Abstract
Ectomycorrhizal fungi (ECMF) play a fundamental role in the nutrient cycle in terrestrial ecosystems, especially in forest ecosystems. In this chapter, the value of ECMF species is reviewed from a global framework, not only to increase the production of edible fruit bodies and biomass of plants but also for the regular practices of reforestation and restoration of ecosystems, with implicit applications in biofertilization, bioremediation, and control of soil pathogens. The valuation of the ECMF in forest management must be considered fundamental for innovation and sustainable development. Ecological functions and bioactive compounds of the ECMF of interest to mankind are briefly reviewed. The direct implications of the ECMFs in forestry are described. To do so, its role as a biotechnological tool in forest nursery production is briefly analyzed, as well as the role of MHB bacteria (mycorrhizal helper bacteria). Subsequently, the direct role as biofertilizers of the ECMF in forest management is discussed: reforestation, plantation management, and ecosystem restoration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Certain groups of fungi establish a symbiotic relationship with the roots of plants, called mycorrhizae. Frank established two large subdivisions of mycorrhizae, ecto- and endomycorrhizae (Smith and Read 2008). Ectomycorrhizal fungi (ECMF) form mantle and Hartig network of intercellular hyphae in the roots of forest species. The arbuscular mycorrhizal fungi (AM) form arbuscules, vesicles that are more variable than that of the ECMF, since it forms a symbiosis with trees and herbaceous plants. Endomycorrhizae are classified as arbuscular mycorrhizae, ericoid mycorrhizae, arbutoid mycorrhizae, monotropoid mycorrhizae, ectendomycorrhizae, or orchid mycorrhizae. Each of these categories is characterized by the invasion of plant root cells by fungal hyphae, but differs in the nature of intracellular hyphal development (Peterson et al. 2004; Sharma 2017).
Ectomycorrhizal fungi are predominantly Basidiomycetes and some Ascomycetes. In these symbiotic structures, the Hartig network is the interface for the metabolic exchange between the fungus and the root. The mycorrhizal mantle is connected to the filaments of fungi that extend into the soil (extraradical mycelium), directly involved in the mobilization, absorption, and translocation of soil nutrients and water to the roots (Suz et al. 2012). More than 7000 species of fungi form ectomycorrhizae (Rinaldi et al. 2008), many of them with important commercial trees such as poplar, birch, oak, pine, and spruce (Wiensczyk et al. 2002). The reproductive structures (fruiting bodies) of the macromycetes are known as mushrooms when they grow in the soil and, like truffles, when they grow underground.
The community of mycorrhizal fungi can be determinant in the structure of the plant community (Fitter 2005), therefore, the identification of the mycobiont partner and its functional structure (Agerer 2001) are fundamental to understand the ecological importance of this symbiotic relationship. ECMF diversity studies were initially based on studies of fruiting bodies and, more recently, on the direct identification of ectomycorrhizae (Horton and Bruns 2001).
Most of the cultivated species of edible fungi are saprophytes, and only some of them are ECMF (Savoie and Largeteau 2011). The tickets (Boletus edulis), Chanterelles (Cantharellus spp.), the matsutake mushroom (Tricholoma matsutake), and the truffle (many species of the Tuber genus) are some ECM fungi for which the crop has been studied (e.g., Chang and Hayes 1978; Chevalier 1998; Bencivenga 1998). The black truffle or Périgord, Tuber melanosporum, is widely grown, while other species of ECM mushrooms have not yet been cultivated, including fungi porcini (Boletus edulis S.) and the high-priced Italian fungus, white truffles (T. magnatum).
4.2 Evaluating ECMF
Forest ecosystems and mycelial networks of ectomycorrhizal fungi play an important role in biogeochemical cycles, biodiversity, climatic stability, and economic growth (Smith and Read 2008). Ectomycorrhizal fungi not only promote the growth and health of host plants but also form vast metabolic networks that may be of critical value to ecosystem functions (Leake et al. 2004; Courty et al. 2010).
Ectomycorrhizal fungi are also important drivers for sustainable innovation in different fields of research (Azul et al. 2014), such as the food industry, biotechnology, biomedicine, and agroforestry (Donnini et al. 2013). These are desirable areas of innovation, given the threats to native forests around the world from poor management, soil degradation, pollution, water scarcity, fire, and the spread of invasive species and diseases (FAO 2010). The relationships between the various native edible ECM fungi have been, until relatively recently, insufficiently considered in the strategies of forest management (Dahlberg et al. 2010), and the role of ECMF has been underestimated in bio-industrial innovation. Some authors have presented several examples of representative models of the valuation of the ECMF from a holistic conception (Suz et al. 2012; Azul et al. 2014).
Some of the intrinsic values of the ECMF to human activity are the food (gastronomy, local, and international markets); the value of the landscape; the popular culture; the ecological tourism, as indicators of environmental quality; and the multifunctionality.
So far, different bioactive compounds have been identified from ECM fungi with different biological activities, applications, or properties: low molecular weight organic compounds, which may be used in the food industry to mimic mushroom flavors (Mizuno and Kwai 1992), which may have anticancer properties (Wang et al. 2003) or antioxidant activity (Reis et al. 2011); polysaccharides, which may be included in diabetic diets or to present immunosuppressive and anticancer activity (Hu et al. 1994); fatty acids and other lipids, which may have antioxidant, anti-inflammatory, anticancer (Reis et al. 2011), or immunosuppressive activity (Kreisel et al. 1990); enzymes, which may have application in the paper industry, textile industry, and detergent production (Campbell and Bedford 1992); or enzymes which may have application in environment-contaminant degradation (Pointing and Vrijmoed 2000), paint decoloration (Casieri et al. 2010), food industry (Gupta et al. 2003), cosmetic industry (Liese et al. 2000), etc.; terpenoids, with anticancer activity; and, finally, phenolic compounds, which define organoleptic properties fungi (Ribeiro et al. 2006).
