Abstract
Breast cancer and specifically metastatic breast cancer (mBC) constitutes a major health burden worldwide with the highest number of cancer-related mortality among women across the globe. Despite having similar subtypes, breast cancer patients present with a spectrum of aggressiveness and responsiveness to therapy due to cancer heterogeneity. Drug resistance and metastasis contribute to therapy failure and cancer recurrence. Research in the past two decades has focused on microRNAs (miRNAs), small endogenous non-coding RNAs, as active players in tumorigenesis, therapy resistance and metastasis and as novel non-invasive cancer biomarkers. This is due to their unique dysregulated signatures throughout tumor progression and their tumor suppressive/oncogenic roles. Identifying miRNAs signatures capable of predicting therapy response and metastatic onset in breast cancer patients might improve prognosis and offer prolonged median and relapse-free survival rate. Despite the growing reports on miRNAs as novel non-invasive biomarkers in breast cancer and as regulators of breast cancer drug resistance or metastasis, the quest on whether some miRNAs are capable of regulating both simultaneously is inevitable, yet understudied. This chapter will review the role of miRNAs as biomarkers and as active players in inducing/reversing anti-cancer drug resistance, driving/blocking metastasis or regulating both simultaneously in breast cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Breast cancer
- Metastatic breast cancer
- miRNA
- Drug resistance
- Metastasis
- Biomarker
- Prognostic
- Therapy-predictive
- Multi-drug resistance
18.1 Introduction
18.1.1 Overview on Breast Cancer and Its Metastasis
Breast cancer is a global public health burden, constituting the highest cancer incidence in females and the second most common cancer diagnosed worldwide, with around 1.7 million new cases each year. In the U.S., almost one in eight females fall victims of invasive breast cancer throughout their lifetime [1]. Recent statistics by the American Cancer Society reported breast cancer to be amongst the three most commonly diagnosed female cancers, along with lung and bronchus cancer and colorectal cancer, all of which comprise 50% of all female cancer cases and contribute to most cancer deaths in women [1]. Breast cancer is a heterogeneous disease with various subtypes, conventionally classified according to histology (most common types are ductal carcinoma in situ, invasive ductal carcinoma and invasive lobular carcinoma), immunopathology (estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2) status) and molecular signature (luminal A, luminal B, triple-negative/basal-like, HER2-enriched or normal-like) [2,3,4]. Despite having similar subtypes, breast cancer patients present with a spectrum of aggressiveness and responsiveness to therapy [5]. This questioned the efficacy of the mentioned conventional classification methods and the available prognostic and diagnostic tests for breast cancer. Hence, recent studies are focusing on complementing the conventional breast cancer classification tools using patients’ distinct signature of microRNAs (miRNAs), small non-coding single-stranded nucleotides [6]. For instance, Bhattacharyya et al. [7] used fivefold cross-validation techniques in an attempt to sub-classify breast cancer using miRNA signatures compared to pre-existing clinical records. Their results not only validated that miRNA can corroborate the conventional molecular subtype classification, but also proposed the existence of further subtypes through using hierarchical clustering.
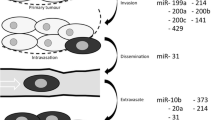
Metastatic breast cancer (mBC) is the most aggressive form of breast cancer, which affects 10–15% of patients within 3 years from diagnosis and is characterized by increased tumor burden and its spread to distal regions [8]. The metastatic cascade begins with tumor dissemination, denoted by local invasion of neighboring tissues, intravasation into the blood or lymph, persistence of the escaped cells in the circulation and subsequent extravasation. It is then followed by colonization, where the escaped cells adjust to the new microenvironment [9]. Epithelial to mesenchymal transition (EMT), an inherent developmental process, necessary for the proper morphogenesis of tissues, is one key step in driving invasion and metastasis. Epithelial cancer cells devise EMT to provoke motility, migration and invasion, switch to a mesenchymal phenotype to lose epithelial polarity and cellular interactions [10]. Developing better diagnostic and therapeutic interventions for mBC is fundamental. miRNAs play key regulatory roles along all stages of the metastatic cascade whereby McGuire et al. [11] summarized the different miRNAs in mBC implicated in invasion (miR-199a, miR-214, miR-200a/b/c, miR-141 and miR-429), dissemination (miR-31), extravasation (miR-10b, miR-373, miR-20a, miR-214 and miR-31) and proliferation (miR-10b, miR-34a, miR-155, miR-200a/b/c, miR-141 and miR-429).
Drug resistance and metastasis continue to pose a challenge in breast cancer and mBC treatment due, in part, to the limitations that entail the available prognostic and diagnostic tests, which range from having low sensitivity, to being highly invasive, to yielding high false positive rates and over-diagnosis [12]. For instance, first, the use of serum carbohydrate antigens such as carcinoembryonic antigen (CEA) and cancer antigen 153 (CA153) as biomarkers is limited by its low sensitivity [13]. Second, there exists few available multi-gene expression DNA microarrays based testing, like Oncotype DX, MammaPrint, Veridex 76-gene and MapQuant Dx. Oncotype DX test, which estimates the recurrence likelihood through assessing 16 cancer-related genes, 5 reference genes and whether patients are eligible for chemotherapy [14], MammaPrint, a prognostic test that analyzes 70 genes and identifies patients with stage 1 or 2, node negative, invasive breast cancer <5 cm in size. Veridex 76-gene signature, a diagnostic test that predicts distant metastasis in ER-positive (ER+) patients within 5 years of diagnosis through a signature of 60 genes for ER+ patients and 16 for ER-negative (ER-) patients [15]. MapQuant Dx, which further classifies grade II tumors into grade I-like (low chance of distant relapses) and grade III-like (clinically similar to grade III) and predicts chemotherapeutic benefit, but can only be used as prognostic tool for ER+ tumors [15]. However, all these tests necessitate patient tissue samples, and thus are highly invasive. Third, mammograms not only exhibit high false positive rates and are incapable of detecting mBC and cause over-diagnosis, but patients below the age of 40 are not recommended to undergo mammography screening because of their dense breast tissue architecture [16, 17]. Additionally, the conventional diagnostic tool for mBC, sentinel lymph node biopsy (SLNB), only detects local but not distal metastasis [11]. Thus, the limitations of the conventional classification tools along with the unavailability of non-invasive, highly sensitive and highly specific mBC diagnostic, prognostic and therapy predictive tests called for the investigation of miRNAs; to better understand their regulatory role in drug resistance and distant metastasis and their biomarker potential.
18.1.2 miRNAs Biogenesis
miRNAs are small (16–29 nucleotides) endogenous, non-coding, single-stranded RNAs that negatively regulate gene expression at the post-transcriptional level [6]. Around half of miRNAs exist in clusters with other miRNAs and are transcribed as polycistronic precursor miRNAs, while others reside within exons and the 3′-untranslated region (UTR) of mRNAs [18]. Various miRNAs promoters transcribe their own miRNAs in intergenic sites, whereas, the majority of miRNAs are transcribed by their host gene promoters when they reside in the introns of protein coding or non-coding host genes. miRNAs biogenesis undertakes a sequence of processes, where RNA polymerase II/III first transcribes miRNAs into primary transcripts (pri-miRNAs) which are several kilobases long. pri-miRNAs in the nucleus are then cleaved by RNase III endonuclease Drosha and DGCR8 protein into intermediate (60–70 nucleotide-long stem loop) precursor miRNAs (pre-miRNAs). The latter are exported to the cytoplasm via exportin-5 (XPO5) complexed with Ran-GTP, and undergo cleavage into mature length by Dicer, another RNase III endonuclease together with the double-stranded RNA-binding protein TRBP [19]. At this stage, the mature miRNA strand unwinds from its complementary strand (passenger strand), and is normally targeted for degradation. The mature strand gets presented onto the RNA-induced silencing complex (RISC), a ribonucleoprotein complex comprised of the mature miRNAs and Argonaute (AGO2) proteins [19, 20]. However, recent studies have shown that the passenger strand can also be loaded onto RISC and therefore can have regulatory function [21]. The RISC complex preferentially binds the seed-matching sequence of the 3′-UTR of target protein-coding mRNA genes, whereby perfect complementarity leads to mRNA degradation by AGO2 via the induction of RNA-mediated interference (RNAi) pathway. Imperfect complementarity leads to translational repression of the target mRNA or mRNA degradation as a result of deadenylation by the CAF1-CCR4-NOT1 de-adenylase complex and subsequent de-capping of the target mRNA [22, 23] (Fig. 18.1). Although miRNAs are known to silence their target mRNA, studies shed light on few miRNAs that promote the expression of their target mRNA, a mechanism termed “RNA activation” (RNAa), majorly attributed to epigenetic regulation of AGO2 [24]. A single miRNA can regulate many genes, which normally exhibit cellular regulatory roles including proliferation, metabolism, differentiation, cell death or aging [25]. Various miRNAs exhibit oncogenic or tumor suppressive roles during cancer pathogenesis, and hence, play part in cancer progression, drug resistance or metastasis. However, deciphering miRNAs that concurrently control drug resistance and metastasis is fundamental, yet understudied [26]. Due to their dysregulation along the different stages of tumorigenesis and their presence and stability in bodily fluids, miRNAs have gained much attention in the past decade. Several studies reported miRNAs diagnostic, prognostic and therapy predictive biomarker potential in breast cancer and others discuss their role as active players in drug resistance and metastasis, all of which will be discussed thereof.
