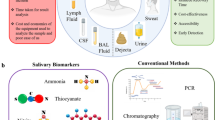
Abstract
Microfluidics, a technology that manipulates fluids into channels smaller than one cell to several millimeters, is an emerging new era in dental/oral and medical research. It has the potential to integrate complex systems in a miniaturized state by controlling the flow rate, providing a dynamic environment, conducting experiments in parallel, and monitoring analytes at cellular scale. This chapter highlights the applications of microfluidics using oral factors, such as saliva. In this area, point-of-care (POC) diagnostic systems have been made based on saliva as an easy, accessible, and sophisticated diagnostic fluid, which are reviewed in this chapter. Saliva testing has the potential to monitor some overall systemic illnesses, as well as oral diseases, which can be integrated in microfluidic devices. This chapter evaluates such microfluidics-based methods to save time and cost over traditional ones.
More specifically, microfluidic systems can be employed for detecting pathogenic bacteria and other analytes of interest in physiological disorders, controlled release of drugs, biofilm bacterial formation, and early detection of cancers—including oral cancers. In recent years, these systems have even exploited microelectronics, biotechnologies, and integration into smartphones to provide portable and miniaturized analytical devices, all of which are discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microfluidics
- Using oral factors
- Saliva-based microfluidics
- Microfluidic paper-based device
- Smartphone-based microfluidics
- Oral cancer detection
- Biofilm
1 Introduction
Medical devices are essential for a proper diagnosis by healthcare providers. Advances in medical devices and diagnoses in recent years have increased life expectancy by up to 5 years and reduced many common diseases [1]. However, people in developing countries do not have access to many new and expensive medical diagnostic technologies. For people who live far from medical laboratories, in developed countries as well as developing countries, it is difficult to go to a well-equipped lab for continuous (periodic) checkups. Microfluidic systems are designed to process the measurements of small volumes of liquids without the need for an expert, with relatively high sensitivity and speed. These unique features require creation of portable point-of-care (POC) medical diagnostic tools [2]. The advancement of microfluidic devices, in conjunction with lower price for POC applications, may provide instruments that can better serve the general population.
Microfluidic technologies , which are used in lab-on-a-chip (LOC) or miniaturized-total-analysis systems, allow for many different laboratory operations to be carried out on a small-scale portable chip [3]. Some microfluidic studies provide methods for shifting traditional macroscale assays to microscale portable assays [4]. Microfluidics has revolutionized POC diagnostic tools in the realms of medical products, biology, bioengineering, microbiology, and chemistry. Revolution in these fields can be considered similar to the revolution of integrated circuits in the electronics industry, a keystone of modern electronics [5]. Microfluidics is currently attempting to be implemented for use in monitoring devices for patients, detection of bacteria, disease diagnostics, drug delivery systems, and microbial studies.
There are many advantages of implementing microfluidics. Some major advantages include, but are not limited to, reducing sample volume, reducing consumption of reagent, reducing consumption of dangerous materials and infectious sample, decreasing risk of contamination, possibility of performing experiments in parallel, controlling the flow rate and providing a dynamic environment, being timesaving, and having low cost. Ultimately, microfluidic devices bring the capabilities of advanced analytical techniques to deprived areas of the developing world where common analytical labs do not exist [5, 6]. Microfluidic systems bring the laboratory to the patient and enable in situ monitoring of analytes.
Microfluidics has advantages over more traditional methods, such as enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR); these are used for the detection of proteins and small molecules and deoxyribonucleic acid (DNA), respectively. These methods are costly, laborious, and time-consuming. The ELISA requires multiple steps of washing and incubation, while PCR requires thermal cycling. Both techniques should be performed by trained personnel and require a large sample size [7].
This chapter aims to provide a review of microfluidics that has been developed using oral factors. Most studies that have benefited from microfluidics in this field have used saliva as a diagnostic fluid . Using saliva as a diagnostic fluid is advantageous because it does not require highly trained personnel and its collection is noninvasive. Saliva samples can be easily stored, and salivary biomarkers can be used to evaluate some diseases and physiological conditions and/or progression of diseases [8]. The primary focus of this paper is saliva-based studies, and this has been subdivided according to the microfluidic technique: on microfluidic chip, paper-based microfluidic devices, and smartphone-based microfluidic devices. The last part of this study explores the application of microfluidics in early diagnosis of cancer and biofilm studies.
2 Saliva as a Diagnostic Tool
The technology of using saliva instead of blood for the diagnosis of diseases is rapidly developing. Interestingly, most studies that use saliva as a diagnostic fluid are typically associated with systemic diseases, rather than localized oral diseases [9, 10]. If the relationship between systemic diseases and the oral cavity is better understood, it will strengthen the collaboration between medicine and dentistry. Since the oral cavity is readily accessible, saliva and mucosal biopsy (transudate) are used for oral diagnostics [11, 12]. Most of the analytes found in the blood are also secreted into the salivary fluid, specifically inorganic and organic materials (carbohydrates, glycoproteins, lipids, etc.), drugs, and their metabolites. Moreover, the collection method of saliva is easy, inexpensive, noninvasive, and safe for patients and healthcare professionals [10]. In addition to its other advantages, this approach requires small amount of saliva. It is rapid, portable, and appropriate for POC testing [8, 13].