4.3 Ecological Functions of ECMF
Some of the traditionally known functions of the ECMF in the ecosystem are:
-
Increase in the water and nutrient supply, extending the volume of land accessible to the plants. Different fungal species (drought-sensitive hydrophilic or drought-tolerant hydrophobic) can have different effects on hydraulic redistribution patterns (Prieto et al. 2016).
-
Increase in the plant’s nutrient supply, assimilating nutrients in the ways that would not normally be available to plants.
-
The mechanisms of improvement in the absorption of P would be: extension of extramatrical hyphae and Pi transfer (inorganic), Pi transporters in the fungus/soil interface; mobilization of organic P (labile), emission of phosphatases; and mobilization of insoluble mineral Pi, emission of low molecular weight organic acids.
-
The mechanisms of improvement in N nitrogen absorption would be intervention in the mineral N cycle (NH4+, NO3−) and assimilation of organic N (emitting proteases, chitinases, others).
-
Colonization of the root by ECMF can provide protection against soil pathogens.
-
The non-nutritive benefits to plants due to changes in water relations, the level of phytohormones, the assimilation of carbon, etc., have already been verified.
-
Carbon is transferred through the fungal mycelium of ECMF that connects different species of plants. This can reduce competition among plants and contribute to the stability and diversity of ecosystems.
-
Epigeous and hypogeal sporocarps of ECMF are important food sources for placental and marsupial mammals. The mycorrhizal roots, the mycelium, and the fruiting bodies of the fungi are important as food sources and habitats for invertebrates.
-
Mycorrhizae influence the microbial populations of the soil and the exudates in the mycorrhizosphere and hyphosphere.
-
The hyphal network produced by ECM fungi significantly alters and improves the structure of the soil.
-
Mycorrhizal fungi contribute to the storage of carbon in the soil by altering the quantity and quality of organic matter in the soil.
-
Enhancing plant tolerance to (biotic and abiotic) stresses.
Recent advances in the knowledge of nutrient translocation processes in the fungus-plant and fungus-soil interaction are especially interesting, in particular, the priority role of transporters of P, N, and C (Bonfante and Genre 2010). The inorganic P and mineral or organic forms of N, such as NH4+, NO3−, and amino acids (AA), are absorbed by specialized transporters located in the fungal membrane in the extraradical mycelium. NH3+/NH4+ and inorganic P (from polyphosphates) are imported from the symbiotic interface to the cells of the plant through selective transporters. Transporters of hexoses import carbon of plant origin into the fungus, while the transporter proteins that participate in the export of nutrients from the plant or the fungus have not yet been identified. The nutritional strategies seem to be different between symbiotic and pathogenic fungi, for example, in the translocation of C. Even different transport strategies have been found between ECMF symbionts belong to Ascomycota and Basidiomycota. The understanding of the different systems of transporters or nutrient channels involved both at the level of the extraradical mycelium and at the level of the symbiotic interface will clarify in the future the processes of nutrition in the plant-fungus and fungus-soil interaction.
4.4 ECMF Genomic Studies
So far, genome sequencing of two ECMF (ectomycorrhizae), the Laccaria bicolor and Tuber melanosporum (black truffle), helps in the identification of factors that regulate the development of mycorrhiza and its function in the plant cell (Bonfante and Genre 2010). The study of symbiotic and transcriptomic genomes will provide in the future, among others, the following lines of knowledge:
-
A better understanding of the mesocosm of the tree (i.e., the interactions of the host plant with its courtship of endophytes, symbiotics, and pathogenic microorganisms).
-
A basis for the study of the crosstalk of encoded proteins between symbiotic partners that involve mycorrhizal effectors.
-
A molecular definition of the mechanisms that lead to the initiation of the carpophore and its development.
-
The metabolic pathways that control the transport and assimilation of nutrients in the symbiosis and in the body of fructification.
-
Bioinformatic exploration of important symbiotic gene networks and major transcriptional factors—the mycorrhizal genetic landscape.
-
Comparative transcriptomics with other economically important saprobionts, and with pathogenic fungi (Martin and Bonito 2012).
4.5 ECMF Selection Criteria for Sustainable Development
Some of the most common criteria considered for the selection of a most valued species or strain of ECMF (some of them implicit in others) are the abiotic criteria like climatic conditions, such as temperature, insolation, and humidity and improvement of soil properties, such as texture and permeability, abiotic soil stress mitigation, soil contamination mitigation, soil metal mobilization, or nutrient cycling. There may also be criteria regarding the host, such as the plant/fungus specificity, the improvement of plant health, or the increase in the biomass of the plant. The criteria regarding the fungus include abundance, effectiveness, propagules’ competitiveness, fungus growth rate, or edibility. The other criteria may be the conservation of native biodiversity, the functioning of the ecosystem, human health, food, nutraceutical value, etc. (Suz et al. 2012; Azul et al. 2014).
4.6 Applications: ECMF and Forestry
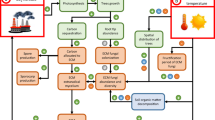
Since the late 1950s, mycorrhizal fungi were utilized as biofertilizers to promote plant growth, because of their ability to increase the plant uptake of P, N, mineral nutrients, and water (Feldmann et al. 2009; Koide and Mosse 2004; Miransari 2011). Much of our understanding of the functions of ECMF has come from research directed toward practical application in forestry (Fig. 4.1). Although successful inoculation of tree seedlings (already planted) in field has been known, nursery inoculation is more common. Seedlings inoculated in nursery can establish a healthy ECMF system before outplanting.
4.7 ECMF in Forest Nurseries
The challenge in the controlled synthesis of the ectomycorrhizal symbiosis is to produce quality mycorrhizal plant, only colonized by the desired fungus. Accurate identification of the inoculum used and avoiding contamination during the growth of the inoculated plants are essential parts of the production process to avoid the introduction of unwanted species and to avoid the mixing of their genetic material with indigenous species (Murat and Martin 2008).
