Abstract
Arsenic (As)-tainted agricultural land and drinking water is a global issue due to its extremely toxic and carcinogenic behavior for various life forms. Arsenic creates morphological disorder in plants by altering their physiology and metabolism. Recent solution culture and soil–plant system researches on As uptake and metabolism indicate that different forms of As exert toxicity in species-specific manner through inflection of diverse metabolites from crucial biological pathways. Various plant species adapt an array of genomic and proteomic approaches for As tolerance and detoxification which is manifested through stress-responsive metabolites. Eventually, the increased level of targeted metabolites during As stress plays a significant role for recovering the altered metabolism in plants. The review highlights the utility of modulated metabolites to understand their role for As tolerance and detoxification in As-stressed plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Progressively increasing contamination of arsenic (As) is a dreadful situation these days, which adversely influences all components of the environment. Arsenic is introduced in the environment through natural processes (volcanic eruptions, weathering of rocks, minerals, and fossil fuels) and anthropogenic activities (metal mining and smelting, agrochemicals, industrial effluents, and phosphate fertilizers), with its high level for the most part of India, Bangladesh, and China (Mondal et al. 2006, Tripathi et al. 2012a, b). The worst conditions of As contamination came across in Bangladesh and West Bengal (India) due to natural processes (Smith et al. 2000, Nordstrom 2002, Brammer and Ravenscroft 2009).
The subsurface mobilization of As is caused by the combination of chemical, physical and microbial factors, and various theories have been proposed to explain the mechanism of As mobilization (Mondal et al. 2006; Drewniak et al. 2012). Of these, the important theories are the pyrite oxidation and oxyhydroxide reduction (Hossain 2006). Flooding induces reducing (anaerobic) conditions in soils; hence, adsorbed arsenate [As(V)] is reduced to arsenite [As(III)] by anaerobic metal-reducing bacteria released by mediating reductive dissolution of As-rich Fe(III) oxyhydroxides (Islam et al. 2004). Besides natural processes, the global input of As to soil by human activities was estimated about 52,000 to 1,12,000 tons per year in earlier decade (Nriagu and Pacyna 1988).
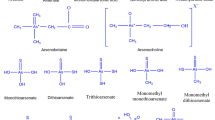
Inorganic arsenic (iAs) forms such as As(V) and As(III) are class 1 carcinogens (IARC 2004). It is often implicit that organic forms, dimethylarsonic acid [DMA(V)] and monomethylarsonic acid [MMA(V)], are lesser toxic to humans than iAs (Schoof et al. 1999). However, reduced trivalent intermediate species of DMA and MMA are actually more cytotoxic and genotoxic than iAs (Thomas et al. 2001). There is a widespread chronic iAs poisoning in regions of South and Southeast Asia, South America, and elsewhere, due to the consumption of drinking water extracted from shallow underground aquifers with geogenically elevated iAs (Naujokas et al. 2013). According to the World Health Organization (WHO), the permissible limit of As in drinking water is 10 μg L−1. Besides As-contaminated drinking water, plant-based foods are also recognized as an important source of iAs (Williams et al. 2005; Meharg et al. 2009).
Rice is specifically a problem regarding the entry of As into the food chain, owing to a combination of the anaerobic growing conditions and specific plant physiological characteristics. Nearly half the world’s population relies on rice for subsistence diet. Rice contributes about 50% iAs intake to the populations exposed to high level of iAs in drinking water in South Asia (Meharg et al. 2009, Tripathi et al. 2012a, b). However, the excessive use of methylated arsenical pesticides in the USA has led to As buildup in soil as well as in rice grains (Williams et al. 2005). Besides, As stress also affects the productivity of the crop plants with high As accumulation.
In the view of past and recent researches, the studies of As uptake and metabolism have been taken into consideration in this review. Further, As toxicity and tolerance mechanisms are discussed for better understanding of the affected biological pathways and responsible metabolites insides the plants.
2 Arsenic Acquisition and Translocation in the Plant
To understand and control the As hazard from contaminated soils, it is crucial to know how various species of As are taken up by plants. Iron plays a pivotal role in the biogeochemical cycle of As, with Fe oxyhydroxides on soil particulate surfaces, or root surfaces of wetland plants as iron plaque, serving as strong adsorbent for As. Reductive dissolution of Fe oxyhydroxides under reducing environment releases the adsorbed As, leading to enhanced As availability to plants. There are significant varietal differences in the amount of iron plaque formation and, consequently, As sequestration in the iron plaque (Tripathi et al. 2014). Varieties that sequester more As in iron plaque translocate less As to aboveground tissues (Liu et al. 2004). Rice cultivars with high porosities tended to possess higher rates of radial oxygen loss and had higher capacities for limiting the transfer of As to aboveground tissues due to formation of more Fe plaques (Wu et al. 2012). Nonetheless, there are various factors other than the amount of iron plaque that may affect the uptake of As, e.g., the plant species, ionic species and their concentrations, pH of the rhizosphere, or the presence of other elements.
The mobility of As in the plants varies with As speciation as well as physicochemical characteristics of the soil (Marin et al. 1992). The most available and predominant forms of As are As(III), As(V), MMA, and DMA for plant uptake. The rate of uptake of different As species varies in the order of As(III) > As(V) > MMA(V) > DMA(V) in most of the plant, but exceptionally in rice the order is As(III) > MMA(V) > As(V) > DMA(V) (Raab et al. 2007). Differential uptake pathways responsible for the uptake of inorganic and organic species of As are described below.
2.1 Transport of Inorganic Arsenic
Arsenic mobility is dependent on the soil pH. Plant roots assimilate As(V) via phosphate transporters. The chemical analogs of inorganic phosphate (Pi) ions, such as H2AsO4−1, gradually increase at pH range of 3–6; while, H2AsO4−1 and HAsO4−2 species are significantly present at the pH between 7 and 8. Above pH 8 and below 11, the most predominant chemical form is HAsO4−2.
Plants have both low- and high-affinity Pi transport systems. High-affinity transport is mediated by PHT (phosphate transport) 1 proteins. Competition of As(V) with Pi for entry into the cell through both of these transport systems has been demonstrated in numerous plants, monocots and dicots, and both As-hyperaccumulators and non-hyperaccumulators (Ullrich-Eberius et al. 1989, Meharg and Macnair 1990, Tu and Ma 2003). Research validated that phosphate reduces As(V) uptake in plants (Ullrich-Eberius et al. 1989). The presence of minimum 13 and 9 members of phosphate transporter (Pht1) family was reported in rice and Arabidopsis, respectively (Zhao et al. 2010, Tripathi et al. 2012a, b). Due to sharing of same transport system, the toxicity of As(V) is lower under high P conditions (Tripathi et al. 2012a, b). Thus, Pi fertilization can protect plants, including the hyperaccumulator P. vittata, from As(V) toxicity (Tu and Ma 2003). In roots, the major part of As(V) is reduced to As(III), and the rest is further loaded into the xylem vessels by PHT proteins (Zhao et al. 2010). Therefore, some amount of As(V) is also present in shoots along with As(III) and other organic arsenicals (Zhao et al. 2010).