2.1 Detection of Disease on Microfluidic Chips
Herr et al. [13] demonstrated a microfluidic device to detect biomarkers that are responsible for the progression of periodontal disease. In this study, they first bound special antigens that exist primarily in saliva samples with antibodies labeled by fluorescent receptors. Then, they separated antibody-bound antigens using electrophoretic assays. Detection was made using diode lasers.
Malamud [11] offered the feasibility of employing microfluidic devices with saliva samples for detection of diseases like tuberculosis (TB), HIV, and malaria.
Chen et al. [14] made a platform for nucleic acid extraction from bacteria and miniaturized the PCR process into a microfluidic device. In the final step, DNA was labeled with upconverting phosphor to detect on the lateral flow strip.
Chen et al. [12], in an additional study, utilized a similar platform for the diagnosis of HIV infection as a model to investigate the simultaneous detection of both viral RNA and human antibodies against HIV. Highly sensitive reporter upconverting phosphor and high salt lateral flow (HSLF) assays were employed for antibody detection. Three years later, Chen et al. designed a microfluidic device for early detection of HIV disease in the “seroconversion window.” During the seroconversion period , the viral load of HIV is highest, meaning the antibody is present but not detectable [15].
Since thiocyanate is considered an important biomarker in human health evaluation, finding a fast and reproducible way to analyze thiocyanate in bodily fluids is of interest. Wu et al. [16] developed a droplet microfluidic device for thiocyanate detection in real human saliva or serum using the surface-enhanced Raman scattering (SERS) technique. However, Pinto et al. [17] combined the immunoassay on a polydimethylsiloxane (PDMS) microfluidic chip and employed the complementary metal-oxide-semiconductor (CMOS) optical absorption system to detect the amount of cortisol in human saliva (Fig. 16.1).
Table 16.1 summarizes the studies of saliva on chip-based microfluidics for detection of infectious diseases. Other studies in this area have been done using filter paper or smartphone technology, which is explained in Sects. 2.2 and 2.3 in greater detail.
2.2 Microfluidic Paper-Based Analytical Devices (μPADs)
Paper-based systems are considered an alternative rather than the main analytical tools, which are practical for POC testing . These devices have gained popularity due to their ease of use, ease of fabrication, and low cost.
μPADs are built on a filter paper, which explains how they got the name lab-on-a-paper devices, whereas lab-on-a-chip (LOC) devices are usually made of polydimethylsiloxane (PDMS). Furthermore, μPADs do not require any external forces because of capillary phenomenon, while LOC devices require external forces for the movement of fluids. This system has been of interest due to the hydrophilic nature of filter paper, passive fluid movement, biocompatibility, and disposability. Various methods have been introduced for the fabrication of paper-based devices, such as lithography, three-dimensional (3D) printing, wax jetting, wax smearing, PDMS screen printing, permanent marker pen, wax printing, ink-jet printing, eyeliner pencil, cutter printer, hydrophobic sol-gel fabrication, and scholar glue spray method [18]. μPADs employ different detection methods, such as colorimetric, luminescence, and electrochemical detection. The most common way of detection in these microfluidic systems is colorimetric readouts .
Colorimetric detection is based on visual comparison and determines the concentration of analytes by means of color reagents. There are various types of luminescence. Both fluorescence and chemiluminescence (CL) are common types of luminescence that have been successful in paper-based devices. Unlike fluorescence, CL relies on producing photons as a by-product of a chemical reaction without requiring a light source [19, 20].
Electrochemical detection is based on the measurement of electrical parameters in the sample [21]. In this method, electrochemical analytical devices should be connected to a smartphone to measure changes from one of the input/output ports, such as the micro-USB.
Bhakta et al. [22] developed a μPAD with the wax printing method to identify and quantify levels of nitrite in saliva. It has been proven that nitrite concentration in saliva is associated with clinical symptoms of periodontitis (bleeding, swelling, and redness) [23]. In this approach, the main channel of the μPAD is placed vertically in contact with the saliva sample. The sample is taken up by capillary action and driven into the four branched channels and testing zones (Fig. 16.2). The nitrite in the saliva is combined with the Griess reagent, and this leads to magenta azo compound formation. The Griess reagent is made by mixing sulfanilamide in 5% (v/v) phosphoric acid solution and 0.1% (w/v) naphthylethylenediamine dihydrochloride in water. Color intensity is associated with the concentration of nitrite in the sample and can be representative of the progression of periodontitis [22].
μPAD designed for nitrite detection in human saliva [22]
Nitrite is also known as a helpful biomarker for patients with end-stage renal disease to detect progression of hemodialysis. It has been reported that the concentration of nitrite in the patient’s saliva reflects the amount of nitrite in the blood and is suitable for POC application [24]. Salivary nitrite concentrations were measured in a study by Blicharz et al. with test strips. They showed nitrite concentrations decreased after dialysis and found this method to be a noninvasive and beneficial way of monitoring renal disease status [25].