The appropriate selection of suitable plant-host species is essential for the success of mycorrhization (Olivier 2000). Relatively fast-growing fungi are generally preferred for inoculation because of their short incubation period. Unfortunately, many otherwise desirable ECMF grow slowly. According to Marx (1980), fresh cultures are preferred to cultures repeatedly transferred and stored for several years. He further suggested passing important fungus cultures through a host inoculation and mycorrhiza formation followed by re-isolation, every few years, to maintain mycorrhiza-forming capacity. Moreover, fungi which produce large hyphal stands of rhizomorphs in culture of soil may be superior in soil exploration and mineral uptake to those which lack rhizomorphic growth. On the other hand, the fruiting of the ECMF species is not based solely on the mycorrhizal state of the seedlings. After planting, in addition to the presence of indigenous competitors, the biotic and physicochemical characteristics of the soil also influence the persistence and spread of the cultivated fungus (Hortal et al. 2009).
The type of ECMF material used for inoculation can affect the success of a mycorrhizal inoculation program. In addition to remaining viable during storage and transport, the inoculant must also maintain its infectivity for several months after its introduction (Rossi et al. 2007).
There are three main sources of fungal inoculum:
-
(a)
The use of the soil or humus collected from the area in which the mycorrhizal seedlings are going to be planted: Its main disadvantage is the lack of control of the species of ECMF present in the soil or of microorganisms and harmful germs. It is widely used in developing countries, although it is currently discarded in mycorrhization programs. Also, planting mycorrhizal “nurse” seedlings or incorporating chopped roots of ECMF hosts into nursery beds as a source of fungi for neighboring young seedlings has been successful (Sim and Eom 2006).
-
(b)
The use of spores of fruit bodies collected in the field: The main advantages are that the spores do not require the extension of the aseptic culture and that the spore inoculum is not heavy (Marx and Cordell 1989). Most of the recent research has been with P. tinctorius; however, inoculation with Rhizopogon species also appears promising. Abundant Rhizopogon mycorrhizae formed on seedlings produced from the coated seed of P. radiata with basidiospores of Rhizopogon luteolus (Sharma 2017). However, it has three main drawbacks: (A) significant quantities of fruiting bodies are required and may not be available each year; (B) the success of the inoculation is highly dependent on the viability of the spores; and (C) the lack of genetic definition. Freeze drying and storage at a low temperature in the dark is helpful to maintain its viability. The spores can be mixed with physical supports before the soil inoculation, suspended in water and soaked in the soil, sprinkled, sprayed or pelleted and emitted to the ground, encapsulated or coated on the seeds and they can be embedded in hydrocolloid chips (Marx and Cordell 1989).
-
(c)
Mycelial inoculum: It is the use of hyphae as an inoculum in a solid or liquid medium or substrate. Fungal hyphae are cultivated mainly from sterile parts of fruiting bodies, less frequently from mycorrhiza due to their low (approx. 5–20%) success rate (Molina and Palmer 1982) and rarely from sclerotia (Trappe 1969) or sexual spores (Fries and Birraux 1980). It is considered the most appropriate method since it allows the selection of particular strains of a fungus previously tested for its ability to promote the growth of plants (Marx 1980). Many species do grow well in culture, e.g., most species of Suillus, Hebeloma, Laccaria, Amanita, Rhizopogon, and Pisolithus. Liquid substrates have the advantage over solids because they are easily mixed, and they produce more uniform conditions for crop growth, but the risk of bacterial contamination and costs are higher (Rossi et al. 2007). On the other hand, the main advantages of the solid medium (Cannel and Moo-Young 1980) are the reduction of bacterial contamination due to the lower water content, the low costs of the equipment, and the simplified design of the bioreactors. The main drawback of the use of mycelial inocula is that several species of ECMF are difficult to grow under laboratory conditions, or growth is very slow (due to the absence of their symbiont), and it is not always easy to produce large amount of inoculum viable for large-scale nursery inoculation programs. Some advances have been made using mycelium encapsulated in “beads” of calcium alginate (Le Tacon et al. 1983), but they have to be refrigerated. Inoculant beads can remain viable for several months under refrigeration, although the results vary between fungal species. For several species, the mycelial inoculum has been tested with trees of economic interest. This technique has great potential for the inoculation of seedlings in reforestation programs. For example, Rossi et al. (2007) designed a bioreactor with the capacity to produce inoculum for 300,000 seedlings, enough to reforest 200 ha. Based on a global demand of 3.0 billion cubic meters of wood, an estimated 4.3 tons of mycelium would be needed to inoculate 12 billion seedlings (5 g of dry mycelium per plant, Rossi et al. 2007). An advantage of alginate gel is the possibility of preparing a multimicrobial inoculant.
4.7.1 Mycorrhizal Helper Bacteria
The concept of “mycorrhizal helper bacteria” (MHB) was introduced in a “Tansley Review”—Helper Bacteria: a new dimension of mycorrhizal symbiosis (Garbaye 1994)—which has led to new research in the plant-fungus model system, as for the meaning of these bacteria that promote the formation of mycorrhizae and cause many physiological effects of mutualistic interaction.
In general, the ability of some microorganisms to influence the formation and functioning of the symbiosis is known, through activities of various kinds such as the activation of infective propagules of the fungus in presymbiotic stages (Azcón-Aguilar and Barea 1996), facilitating the formation of entry points in the root (Linderman 1988), and increase the growth rate (Carpenter-Boggs et al. 1995). The MHB improve mycorrhiza formation, although the same MHB can benefit mycorrhization for certain fungi and be negative for others (Garbaye and Duponnois 1992). The above reflects the fungal specificity by isolate, which exemplifies the genetic distance between isolates of different origin.