Recent studies have shown that a number of plant aquaporins belonging to the NIP (nodulin26-like intrinsic protein) subfamily are permeable to As(III), which is present predominantly as the undissociated neutral molecule (arsenous acid) at pH < 8 (Ma et al. 2008). NIP aquaporins also mediate transport of a range of small neutral molecules including ammonia, urea, boric acid, and silicic acid (Wallace et al. 2006). In yeast, a variety of proteins like hexose permeases are also involved majorly for As(III) transport (Finnegan and Chen 2012). While in plants, the role of yeast hexose permeases like proteins is still unknown if they provide a path for As(III) entry into plant cells. However, various plasma intrinsic proteins (PIPs) like OsPIP2;4, OsPIP2;6, and OsPIP2;7 are also suggested to transport As(III) in plants (Mosa et al. 2012). In rice, two silicon (Si) transporters, Lsi1 (OsNIP2;1 aquaporin) an influx transporter and Lsi2 (an efflux transporter), play a major role in the uptake of As(III) (Ma et al. 2008). The localization of OsLsi2 to the proximal side of epidermal and endodermal cells (Ma et al. 2007) and OsLsi1 to the distal side of the same cells (Ma et al. 2006) is an elegant example of the heterogeneous distribution of proteins in a membrane. Thus, usage of highly efficient Si uptake pathway for passage of As(III) might be explaining why rice is efficient in accumulation of As than other cereals (Williams et al. 2005). Thus, Si fertilization may be an effective strategy to decrease As accumulation in rice grown in As-contaminated soil (Tripathi et al. 2013b). Lsi2 plays an important role in the root to shoot transport of As(III) and its accumulation in the rice grain. Lsi2 has a low degree of homology (18%) with the As(III) efflux transporter ArsB in Escherichia coli (Ma et al. 2007). Mutants of rice defective in silicon efflux transporter (Lsi2) showed significantly decreased transportation of As(III) to the xylem and accumulation in shoots and grains transport (Zhao et al. 2010). In most plant species analyzed, As(III) dominates in the xylem sap, also even when As(V) is supplied to plant roots due to high capacity of roots for As(V) reduction.
2.2 Transport of Organic Arsenic
The mobility of organic As MMA(V) and DMA(V) within the plant appears to be substantially greater than that of As(III) or As(V) (Raab et al. 2007, Li et al. 2009). Interestingly, Lsi1 also contributes to the uptake of undissociated pentavalent MMA and DMA (Li et al. 2009); the rice lsi1 mutant has lost 80% and 50% of the uptake capacity for MMA and DMA, respectively, compared with the wild-type rice. MMA and DMA have lower dissociation constants than As(III). In contrast to As(III), the rice Lsi2 is not involved in the efflux of MMA or DMA toward the stele, possibly because most MMA and DMA are dissociated at the cytoplasmic pH (Li et al. 2009). Inadequate information is available about phloem loading and unloading of As. Using rice panicles excised below the flag leaf node, Carey et al. (2010) found that DMA was transported to the immature grain approximately 30 times more efficiently than As(III). To ensure the As(III) and DMA loading in grain through phloem, stem girding was applied and further tenfold and 50% decreased was obtained for As(III) and DMA transport, respectively. The outcome implies that As(III) is transported to rice grain primarily through the phloem, while both the phloem and xylem are contributed equally for DMA transport to grain.
3 Arsenic Detoxification Mechanism in Plants
Plants are able to detoxify As by adopting a range of detoxification strategies. The first step involves As reduction, synthesis of metal binding peptides such as metallothioneins (MTs) and phytochelatins (PCs) to make complex with As and their compartmentalization/vacuolar sequestration to keep the free As concentration at its minimum level (Zhao et al. 2010, Tripathi et al. 2012a, b). The second step is the activation of antioxidant defense system to counteract the As-induced oxidative stress (Zhao et al. 2010).
Arsenic is mainly taken by terrestrial plants as As(V) or As(III) (Meharg and Hartley-Whitaker 2002), followed by rapid reduction of As(V) to As(III) through arsenate reductase (AR) enzyme or via reducing agents such as GSH and ascorbic acid (Meharg and Hartley-Whitaker 2002). Both roots and shoots of rice exhibit As(V) reduction activities (Duan et al. 2007), but roots may be quantitatively more important because As(III) is the main form found in the xylem sap of a number of plant species (Zhao et al. 2003). PCs have a high affinity for As(III), but not for As(V). PCs are thought to have a fundamental role in As detoxification in plants (Schmöger et al. 2000). Arsenic is a strong inducer of PC synthesis and combines rapidly with sulfhydryl-rich protective molecules like GSH and PC (Schmöger et al. 2000). They are synthesized posttranslationally from GSH by the transpeptidation of γ-glutamyl-cysteinyl dipeptides through the action of the constitutive enzyme PC synthase (Grill et al. 2006). The PC–As(III) complexes are likely to be stored in vacuoles. The yeast vacuolar transporter Ycf1p, a member of the ATP-binding cassette (ABC) superfamily, confers As(III) resistance by transporting the conjugated As(III)–(GS)3 complex into the vacuole (Ghosh et al. 1999). In Arabidopsis, this transport occurs via the ABC transporters MRP1/ABCC1 and MRP2/ABCC2 (Song et al. 2010). Homologs of Arabidopsis ABCC1 and ABCC2 are found throughout the plant kingdom (Mendoza-Cózatl et al. 2011). A homolog of ABCC2 was among several ABC transporters to be upregulated at the transcript level in response to As in rice (Norton et al. 2008a, b). Thus, in many plants, As(III) present in root cells is rapidly complexed to PC and sequestered in the vacuole, severely restricting the transport of As from the root (Mendoza-Cózatl et al. 2011) and preventing its interaction with cellular metabolism.
Another probable constitutive mechanism of As detoxification is the efflux of As(III) to the external medium. Recently, Indriolo et al. (2010) characterized two genes from P. vittata (ACR3 and ACR3;1), which encode proteins similar to the ACR3 As(III) effluxer of yeast. In another study, examining heterologous expression of the yeast As(III) efflux system, ACR3 was cloned from yeast and transformed into wild-type and nip7;1 Arabidopsis (Ali et al. 2012). At the cellular level, all transgenic lines showed increase tolerance to As(III) and As(V) and a greater capacity for As(V) efflux. Expressing Saccharomyces cerevisiae ScACR3 in rice enhanced As(III) efflux and also reduced As accumulation in rice grains (Duan et al. 2012). Rice transgenics overexpressing As(III)-S-adenosyl methyltransferase (arsM) has been found to methylate As and gave tenfold higher volatile arsenical, maintaining low As levels in rice seed along with MMA(V) and DMA(V) in the roots and shoots of transgenic rice (Meng et al. 2011).
4 Consequences of Arsenic Toxicity on Plant Metabolism
The mode of toxicity differs involving As species; As(V) interferes with phosphate metabolism such as phosphorylation and ATP synthesis, whereas As(III) binds to vicinal sulfhydryl (-SH) groups of proteins affecting their structures or catalytic functions (Hughes 2002). In plant tissue, As(V) is rapidly reduced to As(III) and normally itself not have high enough cytoplasmic concentrations to exert toxicity (Bertolero et al. 1987). As chemical analog of phosphate, As(V) can disrupt several cellular biochemical reactions which used phosphate as a substrate (Gresser, 1981). Arsenate can be translocated through cellular membranes by PHT proteins, creating imbalances in phosphate supply. Possible As(V)-susceptible biological reactions include central to cellular metabolism, i.e., glycolysis, oxidative phosphorylation, biosynthesis of phospholipid, nucleic acids retrieval, and cellular signaling including protein phosphorylation/dephosphorylation.