Formaldehyde and acetaldehyde are well known as carcinogenic agents. Smoking is one of the most important sources of aldehyde contamination in the indoor air. Additionally, it has been shown that consuming alcoholic beverages has the same carcinogenic effect due to the transformation of ethanol into acetaldehyde [26]. Ramdzan et al. [27] employed a two-layer configuration paper-based microfluidic device for determining salivary aldehydes. This study revealed that this paper-based technique could be a successful alternative to the costly and large-scale analytical tools [e.g., high-performance liquid chromatography (HPLC) or gas chromatography (GC)] that determine aldehydes in biological fluids, such as salivary fluid. In addition, the sensitivity of this technique is comparable to that of the aforementioned methods [27].
One of the more basic of the “paper-based devices” is lateral flow immunoassay (LFI) . LFI has been employed with smartphones as a new developing field in recent literature. This situation is discussed in the next section.
2.3 Smartphone-Based Microfluidic Devices
Integration between microfluidic devices and smartphones introduces a new world and has the potential to revolutionize the smartphone industry. Smartphone-based diagnostics provides a user-friendly, easily accessible, miniaturized, and portable technology [28].
In this approach, the phone is the main device that performs the entire analytical process, including data acquisition, analysis, and result readout. Smartphone, like a computer, has a processor (CPU) and hardware for receiving, sending, and processing a variety of signals. Therefore, it has the ability to receive data from sensors and control various actuators or send data to and receive data from other devices, by wire or wirelessly. This allows a variety of applications on the smartphone to run in order to analyze data and check the biological samples.
Many studies in this area utilize smartphone cameras to receive information from samples. Different principles for the development of biosensors have been used. Biosensors in these systems work based on color, luminescence, or electrochemical detection. Smartphone microfluidic systems that employ saliva as a diagnostic fluid often use colorimetric and luminescence techniques.
It is almost impossible for the human eye to quantize the color variation or the light emitted from the luminescence samples [29]. Therefore, an accessory is needed to standardize the effective factors to eliminate quantization errors. In these methods, a dark chamber is required to avoid ambient light interfering with measurements. Different techniques are considered to standardize the camera flashlight. A holder is needed for connecting the dark chamber to the smartphone [20]. Another detection method that has been mentioned in the articles using smartphones is electrochemical sensors (amperometric, impedimetric, and potentiometric sensors). This method uses different sample mediums, such as saliva, blood, water, food, and urine [19].
Monitoring pH in saliva is important for investigating the person’s diet and its relationship with enamel decalcification. Monitoring pH in sweat helps explain the risk of dehydration during physical activities. Oncescu et al. [30] designed a software and a hardware accessory installed on a smartphone. After collecting a saliva or sweat sample, the test strip was inserted into the device, and the pH analysis was carried out using a mobile platform. The flash on the smartphone’s camera was used to illuminate the back of the test strip, exposing the pH indicator. Colorimetry was used to understand the pH of either saliva or sweat sample.
Other studies reported using a smartphone for detection of bile acid in serum and oral fluid [31], salivary cortisol [31,32,33,35], L-lactate [20, 36, 37], glucose [38, 39], Zika virus (ZIKV) [40, 41], drugs related to driving under influence (DUI) cases [41,42,43,44,46], ovulation time [47], and others [48, 49] in human saliva (Table 16.2). However, there are some published papers that utilize blood as the diagnostic fluid with smartphone technology.
Nowadays, stress is one of the major causes of psychiatric disease. Stressful daily routines can lead to additional mental health problems, such as depression, anxiety, low self-esteem, and more. Thus, many efforts have been made to design stress monitoring tools [17, 32]. Cortisol is one of the most well-known biomarkers of stress. The main advantages of using saliva as a diagnostic fluid to measure cortisol levels are its easy, noninvasive, and rapid collection method. This itself reduces a potential stress response and allows continuous monitoring of stress level. Cortisol has two active states: free active state and protein-bonded state. Cortisol in the saliva is in the free active state, meaning that it is detectable, unlike what exists in the blood—where about 90% of it is protein-bonded [17, 50]. This is a major advantage of using saliva over blood for detection of cortisol.
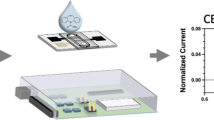
Choi et al. [32] and Zangheri et al. [33] have demonstrated a smartphone-based system with lateral flow immunoassay for quantitative measurement of salivary cortisol. Choi et al. [32] compared the changes in the hue (color) and brightness (lightness or darkness) values with cortisol concentration. Zangheri et al. [33] immobilized anti-cortisol antibodies in rabbits (T-line) and anti-peroxidase antibodies in rabbits (C-line). These antibodies were placed on nitrocellulose membranes to make LFI test strips for their experiment. They measured the intensity of the chemiluminescence signal of the T-line for quantification of the amount of cortisol. It was found that the intensity of the chemiluminescence and cortisol in the sample were inversely correlated. Chemiluminescence signal of the C-line was also used to evaluate the validity of the test (Fig. 16.3).
(a) 3D printed smartphone accessory. (b) 3D printed cartridge hosting the LFI strip with control (C-line) and test lines (T-line). (c) Smartphone-based microfluidic device for measuring salivary cortisol [33]
At first, Zika virus appeared to only cause mild disease, but the outbreak in Brazil in 2015 showed the relationship between the virus and severe fetal abnormalities, called congenital Zika syndrome. In search of diagnostic technologies, Priye et al. [40] designed a study to detect the virus using similar cases. Unlike most papers, in this research, a microcontroller was used to monitor and control the temperature of a dark plastic enclosure containing the samples. This microcontroller, an Arduino, was wirelessly connected to the smartphone and was able to control the RGB LED light source placed in this box. The smartphone application with a simple graphical user interface (GUI) was created to actuate the LED excitation source and the isothermal heater through the microcontroller and display a live camera feed. It also provided other features and facilities for the user. Finally, the test results were displayed on the final screen after processing.