Among the mechanisms presented by the MHB are:
-
(a)
Promotion of the establishment of the symbiosis by stimulation of the mycelial growth. The germination of spores and mycelial growth are improved by the production of growth factors (Keller et al. 2006).
-
(b)
Increased contact and colonization root-fungi surfaces: increasing of lateral root number by the production of phytohormones (Bending et al. 2002) and the improvement of radical colonization by induction of flavonoid production (Xie et al. 1995).
-
(c)
Reduction of the impact of adverse environmental factors on the mycelium of the mycorrhizal fungus. Bacteria can detoxify soils, restoring their conductivity, similarly freeing them from contamination generated by heavy metals (Brulé et al. 2001) and reducing the concentrations of phenolic antagonist compounds produced by the same mycorrhizal fungi (Duponnois and Garbaye 1990). The rhizospheric microorganisms also have an effect on the growth of the plants, reaching a synergistic effect, where the presence of the micro-fungus and the other microorganism produce an increase in the growth, vigor, and protection of the plant (Domínguez et al. 2012). These effects are based on activities such as the acquisition of nutrients, inhibition of the growth of pathogenic fungi (Budi et al. 1999), and improvement of the root ramification (Gamalero et al. 2004).
In recent years, a potential capacity of bacteria associated with ectomycorrhizae to fix atmospheric nitrogen has been suggested (Frey-Klett et al. 2007). Several studies suggest a real possibility that the bacteria present in mycorrhizal tissues contribute to the nutritional needs of both the fungus (ascocarp development) and consequently of the plants, by providing them with available nitrogen derived from atmospheric nitrogen (N2).
MHB belong to a wide range of genera (Burkholderia, Paenibacillus, Poole et al. 2001; Pseudomonas, Bacillus, Duponnois and Garbaye 1991; Streptomyces, Maier et al. 2004).
However, the molecular mechanisms by which MHB induce the growth of ECMF are not well described. Recently, changes in expression of genes involved in the development of certain ECMF have been studied at the molecular level in confrontations with MHB (Schrey et al. 2005; Riedlinger et al. 2006; Deveau et al. 2007; Zhou et al. 2014). Research in mycorrhizae should, therefore, strive towards an improved understanding of the functional and molecular mechanisms involved in interactions in the mycorrhizosphere, in order to develop ad hoc biotechnology that allows the application of optimized combinations of microorganisms as effective inoculators within sustainable systems of plant production (Artursson et al. 2006).
4.7.2 Polymicrobial Formulations
Polymicrobial formulations containing a diverse mixture of beneficial rhizosphere microorganisms with multiple functionalities is attractive because combining different classes of soil organisms can take advantage of multiple plant growth-promoting mechanisms and could be applied to multiple crops (Avis et al. 2008; Gravel et al. 2007; Hayat et al. 2010; Malusa et al. 2012; Vestberg et al. 2004). A key concept in constructing effective polymicrobial multifunctional formulations is the selection and use of a right combination of rhizosphere bacteria and fungi that are mutually compatible, have complementary functionalities, effectively colonize the rhizosphere of the crop(s) of interest, and bring about a synergistic promotion of growth and yield of crop(s) (Avis et al. 2008; Azcón-Aguilar et al. 2009; Barea et al. 2005; Hata et al. 2010). It is to be expected that well-designed multifunctional formulations such as the one described would be a welcome addition to the fast-growing inoculant enterprises worldwide. Such an inoculant is also expected to be eco-friendly and suitable for organic farming and other integrated production systems, where synthetic fertilizer inputs are not allowed or restricted by law. However, construction of such complex formulations is technically demanding (Reddy and Saravanan 2013).
Ectomycorrhizal fungi exhibit synergistic interactions with other plant-beneficial organisms such as symbiotic N2-fixers. For example, ectomycorrhizal symbiosis enhanced the efficiency of inoculation of two Bradyrhizobium strains on the growth of legumes (Andre et al. 2005). It is also of interest that similar synergies were seen when AMF (Glomus mosseae), ECM fungus (Pisolithus tinctorius), and Bradyrhizobium sp. were used together to inoculate Acacia nilotica; enhancement of N2 fixation, growth, and dry biomass were observed when all three organisms were present (Saravanan and Natarajan 1996, 2000).
Also, using plant growth-promoting microorganism (PGPM) strains that form stable and effective biofilms could be a strategy for producing commercially viable inoculant formulations (Malusa et al. 2012; Seneviratne et al. 2008). A majority of plant-associated bacteria found on roots and in the soil are found to form biofilms (Ude et al. 2006). Bacterial, fungal, and bacteria/fungal biofilms were suggested as possible inoculants. This is a novel and interesting idea, but to what extent this approach would be practiced remains to be seen (Reddy and Saravanan 2013).
4.8 Application of ECMF in Forest Management
The inoculation of ECMF can be done not only with the objective of producing edible carpophores but also because of its considerable value in forest management (Fig. 4.1); in particular, they have had great importance in reforestation programs where it was expected that the quality and economic productivity of the plantations would increase (Garbaye 1990). The success of the plantations with mycorrhized seedlings from the nursery depends on their ability to quickly access the nutrients and water available within the soil matrix (Duñabeitia et al. 2004).
In mycorrhizal plantations (productive or conservation forest reforestations), a consequence of the recognition of the advantages of fungal diversity in ecosystems will be an increase in the refusal to introduce potentially dominant species in mixed communities. On the other hand, unfortunately, it seems that many of those fungi selected for optimal colonization in the nursery have been poor competitors in the field, especially when the planting sites contained indigenous populations of mycorrhizal fungi. There are several possible explanations for the inoculation failure (from the nursery) to produce beneficial effects in the planting sites. Probably, among the most important of these is the inability of inoculum introduced to persist in the roots of the plant after the transfer of the nursery to the field. The soil conditions experienced in the nursery and with the plant growing in a container are very different from those of most of the planting sites; in addition, the raising, storage, and transport of seedlings can reduce the vigor of fine roots and their fungal associates. Species such as Pisolithus tinctorius (15 sub spp), in circumstances such as degraded environments, with absence or scarcity of autochthonous mycorrhizal populations, have achieved the greatest success in inoculation programs (McAfee and Fortin 1986).