Canonical miRNA biogenesis. Canonical miRNA biogenesis starts by RNA polymerase II/III transcribing miRNAs into primary transcripts (pri-miRNAs) in the nucleus, which are then cleaved by Drosha and DGCR8 protein into intermediate precursor miRNAs (pre-miRNAs). Pre-miRNAs are exported to the cytoplasm via exportin-5 (XPO5) complexed with Ran-GTP, and undergo cleavage into mature length by Dicer complexed with TRBP. The mature miRNA strand is unwound from its complementary strand (passenger strand), which gets degraded, while the mature strand is presented onto the RNA-induced silencing complex (RISC), comprised of the mature miRNAs and AGO2 proteins. The RISC complex preferentially binds the seed-matching sequence of the 3′-UTR of target protein-coding mRNA genes and perfect complementarity leads to mRNA degradation while imperfect complementarity leads to translational repression or deadenylation, which results in degradation of the target mRNA. (Modified from: Winter et al. [19])
18.1.3 Circulating miRNAs Origin and Function
miRNAs were first detected in bodily fluids by Chim et al. [27] who discovered circulating placental miRNA in pregnant women plasma. Soon after, Lawrie et al. [28] identified the first miRNA signature in patients with diffuse large B-cell lymphoma with elevated serum miR-155, miR-210 and miR-21. Circulating miRNAs were then found in blood and plasma, colostrum and breast milk, tears, bronchial lavage and in amniotic, peritoneal, seminal, pleural and cerebrospinal fluids [29]. Previous studies by Lima et al. [30] related the stability of miRNAs in the circulation to their encapsulation in microvesicles or in exosomes. Turchinovich et al. [31] showed that some circulating miRNAs are generated from dead cells, while Merkerova et al. [32] attributed a portion of circulating miRNAs to have originated from blood or immune cells. Recent studies mainly attribute the origin of circulating miRNAs to their passive out-flow from dead cells or active secretion in exosome by tumor and other cell types. Wu et al. [33] characterized exosome-derived miR-19a as a key player in breast cancer metastasis to the bones through facilitating breast cancer and osteoclast cellular communication. Zhong et al. [34] argued that drug resistance can be transmitted from resistant to sensitive breast cancer cells through exosomal miRNA discharge. Similarly, Le et al. [35] showed that transferring cells expressing miR-200 and extracellular vesicles from tumors into murine and human cancer xenografts resulted in acquisition of metastatic potential in weakly metastatic cells, both locally and distally.
Circulating miRNAs harbor a plethora of non-invasive biomarkers and warrant more extensive investigation due to their ease of accessibility in bodily fluids, stability, resistance to RNase digestion and extreme conditions and withstanding long storage [33, 36]. Many studies bid the urge of using miRNAs as novel non-invasive biomarkers for prognosis, diagnosis and therapy prediction in breast cancer and mBC [12, 33] while others discuss the role miRNAs play in controlling metastasis [37,38,39,40,41] inducing and/or reversing breast cancer drug resistance [26, 42, 43], setting new patient selection criteria for clinical trials [44], characterizing new breast cancer subtypes [7] and identifying miRNAs that have both therapy-sensitizing and metastasis blocking roles in breast cancer [26]; most of which will be discussed hereafter. This chapter will highlight the biomarker roles of miRNAs in breast cancer and mBC and will review the regulatory role of miRNAs in causing or reversing drug resistance, metastasis, or both simultaneously.
18.2 miRNAs as Diagnostic, Prognostic and Therapy Predictive Biomarkers in Breast Cancer
McGuire et al. [11] reviewed the essential role circulating miRNAs play as mBC diagnostic or prognostic biomarkers in discriminating non-metastatic from metastatic tumors to guide mBC early diagnosis and monitor disease progression. For instance, circulating miR-10b, miR-34a and miR-155 were elevated in mBC patients [45] and circulating miR-10b and miR-373 [46] as well as miR-20a and miR-214 [47] were upregulated in patients with lymph node positive breast cancer as opposed to patients with no lymph node involvement. Moreover, miR-10b has been reported as a potential mBC biomarker to the brain and bones [48, 49] while miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-210, miR-375 and miR-801 were significantly upregulated in plasma of mBC patients with circulating tumor cells, CTC [50]. Upregulation in miR-105 predicted metastasis in early onset breast cancer [51], while elevation in miR-17 and miR-155 discriminated metastatic from non-metastatic breast cancers [52]. Moreover, metastasis as a result of primary breast tumors correlated with over-expression of miR-34a and miR-155 in the serum, while upregulated miR-34a predicted increased aggressiveness [53].
Nassar et al. [12] shed light in their review on prognostic miRNAs in breast cancer in terms of predicting the overall survival (OS), disease outcome and recurrence in patients. Out of the prognostic biomarkers, miR-106b, found in serum and tissues, predicted risk of high recurrence and shorter OS [54], while miR-122, which was over-expressed in serum of relapsed patients, served as metastasis predictive miRNA. Sahlberg et al. [55] reported that miR-18b, miR-103, miR-107 and miR-652 predicted recurrence and decreased OS in triple negative breast cancer (TNBC) patients. Recent study by Halvorsen et al. [56] was the first to characterize miRNAs profiled from tumor interstitial fluid (TIF) as prognostic and diagnostic biomarkers and as potential bridges between tumor cells and their micro-environment. The authors profiled TIF, normal interstitial fluid, tumor tissues and serum samples from breast cancer patients and a corresponding validation cohort. The results identified upregulation of 266 miRNAs in TIF, of which 61 were present in more than three quarters of the serum samples. Seven miRNAs of the latter predicted poor survival rate and 23 miRNAs were linked to immune cells and adipocyte existence in the serum. Furthermore, Lánczky et al. [57] devised an integrated platform that can search for all documented miRNAs through GEO, EGA, TCGA and PubMed database to arrive at survival analysis capable of predicting the efficiency of miRNAs acting as prognostic biomarkers. Importantly, via this platform, miR-210, miR-328, miR-484 and miR-874 were shown to be capable of predicting prognosis or risk of recurrence [11].
Dysregulation of miRNAs could also predict the therapy outcome and patient’s sensitivity or resistance to a specific treatment, which is the leading cause of recurrence and poor prognosis in breast cancer patients [58]. Chen et al. [59] showed in breast cancer formalin-fixed paraffin-embedded (FFPE) tissues that miR-222, miR-29a, miR-34a, miR-423, miR-140, miR-3178, miR-574, miR-6780b and miR-744 were significantly associated with drug resistance and that miR-222, miR-29a, miR-140, miR-574, miR-6780b, miR-7107 and miR-744 were correlated with poor prognosis. Moreover, some miRNAs were associated with radioresistance, like the over-expression of miR-21, miR-144 and miR-27a and the down-regulation of miR-205, miR-200c and miR-302 [12]. Gasparri et al. [53] reviewed urinary miRNAs in breast tumors, wherein miR-125b predicted resistance to chemotherapy while miR-21, miR-34a, miR-125b, miR-155, miR-195, miR-200b, miR-200c, miR-375 and miR-451 were specific to breast cancer patients and were capable of predicting therapy outcome [60]. Other miRNAs offer potential therapeutic roles in addition to their therapy predictive roles, like the case with miR-200 family, which inhibits angiogenesis through targeting EMT [61]. However, drug resistance remains the leading cause of therapy failure, cancer recurrence and metastasis in breast cancer patients, and thus, understanding its underlying mechanisms along with miRNAs regulatory function holds major promises.
18.3 miRNAs and Drug Resistance in Breast Cancer
18.3.1 Mechanisms of Drug Resistance in Breast Cancer
The conventional treatment regimens for breast cancer, and mBC, include a combination of surgery with chemotherapeutic agents [mostly anthracyclines (doxorubicin and epirubicin), taxanes (paclitaxel and docetaxel), fluorouracil (5-FU) and cyclophosphamide], hormonal therapies [estrogen antagonists: tamoxifen, toremifene and fulvestrant that compete with estrogen to bind and block its receptor or aromatase inhibitors (AIs): letrozole, anastrozole, exemestane, which stop estrogen production], targeted therapy (trastuzumab against HER2+) or a combination thereof. Despite the available treatment regimens, breast cancer drug resistance is amongst the leading causes of therapy futility, cancer recurrence and distant metastasis worldwide [11, 26, 42, 62]. One in two breast cancer patients are expected to present with therapy failure or acquire chemotherapy resistance with aggressive malignancy [63, 64]. Anti-cancer therapy resistance can be classified into intrinsic and acquired, wherein pre-existing resistance mechanisms render the patient unresponsive or resistant to cancer therapy (intrinsic resistance), while acquired selection pressure along the course of treatment might tilt the balance from initially-responsive to resistant variants (acquired resistance) [65]. Various mechanisms contribute to cancer drug resistance including reduction in the intracellular drug concentrations brought by aberrant drug transport and metabolism (less drug reaching the cells or higher drug efflux), deregulation in cell cycle, apoptosis and/or DNA repair machineries, overexpression of oncogenic signaling pathways responsible for tumor transformation, dysregulation in DNA methylation and histone modifications and changes in drug target expression and/or availability [20, 65]. All of which have been implicated in breast cancer and have been shown to be regulated in part by miRNAs. In this regard, miRNAs have been studied as potential biomarkers, to predict treatment response and as master regulators in chemotherapy, hormonal, targeted and radiotherapy resistance.