Internet of Medical Things (IoMT)—a new technology to connect the medical devices to the healthcare providers—is revolutionizing the medical world. This technology offers new paradigms of diagnosis, treatment, and healthcare services in a timely and cost-effective manner. In addition, it may be helpful in epidemiology studies [51]. However, current IoMT devices are limited to monitor physiological information, such as heart rate and blood pressure. Song et al. [41] demonstrated a IoMT POC device based on a smartphone for molecular diagnostics, providing quantitative detection for Zika virus (ZIKV) and HIV, transferring test results to the doctor’s office, and allowing communication of data.
Calabria et al. [36] demonstrated “wafer-like” structures as a functionalized paper of cellulose that contained reagents and enzymes to measure accurate L-lactate levels in saliva. It was shown that smartphones could also detect L-lactate based on the hue saturation value (HSV) for rapid colorimetric measurement.
In another study by Roda et al. [20], a chemiluminescence biosensor was introduced, which is comprised of a disposable minicartridge with two reaction chambers. Inside these chambers are nitrocellulose disks that contain the enzymes needed for reaction. Each of chambers were connected to two reservoirs containing reagents using channel.
In 2019, Potluri et al. [47] introduced a low-cost smartphone-based device beneficial for couples interested in planning pregnancy. Studies have shown that during the follicular phase of the menstrual cycle, increased level of estradiol in the blood results in an increase in the salivary electrolytes, which can cause a crystallized pattern like fern leaves in air-dried saliva. This pattern may be used for ovulation detection. They utilized a neural network which is trained with salivary ferning images. Artificial intelligence (AI) was employed for detection of ovulation period from air-dried saliva samples.
3 Detection of Oral Cancer
Cancers of the oral cavity comprise approximately 40% of head and neck cancers and affect several areas, such as the tongue, gums, lips, palate, floor of the mouth, and buccal mucosa [55]. Despite recent advances in diagnostics, therapeutics, and surgical procedures, the success rate of treating oral squamous cell carcinoma (OSCC) still remains very poor [56].
Dentists and oral hygienists play a key role in early detection of oral cancers. They ought to be screening for suspicious malignant lesions and determine whether a tissue biopsy is necessary. However, while they do not want delay in any necessary procedure, they tend to diminish the unwarranted biopsy and patients’ inconvenience [57]. Moreover, considering aggressive treatment of OSCC can lead to a severe deformity or death, its treatment is controversial. Most patients with this cancer are either undertreated or overtreated. Often, OSCC is diagnosed at an advanced stage. This limits treatment options and is a big reason why the survival rate of patients is so low [5].
Early diagnosis of cancerous or premalignant lesions significantly increases the likelihood of patient survival. Early detection does not automatically mean success. However, it can lead to frequent visits by the patient, change in diet, stopping smoking and alcohol consumption, removing lesions, and taking the required medications. Due to the fact that OSCC has a high mortality rate, better screening methods need to be implemented to detect this cancer early [5, 58].
Some studies demonstrated cell-based microfluidic sensors to detect cancer biomarkers. Cytology , which is the easiest way to screen premalignant and cancerous lesions, was employed to capture cells from the targeted tissues. Expression of cancer-specific biomarkers could be used to discriminate cancer cells from normal cells [56, 59, 60]. This review focuses on saliva-based microfluidic devices for screening of cancers.
In a study conducted by De et al. [61], a LOC microfluidic system was employed for hypermethylated DNA extraction and enrichment, which was suitable for processing small biological samples such as saliva and urine. This device was demonstrated to identify the patterns of hypermethylation parts in tumor-suppressor genes with the potential for the early detection and diagnosis of cancers.
Zilberman et al. [62, 63] introduced a new platform to assist stomach cancer diagnosis. The underlying cause of the stomach cancer is the infection by a gram-negative bacterium Helicobacter pylori. This bacterium converts urea into ammonia (NH3) and carbon dioxide (CO2) by secreting the urease enzyme, resulting in high levels of these substances in the body fluids and breath. They demonstrated a microfluidic optoelectronic sensor to detect the amount of salivary NH3 and CO2. Using saliva for screening cancers is a convenient and noninvasive approach compared to traditional endoscopy method. Using the proposed method of Zilberman et al. can eliminate the need for trained personnel and high costs of endoscopy.
Biomarkers , which represent normal biological and pathological procedures, may provide helpful information for detection, diagnosis, and prognosis of the diseases. More than 100 biomarkers (DNA, RNA, mRNA, protein markers) have already been recognized in oral fluid, including cytokines (IL-8, IL-1b, TNF-a), p53, Cyfra 21-1, defensin-1, and tissue polypeptide-specific antigen [64].
Lin et al. [65] developed an automated microfluidic chip using magnetic bead-based immunoassays, well suited for anti-p53 quantification in saliva. The proposed device can considerably reduce the time for conventional immunoassay, which takes about 3 hours, to approximately 60 minutes.
