of anandamide, potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig basilar artery. Naunyn Schmiedeberg’s Arch Pharmacol 361(5):535–542
Niederhoffer N, Szabo B (1999) Involvement of CB1 cannabinoid receptors in the EDHF-dependent vasorelaxation in rabbits. Br J Pharmacol 126(6):1383–1386
Mulvany MJ, Halpern W (1977) Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41(1):19–26
Lu X, Kassab GS (2011) Assessment of endothelial function of large, medium, and small vessels: a unified myograph. Am J Physiol Heart Circ Physiol 300(1):H94–H100
Falloon BJ, Stephens N, Tulip JR, Heagerty AM (1995) Comparison of small artery sensitivity and morphology in pressurized and wire-mounted preparations. Am J Phys 268(2 Pt 2):H670–H678
Pratt PF, Hillard CJ, Edgemond WS, Campbell WB (1998) N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Phys 274(1 Pt 2):H375–H381
Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK et al (2003) Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol 63(3):699–705
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V et al (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400(6743):452–457
Begg M, Baydoun A, Parsons ME, Molleman A (2001) Signal transduction of cannabinoid CB1 receptors in a smooth muscle cell line. J Physiol 531(Pt 1):95–104
White R, Hiley CR (1998) The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br J Pharmacol 125(3):533–541
Weresa J, Pedzinska-Betiuk A, Kossakowski R, Malinowska B (2018) Cannabinoid CB1 and CB2 receptors antagonists AM251 and AM630 differentially modulate the chronotropic and inotropic effects of isoprenaline in isolated rat atria. Pharmacol Rep 71(1):82–89
Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C et al (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350(2-3):240–244
Kozlowska H, Baranowska M, Schlicker E, Kozlowski M, Laudanski J, Malinowska B (2007) Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J Hypertens 25(11):2240–2248
Ho WS, Hiley CR (2003) Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol 138(7):1320–1332
Chaytor AT, Martin PE, Evans WH, Randall MD, Griffith TM (1999) The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J Physiol 520(Pt 2):539–550
Bondarenko AI, Panasiuk O, Drachuk K, Montecucco F, Brandt KJ, Mach F (2018) The quest for endothelial atypical cannabinoid receptor: BKCa channels act as cellular sensors for cannabinoids in in vitro and in situ endothelial cells. Vasc Pharmacol 102:44–55
Bukoski RD, Batkai S, Jarai Z, Wang Y, Offertaler L, Jackson WF et al (2002) CB1 receptor antagonist SR141716A inhibits Ca2+-induced relaxation in CB1 receptor-deficient mice. Hypertension 39(2):251–257
Raffa RB, Ward SJ (2012) CB(1)-independent mechanisms of Delta(9)-THCV, AM251 and SR141716 (rimonabant). J Clin Pharm Ther 37(3):260–265
Hoddah H, Marcantoni A, Comunanza V, Carabelli V, Carbone E (2009) L-type channel inhibition by CB1 cannabinoid receptors is mediated by PTX-sensitive G proteins and cAMP/PKA in GT1-7 hypothalamic neurons. Cell Calcium 46(5–6):303–312
Carpi S, Fogli S, Romanini A, Pellegrino M, Adinolfi B, Podesta A et al (2015) AM251 induces apoptosis and G2/M cell cycle arrest in A375 human melanoma cells. Anti-Cancer Drugs 26(7):754–762
White R, Ho WS, Bottrill FE, Ford WR, Hiley CR (2001) Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol 134(4):921–929
Ishioka N, Bukoski RD (1999) A role for N-arachidonylethanolamine (anandamide) as the mediator of sensory nerve-dependent Ca2+-induced relaxation. J Pharmacol Exp Ther 289(1):245–250
Baranowska-Kuczko M, Kozlowska H, Kozlowski M, Schlicker E, Kloza M, Surazynski A et al (2014) Mechanisms of endothelium-dependent relaxation evoked by anandamide in isolated human pulmonary arteries. Naunyn Schmiedeberg’s Arch Pharmacol 387(5):477–486
Baranowska-Kuczko M, MacLean MR, Kozlowska H, Malinowska B (2012) Endothelium-dependent mechanisms of the vasodilatory effect of the endocannabinoid, anandamide, in the rat pulmonary artery. Pharmacol Res 66(3):251–259
Stanley CP, Hind WH, Tufarelli C, O’Sullivan SE (2016) The endocannabinoid anandamide causes endothelium-dependent vasorelaxation in human mesenteric arteries. Pharmacol Res 113(Pt A):356–363