18.3.2 Role of miRNA in Chemotherapy and in Multidrug Resistance
Breast cancer drug resistance poses a threat through therapy failure, cancer recurrence and distant metastasis. One major hurdle in chemotherapeutic response is cancer cells acquisition of multidrug resistance (MDR), a phenomenon cancer cells develop upon exposure to one chemotherapeutic agent that renders them unresponsive and resistant to various drugs, subjecting breast cancer patients to treatment futility, poor prognosis and cancer-related deaths [66]. MDR is classified into non-classical and transport-based classical MDR phenotypes. Changes in enzymatic activity of glutathione S-transferase and topoisomerase or alteration in apoptotic proteins are responsible for the non-classical phenotype, while reduced uptake of the drug by cancer cells or increased drug efflux out of the cell represent classical MDR.
The major players in classical MDR are comprised of one or more ATP binding cassette (ABC) transporters, which are ABCB1 (MDR-1/P-pg), multidrug resistance-associated protein ABCC1 (MRP-1) and breast cancer-resistant protein ABCG2 (BCRP), all of which possess hydrophobic elements that compete with drug transport across the cellular membrane [66]. For instance, an upregulation of miR-130 and consequent downregulation of PTEN was detected in tumor tissues as compared to normal adjacent tissues as well as in MCF-7 breast cancer cells resistant to adriamycin (MCF-7/ADR) as compared to sensitive MCF-7 and MCF-10A cells, a non-malignant breast epithelial cell line [43]. Increased drug resistance and proliferation and decreased apoptotic levels were observed upon over-expression of miR-130b in MCF-7/ADR cells, while downregulation of miR-130b showed opposite patterns. The authors also noted along with the downregulation of PTEN an induction of MDR through activation of the PI3K/Akt pathway and linked it to the upregulation of miR-130b, which in turn induced proliferation and apoptosis. Another example of MDR was reported in doxorubicin-resistant (MCF-7/DOX) breast cancer cells that exhibited low levels of miR-451 compared to DOX-sensitive cells and resulted in increased MDR1 levels, hence increased DOX resistance [67]. By rescuing the levels of miR-451, DOX sensitivity increased through bypassing MDR. Furthermore, MRP-1-mediated MDR can be regulated by miR-326, particularly, in VP-16 (Etoposide)-resistant MDR cell line (MCF-7/VP), where MRP-1 was the only over-expressed ABC transporter protein [66]. A downregulation of miR-326 and up-regulation of MRP-1 were reported in MCF-7/VP cells as well as in different tissues of advanced breast cancer, while a decrease in MRP-1 expression and an increase in VP-16 and DOX sensitivity were identified upon transfection with miR-326 mimics [67].
In non-classical MDR, Glutathione S-transferase P1 (GSTP1) was studied in tissue samples and exosomes from sera of patients with advanced breast cancer pre-and-post anthracycline/taxane-based neoadjuvant chemotherapy to reduce the tumor burden and block metastasis [68]. GSTP1, a member of the phase II metabolic enzymes, can drive chemoresistance through conjugating various anti-cancer drugs with glutathione, resulting in their detoxification. After therapy, levels of GSTP1 were elevated in advanced patients compared to responsive patients with partial re-localization of cellular GSTP1 to the cytoplasm, both in tissue and exosomal samples. The same pattern was seen in the exosomal marker, tumor susceptibility gene 101 protein (TSG101). This proposed the use of GSTP1-containing exosomes in predicting/transferring chemo-resistance. Therefore, future studies could develop exosomal miRNA biomarkers for MDR prediction, to prevent chemoresistance beforehand, and anti-cancer treatments could govern a merge between the already available therapies and ones that take into consideration preventing/reversing MDR, including exosomal miRNAs [69].
As for the role of miRNAs in chemoresistance, miRNAs are shown either to exhibit a confirmed involvement in chemoresistance, thus increasing the value of IC50 in vitro or drug resistance in vivo or to serve as a biomarker of chemoresistance [65]. For instance in vitro analysis showed that miR-451 was downregulated in MCF-7/DOX-resistant breast cancer cells and was involved in DOX-resistance through targeting P-glycoprotein (MDR1 gene) [67], while the up-regulation of miR-221-222 served as biomarkers for Tamoxifen resistance via targeting p27(Kip1) in MCF-7 and T47D cells [70]. The same pattern was seen with miR-449a/b upregulation in Tamoxifen resistance in frozen breast cancer tissues. In addition, an increase in miR-449a/b levels was shown in tamoxifen-sensitive ZR75 cells while decreased levels of miR-449a/b conferred chemo-resistance in tamoxifen-resistant AK47 cells and other resistant cell lines, which is possibly a consequence of repression of miR-449a/b through DNA methylation [71].
Moreover, aggressive TNBC cells exhibited higher survival and metastatic potential as a result of miR-181a upregulation upon Dox treatment, which is in line with the poor disease free survival and overall survival noticed in TNBC patients that have high levels of miR-181a upon DOX treatment [42]. Hong et al. [72] discussed one of the most studied miRNAs in breast cancer, oncomiR miR-21. miR-21 upregulation infers chemoresistance, possibly through either enhancing proliferation and suppressing tumor suppressor programmed cell death 4 (PDCD4), thus inhibiting apoptosis, or through repressing PTEN, therefore boosting growth and invasion [73, 74]. A combination therapy of miR-21 inhibitors with paclitaxel was shown to be more efficacious than paclitaxel alone [75]. Zhou et al. [76] characterized a crucial role upregulation of miR-125b plays in paclitaxel-resistant breast cancer cells, by directly downregulating pro-apoptotic Bcl-2 antagonist killer 1 (Bak1), which in turn is partially responsible for paclitaxel cellular uptake. Rescuing the sensitivity of breast cancer cells to paclitaxel was attained through re-expressing Bak1, or inhibiting miR-125b.
18.3.3 Role of miRNAs in Hormonal Therapy Response/Resistance
miRNAs also play important regulatory roles in hormonal and targeted therapy resistance that might also be breast cancer sub-type specific. As for ER+ breast tumors, treatment regimens typically rely on decreasing (both endogenous or circulating) estrogen levels or on blocking ER using tamoxifen. Although tamoxifen is widely used, ER- breast cancer patients, which comprise 20–30% of breast cancer cases, cannot benefit from this endocrine therapy, neither do a large number of ER+ patients that display intrinsic resistance to endocrine therapy. Unfortunately, most of the patients who primarily respond to endocrine therapy acquire resistance along the way due to the evasion of cancer cells to endocrine regulatory effect by means of estrogen-independent ER constitutive activation, estrogen/ER-independent growth pathway activation, EMT or miRNAs aberrant expression. While remission is documented in post-menopausal women who receive aromatase inhibitors or other post-tamoxifen therapies, the majority fall victims to relapse and metastasis. This reflects one of the limitations in the conventional staging tools that are incapable of stratifying patients with more stringent differential prognosis and predicting their likelihood to respond to endocrine therapy. Thus, the latter is now accompanied by further cancer subtype classification methods such as the Oncotype DX and MapQuant Dx, which should also be coupled by characterizing the patient’s miRNAs signature for enhanced therapy response prediction [77].
miRNAs regulatory role was studied in three tamoxifen-resistant breast cancer cell lines (TamRs) and their tamoxifen-sensitive counterparts in a pursuit to interpret the molecular machineries behind tamoxifen resistance [78]. Out of the 131 dysregulated miRNAs in TamRs, 22 miRNAs showed comparative expression levels among all TamRs, and were shown to affect common underlying pathways, despite regulating different target genes. Of the regulated gene targets ESR1, PGR1, FOXM1 and 14-3-3 family genes were noted. Integrational and functional analysis revealed two significantly upregulated target genes, SNAI2 (a member of the Snail superfamily which can repress E-cadherin, plays a role in EMT and has an anti-apoptotic activity) and FYN (a proto-oncogene tyrosine-protein kinase and a member of the Src family of kinases) in all TamRs, with the downregulation of their regulatory miRNAs and a growth regulatory effect on TamRs. To corroborate the results, combination of miR-190b and miR-516a-5p expression (out of the 131 dysregulated miRNAs in TamRs) exhibited a therapy predictive role in ER+ breast cancer patient cohort who underwent adjuvant tamoxifen treatment. Moreover, transfection of miR-101 in tamoxifen sensitive MCF-7 cells rendered them resistant to tamoxifen and enhanced their growth, independent of estrogen, via AKT activation and Magi-2 suppression [79].
18.3.4 Role of miRNAs in Targeted- and Immune-Therapy Response/Resistance
Trastuzumab (HER2 monoclonal antibody) resistance is correlated with poor prognosis in HER2+ breast cancer patients [80]. Downregulation of tumor suppressor PTEN, a key regulator of apoptosis and cell invasion, is related to the up-regulation of miR-21. Treatment of breast cancer cells that are resistant to Trastuzumab therapy with antisense oligonucleotides against miR-21 re-sensitized cells through prompting cell death and arresting cell cycle [81]. Moreover, overexpression of miR-125a and miR-125b in SKBR3 cell lines, which overexpress HER2 (ErbB2), efficiently decreased mRNA and protein levels of ErbB2 and ErbB3. It also suppressed anchorage-dependent growth, migration and invasion, subsequently, suppressing MAPK and PI3K/Akt pathways [82]. This is of importance since many studies have been working on the inhibition of PI3K/Akt/mTOR pathway in an effort to target ErbB2 overexpression for the treatment of HER2+ tumors [83]. Studies have also characterized the miRNAs profile specific to HER2 status in breast tumors represented by miR-520d, miR-181c, miR-302c, miR-376b, miR-30e as well as let-7f, let-7g, miR-107, mir-10b, miR-126, miR-154 and miR-195 [84, 85].