In a study by Dong et al. [66], an optical microfluidic biosensor has demonstrated good analytical performance for salivary protein biomarkers (IL-8, IL-1β, and MMP-8) to differentiate patients with oral cancer from healthy people.
A microfluidic paper-based electrochemical DNA biosensor was fabricated by Tian et al. [67] to detect epidermal growth factor receptor (EGFR) mutations in DNA extracted from non-small cell lung cancer patients’ saliva samples (Fig. 16.4).
Schematic representation of a microfluidic device including inlets and outlets, which embeds microwells occupied with organic dye-doped ion-exchange polymer microbeads. The response of this optoelectronic sensor is monitored by a USB spectrometer [63]
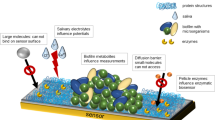
4 Biofilm Formation
Most of the bacteria tend to switch from a free-floating planktonic state to a sticky organized biofilm state. Forming the biofilm protects the bacteria from harmful conditions (e.g., nutrient deficiency, pH alterations, antibacterial agents, and shear stress) in the host, and the bacteria benefit from cooperating with each other.
The microfluidic systems provide a closed system where bacteria can exist in biofilms and interact with hydrodynamic environments. Microfluidic systems can be beneficial for studying biofilm formation mechanisms and solving problems associated with biofilm. Importantly, they allow for the flow of fluids to be controlled. The small scale of these devices makes it easy to cultivate bacteria and form biofilms because it is easy to set up a variety of conditions. In addition, their transparency makes it possible to observe the development of biofilms [68].
It has been reported that most bacteria utilize a form of cell-to-cell communication named quorum sensing (QS) for their initial colonization and development of biofilm communities. This allows each bacterial species to adjust the expression of target genes depending on the environment and physiological needs. Therefore, it is important to understand the role of various QS signals in biofilm formation. Kim et al. developed a microfluidic device to investigate QS in oral biofilm formation [69].
Streptococcus mutans is the main cause of dental caries [70]. Environmental factors (e.g., shear rate, nutrients, and pH) greatly affect the biofilm formation of S. mutans on the tooth surface, and monitoring of these factors and investigation of their effects are of interest to researchers [71]. S. mutans plays an important role in the development of the exopolysaccharide (EPS) matrix in biofilm formation on the tooth surface of the tooth [70].
Quantitative analysis of EPS can be investigated using microliter plate-based assay, which is recognized as a reliable technique. However, this assay is performed in static conditions. Microfluidic devices have the advantage of providing a dynamic flow system that mimics the environment of the oral cavity [72].
In a study conducted by Shumi et al., the effect of the environmental factors on biofilm formation of S. mutans was examined in a microfluidic device with glass beads. In this study, glass beads mimicked the interproximal space between teeth. They found production of EPS and attachment of S. mutans to the surface of the glass beads happened in the presence of sucrose [71]. In another study, Shumi et al. designed a microfluidic platform with a funnel that provided various ranges of shear stresses.
Sucrose-dependent and sucrose-independent aggregates of S. mutans were evaluated under different flow rates and times. The findings showed S. mutans colonies that were sucrose-dependent strongly adhered to the funnel wall. Detachment of sucrose-dependent colonies from the funnel wall was possible only with high shear stress, while in the case of sucrose-independent colonies, they were easily separated from the funnel by a slight shear stress [73].
The dental plaque on the tooth’s surface is a multispecies biofilm, containing over 500 species of bacteria. The bacteria in biofilms are less susceptible to antibacterial agents than planktonic cells [74, 75]. To evaluate the effects of antibacterial agents on bacteria, it is important to create biofilms that contain the species present in the oral cavity. Nance et al. and Samarian et al. simulated the condition of the oral cavity with a commercially available BioFlux microfluidic system (Fluxion Biosciences, Inc., Alameda, CA 94502, USA) [6, 75]. This system is useful for overcoming limitations, such as using less materials and not requiring artificial lab media, especially since it can be costly and time-consuming to run culture-based studies. A lot of media are required in tests that are conducted in flow cell studies. Using this high-throughput system allows multiple experiments to be done in parallel. Finally, this system is able to combine imaging systems to allow precise investigation of biofilm testing [6, 76, 77].
Nance et al. investigated the effect of different concentrations of antimicrobial agent (cetylpyridinium chloride) using confocal laser scanning microscope (CLSM) and 3D imaging software. Some studies have utilized a germane environmental situation to resemble the real-world environment. The use of human saliva in this method has become popular for investigating biofilms. Cell-free saliva (CFS) plays the role of growth medium, and the untreated bacterial cell-containing saliva (CCS) plays the role of inoculum that contains bacterial species in the oral cavity. The formed multispecies biofilm is washed and stained for in situ confocal laser scanning microscopy [6, 75, 77].
Most studies examine biofilms in a static state. In the oral cavity, designing continuous fluid flow is preferred to mimic formed biofilm in the body. In the case of peri-radicular lesions (non-ideal root canal therapy or pulpal necrosis), there is a fluid exchange inside and outside the root canal space that provides feeding and flow for the growth of bacteria. Cheng et al. compared the bactericidal effect of several root canal irrigants against Enterococcus faecalis biofilm, recognized as the most important persistent endodontic infection. They evaluated E. faecalis biofilm in flow state as well as static state [78].
