Abstract
Circadian rhythm (CR) is an important regulator of numerous basic functions of the living organisms such as carbon metabolism, gene expression and regulation, growth and reproduction. It is widely accepted, and several research activities prove its implication on health and disease especially in humans and plants including microbes associated with it. CR is reported to regulate circadian clock which is subjected to extensive natural variation during day and night, light intensity, availability of nutrients, stress and other factors. CR varies within and between species; this underlies the importance of understanding the phenomenon at the individual level to develop disease management strategies or production of microbial formulations used for growth promotion. In plants, rhizosphere microorganisms extensively depend on the root exudates, and its composition is reported to alter with CR in response to external stimuli including global warming and pollution. These microbes play an important role in plant growth and its environmental fitness and hence the concept of plant growth-promoting rhizobacteria (PGPR) came to existence. However, even today circadian clock regulating interaction of PGPR with plants is not extensively studied, and hence most of the time, microbes developed in the laboratory fail to perform in the field level. The world is awaiting another green revolution to feed the growing population with bitter experience of the previous revolution. It is the right time to understand the circadian clock at the species level and to develop suitable formulations to exploit the beneficial aspect of plant-microbe interaction to achieve high yield in the agricultural fields as a part of the sustainable agriculture. Understanding the CR in plant-pathogen interaction will also help to develop suitable treatment strategies to overcome the yield loss due to infection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Genes not only inherit the capacity of the organisms to clone but also the capacity of the generations to endure environmental changes referred to as chronon, which means the cyclical, irreversible, recursive and chronological expression of genes as a function of biological time. The stimulation of these constitutive biological rhythms of the living organisms defines its fitness to the environmental variations. Halberg et al. (1959) referred this rhythm as circadian (daily clock phenomenon) derived from the Latin word circa for “about” and dies for “day”. It is defined as the biological activities with a frequency of one activity cycle every 24 h (Halberg et al. 1977).
Linnaeus (1770) is the pioneer in studying the plant behaviour in response to time. He observed the periodical movement of flowers in response to external conditions such as temperature and change in light. His observations on timely response of different varieties of flowers recorded using garden clock helped in developing a concept of unique rhythms in many species. He named it as sleep of plant analogous to that of animals. Even though these observations are connected with plant response to external stimuli in time scale, detailed research on this concept was taken up later to prove it.
Animals also respond to this clock and select the feed accordingly based on the variation in the plant metabolites. Related to this, an interesting study on feeding habit of olive baboon was reported by Adeola et al. (2014). These animals showed different choices of feeding during wet and dry season. Among the plants used for consumption, 7 plants, viz. Andropogon gayanus, Strychnos spinosa, Nuclear larifiora, Vitellaria paradoxa, Ficus sycomorus, Annona senegalensis and Tamarindus indica, were consumed in wet season with 303 feeding events, while other 10 plants Detarium macrocarpum, Gardenia sotoemsis, Parkia biglobosa, Piliostigma thonningii, Pterocarpus erinaceus, Prosopis africana, Ficus sycomorus, Ximenia americana, Annona senegalensis and Vitex doniana were consumed with 315 feeding events during dry season. It is a clear indication that the plant with higher nutritional quality was consumed by the animals. The change in feeding habit also indicates that the plants are subjected to seasonal variation due to which the nutritional composition also alters. It is a best example for how animals choose their feeding to satisfy the nutritional balance. This change in feeding habit also indicates change in plant metabolism in response to seasonal variation and provides clear evidence that plant physiology is altered with season and time.
Present-day advanced research is providing more insights into this concept, the broader understanding of this phenomenon and its widespread application in several aspects of plant growth and adaptability. The study on this behaviour needs accurate observations and mathematical interpretation of numerous experimental data recorded in different intervals of day and night. Recording the biological fluctuations or variability in measurements of hormone and pigment concentrations, membrane transport rates, growth, ion fluxes, protein production, etc. underlies the basic understanding of rhythms.
14.2 Rhizosphere Microflora and Root Exudates
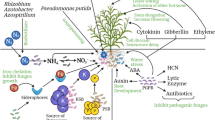
Soil being a natural media supports plant-microbe interaction. Beneficial microorganisms such as asymbiotic and symbiotic nitrogen-fixing microorganisms, ecto- and endomycorrhizal fungi and plant growth-promoting rhizobacteria including K and P solubilizers play a vital role in plant growth. Soil microbes also exhibit antifungal activity, produce volatile organic compounds and induce systemic resistance in plants. To maintain these microorganisms in the vicinity of the root, plants release 5–10% of net photosynthate by roots, and this percentage increases when it is grown in nonsterile system (Barber and Martin 1976). This indicates that the structure and diversity of the rhizosphere microflora vary among plant species and over time (Baudoin et al. 2002). It is also interesting to note that different root zones of the same plant choose colonization of specific microbial communities by releasing specific substrates which varies from simple sugar to complex aromatic compounds (Kamilova et al. 2006). Composition of the root exudates hence is an important selection force for beneficial plant-microbe interaction. It comprises phenolics, sugars, amino acids and secondary metabolites of low molecular weight and polysaccharides, proteins and other biomolecules of high molecular weight (Abbot and Murphy 2003; Walker et al. 2003). These biomolecules are often less diverse but available in larger proportion in the exudates, and polysaccharides in general decide the association of heterotrophic rhizobacteria with rhizosphere and rhizoplane. Glycosides and hydrocyanic acid are considered as toxic metabolites of root origin which is known to inhibit the growth of pathogens (Rangaswami 1988).