Grainger J, Boachie-Ansah G (2001) Anandamide-induced relaxation of sheep coronary arteries: the role of the vascular endothelium, arachidonic acid metabolites and potassium channels. Br J Pharmacol 134(5):1003–1012
Ho WS, Randall MD (2007) Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol 150(5):641–651
Kagota S, Yamaguchi Y, Nakamura K, Sugiura T, Waku K, Kunitomo M (2001) 2-Arachidonoylglycerol, a candidate of endothelium-derived hyperpolarizing factor. Eur J Pharmacol 415(2-3):233–238
Mechoulam R, Fride E, Ben-Shabat S, Meiri U, Horowitz M (1998) Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur J Pharmacol 362(1):R1–R3
Stanley CP, O’Sullivan SE (2014) Cyclooxygenase metabolism mediates vasorelaxation to 2-arachidonoylglycerol (2-AG) in human mesenteric arteries. Pharmacol Res 81C:74–82
Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP (2005) Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol 568(Pt 2):539–551
Al Suleimani YM, Al Mahruqi AS, Hiley CR (2015) Mechanisms of vasorelaxation induced by the cannabidiol analogue compound O-1602 in the rat small mesenteric artery. Eur J Pharmacol 765:107–114
Al Kury LT, Voitychuk OI, Yang KH, Thayyullathil FT, Doroshenko P, Ramez AM et al (2014) Effects of endogenous cannabinoid anandamide on voltage-dependent sodium and calcium channels in rat ventricular myocytes. Br J Pharmacol 171(14):3485–3498
Chemin J, Monteil A, Perez-Reyes E, Nargeot J, Lory P (2001) Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J 20(24):7033–7040
Lagaud GJ, Randriamboavonjy V, Roul G, Stoclet JC, Andriantsitohaina R (1999) Mechanism of Ca2+ release and entry during contraction elicited by norepinephrine in rat resistance arteries. Am J Phys 276(1 Pt 2):H300–H308
Zhang J, Ren C, Chen L, Navedo MF, Antos LK, Kinsey SP et al (2010) Knockout of Na+/Ca2+ exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2+ channel current and lowers blood pressure. Am J Physiol Heart Circ Physiol 298(5):H1472–H1483
Parmar N, Ho WS (2010) N-arachidonoyl glycine, an endogenous lipid that acts as a vasorelaxant via nitric oxide and large conductance calcium-activated potassium channels. Br J Pharmacol 160(3):594–603
Ho WS, Yeung SYM (2009) Novel G-protein-coupled receptors in rat arteries: potential targets for N-arachidonoyl glycine? Proc Br Pharmacol Soc 7(4):060P
Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S et al (2006) N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A 103(7):2428–2433
Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM et al (2005) Evidence for novel cannabinoid receptors. Pharmacol Ther 106(2):133–145
Bondarenko AI (2014) Endothelial atypical cannabinoid receptor: do we have enough evidence? Br J Pharmacol 171(24):5573–5588
MacIntyre J, Dong A, Straiker A, Zhu J, Howlett SE, Bagher A et al (2014) Cannabinoid and lipid-mediated vasorelaxation in retinal microvasculature. Eur J Pharmacol 735C:105–114
O’Sullivan SE, Kendall DA, Randall MD (2004) Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA). Br J Pharmacol 141(5):803–812
Hoi PM, Visintin C, Okuyama M, Gardiner SM, Kaup SS, Bennett T et al (2007) Vascular pharmacology of a novel cannabinoid-like compound, 3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16) in the rat. Br J Pharmacol 152(5):751–764
Bol M, Leybaert L, Vanheel B (2012) Influence of methanandamide and CGRP on potassium currents in smooth muscle cells of small mesenteric arteries. Pflugers Arch 463(5):669–677
Sade H, Muraki K, Ohya S, Hatano N, Imaizumi Y (2006) Activation of large-conductance, Ca2+-activated K+ channels by cannabinoids. Am J Phys Cell Physiol 290(1):C77–C86
Bondarenko AI, Panasiuk O, Okhai I, Montecucco F, Brandt KJ, Mach F (2017) Direct activation of Ca2+- and voltage-gated potassium channels of large conductance by anandamide in endothelial cells does not support the presence of endothelial atypical cannabinoid receptor. Eur J Pharmacol 805:14–24
Baker D, Pryce G, Visintin C, Sisay S, Bondarenko AI, Ho WSV et al (2017) Big conductance calcium-activated potassium channel openers control spasticity without sedation. Br J Pharmacol 174(16):2662–2681
Dopico AM, Bukiya AN (2014) Lipid regulation of BK channel function. Front Physiol 5:312
Bukiya AN, Durdagi S, Noskov S, Rosenhouse-Dantsker A (2017) Cholesterol up-regulates neuronal G protein-gated inwardly rectifying potassium (GIRK) channel activity in the hippocampus. J Biol Chem 292(15):6135–6147