In the study conducted by Lam et al., a high-throughput microfluidic system with the name of “artificial teeth” was introduced. This device had different layers of channels (e.g., water jackets, gas control, upper and lower flow control), which could provide complex and dynamic environmental situations and acquire quantitative characteristics about thickness and cell viability of dental bacteria [79]. The summary of microbial studies using a microfluidic device is provided in Table 16.3 (Fig. 16.5).
5 Summary
Microfluidics is an attractive, rapidly developing, and promising technology that may enable detection of certain diseases, such as diabetes and periodontal disease. Microfluidics can also be exploited as a diagnostic tool for early detection of complex diseases, such as cancers and viral infections, like HIV. Among the microfluidic systems used in the biomedical field, μPADs have become the most commercially available system due to their low manufacturing cost, as well as not requiring complex accessories during tests. This developing approach faces many challenges requiring standardization to verify its reproducibility and reliability.
For future development, it is expected to involve other technological devices—such as smartphones or tablets with microfluidics—to analyze the physiological state of humans using a drop of saliva or other biological fluids. Considering how advanced smartphones have become and how rapidly they are developing, the combination of these technologies will allow continuous and easy health monitoring of individuals or target groups. The results can then be sent directly to the patient’s physician or healthcare provider. The ramifications of this technology are powerful; it has the potential to make major improvements in the quality of life of people in modern and developing societies.
References
Guan, A., et al. (2017). Medical devices on chips. Nature Biomedical Engineering, 1(3), 1–10.
Yager, P., et al. (2006). Microfluidic diagnostic technologies for global public health. Nature, 442(7101), 412–418.
Gupta, S., et al. (2016). Lab-on-chip technology: A review on design trends and future scope in biomedical applications. International Journal of Bio-Science and Bio-Technology, 8(5), 311–322.
Sackmann, E. K., Fulton, A. L., & Beebe, D. J. (2014). The present and future role of microfluidics in biomedical research. Nature, 507(7491), 181–189.
Ziober, B. L., et al. (2008). Lab-on-a-chip for oral cancer screening and diagnosis. Head & Neck, 30(1), 111–121.
Nance, W. C., et al. (2013). A high-throughput microfluidic dental plaque biofilm system to visualize and quantify the effect of antimicrobials. Journal of Antimicrobial Chemotherapy, 68(11), 2550–2560.
Lim, W. Y., Goh, B. T., & Khor, S. M. (2017). Microfluidic paper-based analytical devices for potential use in quantitative and direct detection of disease biomarkers in clinical analysis. Journal of Chromatography B, 1060, 424–442.
Meagher, R., & Kousvelari, E. (2018). Mobile oral heath technologies based on saliva. Oral Diseases, 24(1–2), 194–197.
Arunkumar, S., et al. (2014). Developments in diagnostic applications of saliva in oral and systemic diseases-A comprehensive review. Journal of Scientific and Innovative Research, 3(3), 372–387.
Chojnowska, S., et al. (2018). Human saliva as a diagnostic material. Advances in Medical Sciences, 63(1), 185–191.
Malamud, D. (2013). The oral-systemic connection: role of salivary diagnostics. In Sensing Technologies for Global Health, Military Medicine, and Environmental Monitoring III. International Society for Optics and Photonics.
Chen, Z., et al. (2013). Development of a generic microfluidic device for simultaneous detection of antibodies and nucleic acids in oral fluids. BioMed Research International, 2013, 1–12.
Herr, A. E., et al. (2007). Integrated microfluidic platform for oral diagnostics. Annals of the New York Academy of Sciences, 1098(1), 362–374.
Chen, Z., et al. (2007). A microfluidic system for saliva-based detection of infectious diseases. Annals of the New York Academy of Sciences, 1098(1), 429–436.
Chen, Z., et al. (2016). A rapid, self-confirming assay for HIV: Simultaneous detection of anti-HIV antibodies and viral RNA. Journal of AIDS & Clinical Research, 7(1), 1–8.
Wu, L., et al. (2014). Rapid and reproducible analysis of thiocyanate in real human serum and saliva using a droplet SERS-microfluidic chip. Biosensors and Bioelectronics, 62, 13–18.
Pinto, V., et al. (2017). Microfluidic immunosensor for rapid and highly-sensitive salivary cortisol quantification. Biosensors and Bioelectronics, 90, 308–313.
Sriram, G., et al. (2017). Paper-based microfluidic analytical devices for colorimetric detection of toxic ions: A review. TrAC Trends in Analytical Chemistry, 93, 212–227.
Roda, A., et al. (2016). Smartphone-based biosensors: A critical review and perspectives. Trends in Analytical Chemistry, 79, 317–325.
Roda, A., et al. (2014). A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst, 139(24), 6494–6501.
Snober, A., Minh-Phuong, N., & Abdennour, A. (2016). Paper-based chemical and biological sensors: Engineering aspects. Biosensors and Bioelectronics, 77, 249–263.
Bhakta, S. A., et al. (2014). Determination of nitrite in saliva using microfluidic paper-based analytical devices. Analytica Chimica Acta, 809, 117–122.