In the case of an artificially mycorrhized plant with edible ECM fungi of interest, such as Tuber melanosporum (black truffle), the establishment of plots has always had the main objective of producing fruiting bodies, leaving in the background the contribution of ecological functions of the symbiosis (in the plant, soil, and, in general, the ecosystem, Domínguez et al. 2006). The example of mycorrhizal plantations for truffle production has been generally successful (Olivier et al. 1996), obtaining productions from 6 to 7 years of implantation.
In restoration of ecosystems, the biofertilization, bioremediation, or the control of soil pathogens are prominent roles of the mycorrhizal forest plants. Degraded ecosystems are the result of a wide range of characteristics and factors related to unfavorable land management or industrial activities. Environmental degradation of the soil is increasing worldwide at an alarming rate due to erosion, acidity, salinization, compaction, the depletion of organic matter, and water scarcity. In a healthy ecosystem, there is a balanced microbiota of the soil, in such a way that the potential of pathogenic and mycorrhizal fungi coexists in apparent harmony. Ectomycorrhizal fungi can survive in extreme habitats with high or low temperature (Tibbett and Cairney 2007; Geml et al. 2011), salt and metal concentration (Colpaert et al. 2011), drought (Azul et al. 2010), and other circumstances related to the degradation of the ecosystem. The importance of ECM fungi in the balance of the ecosystem can be enormous, since they can be used to increase the tolerance of plants against abiotic stresses, especially their capacity to fix heavy metals or to degrade a wide variety of persistent organic compounds; to interact with soil bacteria; to attack fungi, bacteria, and pathogenic nematodes; and to improve the vegetative growth and the nutritional status of its symbiont plant. In addition, the extraradical mycelium of the ECM fungi provides a direct pathway for the translocation of photosynthesized carbon to microsites in the soil and a large surface area for interaction with other microorganisms (Sun et al. 1999; Suz et al. 2012). Very little is known about how the tolerance of fungi to metals affects the transfer of metal to the host plant. The ability to accumulate metals depends not only on the inter- and intraspecific variation of the sensitivity of mycorrhizal fungi to metal but also on environmental factors (Suz et al. 2012). Meharg and Cairney (2000) revised potential ways in which ectomycorrhizal fungi might support rhizosphere remediation of persistent organic pollutants (POPs). Recently, the importance of low molecular weight organic acids and metal-chelating agents (such as siderophores) from ECMF in the fixation of metal ions and their transmission or not to the root of the host plant has been described (Machuca 2011).
4.9 Conclusions
Research on ectomycorrhizae should focus on better understanding the functional and molecular mechanisms involved in interactions in the mycorrhizosphere. It should aim to find the appropriate technology for the commercial techniques of multiplication and large-scale inoculation of the mycorrhizal inoculum and the application of optimized combinations of plant-microorganisms, adopted under well-defined environmental and soil conditions. The role of ECMF as biofertilizers in reforestation and environmental restoration has been fundamental up to now, and its importance in the balance of the ecosystem can be enormous, increasing the tolerance of plants against biotic and abiotic stress.
References
Agerer R (2001) Exploration types of ectomycorrhizae. A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11:107–114
Andre S, Galiana A, Le Roux C, Prin Y, Neyra M, Duponnois R (2005) Ectomycorrhizal symbiosis enhanced the efficiency of inoculation with two Bradyrhizobium strains and Acacia holosericea growth. Mycorrhiza 15:357–364
Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ Microbiol 8:1–10
Avis TJ, Gravel V, Autoun H, Tweddel RJ (2008) Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol Biochem 40:1733–1740
Azcón-Aguilar C, Barea JM (1996) Arbuscular mycorrhizas and biological control of soil-borne plant pathogens – an overview of the mechanisms involved. Mycorrhiza 6:457–464
Azcón-Aguilar C, Barea JM, Gianinazzi S, Gianinazzi-Pearson V (eds) (2009) Mycorrhizas functional processes and ecological impact. Springer, Berlin
Azul AM, Sousa JP, Agerer R, Martín MP, Freitas H (2010) Land use practices and ectomycorrhizal fungal communities from oak woodlands dominated by Quercus suber L. considering drought scenarios. Mycorrhiza 20:73–88
Azul AM, Nunes J, Ferreira I, Coelho AS, Verissimo P, Trovao J, Campos A, Castro P, Freitas H (2014) Valuing native ectomycorrhizal fungi as a mediterranean forestry component for sustainable and innovative solutions. Botany-Botanique 92(2):161–171
Barea JM, Azcón R, Azcón-Aguilar C (2005) Interactions between mycorrhizal fungi and bacteria to improve plant nutrient cycling and soil structure. In: Varma A, Buscot F (eds) Microorganisms in soils: roles in genesis and functions, vol 3. Springer, Heidelberg, pp 195–212
Bencivenga M (1998) Ecology and cultivation of Tuber magnatum Pico. In: Proceedings of the first international meeting on ecology, physiology and cultivation of edible mycorrhizal mushrooms. Swedish University of Agricultural Sciences, Uppsala, Sweden, 3–4 July