Interestingly, not only do miRNAs take part in drug resistance of different breast cancer types, but they can also help cancer cells escape immunosurveillance and acquire therapy resistance in aggressive breast tumors via regulating apoptosis and immune detection. Elevated levels of miR-519a-3p in breast cancer is correlated to poor survival and breast cancer resistance through regulating TRAIL-R2, FasL and granzyme B/perforin and enhancing apoptosis. By directly repressing TRAIL-R2 and caspase-8 and indirectly repressing caspase-7, miR-519a-3p increases breast cancer cell resistance to therapy and hinders their responsiveness to apoptotic stimuli. As for its role in evading immunosurveillance, miR-519a-3p impairs the recognition of tumor cells by natural killer (NK) cells by means of decreasing the expression of NKG2D ligands ULBP2 and MICA present on tumor cell surface, necessary for cancer cell recognition [86].
18.3.5 Role of miRNAs in Radioresistance
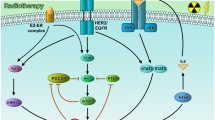
miRNAs also play part in breast cancer radioresistance. For instance, miR-21 over-expression plays a major role in radioresistance in breast cancer cells through inducing DNA damage-G2 checkpoint upon irradiation, subsequently, aiding tumor cell survival [87]. A transient upregulation of miR-21 in radioresistant T47D breast cancer cells was reported upon 5 Gy irradiation compared to a downregulation in radiosensitive MDA-MB-361 cells. Inhibiting miR-21 pre-irradiation resulted in DNA damage-G2 checkpoint decrease and increase in apoptosis both in T47D cells (7–27%) and in MDA-MB-361 cells (18–30%). In a validation cohort of 86 invasive breast cancer patient samples and their normal adjacent tissues, miR-21 was overexpressed in the cancerous tissues and associated with decreased metastases-free survival. This proposed the potential of combining anti-miR-21 with radiotherapy to avoid radioresistance. Moreover, downregulation of miR-302 correlated with radioresistance, because rescuing of its expression in breast cancer cells increased their radiosensitivity. miR-302 acts as a key player in sensitizing radioresistant breast cancer cells to radiotherapy through downregulating key regulators in radioresistance, AKT1 and RAD52, both in vitro and in vivo [88].
Thus, studying the miRNome of breast cancer patients will help discover predictive biomarkers to circumvent unnecessary toxic treatments, and using miRNAs in combination with conventional therapy may reverse subsequent drug resistance. Deciphering the roles miRNAs play in drug (chemo/hormonal/targeted/radio therapy) resistance across different breast cancer types is of great importance.
18.4 miRNAs and Metastasis in Breast Cancer
18.4.1 Circulating miRNAs as Biomarkers for Metastasis in Breast Cancer
Breast cancer morbidity and mortality is generally consequent to distant metastasis rather than the primary tumor per se, constituting 90% of mortality in solid tumors [89, 90]. mBC usually manifests in the lungs, liver, brain or bones. Almost half of mBC patients suffer from distal metastasis to the bones, the most common site, followed by lungs, liver and brain, respectively. Moreover, breast cancer relapse as a result of therapy failure results in metastasis, whereby around 22% of relapsed patients present with various metastatic sites. Different breast cancer molecular subtypes metastasize into distinct sites. For instance, luminal A, B and HER2+ breast cancers metastasize mostly to the bones while basal breast cancers metastasize mainly to the lungs. While luminal tumors rarely metastasis to the brain, HER2+ cancers do [11].
Despite the significant drop in deaths from breast cancer in the last two decades, the majority of female cancer mortality is attributed to breast cancer, specifically mBC. The continuous follow-up on patient’s prognosis, through predicting progression-free survival (PFS) and OS tailored to a patients’ unique profile is key for personalized medicine, which can increase patient’s overall quality of life. CTC are FDA-approved mBC prognostic markers. To date, clinicopathological characteristics including patient’s age at diagnosis, size of the tumor, number and types of metastatic sites, receptor status, distant disease-free survival (DDFS), among others are used for metastasis and patient survival prediction. Circulating miRNAs are promising biomarkers for mBC. For instance, elevated levels of miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-375, miR-210 and miR-801 not only predicted mBC onset, but also correlated with CTC status and predicted PFS, OS and metastasis 2 years prior to onset [39]. Markou et al. [40] studied the expression level of a panel of miRNAs (miR-21, miR-146a, miR-200c and miR-210) in primary breast tumors from formalin-fixed, paraffin-embedded tissues (89 FFPE samples) compared to normal breast tissues (30 samples) and in CTCs as well as in the plasma of mBC patients (55 donors) compared to healthy subjects (20 donors). CTCs, plasma and primary tumor tissues were studied concurrently from more than half [30] of the metastatic patients under study. Results revealed a differential expression in all metastatic miRNAs between the normal and mBC tissues and an upregulation of all metastatic miRNAs in CTC and matching plasma samples (especially miR-21 in CTCs). More so, overexpression of miR-21 and miR-146a and down-regulation in miR-200c and miR-210 were noted in tumor tissues, while miR-21, miR-146a, and miR-210 were exclusively dysregulated in plasma of breast cancer patients, but not healthy subjects. Another study characterized the miRNome of 40 mBC patients, confirmed it in another patient cohort and found a panel of 16 prognostic miRNAs that correlated with overall survival, of which 11 related to progression-free survival [39]. Importantly, 6 miRNAs (miR-200a, miR-200b, miR-200c, miR-210, miR-215 and miR-486-5p) were identified as early detection markers for metastasis, up to 2 years before its clinical manifestation. Thus, identifying miRNAs signature capable of predicting metastatic onset might offer prolonged median and relapse-free survival rates and might enhance prognosis in breast cancer and mBC patients.
18.4.2 miRNAs as Active Players/Regulators of Breast Cancer Along the Metastatic Cascade
miRNAs, through regulating genes involved in breast tumorigenesis, have been reported to play crucial roles in the genetic and epigenetic alterations along the metastatic cascade. miRNAs can play a dichotomous role as metastasis promoters, like the scenario with miR-373, miR-151, miR-520, miR-143 or miR-10b or as metastasis suppressors, as with miR-9, miR-139, miR-335, miR-125 or miR-206 [89]. Ma et al. [91] correlated the increase in migration and invasion in mBC cells to the upregulation of miR-10b, which is transcriptionally controlled by TWIST, basic helix–loop–helix protein. Recent studies showed that restoration of tumor-suppressor miR-340 in metastatic MDA-MB-231 cells drastically suppressed migration, invasion and metastasis through targeting the Wnt signaling pathway [38].
miRNAs contribute to metastasis first by priming cells to adopt an EMT phenotype, thus rendering them more motile and invasive. EMT regulatory miRNAs are miR-7, miR-124, miR-145, miR-200 family, miR-205, miR-375 and miR-448 [92]. EMT is characterized by the loss of cells to their epithelial features like apical-basal polarity and tight cell-cell adhesion and the subsequent acquisition of mesenchymal ones via development of extensions, loosened cell-cell adhesion and actin cytoskeletal reorientation. Key players in EMT are Snail (SNAI1), Slug, ZEB (ZEB1 and ZEB2/SIP1) and TWIST1 and E47, all of which act towards the suppression of E-cadherin. miRNAs not only control the initial step of metastasis, EMT, but they also contribute to intravasation of cancer cells into the circulation and the successive extravasation and survival in the metastatic sites. For instance, in SUM149 breast cancer cells, miR-9 was shown to regulate E-cadherin coding gene, CDH1, thus increased EMT, cell motility and invasiveness [93]. In ER- breast cancer cells, miR-520/373 family repressed invasion and intravasation in vitro and in vivo, respectively. Moreover, in patients with ER- breast tumors, miR-520c suppression was indicative of lymph node metastasis. After cancer cells undergo EMT, intravasation, extravasation and manage to survive and disseminate to the appropriate distal organ, the final step towards metastasis is the cells’ proper colonization at the metastatic site. This is defined as the well-known seed-and-soil hypothesis, implying that cancer cells or “seed” grow in fertile or appropriate tumor microenvironment, the “soil” [9]. For instance, miR-200 through directly regulating the metastasis suppressor Sec23a contributes to breast tumor cells colonization [94].
McGuire et al. [11] summarized different miRNAs implicated in invasion (miR-199a, miR-214, miR-200a/b/c, miR-141, miR-429), dissemination (miR-31), extravasation (miR-10b, miR-373, miR-20a, miR-214, miR-31) and proliferation (miR-10b, miR-34a, miR-155, miR-200a/b/c, miR-141, miR-429). Moreover, antagonistic effects of miRNAs were studied for miR-214 and miR-148b that act as pro-metastatic and anti-metastatic miRNAs in mBC dissemination through dictating the interactions between tumor and endothelial cells. Metastatic dissemination was blocked through dual alteration; downregulating miR-214 and upregulating miR-148b, resulted in downregulation of cell adhesion genes ITGA5 and ALCAM, subsequently blocked tumor escape through blood endothelial vessels in vitro, in vivo and in primary breast cancer patient samples [95].
miR-22/SIRT1 (Sirtuin1) axis was linked to breast cancer growth and metastatic suppression and proposed it as a potential therapeutic target against mBC [96]. Notably, miR-22 directly suppresses SIRT1 in MCF-7 breast cancer cells. The suppression of miR-22 and significant upregulation of SIRT1 was revealed in breast cancer tissues as compared to normal tissues and in stage III-IV breast tumors as compared to stage I-II breast tumors. Thus, miR-22 downregulation was indicative of poor differentiation, metastasis and progressive breast cancer stages. On the contrary, overexpression of miR-22 attenuated proliferation, migration and invasion in MCF-7 cells, while overexpressing SIRT1 reversed the tumor-suppressive and metastasis-suppressive role of up-regulated miR-22 in the cells. Moreover, Li et al. [97] related breast cancer metastatic initiation to the downregulation of miR-452 and the resulting upregulation in RAB11A, both in breast cancer tissues and cell lines. miR-452 acts a tumor suppressor through downregulating RAB11A and is responsible for suppressing migration and invasion in breast cancer. In addition, by upregulating the pro-metastatic gene RhoA, miR-155 promoted EMT, cell migration and invasion [98], while miR-31 blocked metastasis by inhibiting RhoA and disabling cancer cells from exiting the primary tumor site, disseminating and/or surviving in distal sites [99, 100]. miR-31 targets also include Frizzled3 (Fzd3), integrin α-5 (ITGA5), myosin phosphatase-Rho interacting protein (M-RIP), matrix metallopeptidase 16 (MMP16), radixin (RDX), as well as PKCε, which deregulates NF-κB signaling pathway, increase apoptosis and enhances MCF-10A and MDA-MB-231 cells radiosensitivity [101, 102]. In MCF-7 cells, upregulation of miR-17-5P increased invasiveness and migration via targeting HBP1/β-catenin pathway [103]. In addition, miR-145, through regulating c-Myc and mucin and downregulating c-Myc downstream targets like cyclin D1 and elF4E plays a role in cancer cell motility and cell cycle progression [79].