Bukiya AN, Osborn CV, Kuntamallappanavar G, Toth PT, Baki L, Kowalsky G et al (2015) Cholesterol increases the open probability of cardiac KACh currents. Biochim Biophys Acta 1848(10 Pt A):2406–2413
Kannan KB, Barlos D, Hauser CJ (2007) Free cholesterol alters lipid raft structure and function regulating neutrophil Ca2+ entry and respiratory burst: correlations with calcium channel raft trafficking. J Immunol 178(8):5253–5261
Morales-Lazaro SL, Rosenbaum T (2017) Multiple mechanisms of regulation of transient receptor potential ion channels by cholesterol. Curr Top Membr 80:139–161
Beech DJ, Bahnasi YM, Dedman AM, Al-Shawaf E (2009) TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium 45(6):583–588
Dopico AM, Bukiya AN, Singh AK (2012) Large conductance, calcium- and voltage-gated potassium (BK) channels: regulation by cholesterol. Pharmacol Ther 135(2):133–150
Bukiya AN, Belani JD, Rychnovsky S, Dopico AM (2011) Specificity of cholesterol and analogs to modulate BK channels points to direct sterol-channel protein interactions. J Gen Physiol 137(1):93–110
Dainese E, Oddi S, Maccarrone M (2010) Interaction of endocannabinoid receptors with biological membranes. Curr Med Chem 17(14):1487–1499
Di Scala C, Fantini J, Yahi N, Barrantes FJ, Chahinian H (2018) Anandamide revisited: how cholesterol and ceramides control receptor-dependent and receptor-independent signal transmission pathways of a lipid neurotransmitter. Biomolecules 22:8(2)
Di Scala C, Mazzarino M, Yahi N, Varini K, Garmy N, Fantini J et al (2017) Anandamide-ceramide interactions in a membrane environment: molecular dynamic simulations data. Data Brief 14:163–167
McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM et al (2010) N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci 11:44
Yin H, Chu A, Li W, Wang B, Shelton F, Otero F et al (2009) Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem 284(18):12328–12338
Lu VB, Puhl HL 3rd, Ikeda SR (2013) N-Arachidonyl glycine does not activate G protein-coupled receptor 18 signaling via canonical pathways. Mol Pharmacol 83(1):267–282
Finlay DB, Joseph WR, Grimsey NL, Glass M (2016) GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-arachidonoyl glycine. PeerJ 4:e1835
El-Remessy AB, Rajesh M, Mukhopadhyay P, Horvath B, Patel V, Al-Gayyar MM et al (2011) Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia 54(6):1567–1578
Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B et al (2012) Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61(3):716–727
Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Mackie K, Pacher P (2010) Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol 160(3):688–700
Mach F, Steffens S (2008) The role of the endocannabinoid system in atherosclerosis. J Neuroendocrinol 20(Suppl 1):53–57
Lepicier P, Lagneux C, Sirois MG, Lamontagne D (2007) Endothelial CB1-receptors limit infarct size through NO formation in rat isolated hearts. Life Sci 81(17-18):1373–1380
Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M et al (2009) CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol 46(5):612–620
Hajrasouliha AR, Tavakoli S, Ghasemi M, Jabehdar-Maralani P, Sadeghipour H, Ebrahimi F et al (2008) Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. Eur J Pharmacol 579(1-3):246–252
Gonzalez C, Herradon E, Abalo R, Vera G, Perez-Nievas BG, Leza JC et al (2011) Cannabinoid/agonist WIN 55,212-2 reduces cardiac ischaemia-reperfusion injury in Zucker diabetic fatty rats: role of CB2 receptors and iNOS/eNOS. Diabetes Metab Res Rev 27(4):331–340
Choi IY, Ju C, Anthony Jalin AM, Lee DI, Prather PL, Kim WK (2013) Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am J Pathol 182(3):928–939
Yu SJ, Reiner D, Shen H, Wu KJ, Liu QR, Wang Y (2015) Time-dependent protection of CB2 receptor agonist in stroke. PLoS One 10(7):e0132487
Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S et al (2010) Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med 48(3):457–467
Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C et al (2005) Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 434(7034):782–786
Montecucco F, Di Marzo V, da Silva RF, Vuilleumier N, Capettini L, Lenglet S et al (2012) The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. Eur Heart J 33(7):846–856