The mode of action of As(III) differs substantially from that of As(V). As(III) is a thiol-reactive compound that can bind up to sulfhydryl groups such as glutathione (GSH) or phytochelatin (PC), thiol-containing proteins, and cofactors. The binding of As(III) to proteins can have profound effects on their folding (Cline et al. 2003). In various systems, proteins that are known to bind As(III) include transcription factors, signal transduction proteins, proteolytic proteins, metabolic enzymes, redox regulatory enzymes, and structural proteins. Compared to As(III), MMA(III) is a more potent inhibitor of various enzymes (Styblo et al. 1997). MMA(III) and DMA(III) both (low to mid micromolar concentration) were able to displace Zn2+ from a zinc-finger protein (Schwerdtle et al. 2003), an important class of proteins involved in gene expression and DNA repair.
A number of hydroponic studies infer that As phytotoxicity depends on the chemical species supplied to the plant, but the most phytotoxic form of As was still unidentified (Abbas and Meharg 2008). The inconsistent order of phytotoxicity of different As species might be a sign that As reacts in a different way with the offered nutrients or responds to plant species-specific manner.
At higher concentrations, As interferes with plant metabolic processes and can inhibit growth, often leading to death (Jiang and Singh 1994). Arsenic exposure could hamper normal growth of plants with toxicity symptoms such as biomass reduction, loss of yield and fruit production, and morphological changes (Mokgalaka-Matlala et al. 2008, Srivastava et al. 2009). Consequences of arsenic toxicity have been depicted in Fig. 1 which showed the alteration on growth, physiology, and plant metabolism. The severity of straight head disease (sterility of the florets/spikelets) was increased in rice grown in As-spiked soil solution (Zhao et al. 2010).
Interveinal necrotic (Singh et al. 2006) and whitish chlorotic symptoms (Shaibur et al. 2008) have been reported in As-stressed plants. Arsenic damages the chloroplast membrane and disorganizes the functions of integral photosynthetic process (Stoeva and Bineva 2003). Rate of CO2 fixation and functional activity of photosystem II (PSII) were reduced in oat plants growing under As stress (Stoeva and Bineva 2003). As a consequence leaf gas exchange, the chlorophyll fluorescence ratio Fv/Fm, leaf photosynthesis activity, carotenoid content, and the functional activity of PSII decreased (Georgieva and Yordanov 1993, Stoeva and Bineva 2003).
High concentrations of As may interfere with plant metabolism and membrane stability index, impair macro- and micronutrient uptake, or may simply compete with essential plant nutrients (Meharg and Macnair 1990); for instance, As is known to compete with plant P uptake (Mokgalaka-Matlala et al. 2008).
Arsenic exposure generates reactive oxygen species (ROS) in plant tissues and induces oxidative stress such as lipid peroxidation of biomolecules, ultimately resulting in death of the plants (Ahsan et al. 2008). Based on the chemical and physical properties, As causes toxicity in three different ways: (a) enhanced production of reactive oxygen species (ROS) such as superoxide radicals (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•), (b) blocking of essential functional groups in biomolecules, and (c) displacement of essential metal ions from biomolecules. Arsenic stimulates generation of ROS, either by direct electron transfer or as a consequence of metal mediated inhibition of metabolic reactions (Zhao et al. 2010).
Under normal cellular conditions, ROS homeostasis is precisely balanced. At low level of environmental stresses such as temperature and light, minute ROS imbalances occur that can act as signals of cellular status and are easily managed by pre-existing antioxidant defense mechanisms (Van Breusegem and Dat 2006). However, under stronger stresses, such as As exposure, where ROS generation increases, these defense mechanisms may be harassed, leading to cellular damage and death (Van Breusegem and Dat 2006).
ROS are capable of causing considerable cellular damage through oxidation of lipids, proteins, amino acids, and nucleic acids (Møller et al. 2007). Exposure of protein to OH• radical produces dimers, trimers, and tetramers by covalent cross-linking due to formation of intermolecular bityrosine. Exposure to OH•, O2•−, and O2 further leads to fragmentation or aggregation of protein. Arsenic may also affect the protein synthesizing machinery of the cells by their effect on enzymes of nitrogen metabolism (Schmidt et al. 2005). Lipid peroxidation not only compromises cellular function but leads to the production of lipid-derived radicals (Van Breusegem and Dat 2006, Møller et al. 2007). The molecular targets that are most sensitive to the ROS produced by As exposure are not yet clear, although there are many candidates.
These insights provide the facts that As can affect growth and productivity due to excess of morphological, physiological, biochemical, and molecular alterations inside the plants. However, tolerant plant species adapt an array of detoxification strategies, viz., genomic, proteomic, and metabolomic approaches to overcome the As stress. A lot of reviews on As stress in plants reflect the genomic and proteomic modulation in detail. Thus, in the next section we summarize the role of various metabolites modulated during As stress in plants.
5 Metabolome Modulation During Arsenic Stress and Tolerance in Plants
Metabolomics refers to the identification and quantification of all low-molecular-weight metabolites required by the organisms during developmental stages (Arbona et al. 2013), and some metabolites have been reported to be involved under heavy metal stress tolerance strategies. In the following section, we discuss the role of metabolomics under arsenic stress. Despite the fact that transcriptomic approaches provide almost complete coverage and proteomics approaches are now capable of detecting most of the cellular protein complement, metabolomics is currently capable of determining only a small fraction of the metabolites found in any one cell. The more challenging aspect of metabolomic technologies is the refined analysis of quantitative dynamics in biological systems. For metabolomics, gas and liquid chromatographies coupled with mass spectrometry are well suited for analysis of high sample numbers in reliable measurement times with respect to both technical accuracy and the identification and quantification of small-molecular-weight metabolites. However, to the best of our knowledge, a very limited study has been performed to recognize the modulation of differential metabolomic pathway during As stress. Thus, an account about the metabolites involved in antioxidant systems, thiol metabolism, amino acid pool, carbohydrate metabolism, hormonal balance, and others has been particularly given below during As stress (Fig. 2).
5.1 Thiolic Content and Related Enzymes
The central role played by the binding of As(III) to sulfhydryl groups in GSH and PC in the detoxification of the metalloid indicates a critical importance for sulfur metabolism in determining plant survival in As-contaminated soils. The biosynthesis of GSH and PC that is typically induced by As exposure requires adequate supplies of the GSH building blocks glutamic acid (Glu), cysteine (Cys), and glycine (Gly). In both shoots and roots of wild-type Arabidopsis, the mass ratios of free Glu/Gly/Cys were about 20:3:1 (Muñoz-Bertomeu et al. 2009). Thus, at least in Arabidopsis, Cys is by far the limiting substrate for GSH biosynthesis. However, other studies indicate that As(V) exposure can decrease cellular Cys pools (Sung et al. 2009) and that under some growth conditions, it is possible that the synthesis of PC can deplete GSH pools, decreasing the antioxidant capacity of the cell (De Vos et al. 1992). The Cys content increased in some aquatic plants such as H. verticillata, rootless plant C. demersum, and crop plant B. juncea during As stress (Srivastava et al. 2007, 2009, Mishra et al. 2008, Tripathi et al. 2012a, b). Before sulfate acquired from the soil can be used for the biosynthesis of Cys, and thus the biosynthesis of GSH and PC, it must be reduced via sulfite to sulfide (Takahashi et al. 2011). In a sulfur metabolome study, high-resolution Fourier transform mass spectrometric approach in combination with stable isotope labeling was used in Arabidopsis thaliana (Gläser et al. 2014), and about approximately 140 sulfur metabolites that have not been assigned to the databases to date were identified. Further, unraveling of unidentified sulfur metabolite during As stress is of prime importance in plants.