Allaker, R., et al. (2001). Antimicrobial effect of acidified nitrite on periodontal bacteria. Molecular Oral Microbiology, 16(4), 253–256.
Klasner, S. A., et al. (2010). Based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Analytical and Bioanalytical Chemistry, 397(5), 1821–1829.
Blicharz, T. M., et al. (2008). Use of colorimetric test strips for monitoring the effect of hemodialysis on salivary nitrite and uric acid in patients with end-stage renal disease: A proof of principle. Clinical Chemistry, 54(9), 1473–1480.
Demkowska, I., Polkowska, Ż., & Namieśnik, J. (2010). Formaldehyde in human saliva as an indication of environmental tobacco smoke exposure. Polish Journal of Environmental Studies, 19(3), 573–577.
Ramdzan, A. N., et al. (2016). Development of a microfluidic paper-based analytical device for the determination of salivary aldehydes. Analytica Chimica Acta, 919, 47–54.
Yang, K., et al. (2016). Novel developments in mobile sensing based on the integration of microfluidic devices and smartphones. Lab on a Chip, 16(6), 943–958.
Deeb, S. (2005). The molecular basis of variation in human color vision. Clinical Genetics, 67(5), 369–377.
Oncescu, V., O’Dell, D., & Erickson, D. (2013). Smartphone based health accessory for colorimetric detection of biomarkers in sweat and saliva. Lab on a Chip, 13(16), 3232–3238.
Roda, A., et al. (2014). Integrating biochemiluminescence detection on smartphones: Mobile chemistry platform for point-of-need analysis. Analytical Chemistry, 86(15), 7299–7304.
Choi, S., et al. (2014). Real-time measurement of human salivary cortisol for the assessment of psychological stress using a smartphone. Sensing and Bio-Sensing Research, 2, 8–11.
Zangheri, M., et al. (2015). A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosensors and Bioelectronics, 64, 63–68.
Choi, S., et al. (2017). Relationship analysis of speech communication between salivary cortisol levels and personal characteristics using the Smartphone Linked Stress Measurement (SLSM). BioChip Journal, 11(2), 101–107.
Rey, E., et al. (2018). Personalized stress monitoring: A smartphone-enabled system for quantification of salivary cortisol. Personal and Ubiquitous Computing, 22(4), 867–877.
Calabria, D., et al. (2017). Smartphone–based enzymatic biosensor for oral fluid L-lactate detection in one minute using confined multilayer paper reflectometry. Biosensors and Bioelectronics, 94, 124–130.
Yao, Y., et al. (2017). An electrochemiluminescence cloth-based biosensor with smartphone-based imaging for detection of lactate in saliva. Analyst, 142(19), 3715–3724.
Soni, A., & Jha, S. K. (2017). Smartphone based non-invasive salivary glucose biosensor. Analytica Chimica Acta, 996, 54–63.
Jia, Y., et al. (2018). Based graphene oxide biosensor coupled with smartphone for the quantification of glucose in oral fluid. Biomedical Microdevices, 20(4), 89.
Priye, A., et al. (2017). A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Scientific Reports, 7, 44778.
Song, J., et al. (2018). Smartphone-based mobile detection platform for molecular diagnostics and spatiotemporal disease mapping. Analytical Chemistry, 90(7), 4823–4831.
Jung, Y., et al. (2015). Smartphone-based colorimetric analysis for detection of saliva alcohol concentration. Applied Optics, 54(31), 9183–9189.
Carrio, A., et al. (2015). Automated low-cost smartphone-based lateral flow saliva test reader for drugs-of-abuse detection. Sensors, 15(11), 29569–29593.
Lee, J.-R., et al. (2016). Small molecule detection in saliva facilitates portable tests of marijuana abuse. Analytical Chemistry, 88(15), 7457–7461.
He, M., et al. (2016). Portable upconversion nanoparticles-based paper device for field testing of drug abuse. Analytical Chemistry, 88(3), 1530–1534.
Kim, H., et al. (2017). Colorimetric analysis of saliva–alcohol test strips by smartphone-based instruments using machine-learning algorithms. Applied Optics, 56(1), 84–92.
Potluri, V., et al. (2019). An inexpensive smartphone-based device for point-of-care ovulation testing. Lab on a Chip, 19(1), 59–67.
Zhang, L., et al. (2015). Smartphone-based point-of-care testing of salivary α-amylase for personal psychological measurement. Analyst, 140(21), 7399–7406.
Soni, A., Surana, R. K., & Jha, S. K. (2018). Smartphone based optical biosensor for the detection of urea in saliva. Sensors and Actuators B: Chemical, 269, 346–353.
Kaushik, A., et al. (2014). Recent advances in cortisol sensing technologies for point-of-care application. Biosensors and Bioelectronics, 53, 499–512.
Haghi, M., Thurow, K., & Stoll, R. (2017). Wearable devices in medical internet of things: Scientific research and commercially available devices. Healthcare Informatics Research, 23(1), 4–15.
Stedtfeld, R. D., et al. (2015). Static self-directed sample dispensing into a series of reaction wells on a microfluidic card for parallel genetic detection of microbial pathogens. Biomedical Microdevices, 17(5), 89.
Zhang, X.-X., et al. (2018). Sensitive paper-based analytical device for fast colorimetric detection of nitrite with smartphone. Analytical and Bioanalytical Chemistry, 410(11), 2665–2669.