Bending GD, Poole EJ, Whipps JM, Read DJ (2002) Characterisation of bacteria from Pinus sylvestris-Suillus luteus mycorrhizas and their effects on root-fungus interactions and plant growth. FEMS Microbiol Ecol 39:219–227
Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant – fungus interactions in mycorrhizal symbiosis. Nat Commun 27:1–48. https://doi.org/10.1038/ncomms1046
Brulé C, Frey-Klett P, Pierrat JC, Courier S, Gérard F, Lemoine MC, Rousselet J, Somer J, Garbaye J (2001) Survival in the soil of the ectomycorrhizal fungus Laccaria bicolor and effect of a mycorrhiza helper Pseudomonas fluorescens. Soil Biol Biochem 33:1683–1694
Budi SW, Van Tuinen D, Martinotti MG, Gianiazzi S (1999) Isolation from the Sorghum bicolour mycorrhizosphere of a bacterium compatible with arbuscular mycorrhiza development and antagonistic towards soil-borne fungal pathogens. Appl Environ Microbiol 65:148–150
Campbell GL, Bedford MR (1992) Enzyme applications for monogastric feeds: a review. Can J Anim Sci 72:449–466
Cannel E, Moo-Young M (1980) Solid-state fermentation systems. Process Biochem 15:24–28
Carpenter-Boggs L, Loynachan TE, Stahl PD (1995) Spore germination of Gigaspora margarita stimulated by volatiles of soil-isolated actinomycetes. Soil Biol Biochem 27:1445–1451
Casieri L, Anastasi A, Prigione V, Varese GC (2010) Survey of ectomycorrhizal, litter-degrading, and wood-degrading Basidiomycetes for dye decolorization and ligninolytic enzyme activity. Antonie Van Leeuwenhoek 98:483–504
Chang ST, Hayes WA (1978) The biology and cultivation of edible mushrooms. Academic Press, New York
Chevalier G (1998) The truffle cultivation in France: assessment of the situation after 25 years of intensive use of mycorrhizal seedlings. In: Proceedings of the first international meeting on ecology, physiology and cultivation of edible mycorrhizal mushrooms, Swedish University of Agricultural Sciences, Uppsala, Sweden, 3–4 July
Colpaert JV, Wevers JHL, Krznaric E, Adriaensen K (2011) How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Ann For Sci 68:17–24
Courty PE, Franc A, Garbaye J (2010) Temporal and functional pattern of secreted enzyme activities in an ectomycorrhizal community. Soil Biol Biochem 42(11):2022–2025
Dahlberg A, Genney DR, Heilmann-Clausen J (2010) Developing a comprehensive strategy for fungal conservation in Europe: current status and future needs. Fungal Ecol 3(2):50–64
Deveau A, Palin B, Delaruelle C, Peter M, Kohler A, Pierrat JC, Sarniguet A, Garbaye J, Martin F, Frey-Klett P (2007) The mycorrhiza helper Pseudomonas fluorescens BBc6R8 has a specific priming effect on the growth, morphology and gene expression of the ectomycorrhizal fungus Laccaria bicolor S238N. New Phytol 175:743–755
Domínguez JA, Selva J, Rodríguez Barreal JA, Saiz de Omeñaca JA (2006) The influence of mycorrhization with Tuber melanosporum in the afforestation of a Mediterranean site with Quercus ilex and Quercus faginea. For Ecol Manag 231:226–233
Domínguez JA, Martin A, Anriquez A, Albanesi A (2012) The combined effects of Pseudomonas fluorescens and Tuber melanosporum on the quality of Pinus halepensis seedlings. Mycorrhiza 22(6):429–436
Donnini D, Gargano ML, Perini C, Savino E, Murat C, Di Piazza S, Altobelli E, Salerni E, Rubini A, Rana GL, Bencivenga M, Venanzoni R, Zambonelli A (2013) Wild and cultivated mushrooms as a model of sustainable development. Plant Biosyst 147(1):226–236
Duñabeitia M, Rodríguez N, Salcedo I, Sarrionandia E (2004) Field mycorrhization and its influence on the establishment and development of the seedlings in a broadleaf plantation in the Basque country. For Ecol Manag 195:129–139
Duponnois R, Garbaye J (1990) Some mechanisms involved in growth stimulation of ectomycorrhizal fungi by bacteria. Can J Bot 68:2148–2152
Duponnois R, Garbaye J (1991) Mycorrhizal helper bacteria associated with the Douglas fir Laccaria laccata symbiosis: effects in aseptic and in glasshouse conditions. Ann For Sci 48:239–251
FAO (Food and Agricultural Organization of the United Nations) (2010) Global forest resources assessment, 2010. Main report. FAO Forestry Paper 163. Food and Agricultural Organization of the United Nations, Rome, Italy
Feldmann F, Hutter I, Schneider C (2009) Best production practice of arbuscular mycorrhizal inoculum. In: Varma A, Kharkwal AC (eds) Symbiotic fungi: principles and practice, vol 18. Springer, Berlin, pp 319–336
Fitter AH (2005) Darkens visible: reflections on underground ecology. J Ecol 93:231–243
Frey-Klett P, Garbaye J, Tarkka M (2007) The Mycorrhiza helper bacteria revisited. Tansley review. New Phytol 176:22–36
Fries N, Birraux D (1980) Spore germination in Hebeloma stimulated by living plant roots. Exp Dermatol 36:1056–1057
Gamalero E, Trotta A, Massa N, Copetta A, Martinotti MG, Berta G (2004) Impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza 14:185–192
Garbaye J (1990) Use of mycorrhizas in forestry. In: Strullu DG (ed) Les mycorhizes des arbres et plantes cultivées. Lavoisier, Paris, pp 197–248
Garbaye J (1994) Helper bacteria: a new dimension to the mycorrhizal symbiosis. New Phytol 128(2):197–210
Garbaye J, Duponnois R (1992) Specificity and function of mycorrhization helper bacteria (MHB) associated with the Pseudotsuga menziesii–Laccaria laccata symbiosis. Symbiosis 14:335–344