Once sulfate is reduced to sulfide, the sulfide is combined with O-acetylserine to form Cys in a reaction catalyzed by O-acetylserine (thiol)-lyase (OAS-TL), also known as Cys synthase. The O-acetylserine is formed from Ser in a reaction catalyzed by Ser acetyltransferase. OAS-TL exists either as a free active homodimer or in association with Ser acetyltransferase as an inactive subunit of the Cys synthase complex (Takahashi et al. 2011). It appears that As(V) and As(III) exposure may cause a downregulation of OAS-TL in As-sensitive plants (Requejo and Tena 2006, Srivastava et al. 2009). Thus, it seems likely that Cys biosynthesis, and, therefore, As detoxification through GSH and PC would be compromised. Indeed, the Cys and GSH levels in an As-sensitive variety of B. juncea were lower upon As exposure than in the untreated control (Srivastava et al. 2009). In contrast, a B. juncea variety with increased tolerance to As showed a more general induction of the sulfate assimilation and GSH biosynthetic pathways (Srivastava et al. 2009), similar to that seen in yeast (Thorsen et al. 2007). A list of As-modulated antioxidants are given in Table 1. In the As-tolerant variety, there was an induction in Cys synthase activity, as well as in the activities of Ser acetyltransferase and γ-ECS, the penultimate enzyme in the biosynthesis of GSH. These increases in enzyme activity were accompanied by increased levels of both Cys and GSH, indicating that increased sulfur metabolism may be a viable mechanism for increasing As tolerance in plants. Arabidopsis may behave similarly, with transcripts for a γ-ECS, GSH synthetase, and PC synthase being induced during As exposure (Sung et al. 2009).
5.2 Antioxidant Enzymes
Arsenic intraconversion inside the plant may produce oxygen imbalance which causes oxidative damage after production of ROS (Tripathi et al. 2012a, b). To combat oxidative stress, various antioxidant enzymes have been increased their activities in the plants (Mishra et al. 2008, Tripathi et al. 2012a, b). The hyperaccumulator plants can accumulate a significantly higher concentration of As in their bodies, but they have evolved a variety of As tolerance mechanisms against higher levels of free radicals (Khan et al. 2009).
Upon exposure to As, the enzymes involved in the balancing of these free radicals include superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and ascorbate peroxidase (APX) (Souri et al. 2018). The antioxidative enzymes are generally electron donors and react with ROS to form a neutral and nontoxic end product. Under biotic or abiotic stress, the activation or the suppression of antioxidant enzymes to scavenge ROS takes place in conjugation with each other. Among these antioxidant enzymes, SOD is a key player that constitutes the first line of defense by dismutation of superoxide free radicals (O2−) into O2 and H2O2 in plants (Tripathi et al. 2017). Arsenic-induced increase in the activity of SOD can be due to an enhanced level of O2− or the direct action of As on SOD gene overproduction.
Souri et al. (2018) observed a significantly higher increase (116%) in SOD activity in Isatis cappadocica, which was exposed to 800 μM As under hydroponic conditions. As-induced SOD activities have been observed in many other studies as well (Singh et al. 2006, Srivastava et al. 2007, Mishra et al. 2008, Tiwari and Sarangi 2017, Tripathi et al. 2017).
Catalase scavenges H2O2 level in peroxisomes in many stress conditions including As stress in an energy-efficient manner (Srivastava et al. 2017). Arsenic stress may modulate CAT activity depending upon the strength, extent, and kind of stress in various plants (Tripathi et al. 2012a, b, 2017). Accordingly, Souri et al. (2018) also observed that increased activity of CAT was responsible for H2O2 detoxification during lower level of SOD in the shoots of I. cappadocica.
Ascorbate peroxidase is a key enzyme of ascorbate–glutathione cycle and plays an important role for H2O2 detoxification (Singh et al. 2006, Tripathi et al. 2013a, b, 2017). The deviation of APX activity during As stress has been well-documented in ameliorating the harmful effects of H2O2 in various plants (Srivastava et al. 2007, Mishra et al. 2008, Souri et al. 2018, Tripathi et al. 2012a, b, 2017).
Glutathione reductase assists plants in the NADPH-dependent reduction of oxidized glutathione to maintain the ratio of GSH/GSSG for proper functioning of the cells (Srivastava et al. 2009, Begum et al. 2016). The As-induced oxidative damage was partly mitigated by induced activity of GR, in order to sustain the balance of GSH (Souri et al. 2018). Similarly, GR overproduction has been noticed in many other plants under As stress (Mishra et al. 2008, Srivastava et al. 2007, 2009, Begum et al. 2016). It is established fact that As significantly affects the GSH level in plants by converting it into PCs. The higher demand for GSH is managed by higher GSH turnover through enhanced GR activities in As-stressed plants (Mishra et al. 2008).
In general, plants exhibit remarkable difference in their antioxidant potential during As stress (Singh et al. 2006, Srivastava et al. 2007, Mishra et al. 2008, Tiwari and Sarangi 2017, Tripathi et al. 2017). As-hyperaccumulators tolerate As stress by activation of antioxidative defense system in order to maintain lower ROS level, further leading to As detoxification and significant accumulation in tissue (Khan et al. 2009). Arsenic exposure also activates the antioxidant machinery in various plant species, such as H. verticillata, C. demersum, B. juncea, O. Sativa, and Glycine max L. (Srivastava et al. 2007, Mishra et al. 2008, Bustingorri et al. 2017, Hasanuzzaman et al. 2017).
5.3 Nonenzymatic Antioxidants (Ascorbate, Polyamines, and Phenols)
Ascorbate and dehydroascorbate (low-molecular antioxidants) act as nonenzymatic antioxidants in glutathione–ascorbate cycle for free radical scavenging. They are measured in various As-stressed plants, and increased ratio of AsA/DAsA was noticed in P. vittata, P. ensiformis, H. verticillata, and O. sativa (Singh et al. 2006, Srivastava et al. 2011, Tripathi et al. 2012a, b). These insights suggest the prominent role of ascorbate for removal of As stress.
The polyamines are also known to involve in ROS scavenging (Drolet et al. 1986) and the diminution of lipid peroxidation (Borrell et al. 1997) to protect the plant under As stress. Mascher et al. (2002) confirmed that levels of polyamines, viz., spermidine and spermine, are raised merely at lower As exposure; however, diamine is induced at higher doses during As(V) exposure in red clover plants (Trifolium pratense). The phenolic content was also showed their variation in rice during As stress (Chauhan et al. 2017). Out of eight, seven phenolic compounds have been identified from roots and shoots of the rice during As–Se interaction. Out of eight, all polyphenols were found to decrease during As stress in shoots and roots except rutin and ferulic acid in roots, which were increased about 25-fold and 87%, respectively. However, quercetin was detected only during As stress in both roots and shoots, while caffeic acid was not detected in shoots during As stress imparting its tissue-specific modulation (Chauhan et al. 2017). Consequently, differential metabolite profiling in plant species denote that plants may modulate their metabolome up to variable degree for As tolerance and toxicity mechanisms.