Moreover, miRNAs not only control metastasis, but they also regulate angiogenesis. Lu et al. [104] investigated the role of the tumor suppressive miR-140-5p in breast cancer in regulating invasion and angiogenesis. Their results showed that miR-140-5p regulates the vascular endothelial growth factor VEGF-A in vitro and in vivo. Similarly, miR-378 and miR-27a have been shown to enhance angiogenesis and tumor cell survival in breast cancer [105, 106]. Moreover, downregulation of miR-140-5p was observed in breast cancer and mBC tissues as compared to their normal counterparts, and thus, might serve as a novel anti-metastatic and anti-angiogenic agent in breast cancer.
18.4.3 Examples of miRNAs Implicated in Common Sites of Breast Metastases
miRNAs can play a role in breast cancer metastasis to distal regions such as brain, bone and lung. Li et al. [37] discussed the importance of deciphering the role miRNAs play in diagnosing and, possibly, treating breast cancers with brain metastasis. This is since (10–30%) of patients with advanced breast cancer suffer from brain metastasis with poor prognosis. The universal gene expression signatures of patients with primary in situ breast carcinoma and patients with brain metastasis were investigated in a pursuit to identify the differential expression patterns in miRNAs, their corresponding mRNA targets and the underlying signaling pathways that might serve as early detection markers for brain metastasis. Results showed a strong correlation between miR-17-5p and miR-16-5p and BCL2, SMAD3 and SOCS1 and subsequent oncogenic pathways like ones concerned with EMT, cell cycle control, adherence junctions and extracellular matrix-receptor communication. A comparison of patient samples to matched breast cancer patients from The Cancer Genome Atlas (TCGA) revealed similar expression levels in 11 miRNAs, wherein miR-17-5p was upregulated in TNBC tissues extracted from the database, with opposing patterns between miR-17-5p levels and overall survival and PTEN and BCL2 levels. Thus, devising a systems-gene expression patterns can better guide clinicians into predicting optimal treatment options specific for patients with breast cancer brain metastasis.
Soria-Valles et al. [107] linked downregulation of miR-21 to matrix metalloproteinase, collagenase-2 (MMP-8), which exhibited a tumor suppressive role and lung metastasis blockage in MDA-MB-231 breast cancer cells. The authors validated the results in vitro and in vivo and related the protective role of MMP-8 to decorin cleavage and inhibition in TGF-β signaling, which in turn downregulates miR-21. This eventually induces tumor suppressors such as programmed cell death 4. An example of miRNAs effect on bone metastasis was discussed in a study on the stimulatory role of TWIST1 on breast cancer intravasation and dissemination to the bones using human osteotropic MDA-MB-231/B02 breast cancer cells and immunodeficient mice [108]. TWIST1 stable transfection in vitro showed enhancement in tumor cell invasion, but not tumor growth, and resulted in upregulation in the pro-invasive miR-10b level. In vivo, TWIST1 transfection caused higher osteolytic lesions, reduced bone volume and caused doubling of the tumor burden. Upon treatment with DOX, TWIST1 was suppressed, and hence, bone metastasis was blocked in vivo. Blocking miR-10b in the mice caused drastic reduction in TWIST1-expressing breast cancer cells found in the bone marrow. Therefore, miR-10b takes part in regulating TWIST1-induced breast cancer bone metastasis.
Bishopric et al. [109] inoculated MDA-MB-231 and MDA-MB-436 breast cancer xenografts into immunedeficient mice mammary fat pads to produce primary tumors and corresponding lymph node, liver, lung and diaphragm metastases. By comparing the miRNAs profiles of the primary and the metastatic tumors, the authors found miR-203 levels, which acts as a tumor suppressor, to be significantly associated with the size of the primary tumor at all metastatic sites. miR-203 acted by directly targeting TWF1 and APBB2. Although miR-203 was shown to be necessary for metastasis growth, its over-expression inhibited metastasis, thus implicating opposing function and a dynamic, context-dependent function of miR-203 along the metastatic cascade.
18.5 miRNAs Role in the Interplay Between Drug Resistance and Metastasis in Breast Cancer
Despite the booming reports on miRNAs that act on drug resistance or metastasis, the quest on whether some miRNAs are capable of regulating both simultaneously is fundamental, yet understudied. The rationale behind this is that the likelihood of recurrence and subsequent distant metastasis in tumor cells increases due to drug resistance. For instance, miR-644a acts pleiotropically through increasing cell death and inhibiting EMT, thus sensitizing various breast cancer subtypes to both hormonal-and-targeted therapeutic agents (like tamoxifen and gefitinib) and blocking metastasis [26]. EMT inhibition was thus proposed as the common underlying mechanism towards drug sensitization and metastasis blockade. Moreover, miR-644a was shown to directly downregulate transcriptional co-repressor C-Terminal Binding Protein 1 (CTBP1), which in turn upregulates wild type-or mutant-p53. The downregulation of CTBP1 retarded growth, metastasis and drug resistance and was validated in miR-644a CRISPR-Cas9 knockouts. Of note, only patients with mutant-p53 and upregulation in CTBP1 exhibited shorter survival, priming CTBP1 to serve as a prognostic marker for p53-mutant patients. This suggested a therapy-sensitizing and metastasis blocking potential through reactivation of miR-644a/CTBP1/p53 axis in breast cancer along with its potential as progression and therapy predictive biomarker. Another study suggested NSC95397, a small molecule capable of obstructing transcriptional repression brought by CTBP1, as an easier drug target than miR-644a [110].
Other examples of the role miRNAs play in regulating EMT in breast cancer, leading to endocrine (hormone) therapy resistance is how breast cancer cells acquired a mesenchymal phenotype due to the upregulation of miR-9 that resulted in E-cadherin repression and vimentin overexpression [77, 93]. Moreover, miRNAs can initiate drug resistance and metastasis concomitant with cancer stem cell (CSC) characteristics. Although CSCs only constitute part of the tumor burden, they are known to initiate growth, metastasis and drug resistance in tumors [89]. While some miRNAs have been reported to control the interplay between cancer stemness and drug resistance, others reported how miRNAs control stem cell and metastatic characteristics of cancer cells via EMT regulation. For instance, compared to non-CSCs extracted from advanced mBC cells, CD24−/CD44+/ESA+ CSC population was capable of driving metastasis. When these CSC metastasize to the bones and brain, downregulation in miR-7 was noted; however, blockade of brain metastasis was possible through re-expressing miR-7 in breast CSCs, which in turn repressed stemness regulatory gene, KLF4 [111]. In addition, miRNAs can play a role in breast cancer drug resistance and metastasis through epigenetics [112]. Thus, studies are investigating the effect of differentially methylated regions (DMRs) of miRNAs loci in invasive breast cancers. For instance, analysis of DMRs and methylation patterns in miR-31, miR-135b and miR-138-1 were correlated with patterns seen in early and late postpartum breast cancer patients [113]. Moreover, a correlation was shown between aggressiveness and advanced breast cancer disease and the methylation of tumor suppressive and DNMT3b targets which are miR-124a-1, miR-124a-2 and miR-124a-3 [114]. However, while aberrant DNA methylation, in part, controls the expression of miRNAs and subsequent downstream pathways, miRNAs can also control some DNA methylators.
In addition, miRNAs can play a role in chemoresistance in aggressive TNBC, which does not respond to any targeted therapies. Niu et al. [115] showed that TNBC cells exhibited higher survival and metastatic potential as a result of miR-181a upregulation upon genotoxic DOX treatment. These results were also noticed in TNBC patients with high levels of miR-181a post-DOX treatment who had poor disease free survival and overall survival. Moreover, chemoresistance was attributed to apoptosis evasion and enhanced invasion in DOX-treated TNBC cells to the direct suppression of BAX by miR-181a. Thus, blocking miR-181a could potentially rescue DOX sensitivity in TNBC cells and alleviate metastasis. A similar pattern was observed in HER2+ breast cancer patients, with noted upregulation in miR-181a, whereby blocking miR-181a re-sensitized breast cancer cells to Trastuzumab and inhibited metastasis. Thus, inhibiting miR-181a could reverse both chemo-and-targeted therapy resistance and block metastasis in TNBC and HER2+ breast tumors, respectively. Moreover, Bai et al. [116] showed increase in EMT and TGF-β signaling with a downregulation of miR-200c in highly invasive, tumorigenic, Trastuzumab-resistant HER2+ breast cancer cells. Re-expression of miR-200c targeted both a TGF-β transcriptional activator ZNF217 and a key player in the TGF-β signaling pathway, ZEB1, thus rescuing Trastuzumab sensitivity and blocking invasion concomitantly. Alternatively, silencing of ZEB1 or ZNF217 or inhibiting TGF-β signaling exhibited same response as restoration of miR-200c in resistant cells, suggesting a miR-200c/ZEB1 and miR-200c/ZNF217/TGF-β/ZEB1regulatory circuits in Trastuzumab resistance and distal metastasis.