Geml J, Timling I, Robinson CH, Lennon N, Nusbaum HC, Brochmann C, Noordeloos ME, Taylor DL (2011) An arctic community of symbiotic fungi assembled by long-distance dispersers: phylogenetic diversity of ectomycorrhizal basidiomycetes in Svalbard based on soil and sporocarp DNA. J Biogeogr 39(1):74–88
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and growth yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida and Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39:1968–1977
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Mycrobial α-amylases: a biotechnological perspective. Process Biochem 38:1599–1616
Hata S, Kobae Y, Banba M (2010) Interactions between plants and arbuscular mycorrhizal fungi. In: Kwang WJ (ed) International review of cell and molecular biology, vol 281. Academic Press, New York, pp 1–48
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Hortal S, Pera J, Parladé J (2009) Field persistence of the edible ectomycorrhizal fungus Lactarius deliciosus: effect of inoculation strain, initial colonization level, and site characteristics. Mycorrhiza 19:167–177
Horton TR, Bruns TD (2001) The molecular revolution in ectomycorrhizal ecology: peeking into the black-box. Mol Ecol 10:1855–1871
Hu HJ, Li PZ, Lin T, Hang BQ, Guo YW (1994) Effects of polysaccharide of Tuber sinense on tumor and immune system of mice. J Chin Pharmaceut Univ 125:289–292
Keller S, Schneider K, Sussmuth RD (2006) Structure elucidation of auxofuran, a metabolite involved in stimulating growth of fly agaric, produced by the mycorrhiza helper bacterium Streptomyces AcH 505. J Antibiot (Tokyo) 59:801–803
Koide R, Mosse B (2004) A history of research on arbuscular mycorrhiza. Mycorrhiza 14:145–163
Kreisel H, Lindeguis U, Hurak M (1990) Distribution, ecology, and immunosuppressive properties of Tricholoma populinum (Basidiomycetes). Zentralbl Mikrobiol 145:393–396
Le Tacon F, Jung G, Mugnier J, Michelot P (1983) Efficiency in a forest nursery of an inoculant of an ectomycorrhizal fungus produced in a fermentor and entrapped in polymetric gels. Ann For Sci 40:165–176
Leake J, Johnson D, Donnelly D, Muckle G, Boddy L, Read D (2004) Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can J Bot 82(8):1016–1045
Liese A, Seelbach K, Wandrey C (2000) Industrial biotransformations. Wiley-VCH, Weinheim (Federal Republic of Germany)
Linderman RG (1988) Mycorrhizal interactions with the rhizosphere micoflora – the rhizosphere effect. Phytopathology 78:366–371
Machuca A (2011) Metal-chelating agents from ectomycorrhizal fungi and their biotechnological potential. In: Rai M, Varma A (eds) Diversity and biotechnology of ectomycorrhizae. Soil biology, vol 25. Springer, Berlin, pp 347–369
Maier A, Riedlinger J, Fiedler HP, Hampp R (2004) Actinomycetales bacteria from a spruce stand: characterization and effects on growth of root symbiotic and plant parasitic soil fungi in dual culture. Mycol Prog 3(2):129–136
Malusa E, Sas-Paszt L, Ciesielska J (2012) Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J 2012:491206. https://doi.org/10.1100/2012/491206
Martin F, Bonito G (2012) Ten years of genomics for ectomycorrhizal fungi: what have we achieved and where are we heading? In: Zambonelli A, Bonito GM (eds) Edible ectomycorrhizal mushrooms. Current knowledge and future prospects. Soil biology, vol 34. Springer, Berlin, pp 383–401
Marx DH (1980) Ectomycorrhizal fungus inoculations: a tool for improving forestation practices. In: Mikola P (ed) Tropical mycorrhiza research. Oxford University Press, London, pp 13–71
Marx DH, Cordell CE (1989) The use of specific ectomycorrhizas to improve artificial forestation practices. In: Whipps JM, Lumsden RD (eds) Biotechnology of fungi for improving plant growth: symposium of the British. Cambridge University Press, Cambridge, pp 1–25
McAfee BJ, Fortin JA (1986) Competitive interactions of ectomycorrhizal mycobionts under field conditions. Can J Bot 64:848–852
Meharg AA, Cairney JWG (2000) Ectomycorrhizas: extending the capacities of rhizosphere remediation? Soil Biol Biochem 32:1475–1484
Miransari M (2011) Soil microbes and plant fertilization. Appl Microbiol Biotechnol 92:875–885
Mizuno T, Kwai M (1992) Chemistry and biochemistry of mushroom fungi. Gakai-shupan Center, Tokyo
Molina R, Palmer JG (1982) Isolation, maintenance, and pure culture manipulation of ectomycorrhizal fungi. In: Schenck NC (ed) Methods and principles of mycorrhizal research. The Americal Phytopathological Society, St Paul, MN, pp 115–129
Murat C, Martin F (2008) Sex and truffles: first evidence of Périgord black truffle outcrosses. New Phytol 180:260–263
Olivier JM (2000) Progress in the cultivation of truffles. In: Van Griensven LJLD (ed) Mushroom science XV: science and cultivation of edible fungi, vol 2. Balkema, Rotterdam (Netherlands), pp 937–942
Olivier JM, Savignac JC, Sourzat P (1996) Truffe et trufficulture. Ed Fanlac, Perigueux, France
Peterson RL, Massicotte HB, Melville LH (2004) Mycorrhizas: anatomy and cell biology. CAB International, Wallingford
Pointing SB, Vrijmoed LLP (2000) Decolorization of azo and triphenylmethane dyes by Pycnoporus sanguineus producing laccase as the sole phenoloxidase. World J Microbiol Biotechnol 16:317–318