5.4 Amino Acids
Amino acids , the building block of protein, are known to chelate metals ion for metal tolerance inside the plants. The role of various essential and nonessential amino acids was explored for As tolerance. Dwivedi et al. (2010) analyzed the amino acid profile in grain of various rice genotypes during As stress. They concluded that most of the essential amino acid (EAA) metabolites such as valine, methionine, leucine, and alanine, and nonessential amino acids (NEAAs), viz., histidine, alanine, proline, glutamic acid, and cysteine, increased in most of the rice genotypes during As(V) exposure. Some other amino acids, viz., proline, glutamic acid, aspartic acid, and alanine, were also increased during As(V) stress in Spinacia oleracea (Pavlik et al. 2010). Among stress-responsive amino acid, proline is the most studied molecule and can function as an osmolyte and free radical scavenger and also can protect cell membrane damage. The level of proline was also elevated in O. sativa during As (III) stress (Mishra and Dubey 2006). The S-containing amino acid, cysteine, plays a central role in As detoxification, used as a primary metabolite for synthesis of GSH and PCs for effective complexation of As. The cysteine content increased in some aquatic plants such as H. verticillata, rootless plant C. demersum, and crop plants B. juncea and O. sativa (Srivastava et al. 2007, 2009, Mishra et al. 2008, Tripathi et al. 2012a, b) during As stress.
5.5 Carbohydrates
The carbohydrate metabolism is negatively affected during As stress in plants, and soluble sugars can be accumulated for As tolerance (Gramss 2012). In rice shoots, the relative amount of nonreducing sugars was decreased than reducing sugars during As stress (Jha and Dubey 2004) which imitates suppression of sucrose formation than accessible hexose monophosphate. The possible cause behind this variation is the reduced activities of starch-degrading enzymes, i.e., α- and β-amylase, and starch phosphorylase leading to As-induced plant toxicity (Jha and Dubey 2004). On the contrary, an increase of about 77–120% in starch phosphorylase activity was reported in O. sativa and Phaseolus aureus seedlings during As exposure, ensuing in a raised level of soluble sugars (Jha and Dubey 2004).
In addition, stimulation of sucrose-hydrolyzing enzymes, viz., acid invertase and sucrose synthase, was studied along with the inhibition of sucrose phosphate synthase activity during As toxicity (Jha and Dubey 2004). Acid invertases are responsible for the sucrose degradation for the formation of glucose and fructose (hexoses), which activity was directly correlated with hexose level (Kaur et al. 2012). The acid invertase and sucrose synthase are the key enzymes for the synthesis of hexoses, which might be shortly oxidized through the glycolytic pathway (Baud and Lepiniec 2009). The metabolic impairments as discussed above will lead to altered plant growth and development under As stress.
5.6 Nitric Oxide
Nitric oxide (NO) is a free radical signaling agent in plants which regulates several cellular functions under normal and stressed situations (Sahay and Gupta 2017). NO is considered as a reactive nitrogen species, and its useful and injurious effects are based upon its level and localization inside plant cells (Sahay and Gupta 2017).
The defensive role of NO against heavy metal stress occurs through unambiguous mechanisms: (i) prevention of Fenton reaction through reaction of NO with OH radicals to form HNO2, thus defending cells against hazardous OH radical; (ii) avoidance of radical-mediated lipid peroxidation through reaction with lipid radicals; (iii) reaction with superoxide radical to form peroxynitrite (ONOO−), which has lesser toxicity and lifespan than highly toxic H2O2; (iv) activation of antioxidants like SOD, CAT, APX, and POX; and (v) working as a signaling molecule for biological reactions leading to variation in gene expression (Saxena and Shekhawat 2013).
As a signaling molecule, NO mediates activation of antioxidants and the biosynthetic pathway of PCs in roots, which facilitate the induced uptake of sulfate by enhanced synthesis of amino acids and nonprotein thiols (Farnese et al. 2013). NO supply improves As tolerance in Luffa acutangula through dawdling As absorption and accumulation by increased cell wall thickness of the root epidermis (Singh et al. 2013). Hasanuzzaman and Fujita (2013) demonstrated that the exogenous NO promoted relative water content, chlorophyll pigment, antioxidant enzymes, and glyoxalase activities in As-treated wheat seedling. In Vicia faba seedlings, NO supplementation resulted in enhanced seed yield, photosynthetic content, plant hormones, and mineral nutrients to combat As toxicity (Mohamed et al. 2016). The internal concentration of NO was also increased during As(V) stress in H. verticillata and O. sativa (Srivastava et al. 2011, Tripathi et al. 2012a, b, 2015). Similarly, NO addition considerably lowered root As uptake and its transport to shoots in rice plants during As(III) treatment (Singh et al. 2017) and improved chlorophyll through increased Fe uptake.
Nitric oxide was effectively known to lessen adverse impact on growth and ROS-imposed injuries during As stress in O. sativa (Singh et al. 2015), Vigna radiate (Ismail 2012), and Phaseolus vulgaris L. (Talukdar 2013). NO pretreatment in plant scavenges ROS entirely for improvement of As tolerance (Farmer and Mueller 2013, Sahay and Gupta 2017).
5.7 Salicylic Acid
Salicylic acid (SA) is categorized in the group of phenolics and acts as endogenous phytohormone, which contributes in growth, photosynthesis, flowering, nitrate metabolism, and ethylene production (Vicente and Plasencia 2011). SA imparts its crucial job for signaling of protective act next to the pathogen attack and also responds to other undesirable surroundings to plants (Pandey et al. 2013).
SA also plays signaling role for abiotic as well as toxic metal stress like As (Wani et al. 2017). In hydroponics, SA application was capable for amelioration of As stress through increased internal concentrations of both SA and NO in rice plants during As exposure suggesting SA-mediated modulation of NO, correlated with increased activity of nitrate reductase (Singh et al. 2017). Furthermore, reduced expression of OsLsi2 gene during SA and NO interaction was accountable for lesser As uptake.
SA supplementation was reported to induce the level of proline, an antioxidant in plants, thus helpful against As and other metals toxicity (Yang et al. 2009). Similarly, exogenous SA imparts its role in pigment synthesis exhibiting better photosynthesis and dynamic growth in plants (Singh et al. 2017). The SA addition was supportive in extenuating chlorosis by enhancing Fe uptake. In another study, 1 mM SA addition showed induction in chlorophyll and protein content along with reduced lipid peroxidation to combat As toxicity in wheat seedlings (Zengin 2015).
SA also may indirectly involve in antioxidant stimulation through NO induction. Application of SA with As was more effective than SA pretreatment in lowering As uptake in rice. Enhanced level of enzymatic and nonenzymatic antioxidants and thiols plays an important role for As tolerance in plants (Singh et al. 2015). Similarly, Saidi et al. (2017) also reported that SA reduced the As-induced oxidative stress through increased synthesis of APX, CAT, and GPX in seedling of Helianthus annuus.