Therefore, it is vital to focus on miRNAs that act both as therapy sensitizers and metastasis blockers, for an optimal understanding of their regulatory role, and for widening their potential use as biomarkers and therapeutic tools against breast cancer. One successful promising example is oncomiR, miR-21, which is almost upregulated in most breast cancers and has been reported to drive both drug resistance and metastasis. Mei et al. [75] potentiated the simultaneous delivery of miR-21 inhibitor and paclitaxel through G5-PAMAM dendrimer, in order to impede both tumor growth and invasiveness in breast cancer. Thus, complimenting conventional therapies with miRNAs inhibitors/mimics holds hope in combating drug-resistance and circumventing metastasis in breast cancer [20].
18.6 Conclusions and Future Directions
We have highlighted thus far the novelty of utilizing miRNAs to serve not only as biomarkers for breast cancer progression, invasiveness, drug resistance and metastasis, but also as potential key players in re-sensitizing breast cancer cells to chemo/targeted/hormonal therapies and/or potentially blocking metastasis. All the mentioned miRNAs from the literature, pooled according to their regulatory role, their biomarker capability, dysregulation pattern, target protein/pathway, sample source, breast cancer type and mode of action are presented in Tables 18.1, 18.2, 18.3, and 18.4. Moreover, QIAGEN’s Ingenuity® Pathway Analysis (IPA®, QIAGEN Redwood City, www.qiagen.com/ingenuity), IPA, has a comprehensive, manually curated content of the Ingenuity Knowledge Base as well as has powerful algorithms that identify regulators, relationships, mechanisms, functions, and pathways relevant to changes observed in an analyzed dataset. A powerful feature of IPA is the MicroRNA Target Filter that finds validated and predicted miRNA-mRNA target pairings based on Ingenuity Expert Finding, Ingenuity ExpertAssist Findings, TargetScan, TarBase and miRecords and allows filtration according to diseases, cell/tissue type, location, molecule type, species, or biological pathways. Thus, through MicroRNA Target Filter, we linked the miRNAs discussed here to validated mRNAs that are part of IPA networks or canonical pathways of interest (i.e., drug resistance, breast cancer, metastasis, EMT). The validated mRNA targets were filtered according to validated databases: Human, Tarbase, miRecords, Ingenuity Expert Findings and Ingenuity ExpertAssist Findings as well as according to mammary cell/tissue type. The predicted and validated targets are tabulated (if any) in Tables 18.2, 18.3, and 18.4.
However, a few caveats are common in most of the mentioned studies, and must be addressed. Markou et al. [40] pointed out the pitfalls underlying the lack of reproducibility across studies performed by different groups on similar patients and cancer profiles. For instance, when comparing the databases of 15 studies characterizing circulating miRNAs profiles of breast cancer patients, very little overlap was detected. The authors attributed the lack of reproducibility to variations in sample origins (plasma, serum, whole blood), variability in cohort population and inconsistencies in sample collection protocols/timings and sample processing. As for the discrepancies in miRNAs profiles reported from the same tumor, it might be partly attributed to the lack of an established endogenous miRNA for normalization [117]. Pichler and Calin [118] addressed few solutions, like the importance of designing larger prospective clinical trials that encompass the published work on candidate diagnostic or prognostic miRNAs and comparing them to the gold standard techniques in a blinded fashion. Most of the current findings were done retrospectively, were prone to error-and-selection bias and lacked long-term follow-up. Cortez et al. [44] stressed on the importance of characterizing specific panels of differentially expressed miRNAs rather than single miRNAs as exclusive biomarker panels to a certain type of cancer, stage (early vs advanced), therapy response, patient outcome, recurrence or metastatic output, which will also account for intra-tumoral and intercellular heterogeneity.
One major area that requires development in miRNA-based therapies is the establishment of stable and effective delivery systems with minimal off-target and adverse effects. As exosomes house miRNAs, they have proven to be efficient in miRNAs delivery to breast cancer cells expressing EGFR. Ohno et al. [119] described how engineering protocols were capable of expressing transmembrane domain of platelet-derived growth factor receptor (PDGF) merged to GE11 peptide, a less mitogenic EGFR binding partner, on donor cells. These exosomes where then able to successfully deliver let-7a miRNA, intravenously, in RAG2−/−mice breast cancer xenografts that exhibited EGFR. Some successful nucleic acid therapies made it to human clinical trials, the first of which was miraversen (www.clinicaltrials.gov, study no. NCT01200420), which was designed to capture miR-122 to inhibit the replication of hepatitis C virus, after it was proven effective in chimpanzees [65]. More than dozens of clinical trials are underway, testing the prognostic, metastatic and therapy predictive potential possessed by some candidate miRNAs, or alternatively, players in the biogenesis pathway of miRNAs (www.clinicaltrials.gov). Adams et al. [8] predicted the potential of using miR-34a for treatment of TNBC based on clinical studies investigating MRX34, an amphoteric liposome coupled with a synthetic miR-34a mimic, for its efficacy against hepatocellular carcinoma in phase I clinical trial. The rationale behind their prediction is that miR-34a was capable of sensitizing TNBC to dasatinib treatment by targeting c-SRC, and thus, administering miR-34a and dasatinib might be worth investigating in aggressive TNBC.
Finally, focus for BC understanding should be on the original molecular triggers for cellular transformation prior to cancer progression, drug resistance and metastasis. In other terms, it is essential to focus research on the basic molecular mechanisms that trigger cancer. Besides understanding the regulatory role of miRNAs in breast cancer, recent studies are focusing on circular RNAs (circRNAs) and their roles in “sponging” microRNAs. circRNAs are a large class of endogenous RNAs that originate from cellular splicing and play regulatory roles in mammalian cells. Sequencing analysis has also shown circRNAs to be dysregulated in cancers (cell lines, patient tissues, plasma and serum) and are characterized by their stability, conserved sequences and presence in the circulation. Thus, sequencing circRNAs along with their downstream miRNAs targets will add one more layer to better understand the drivers of cancer initiation, progression, drug resistance and metastasis and will bring us a step closer towards devising better breast cancer biomarkers [120]. Moreover, the commonly used integrative analysis approach for predicting miRNAs gene and protein targets and networks, known as the systems biology approach, is continuously being updated and developed to accommodate for better prediction of efficacy and activity of candidate miRNAs on a universal scale [89]. Improving the already available breast cancer miRNAs databases to elucidate details on sample sources, miRNAs expression profiles, extraction protocols, their diagnostic, prognostic, therapy predictive, therapeutic and metastatic potential, would lay grounds for better, more reproducible and more tumor-specific miRNAs studies [12]. This, of course, calls for a more integrative understanding of the miRNA–gene and miRNA-protein interaction networks through the development of multi-disciplinary systems biology approaches assimilating genomics, genetics, proteomics and bioinformatics, to better understand and combat cancer initiation, development, progression and recurrence.