Poole EJ, Bending GD, Whipps JM, Read DJ (2001) Bacteria associated with Pinus sylvestris–Lactarius rufus ectomycorrhizas and their effects on mycorrhiza formation in vitro. New Phytol 151:743–751
Prieto I, Roldán A, Huygens D, Alguacil MM, Navarro-Cano JA, Querejeta JI (2016) Species-specific roles of ectomycorrhizal fungi in facilitating interplant transfer of hydraulically redistributed water between Pinus halepensis saplings and seedlings. Plant Soil 406:15–27
Reddy CA, Saravanan RS (2013) Polymicrobial multi-functional approach for enhancement of crop productivity. In: Sariaslani S, Gadd GM (eds) Advances in Applied Microbiology, vol 82. Elsevier, Burlington, MA, pp 53–113
Reis FS, Pereira E, Barros L, Sousa MJ, Martíns A, Ferreira ICFR (2011) Biomolecule profiles in inedible wild mushrooms with antioxidant value. Molecules 16:4328–4338
Ribeiro R, Rangel J, Valentão P, Baptista P, Seabra RM, Andrade PB (2006) Contents of carboxylic acids and two phenolics and antioxidant activity of dried Portuguese wild edible mushrooms. J Agric Food Chem 54:8530–8537
Riedlinger J, Schrey SD, Tarkka MT, Hampp R, Kapur M, Fiedler HP (2006) Auxofuran, a novel metabolite that stimulates the growth of fly agaric, is produced by the mycorrhiza helper bacterium Streptomyces strain AcH 505. Appl Environ Microbiol 72:3550–3557
Rinaldi AC, Comandini O, Kuyper TW (2008) Ectomycorrhizal fungal diversity: separating the wheat from the chaff. Fungal Divers 33:1–45
Rossi MJ, Furigo A, Oliveira VL (2007) Inoculant production of ectomycorrhizal fungi by solid and submerged fermentations. Food Technol Biotechnol 45:277–286
Saravanan RS, Natarajan K (1996) Effect of Pisolithus tinctorius on the nodulation and nitrogen fixing potential of Acacia nilotica seedlings. Kavaka 24:41–49
Saravanan RS, Natarajan K (2000) Effect of ecto- and endomycorrhizal fungi along with Bradyrhizobium sp. on the growth and nitrogen fixation in Acacia nilotica seedlings in the nursery. J Trop For Sci 12:348–356
Savoie JM, Largeteau ML (2011) Production of edible mushrooms in forests: trends in development of a mycosilviculture. Appl Microbiol Biotechnol 89:971–979
Schrey SD, Schellhammer M, Ecke M, Hampp R, Tarkka MT (2005) Mycorrhiza helper bacterium Streptomyces AcH 505 induces differential gene expression in the ectomycorrhizal fungus Amanita muscaria. New Phytol 168:205–216
Seneviratne G, Zavahir J, Bandara W, Weerasekara M (2008) Fungal-bacterial biofilms: their development for novel biotechnological applications. World J Microbiol Biotechnol 24:739–743
Sharma R (2017) Ectomycorrhizal mushrooms: their diversity, ecology and practical applications. In: Varma A, Prasad R, Tuteja N (eds) Mycorrhiza – function, diversity, state of the art. Springer, Berlin, pp 99–131
Sim M-Y, Eom A-H (2006) Effects of ectomycorrhizal fungi on growth of seedlings of Pinus densiflora. Mycobiology 34:191–195
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London, p 787
Sun YP, Unestam T, Lucas SD, Johanson KJ, Kenne L, Finlay RD (1999) Exudation–reabsorption in mycorrhizal fungi, the dynamic interface for interaction with soil and other microorganisms. Mycorrhiza 9:137–144
Suz L, Azul AM, Pino-Bodas R, Martín MP (2012) Ectomycorrhizal fungi in biotechnology: present and future perspectives. In: Kumar A, Prasad RS (eds) Environment and biotechnology. Lambert Academic Publishing, AG & CKG, pp 472–542
Tibbett M, Cairney JWG (2007) The cooler side of mycorrhizas: their occurrence and functioning at low temperatures. Can J Bot 85:51–62
Trappe JM (1969) Studies on Cenococcum graniforme. An efficient method for isolation from sclerotia. Can J Bot 47:1389–1390
Ude S, Arnold DL, Moon CD, Timms-Wilson T, Spiers AJ (2006) Biofilm formation and cellulose expression among diverse environmental Pseudomonas isolates. Environ Microbiol 8:1997–2011
Vestberg M, Kukkonen S, Saari K, Parikka P, Huttunen J, Tainio L et al (2004) Microbial inoculation for improving the growth and health of micropropagated strawberry. Appl Soil Ecol 27:243–258
Wang HX, Ngai HK, Ng TB (2003) A ubiquitin-like peptide with ribonuclease activity against various polyhomoribonucleotides from the yellow mushroom Cantharellus cibarius. Peptides 24:509–513
Wiensczyk AM, Gamiet D, Durall DM, Jones MD, Simard SW (2002) Ectomycorrhizae and forestry in British Columbia: a summary of current research and conservation strategies. BCJ Ecosyst Manag 2:1–20
Xie ZP, Staehelin C, Vierheilig H, Iemkena W, Jabbouri S, Broughton WJ, Vogeli-Lange R, Boller T (1995) Rhizobial nodulation factors stimulate mycorrhizal colonization of nodulating and nonnodulating soybeans. Plant Physiol 108:1519–1525
Zhou AD, Wu XQ, Shen L, Xu XL, Huang L, Ye JR (2014) Profiling of differentially expressed genes in ectomycorrhizal fungus Pisolithus tinctorius responding to mycorrhiza helper Brevibacillus reuszeri MPt17. Biol Sect Cell Mol Biol 69(4):435–442
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Domínguez-Núñez, J.A., Berrocal-Lobo, M., Albanesi, A.S. (2019). Ectomycorrhizal Fungi: Role as Biofertilizers in Forestry. In: Giri, B., Prasad, R., Wu, QS., Varma, A. (eds) Biofertilizers for Sustainable Agriculture and Environment . Soil Biology, vol 55. Springer, Cham. https://doi.org/10.1007/978-3-030-18933-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-18933-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18932-7
Online ISBN: 978-3-030-18933-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)