5.8 Growth Hormones and Brassinosteroids
Plant growth hormones are the bioactive molecules which are responsible for plant growth and development. Various studies reported that plant growth hormones have protective role against heavy metals toxicity such as Cu, Cd, Pb, As, etc. Initial research into plant hormones identified five major classes: auxin, cytokinins, gibberellins, ethylene, and abscisic acid (Thomas and Thomas 1979). This list was later expanded and brassinosteroids, jasmonates, and strigolactones are now considered as major plant hormones. An inadequate knowledge is available on the role of growth hormones in As stress amelioration, which needs to be elucidated through detailed analysis in plant.
The role of auxin hormone was identified during As(III) stress in A. thaliana (Krishnamurthy and Rathinasabapathi 2013). Auxin transporter mutants aux1, pin1, and pin2 showed its sensitive behavior to As(III) stress compared to the wild type (WT). Inhibitors of auxin transport considerably reduced As(III) tolerance in the WT, whereas addition of indole-3-acetic acid enhanced As(III) tolerance of aux1 but lesser than WT. Uptake assays of labeled H3-IAA exhibited As(III)-modulated auxin transport in WT roots. As(III) enhanced the H2O2 levels in WT although not in aux1, signifying a positive role for AUX1 for auxin transport through ROS-mediated signaling (Krishnamurthy and Rathinasabapathi 2013).
The role of cytokinins (CKs) was also investigated in A. thaliana for As tolerance (Mohan et al. 2016). CK signaling mutants and transgenic plants with reduced endogenous CK levels demonstrated tolerance to As(V) stress through induced level of thiol compounds and lowered uptake of As indicating regulatory role of CK for As tolerance (Mohan et al. 2016). The protective role of brassinosteroids (Brs) against As toxicity was also investigated in rice plants (Xu et al. 2018). The pooled effect of 24-epibrassinolide (Br24) or 28-homobrassinolide (Br28) on As uptake was studied in rice seedlings. The finding concluded that Br24 and Br28 may possibly hamper As accumulation and may ameliorate the As toxicity (Xu et al. 2018).
Methyl jasmonate (MJ) is also a significant plant growth regulator, concerned with plant protection against abiotic stresses; nevertheless, its potential function against metal stress is not very implicit. The impact of MJ was analyzed on physiological and biochemical parameters of As-treated (200 μM) Brassica napus (Farooq et al. 2016) showing considerable raise in leaf chlorophyll fluorescence and biomass than As-stressed plants. The findings revealed that MJ scavenge ROS through improved antioxidant defense system, secondary metabolite, and decreased As accumulation.
6 Conclusion
It is apparent from the above critical discussion that As exposure adversely affects plants at biochemical and molecular levels and influences a majority of physiological responses, such as inhibition in overall growth processes, photosynthetic efficiency, and biomass accumulation. Arsenic can induce oxidative stress via the enhanced production and/or inefficient elimination of ROS and consequently damage biomolecules and interferes with various metabolic pathways, either directly or indirectly, by intervening in activities of certain key enzymes.
Plants adapt several strategies to overcome As stress, such as complexation of As with S-peptides and compartmentalization and induction of stress-responsive amino acids and various enzymatic and nonenzymatic antioxidants (phenolics and ascorbate). Moreover, the signaling metabolites such as NO and SA are typically endowed with As tolerance involving different mechanisms. The fate and recognition of various unidentified sulfur metabolites must be studied during As stress in plants. Our knowledge on the role of growth hormones during As stress is scanty, which must be enriched through in-depth research. Besides, potential research is required to unravel the toxic effects of As on seed germination and plant development through metabolome modulation.
References
Abbas MHH, Meharg AA (2008) Arsenate, arsenite and dimethylarsonic acid (DMA) uptake and tolerance in maize (Zea mays L.). Plant Soil 304:277–289
Abercrombie JM, Halfhill MD, Ranjan P et al (2008) Transcriptional responses of Arabidopsis thaliana plants to As(V) stress. BMC Plant Biol 8:87–96
Ahsan N, Lee DG, Alam I et al (2008) Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during As stress. Proteomics 8:3561–3576
Ali W, Isner JC, Isayenkov SV et al (2012) Heterologous expression of the yeast arsenite efflux system ACR3 improves Arabidopsis thaliana tolerance to arsenic stress. New Phytol 194:716–723
Arbona V, Manzi M, Ollas CD et al (2013) Metabolites as a tool to investigate a biotic stress tolerance in plants. Int J Mol Sci 14:4885–4911
Baud S, Lepiniec L (2009) Regulation of de novo fatty acid synthesis in maturing oilseeds of Arabidopsis. Plant Physiol Biochem 47:448–455
Begum MC, Islam MS, Islam M et al (2016) Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol Biochem 104:266–277
Bertolero F, Pozzi G, Sabbioni E et al (1987) Cellular uptake and metabolic reduction of pentavalent to trivalent arsenic as determinants of cytotoxicity and morphological transformation. Carcinogenesis 8:803–808
Borrell A, Carbonell L, Farras R (1997) Polyamines inhibit lipid peroxidation in senescing oat leaves. Physiol Plant 99:385–390
Brammer H, Ravenscroft P (2009) Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Int 35:647–654
Bustingorri C, Noriega G, Lavado RS et al (2017) Protective effect exerted by soil phosphorus on soybean subjected to arsenic and fluoride. Redox Rep 22:353–360
Carey AM, Scheckel KG, Lombi E et al (2010) Grain unloading of arsenic species in rice. Plant Physiol 152:309–319
Chauhan R, Awasthi S, Tripathi P et al (2017) Selenite modulates the level of phenolics and nutrient element to alleviate the toxicity of arsenite in rice (Oryza sativa L.). Ecotox Environ Safe 138:47–55
Cline DJ, Thorpe C, Schneider JP (2003) Effects of As(III) binding on alpha helical structure. J Am Chem Soc 125:2923–2929
De Vos CHR, Vonk MJ, Vooijs R et al (1992) Glutathione depletion due to copper induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98:853–858
Drewniak L, Maryan N, Lewandowski W et al (2012) The contribution of microbial mats to the arsenic geochemistry of an ancient gold mine. Environ Pollut 162:190–201
Drolet G, Dumbroff EB, Legge RL et al (1986) Radical scavenging properties of polyamines. Phytochemistry 25:367–371
Duan GL, Zhou Y, Tong YP et al (2007) A CDC25 homologue from rice functions as an arsenate reductase. New Phytol 174:311–321
Duan G, Kamiya T, Ishikawa S et al (2012) Expressing ScACR3 in rice enhanced arsenite efflux and reduced arsenic accumulation in rice grains. Plant Cell Physiol 53:154–163
Dwivedi S, Tripathi RD, Tripathi P et al (2010) Effect of arsenate exposure on amino acids, mineral nutrient status and antioxidant in rice (Oryza sativa L.) genotypes. Environ Sci Technol 44:9542–9549
Farmer EE, Mueller MJ (2013) ROS-mediated lipid peroxidation and RES-activated signaling. Annu Rev Plant Biol 64:429–450
Farnese FS, de Oliveira JA, Gusman GS et al (2013) Plant responses to arsenic: the role of nitric oxide. Water Air Soil Pollut 224:1660
Farooq MA, Gill RA, Islam F (2016) Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci 7:468