Zhao Y, Yuan Z, Liu Y, Xue J, Tian Y, Liu W et al (2010) Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J Cardiovasc Pharmacol 55(3):292–298
Mukhopadhyay P, Baggelaar M, Erdelyi K, Cao Z, Cinar R, Fezza F et al (2016) The novel, orally available and peripherally restricted selective cannabinoid CB2 receptor agonist LEI-101 prevents cisplatin-induced nephrotoxicity. Br J Pharmacol 173(3):446–458
Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R et al (2012) Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci 32(12):4004–4016
Steffens S, Mach F (2006) Towards a therapeutic use of selective CB2 cannabinoid receptor ligands for atherosclerosis. Futur Cardiol 2(1):49–53
Pacher P, Mechoulam R (2011) Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res 50(2):193–211
Meletta R, Slavik R, Mu L, Rancic Z, Borel N, Schibli R et al (2017) Cannabinoid receptor type 2 (CB2) as one of the candidate genes in human carotid plaque imaging: evaluation of the novel radiotracer [11C]RS-016 targeting CB2 in atherosclerosis. Nucl Med Biol 47:31–43
Rajesh M, Pan H, Mukhopadhyay P, Batkai S, Osei-Hyiaman D, Hasko G et al (2007) Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol 82(6):1382–1389
Biernacki M, Malinowska B, Timoszuk M, Toczek M, Jastrzab A, Remiszewski P et al (2018) Hypertension and chronic inhibition of endocannabinoid degradation modify the endocannabinoid system and redox balance in rat heart and plasma. Prostaglandins Other Lipid Mediat 138:54–63
Fernandez-Rodriguez CM, Romero J, Petros TJ, Bradshaw H, Gasalla JM, Gutierrez ML et al (2004) Circulating endogenous cannabinoid anandamide and portal, systemic and renal hemodynamics in cirrhosis. Liver Int 24(5):477–483
Domenicali M, Ros J, Fernández-Varo G, Cejudo-Martín P, Crespo M, Morales-Ruiz M et al (2005) Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut 54(4):522–527
Yang Y, Lin H, Huang Y, Lee T, Hou M, Wang Y et al (2007) Role of Ca2+-dependent potassium channels in in vitro anandamide-mediated mesenteric vasorelaxation in rats with biliary cirrhosis. Liver Int 27(8):1045–1055
Ho WS, Gardiner SM (2009) Acute hypertension reveals depressor and vasodilator effects of cannabinoids in conscious rats. Br J Pharmacol 156(1):94–104
Wheal AJ, Bennett T, Randall MD, Gardiner SM (2007) Cardiovascular effects of cannabinoids in conscious spontaneously hypertensive rats. Br J Pharmacol 152(5):717–724
Varma DR, Goldbaum D (1975) Effect of delta9-tetrahydrocannabinol on experimental hypertension in rats. J Pharm Pharmacol 27(10):790–791
Wheal AJ, Jadoon K, Randall MD, O’Sullivan SE (2017) In vivo cannabidiol treatment improves endothelium-dependent vasorelaxation in mesenteric arteries of Zucker diabetic fatty rats. Front Pharmacol 8:248
Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K et al (2005) Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke 36(5):1077–1082
Hayakawa K, Mishima K, Nozako M, Hazekawa M, Irie K, Fujioka M et al (2007) Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J Neurochem 102(5):1488–1496
Hayakawa K, Mishima K, Irie K, Hazekawa M, Mishima S, Fujioka M et al (2008) Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology 55(8):1280–1286
Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S et al (2011) Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56(25):2115–2125
Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR et al (2007) Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol 293(1):H610–H619
Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG (2007) Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 150(5):613–623
Acknowledgements
The author gratefully acknowledges financial support from the Austrian Science Fund, FWF, grant # P27238-B27.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bondarenko, A.I. (2019). Cannabinoids and Cardiovascular System. In: Bukiya, A. (eds) Recent Advances in Cannabinoid Physiology and Pathology. Advances in Experimental Medicine and Biology, vol 1162. Springer, Cham. https://doi.org/10.1007/978-3-030-21737-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-21737-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21736-5
Online ISBN: 978-3-030-21737-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)