Abbreviations
- BC:
-
Breast cancer
- CTCs:
-
Circulating tumor cells
- DCIS:
-
Ductal carcinoma in situ
- DFS:
-
Disease-free survival
- ER:
-
Estrogen receptor
- mBC:
-
Metastatic breast cancer
- MDR:
-
Multidrug resistance
- microRNAs:
-
miRNAs or miRs
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- TNBC:
-
Triple negative breast cancer
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci 98(19):10869–10874
Liu Z, Zhang X-S, Zhang S (2014) Breast tumor subgroups reveal diverse clinical prognostic power. Sci Rep 4:4002
Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L et al (2015) Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 24:S26–S35
Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A (2008) Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol 20(4):628–635
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Bhattacharyya M, Nath J, Bandyopadhyay S (2015) MicroRNA signatures highlight new breast cancer subtypes. Gene 556(2):192–198
Adams BD, Wali VB, Cheng CJ, Inukai S, Booth CJ, Agarwal S et al (2016) miR-34a silences c-SRC to attenuate tumor growth in triple-negative breast cancer. Cancer Res 76(4):927–939
Fidler IJ (2003) The pathogenesis of cancer metastasis: the seed and soil hypothesis revisited. Nat Rev Cancer 3(6):453–458
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9(4):265–273
McGuire A, Brown JA, Kerin MJ (2015) Metastatic breast cancer: the potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev 34(1):145–155
Nassar FJ, Nasr R, Talhouk R (2017) MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol Ther 172:34–49
Shao Y, Sun X, He Y, Liu C, Liu H (2015) Elevated levels of serum tumor markers CEA and CA15-3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS One 10(7):e0133830
Dobbe E, Gurney K, Kiekow S, Lafferty JS, Kolesar JM (2008) Gene-expression assays: new tools to individualize treatment of early-stage breast cancer. Am J Health-Syst Pharm 65(1):23–28
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J et al (2015) Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res 5(10):2929
BoydNF G (2007) Mammographic density and the risk and detection of breast cancer. N Engl J Med 356:227–236
Checka CM, Chun JE, Schnabel FR, Lee J, Toth H (2012) The relationship of mammographic density and age: implications for breast cancer screening. Am J Roentgenol 198(3):W292–W2W5
Singh SK, Pal Bhadra M, Girschick HJ, Bhadra U (2008) MicroRNAs–micro in size but macro in function. FEBS J 275(20):4929–4944
Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11(3):228–234
Kutanzi KR, Yurchenko OV, Beland FA, Vasyl’ FC, Pogribny IP (2011) MicroRNA-mediated drug resistance in breast cancer. Clin Epigenetics 2(2):171
Pink RC, Samuel P, Massa D, Caley DP, Brooks SA, Carter DRF (2015) The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecol Oncol 137(1):143–151
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5(7):522–531
Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E (2009) Deadenylation is a widespread effect of miRNA regulation. RNA 15(1):21–32
Vasudevan S, Tong Y, Steitz JA (2007) Switching from repression to activation: microRNAs can up-regulate translation. Science 318(5858):1931–1934
Li M, Li J, Ding X, He M, Cheng S-Y (2010) microRNA and cancer. AAPS J 12(3):309–317
Raza U, Saatci Ö, Uhlmann S, Ansari SA, Eyüpoğlu E, Yurdusev E et al (2016) The miR-644a/CTBP1/p53 axis suppresses drug resistance by simultaneous inhibition of cell survival and epithelial-mesenchymal transition in breast cancer. Oncotarget 7(31):49859
Chim SS, Shing TK, Hung EC, Leung T-Y, Lau T-K, Chiu RW et al (2008) Detection and characterization of placental microRNAs in maternal plasma. Clin Chem 54(3):482–490
Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K et al (2008) Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 141(5):672–675
Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ et al (2010) The microRNA spectrum in 12 body fluids. Clin Chem 56(11):1733–1741
Lima LG, Chammas R, Monteiro RQ, Moreira MEC, Barcinski MA (2009) Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett 283(2):168–175
Turchinovich A, Weiz L, Langheinz A, Burwinkel B (2011) Characterization of extracellular circulating microRNA. Nucleic Acids Res 39(16):7223–7233
Merkerova M, Vasikova A, Belickova M, Bruchova H (2010) MicroRNA expression profiles in umbilical cord blood cell lineages. Stem Cells Dev 19(1):17–26
Wu K, Feng J, Xing F, Liu Y, Sharma S, Watabe K (2017) Exosomal miR-19a: a novel communicator between cancer cell and osteoclast in osteolytic bone metastasis of breast cancer. AACR 77:4940–4940
Zhong S, Chen X, Wang D, Zhang X, Shen H, Yang S et al (2016) MicroRNA expression profiles of drug-resistance breast cancer cells and their exosomes. Oncotarget 7(15):19601–19609
Le MT, Hamar P, Guo C, Basar E, Perdigão-Henriques R, Balaj L et al (2014) miR-200–containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest 124(12):5109
Ma R, Jiang T, Kang X (2012) Circulating microRNAs in cancer: origin, function and application. J Exp Clin Cancer Res 31(1):38
Li Z, Peng Z, Gu S, Zheng J, Feng D, Qin Q et al (2017) Global analysis of miRNA–mRNA interaction network in breast cancer with brain metastasis. Anticancer Res 37(8):4455–4468
Mohammadi-Yeganeh S, Paryan M, Arefian E, Vasei M, Ghanbarian H, Mahdian R et al (2016) MicroRNA-340 inhibits the migration, invasion, and metastasis of breast cancer cells by targeting Wnt pathway. Tumor Biol 37(7):8993–9000
Madhavan D, Peng C, Wallwiener M, Zucknick M, Nees J, Schott S et al (2016) Circulating miRNAs with prognostic value in metastatic breast cancer and for early detection of metastasis. Carcinogenesis 37(5):461–470
Markou A, Zavridou M, Sourvinou I, Yousef G, Kounelis S, Malamos N et al (2016) Direct comparison of metastasis-related miRNAs expression levels in circulating tumor cells, corresponding plasma, and primary tumors of breast cancer patients. Clin Chem 62(7):1002–1011
Peng F, Tang H, Liu P, Shen J, Guan X, Xie X et al (2017) Isoliquiritigenin modulates miR-374a/PTEN/Akt axis to suppress breast cancer tumorigenesis and metastasis. Sci Rep 7:9022
Teoh S, Das S (2017) The role of MicroRNAs in diagnosis, prognosis, metastasis and resistant cases in breast cancer. Curr Pharm Des 23(12):1845
Miao Y, Zheng W, Li N, Su Z, Zhao L, Zhou H et al (2017) MicroRNA-130b targets PTEN to mediate drug resistance and proliferation of breast cancer cells via the PI3K/Akt signaling pathway. Sci Rep 7:41942
Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA (2011) MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol 8(8):467–477
Roth C, Rack B, Müller V, Janni W, Pantel K, Schwarzenbach H (2010) Circulating microRNAs as blood-based markers for patients with primary and metastatic breast cancer. Breast Cancer Res 12(6):R90
Chen W, Cai F, Zhang B, Barekati Z, Zhong XY (2013) The level of circulating miRNA-10b and miRNA-373 in detecting lymph node metastasis of breast cancer: potential biomarkers. Tumor Biol 34(1):455–462
Schwarzenbach H, Milde-Langosch K, Steinbach B, Müller V, Pantel K (2012) Diagnostic potential of PTEN-targeting miR-214 in the blood of breast cancer patients. Breast Cancer Res Treat 134(3):933–941
Ahmad A, Sethi S, Chen W, Ali-Fehmi R, Mittal S, Sarkar FH (2014) Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Am J Transl Res 6(4):384
Zhao F, Hu G, Wang X, Zhang X, Zhang Y, Yu Z (2012) Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J Int Med Res 40(3):859–866
Madhavan D, Zucknick M, Wallwiener M, Cuk K, Modugno C, Scharpff M et al (2012) Circulating miRNAs as surrogate markers for circulating tumor cells and prognostic markers in metastatic breast cancer. Clin Cancer Res 18(21):5972–5982
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR et al (2014) Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25(4):501–515
Eichelser C, Flesch-Janys D, Chang-Claude J, Pantel K, Schwarzenbach H (2013) Deregulated serum concentrations of circulating cell–free microRNAs miR-17, miR-34a, miR-155, and miR-373 in human breast cancer development and progression. Clin Chem 59(10):1489–1496
Gasparri ML, Casorelli A, Bardhi E, Besharat AR, Savone D, Ruscito I et al (2017) Beyond circulating microRNA biomarkers: urinary microRNAs in ovarian and breast cancer. Tumor Biol 39(5):1010428317695525
Zheng R, Pan L, Gao J, Ye X, Chen L, Zhang X et al (2015) Prognostic value of miR-106b expression in breast cancer patients. J Surg Res 195(1):158–165
Sahlberg KK, Bottai G, Naume B, Burwinkel B, Calin GA, Borresen-Dale A-L et al (2015) A serum microRNA signature predicts tumor relapse and survival in triple negative breast cancer patients. Clin Cancer Res 21:1207–1214
Halvorsen AR, Helland Å, Gromov P, Wielenga VT, Talman MLM, Brunner N et al (2017) Profiling of microRNAs in tumor interstitial fluid of breast tumors–a novel resource to identify biomarkers for prognostic classification and detection of cancer. Mol Oncol 11(2):220–234
Lánczky A, Nagy Á, Bottai G, Munkácsy G, Szabó A, Santarpia L et al (2016) miRpower: a web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res Treat 160(3):439–446
Guestini F, McNamara KM, Ishida T, Sasano H (2016) Triple negative breast cancer chemosensitivity and chemoresistance: current advances in biomarkers indentification. Expert Opin Ther Targets 20(6):705–720
Chen X, Lu P, Wang D-D, Yang S-J, Wu Y, Shen H-Y et al (2016) The role of miRNAs in drug resistance and prognosis of breast cancer formalin-fixed paraffin-embedded tissues. Gene 595(2):221–226
Erbes T, Hirschfeld M, Rücker G, Jaeger M, Boas J, Iborra S et al (2015) Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker. BMC Cancer 15:193
Pecot CV, Rupaimoole R, Yang D, Akbani R, Ivan C, Lu C et al (2013) Tumour angiogenesis regulation by the miR-200 family. Nat Commun 4:2427
Sarkar FH, Li Y, Wang Z, Kong D, Ali S (2010) Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resist Updat 13(3):57–66
Ellis LM, Hicklin DJ (2009) Resistance to targeted therapies: refining anticancer therapy in the era of molecular oncology. Clin Cancer Res 15(24):7471–7478
Sorrentino A, Liu C-G, Addario A, Peschle C, Scambia G, Ferlini C (2008) Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol 111(3):478–486
Allen KE, Weiss GJ (2010) Resistance may not be futile: microRNA biomarkers for chemoresistance and potential therapeutics. Mol Cancer Ther 9:3126–3136
Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K et al (2010) Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol 79(6):817–824
Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Vasyl’ FC et al (2008) Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther 7(7):2152–2159
Yang S-J, Wang D-D, Li J, Xu H-Z, Shen H-Y, Chen X et al (2017) Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene 623:5–14
Wu Q, Yang Z, Nie Y, Shi Y, Fan D (2014) Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett 347(2):159–166
Zhao J-J, Lin J, Yang H, Kong W, He L, Ma X et al (2008) MicroRNA-221/222 negatively regulates estrogen receptorα and is associated with tamoxifen resistance in breast cancer. J Biol Chem 283(45):31079–31086
Lau L-Y, 劉麗儀 (2011) Identification of microRNAs associated with tamoxifen resistance in breast cancer. HKU Theses Online (HKUTO)
Hong L, Han Y, Zhang Y, Zhang H, Zhao Q, Wu K et al (2013) MicroRNA-21: a therapeutic target for reversing drug resistance in cancer. Expert Opin Ther Targets 17(9):1073–1080
Wang Z-X, Lu B-B, Wang H, Cheng Z-X, Yin Y-M (2011) MicroRNA-21 modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch Med Res 42(4):281–290
Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K (2012) Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 31(2):149–160
Mei M, Ren Y, Zhou X, Yuan X-B, Han L, Wang G-X et al (2010) Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol Cancer Res Treat 9(1):77–86
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Xi Y et al (2010) Mir-125b confers the resistance of cancer cells to Taxol through suppression of Bak1. AACR 70, abstract 2109
Luqmani YA, Alam-Eldin N (2016) Overcoming resistance to endocrine therapy in breast cancer: new approaches to a nagging problem. Med Princ Pract 25(Suppl. 2):28–40
Joshi T, Elias D, Stenvang J, Alves CL, Teng F, Lyng MB et al (2016) Integrative analysis of miRNA and gene expression reveals regulatory networks in tamoxifen-resistant breast cancer. Oncotarget 7(35):57239–57253
Sachdeva M, Mo Y-Y (2010) miR-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res 2(2):170
Rehman SK, Huang W-C, Yu D (2010) MiR-21 upregulation in breast cancer cells leads to PTEN loss and Herceptin resistance. AACR 70, abstract 4033
Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen J et al (2011) Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem 286(21):19127–19137
Scott GK, Goga A, Bhaumik D, Berger CE, Sullivan CS, Benz CC (2007) Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J Biol Chem 282(2):1479–1486
del Pilar Camacho-Leal M, Sciortino M, Cabodi S (2017) ErbB2 receptor in breast cancer: implications in cancer cell migration, invasion and resistance to targeted therapy. In: Breast cancer-from biology to medicine. InTech
Lowery AJ, Miller N, Devaney A, McNeill RE, Davoren PA, Lemetre C et al (2009) MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neureceptor status in breast cancer. Breast Cancer Res 11(3):R27
Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK et al (2006) Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer 5(1):1–14
Breunig C, Pahl J, Küblbeck M, Miller M, Antonelli D, Erdem N et al (2017) MicroRNA-519a-3p mediates apoptosis resistance in breast cancer cells and their escape from recognition by natural killer cells. Cell Death Dis 8(8):e2973
Anastasov N, Höfig I, Vasconcellos IG, Rappl K, Braselmann H, Ludyga N et al (2012) Radiation resistance due to high expression of miR-21 and G2/M checkpoint arrest in breast cancer cells. Radiat Oncol 7(1):206
Liang Z, Ahn J, Guo D, Votaw JR, Shim H (2013) MicroRNA-302 replacement therapy sensitizes breast cancer cells to ionizing radiation. Pharm Res 30(4):1008–1016
Raza U, Zhang JD, Şahin Ö (2014) MicroRNAs: master regulators of drug resistance, stemness, and metastasis. J Mol Med 92(4):321–336
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127(4):679–695
Ma L, Teruya-Feldstein J, Weinberg RA (2008) Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 455(7210):256
Luqmani YA, Khajah MA (2015) MicroRNA in breast cancer—gene regulators and targets for novel therapies. In: A concise review of molecular pathology of breast cancer. InTech
Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D et al (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12(3):247–256
Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celià-Terrassa T et al (2011) Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med 17(9):1101–1108
Orso F, Quirico L, Virga F, Penna E, Dettori D, Cimino D et al (2016) miR-214 and miR-148b targeting inhibits dissemination of melanoma and breast cancer. Cancer Res 76(17):5151–5162
Zou Q, Tang Q, Pan Y, Wang X, Dong X, Liang Z et al (2017) MicroRNA-22 inhibits cell growth and metastasis in breast cancer via targeting of SIRT1. Exp Ther Med 14:1009–1016
Li W, Li G, Fan Z, Liu T (2017) Tumor-suppressive microRNA-452 inhibits migration and invasion of breast cancer cells by directly targeting RAB11A. Oncol Lett 14(2):2559–2565
Kong W, Yang H, He L, Zhao J-J, Coppola D, Dalton WS et al (2008) MicroRNA-155 is regulated by the transforming growth factor β/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol 28(22):6773–6784
Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szász AM, Wang ZC et al (2009) RETRACTED: a pleiotropically acting MicroRNA, miR-31, inhibits breast cancer metastasis. Cell 137(6):1032–1046
Valastyan S, Chang A, Benaich N, Reinhardt F, Weinberg RA (2010) Concurrent suppression of integrin α5, radixin, and RhoA phenocopies the effects of miR-31 on metastasis. Cancer Res 70(12):5147–5154
Valastyan S, Weinberg RA (2010) miR-31: a crucial overseer of tumor metastasis and other emerging roles. Cell Cycle 9(11):2124–2129
Körner C, Keklikoglou I, Bender C, Wörner A, Münstermann E, Wiemann S (2013) MicroRNA-31 sensitizes human breast cells to apoptosis by direct targeting of protein kinase C ϵ (PKCϵ). J Biol Chem 288(12):8750–8761
Li H, Bian C, Liao L, Li J, Zhao RC (2011) miR-17-5p promotes human breast cancer cell migration and invasion through suppression of HBP1. Breast Cancer Res Treat 126(3):565–575
Lu Y, Qin T, Li J, Wang L, Zhang Q, Jiang Z et al (2017) MicroRNA-140-5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Ther 24:386–392
Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S (2007) The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res 67(22):11001–11011
Lee DY, Deng Z, Wang C-H, Yang BB (2007) MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci 104(51):20350–20355
Soria-Valles C, Gutiérrez-Fernández A, Guiu M, Mari B, Fueyo A, Gomis R et al (2014) The anti-metastatic activity of collagenase-2 in breast cancer cells is mediated by a signaling pathway involving decorin and miR-21. Oncogene 33(23):3054–3063
Croset M, Goehrig D, Frackowiak A, Bonnelye E, Ansieau S, Puisieux A et al (2014) TWIST1 expression in breast cancer cells facilitates bone metastasis formation. J Bone Miner Res 29(8):1886–1899
Bishopric N, Speransky S, Kajan D, Laderian B, Iorns E, Clarke J et al. (2017) Abstract P4-07-03: dynamic regulation of a microRNA-mRNA network during breast cancer metastasis reveals an essential tumor-promoting role for miR-203. AACR 77, abstract P4-07-03
Blevins MA, Kouznetsova J, Krueger AB, King R, Griner LM, Hu X et al (2015) Small molecule, NSC95397, inhibits the CtBP1-protein partner interaction and CtBP1-mediated transcriptional repression. J Biomol Screen 20(5):663–672
Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK et al (2013) miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res 73(4):1434–1444
Zare M, Bastami M, Solali S, Alivand M (2017) Aberrantly miRNA promoter methylation and EMT-involving miRNAs in breast cancer metastasis: diagnosis and therapeutic implications. J Cell Physiol 233:3729–3744
Sasheva P, Grossniklaus U (2017) Differentially methylated region-representational difference analysis (DMR-RDA): a powerful method to identify DMRs in uncharacterized genomes. Plant Epigenetics: Methods Protocol 1456:113–125
Gacem RB, Abdelkrim OB, Ziadi S, Dhiab MB, Trimeche M (2014) Methylation of miR-124a-1, miR-124a-2, and miR-124a-3 genes correlates with aggressive and advanced breast cancer disease. Tumor Biol 35(5):4047–4056
Niu J, Xue A, Chi Y, Xue J, Wang W, Zhao Z et al (2016) Induction of miRNA-181a by genotoxic treatments promotes chemotherapeutic resistance and metastasis in breast cancer. Oncogene 35(10):1302–1313
Bai WD, Ye XM, Zhang MY, Zhu HY, Xi WJ, Huang X et al (2014) MiR-200c suppresses TGF-β signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int J Cancer 135(6):1356–1368
Schwarzenbach H (2017) Clinical relevance of circulating, cell-free and exosomal microRNAs in plasma and serum of breast cancer patients. Oncol Res Treat 40(7–8):423–429
Pichler M, Calin G (2015) MicroRNAs in cancer: from developmental genes in worms to their clinical application in patients. Br J Cancer 113(4):569–573
Ohno S-i, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N et al (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21(1):185–191
Huang G, Li S, Yang N, Zou Y, Zheng D, Xiao T (2017) Recent progress in circular RNAs in human cancers. Cancer Lett 404:8–18
Acknowledgments
The authors would like to acknowledge Prof. Mounir AbouHaidar, Professor of Virology at the University of Toronto Dept. Cell & Systems Biology, for his critical reading of the manuscript and Ms. Nancy Nasr Al Deen for illustrating Fig. 18.1. The authors would also like to acknowledge the support of the Lebanese National Council for Scientific Research (CNRS-L), the University Research Board (URB-AUB) and the International Breast Cancer and Nutrition (IBCN) project. Dr. Rabih Talhouk and Dr. Rihab Nasr are both members of IBCN. Ms. Nataly Naser Al Deen is the recipient of the AUB-CNRS-L scholarship.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Naser Al Deen, N., Nassar, F., Nasr, R., Talhouk, R. (2019). Cross-Roads to Drug Resistance and Metastasis in Breast Cancer: miRNAs Regulatory Function and Biomarker Capability. In: Ahmad, A. (eds) Breast Cancer Metastasis and Drug Resistance. Advances in Experimental Medicine and Biology, vol 1152. Springer, Cham. https://doi.org/10.1007/978-3-030-20301-6_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-20301-6_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20300-9
Online ISBN: 978-3-030-20301-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)