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Plant Physiol 3:1–18
Gasic K, Korban SS (2007) Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol Biol 64:361–369
Georgieva K, Yordanov I (1993) Temperature dependence of chlorophyll fluorescence parameters of pea seedlings. J Plant Physiol 142:151–155
Ghosh M, Shen J, Rosen BP (1999) Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 96:5001–5006
Gläser K, Kanawati B, Kubo T et al (2014) Exploring the arabidopsis sulfur metabolome. Plant J 77:31–45
Gramss G (2012) Potential contributions of oxidoreductases from alfalfa plants to soil enzymology and biotechnology: a review. J Nat Sci Sustain Technol 6:169
Gresser MJ (1981) ADP-arsenate formation by sub mitochondrial particles under phosphorylating conditions. J Biol Chem 256:5981–5983
Grill E, Mishra S, Srivastava S et al (2006) Role of phytochelatins in phytoremediation of heavy metals. In: Singh SN, Tripathi RD (eds) Environmental bioremediation technologies. Springer, Heidelberg, pp 101–145
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22:584–596
Hasanuzzaman M, Nahar K, Rahman A et al (2017) Actions of biological trace elements in plant abiotic stress tolerance. In: Naeem M, Ansari A, Gill S (eds) Essential plant nutrients. Springer, Cham, pp 213–274
Hossain MF (2006) Arsenic contamination in Bangladesh – an overview. Agric Ecosyst Environ 113:1–16
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16
Indriolo E, Na G, Ellis D et al (2010) A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell 22:2045–2057
International Agency of Research and Cancer (2004) IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 84: some drinking-water disinfectants and contaminants, including arsenic. IARC, Vienna
Islam FS, Gault AG, Boothman C et al (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Ismail GSM (2012) Protective role of nitric oxide against arsenic-induced damages in germinating mung bean seeds. Acta Physiol Plant 34:1303–1311
Jha A, Dubey R (2004) Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J Plant Physiol 161:867–872
Jiang QQ, Singh BR (1994) Effect of different forms and sources of arsenic on crop yield and arsenic concentration. Water Air Soil Pollut 74:321–343
Kaur S, Singh HP, Batish DR et al (2012) Arsenic (As) inhibits radical emergence and elongation in Phaseolus aureus by altering starch-metabolizing enzymes vis-à-vis disruption of oxidative metabolism. Biol Trace Elem Res 146:360–368
Khan I, Ahmad A, Iqbal M (2009) Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotox Environ Safe 72:626–634
Krishnamurthy A, Rathinasabapathi B (2013) Auxin and its transport play a role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant Cell Environ 36:1838–1849
Li Y, Dhankher O, Carreira L et al (2004) Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium sensitivity. Plant Cell Physiol 45:1787–1797
Li RY, Ago Y, Liu WJ et al (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150:2071–2080
Liu WJ, Zhu YG, Smith FA et al (2004) Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? J Exp Bot 55:1707–1713
Ma JF, Tamai K, Yamaji N et al (2006) A silicon transporter in rice. Nature 440:68–691
Ma JF, Yamaji N, Tamai K et al (2007) Genotypic difference in silicon uptake and expression of silicon transporter genes in rice. Plant Physiol 145:919–992
Ma JF, Yamaji N, Mitani N et al (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A 105:9931–9935
Marin AR, Masscheleyn PH, Patrick WH (1992) The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant and Soil 139:175–183
Mascher R, Lippmann B, Holzinger S et al (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43
Meharg AA, Macnair MR (1990) An altered phosphate uptake system in arsenate tolerant Holcus lanatus. New Phytol 116:29–35
Meharg AA, Williams PN, Adomako E et al (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43:1612–1617
Mendoza-Cózatl DG, Jobe TO, Hauser F et al (2011) Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol 14:554–562
Meng XY, Qin J, Wang LH et al (2011) Arsenic biotransformation and volatilization in transgenic rice. New Phytol 191:49–56
Mishra S, Dubey RS (2006) Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: role of proline as enzyme protectant. J Plant Physiol 163:927–936
Mishra S, Srivastava S, Tripathi RD et al (2008) Thiol metabolism and antioxidant systems complement each other during arsenate detoxification in Ceratophyllum demersum L. Aquat Toxicol 86:205–215
Mohamed HI, Latif HH, Hanafy RS (2016) Influence of nitric oxide application on some biochemical aspects, endogenous hormones, minerals and phenolic compounds of Vicia faba plant grown under arsenic stress. Gesunde Pflanzen 68:99–107
Mohan TC, Castrillo G, Navarro C et al (2016) Cytokinin determines thiol-mediated arsenic tolerance and accumulation in Arabidopsis thaliana. Plant Physiol 171:1418–1426
Mokgalaka-Matlala NS, Flores-Tavizo’n E, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL (2008) Toxicity of arsenic (III) and (V) on plant growth, element uptake, and total amylolytic activity of mesquite (Prosopis juliflora x P. velutina). Int J Phytoremediation 10:47–60
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Mondal P, Majumder CB, Mohanty B (2006) Laboratory based approaches for arsenic remediation from contaminated water: recent developments. J Hazard Mater 137:464–479
Mosa KA, Kumar K, Chhikara S et al (2012) Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res 21:1265–1277
Muñoz-Bertomeu J, Cascales-Miñana B, Mulet JM et al (2009) Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiol 151:541–558
Naujokas MF, Anderson B, Ahsan H et al (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121:295–302
Nordstrom DK (2002) Public health. Worldwide occurrences of arsenic in ground water. Science 296:2143–2145
Norton GJ, Lou-Hing DE, Meharg AA et al (2008a) Rice-arsenate interactions in hydroponics: whole genome transcriptional analysis. J Exp Bot 59:2267–2276
Norton GJ, Meher NM, Williams PN et al (2008b) Rice-arsenate interactions in hydroponics: a three-gene model for tolerance. J Exp Bot 59:2277–2284
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water, and soils by trace metals. Nature 333:134–139
Pandey P, Srivastava RK, Dubey R (2013) Salicylic acid alleviates aluminum toxicity in rice seedlings better than magnesium and calcium by reducing aluminum uptake, suppressing oxidative damage and increasing antioxidative defense. Ecotoxicology 22:656–670
PavlíK M, Pavlíková D, Staszková L et al (2010) The effect of arsenic contamination on amino acids metabolism in Spinacia oleracea L. Ecotox Environ Safe 73:1309–1313
Raab A, Ferreira K, Meharg AA, Feldmann J (2007) Can arsenic-phytochelatin complex formation be used as an indicator for toxicity in Helianthus annuus? J Exp Bot 58:1333–1338
Requejo R, Tena M (2006) Maize response to acute arsenic toxicity as revealed by proteome analysis of plant shoots. Proteomics 6:S156–S162
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Sahay S, Gupta M (2017) An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide 67:39–52
Saidi I, Yousfi N, Borgi MA (2017) Salicylic acid improves the antioxidant ability against arsenic-induced oxidative stress in sunflower (Helianthus annuus) seedling. J Plant Nutr 40:2326–2335
Saxena I, Shekhawat G (2013) Nitric oxide (NO) in alleviation of heavy metal induced phytotoxicity and its role in protein nitration. Nitric Oxide 32:13–20