Roda, A., et al. (2019). A simple smartphone-based thermochemiluminescent immunosensor for valproic acid detection using 1,2-dioxetane analogue-doped nanoparticles as a label. Sensors and Actuators B: Chemical, 279, 327–333.
Massano, J., et al. (2006). Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics, 102(1), 67–76.
Abram, T. J., et al. (2016). Cytology-on-a-chip’ based sensors for monitoring of potentially malignant oral lesions. Oral Oncology, 60, 103–111.
Lydiatt, D. D. (2002). Cancer of the oral cavity and medical malpractice. The Laryngoscope, 112(5), 816–819.
Pandya, D., et al. (2015). Lab-on-a-chip-oral cancer diagnosis at your door step. Journal of International Oral Health, 7(11), 122–128.
Patel, V., et al. (2013). DSG3 as a biomarker for the ultrasensitive detection of occult lymph node metastasis in oral cancer using nanostructured immunoarrays. Oral Oncology, 49(2), 93–101.
Weigum, S. E., et al. (2010). Nano-bio-chip sensor platform for examination of oral exfoliative cytology. Cancer Prevention Research, 3(4), 518–528.
De, A., et al. (2014). Rapid microfluidic solid-phase extraction system for hyper-methylated DNA enrichment and epigenetic analysis. Biomicrofluidics, 8(5), 054119.
Zilberman, Y., Chen, Y., & Sonkusale, S. R. (2014). Dissolved ammonia sensing in complex mixtures using metalloporphyrin-based optoelectronic sensor and spectroscopic detection. Sensors and Actuators B: Chemical, 202, 976–983.
Zilberman, Y., & Sonkusale, S. R. (2015). Microfluidic optoelectronic sensor for salivary diagnostics of stomach cancer. Biosensors and Bioelectronics, 67, 465–471.
Najeeb, S., Slowey, P. D., & Rehmanjj, I. U. (2018). Role of salivary biomarkers in oral cancer detection. Advances in Clinical Chemistry, 86, 23.
Lin, Y.-H., et al. (2018). Detection of anti-p53 autoantibodies in saliva using microfluidic chips for the rapid screening of oral cancer. RSC Advances, 8(28), 15513–15521.
Dong, T., & Pires, N. M. M. (2017). Immunodetection of salivary biomarkers by an optical microfluidic biosensor with polyethylenimine-modified polythiophene-C70 organic photodetectors. Biosensors and Bioelectronics, 94, 321–327.
Tian, T., et al. (2017). Based biosensor for noninvasive detection of epidermal growth factor receptor mutations in non-small cell lung cancer patients. Sensors and Actuators B: Chemical, 251, 440–445.
Kim, J., Park, H.-D., & Chung, S. (2012). Microfluidic approaches to bacterial biofilm formation. Molecules, 17(8), 9818–9834.
Kim, S. H. (2008). Role of AI-2 in oral biofilm formation using microfluidic devices. College Station: Texas A & M University.
Koo, H., et al. (2010). Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. Journal of Bacteriology, 192(12), 3024–3032.
Shumi, W., et al. (2010). Environmental factors that affect Streptococcus mutans biofilm formation in a microfluidic device mimicking teeth. BioChip Journal, 4(4), 257–263.
Kolenbrander, P., Andersen, R., & Moore, L. (1989). Coaggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas infelix, Selenomonas noxia, and Selenomonas sputigena with strains from 11 genera of oral bacteria. Infection and Immunity, 57(10), 3194–3203.
Shumi, W., et al. (2013). Shear stress tolerance of Streptococcus mutans aggregates determined by microfluidic funnel device (μFFD). Journal of Microbiological Methods, 93(2), 85–89.
Ten Cate, J., & Zaura, E. (2012). The numerous microbial species in oral biofilms: How could antibacterial therapy be effective? Advances in Dental Research, 24(2), 108–111.
Samarian, D. S., et al. (2014). Use of a high-throughput in vitro microfluidic system to develop oral multi-species biofilms. Journal of Visualized Experiments, (94), e52467.
Benoit, M. R., et al. (2010). New device for high-throughput viability screening of flow biofilms. Applied and Environmental Microbiology, 76(13), 4136–4142.
Foster, J. S., & Kolenbrander, P. E. (2004). Development of a multispecies oral bacterial community in a saliva-conditioned flow cell. Applied and Environmental Microbiology, 70(7), 4340–4348.
Cheng, X., et al. (2016). Bactericidal effect of strong acid electrolyzed water against flow enterococcus faecalis biofilms. Journal of Endodontics, 42(7), 1120–1125.
Lam, R. H., et al. (2016). High-throughput dental biofilm growth analysis for multiparametric microenvironmental biochemical conditions using microfluidics. Lab on a Chip, 16(9), 1652–1662.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Salehipour Masooleh, H., Ghavami-Lahiji, M., Ciancio, A., Tayebi, L. (2020). Microfluidic Technologies Using Oral Factors: Saliva-Based Studies. In: Tayebi, L. (eds) Applications of Biomedical Engineering in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-030-21583-5_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-21583-5_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21582-8
Online ISBN: 978-3-030-21583-5
eBook Packages: EngineeringEngineering (R0)