Schmidt AC, Mattusch J, Reisser W et al (2005) Evaluation of the influence of arsenic species on the nitrogen metabolism of a model angiosperm: nasturtium, Tropaeolum majus. Appl Organometal Chem 19:590–599
Schmoger MEV, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122:793–802
Schoof RA, Yost LJ, Eickhoff J et al (1999) Market basket survey of inorganic arsenic in food. Food Chem Toxicol 37:839–836
Schwerdtle T, Walter I, Hartwig A (2003) Arsenite and its biomethylated metabolites interfere with the formation and repair of stable BPDE-induced DNA adducts in human cells and impair XPAzf and Fpg. DNA Repair 2:1449–1463
Shaibur MR, Kitajima N, Sugewara R et al (2008) Critical toxicity of arsenic and elemental composition of arsenic-induced chlorosis in hydroponic Sorghum. Water Air Soil Pollut 191:279–292
Singh N, Ma LQ, Srivastava M et al (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Singh VP, Srivastava PK, Prasad SM (2013) Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol Biochem 71:155–163
Singh AP, Dixit G, Mishra S et al (2015) Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front Plant Sci 6:340
Singh AP, Dixit G, Kumar A et al (2017) A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol Biochem 115:163–173
Smith AH, Lingas EO, Rahman M (2000) Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 78:1093–1103
Song WY, Park J, Mendoza-Cózatl DG et al (2010) Arsenic tolerance in arabidopsis is mediated by two abcc-type phytochelatin transporters. Proc Nat Acad Sci 107:21187–21192
Souri Z, Karimi N, de Oliveira LM (2018) Antioxidant enzymes responses in shoots of arsenic hyperaccumulator, Isatis cappadocica Desv, under interaction of arsenate and phosphate. Environ Technol 39:1316–1327
Srivastava S, D’Souza SF (2009) Increasing sulfur supply enhances tolerance to arsenic and its accumulation in Hydrilla verticillata (Lf.) Royle. Environ Sci Technol 43:6308–6313
Srivastava S, Mishra S, Tripathi RD et al (2007) Phytochelatins and antioxidants systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f.) Royle. Environ Sci Technol 41:2930–2936
Srivastava S, Srivastava AK, Suprasanna P et al (2009) Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J Exp Bot 60:3419–3431
Srivastava S, Suprasanna P, D’Souza SF (2011) Redox state and energetic equilibrium determine the magnitude of stress in Hydrilla verticillata upon exposure to arsenate. Protoplasma 248:805–815
Srivastava S, Sinha P, Sharma YK (2017) Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J Plant Nutr 40:298–306
Stoeva N, Bineva T (2003) Oxidative changes and photosynthesis in Oat plants grown in As-contaminated soil. Bulg J Plant Physiol 29:87–95
Styblo M, Serves SV, Cullen WR et al (1997) Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem Res Toxicol 10:27–33
Sung DY, Kim TH, Komives EA et al (2009) ARS5 is a component of the 26S proteasome complex, and negatively regulates thiol biosynthesis and arsenic tolerance in Arabidopsis. Plant J 59:802–812
Takahashi H, Kopriva S, Giordano M et al (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62:157–184
Talukdar D (2013) Arsenic-induced changes in growth and antioxidant metabolism of fenugreek. Russ J Plant Physiol 60:652–660
Thomas EW, Thomas LR (1979) Botany: a brief introduction to plant biology. Wiley, New York, pp 155–170
Thomas DJ, Styblo M, Lin S (2001) The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol 176:127–144
Thorsen M, Lagniel G, Kristiansson E et al (2007) Quantitative transcriptome, proteome, and sulfur metabolite profiling of the Saccharomyces cerevisiae response to arsenite. Physiol Genomics 30:35–43
Tiwari S, Sarangi BK (2017) Comparative analysis of antioxidant response by Pteris vittata and Vetiveria zizanioides towards arsenic stress. Ecol Eng 100:211–218
Tripathi P, Mishra A, Dwivedi S et al (2012a) Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotox Environ Safe 79:189–198
Tripathi RD, Tripathi P, Dwivedi S et al (2012b) Arsenomics: omics of arsenic metabolism in plants. Front Plant Physiol 3:1–14
Tripathi P, Tripathi RD, Singh RP et al (2013a) Arsenite tolerance in rice (Oryza sativa L.) involves coordinated role of metabolic pathways of thiols and amino acids. Environ Sci Pollut Res 20:884–896
Tripathi P, Tripathi RD, Singh RP et al (2013b) Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol Eng 52:96–103
Tripathi RD, Tripathi P, Dwivedi S et al (2014) Roles for root iron plaque in sequestration and uptake of heavy metals and metalloids in aquatic and wetland plants. Metallomics 6:1789–1800
Tripathi P, Singh RP, Sharma YK (2015) Arsenite stress variably stimulates pro-oxidant enzymes, anatomical deformities, photosynthetic pigment reduction and antioxidants in arsenic tolerant and sensitive rice seedlings. Environ Toxic Chem 9999:1–10
Tripathi P, Awasthi S, Chauhan R, Singh PK, Srivastava S, Tripathi RD (2017) Biochemical and molecular aspects of arsenic tolerance in plants. In: Chandra R, Dubey NK, Kumar V (eds) Phytoremediation of environmental pollutants. CRC Press, Boca Raton, pp 471–486
Tu C, Ma LQ (2003) Interactive effects of pH, arsenic and phosphorus on uptake of As and P and growth of the arsenic hyperaccumulator Pteris vittata L. under hydroponic conditions. Environ Exp Bot 50:243–251
Ullrich-Eberius CI, Sanz A, Novacky AJ (1989) Evaluation of arsenate- and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba G1. J Exp Bot 40:119–128
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
Wallace IS, Choi WG, Roberts DM (2006) The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim Biophys Acta 1758:1165–1175
Wani S, Jan N, Wani TA et al (2017) Optimization of antioxidant activity and total polyphenols of dried apricot fruit extracts (Prunus armeniaca L.) using response surface methodology. J Saudi Soc Agric Sci 16:119–126
Williams PN, Price AH, Hossain SA et al (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39:5531–5540
Wu C, Ye Z, Li H et al (2012) Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? J Experim Bot 63:2961–2970
Xu B, Yu JY, Xie T et al (2018) Brassinosteroids and iron plaque affect arsenic and cadmium uptake by rice seedlings grown in hydroponic solution. Biol Plant 62:362–368
Yang SL, Lan SS, Gong M (2009) Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol 166:1694–1699
Zengin F (2015) Effects of exogenous salicylic acid on growth characteristics and biochemical content of wheat seeds under arsenic stress. J Environ Biol 36:249
Zhao FJ, Wang JR, Barker JHA et al (2003) The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol 159:403–410
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Acknowledgments
Dr. Preeti Tripathi is thankful to SERB, New Delhi, for the award of DST (SERB) Young Scientist in the Year 2015. Dr. Rudra Deo Tripathi is thankful to the Council of Science and Industrial Research, New Delhi, for the award of Emeritus Scientist Scheme-21(0978/13/EMR-2).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tripathi, P., Tripathi, R.D. (2019). Metabolome Modulation During Arsenic Stress in Plants. In: Srivastava, S., Srivastava, A., Suprasanna, P. (eds) Plant-Metal Interactions. Springer, Cham. https://doi.org/10.1007/978-3-030-20732-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-20732-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20731-1
Online ISBN: 978-3-030-20732-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)