Recent studies proved that rhizosphere microbiome associated with plant growth is also influenced by the type of soil, climate change and anthropogenic activities (Igiehon and Babalola 2018). Even plant cultivar which is having variations in single gene is reported to alter the microbiome. Bressan et al. (2009) observed change in rhizosphere microflora between wild-type and transgenic Arabidopsis, due to release of glucosinolates. They revealed that the presence of a single metabolite significantly affected alphaproteobacteria and fungi population in the rhizosphere.
Abiotic factors such as pH, type of soil, availability of oxygen, intensity of light, soil temperature, availability of proper nutrients and even presence of specific microorganisms govern the qualitative and quantitative composition of root exudates. It varies among the plant species, for example, differential exudation pattern was observed in pines and variation in the amount of amino acids in pea and oat root exudates. Diverse carbohydrates are released by young maples compared to mature trees, which exude more and diverse amino acids.
Even the organic acids released in root exudates vary. Study conducted by Schilling et al. (1998) revealed that root exudates of Zea mays found to contain citric acid, where as in Triticum turgidum var. durum L. it was oxalic acid and acetic acid and acetate is a dominant acid released by roots of Linum usitatissimum L. (Cieslinski et al. 1997). This shows composition of root exudates varies with several factors and it is specific to plant species.
Ultimately it is the quantity and quality and type of carbon sources released in root exudates that decide the composition of microbial communities in the rhizosphere (Merbach et al. 1999). It is not only beneficial organisms; even pathogenic fungi such as Rhizoctonia, Fusarium, Sclerotium, Aphanomyces, Pythium, Colletotrichum, Verticillium and Phytophthora are allowed to germinate in response to specific metabolites released by the roots (Vancura 1964). Plants can maintain high number of antagonists by providing specific nutrients required for the growth of these organisms to develop resistance against specific pathogens.
Raja et al. (2006) reported another interesting observation that even rhizosphere microflora influence composition of root exudates. They observed that the composition varies after application of bioinoculants, viz. Azospirillum lipoferum-A2 204, Bacillus megaterium var. phosphaticum and Pseudomonas fluorescens pf-1, into the soil. It was also supported by rRNA gene profiling and community-level physiological profiles conducted by Miethling et al. (2000). Gomes et al. (2001) reported the alterations in rhizosphere microflora even during senescence.
These studies indicate that the interaction between rhizosphere microflora and plant is not simple and it is the interface which is gaining importance nowadays as a hot spot of plant-microbe interactions, whether it is beneficial or pathogenic. As discussed earlier, this interaction is very specific and influenced by several abiotic and biotic factors including light and temperature, which directly alters the composition of the root exudates and through which metabolic exchange between rhizosphere community and roots is also altered (Berg and Smalla 2009; Harmer 2009). Hence it is the right time to study the alterations in the composition of the root exudates in general and rhizosphere microbial population in particular. If it is not done, the beneficial interaction of specific microbes with specific plant root through metabolites is not going to be established, and it may remain as a major setback in developing microbial formulations for generalized field applications. Sustainable agriculture hence may be achievable only through overall information on plant and its response to various environmental signals in the era of drastic climate change.
14.3 Climate Change and Plant Response
Significant statistical change in distribution of weather patterns over an extension period of time, ranging from decades to millions of years, refers to climate change. It is caused by oceanic circulation, variation in solar radiation, plate tectonics, volcanic eruptions and even human interferences. These changes lead to loss of sea ice, increased in sea level, intense heat waves, extended drought periods and increase in tropical storms. Another important drastic change is the increase in global surface temperature in range of 1.8–3.6 °C by 2100 as a result of increased CO2 levels derived from both anthropogenic and natural sources (IPCC 2007).
The world is witnessing drastic environmental fluctuations such as local cooling, increased global temperature, shifting of vegetation and extreme weather due to climate change. Is it not influencing the CR, plant physiology and root exudation? Scientific reports support the influence of altered environmental conditions on all these plant processes. Especially elevated CO2 increases carbon allocation to root zone and also alters the composition of the root exudates (Fig. 14.1). It is also influenced by C/N ratio, nutrient availability, elevated temperature and drought (Kandeler et al. 2006; Haase et al. 2008). Hence Drigo et al. (2008) opine that the climate change substantially impacts the diversity and activities of microorganisms leading to impaired beneficial effects of these organisms on plant growth and health. It is the right time to develop a strategy to develop holistic approach involving all the factors influencing the composition of root exudates to favour the growth of the beneficial rhizosphere microflora and antagonists to confer resistance to plant pathogens.
Also, these alterations indirectly alter the nature of soil and hence are known to influence the rhizosphere microbiome. Increase in CO2 levels, one of the causes of global warming, is known to alter the root exudation patterns which in turn decide the soil food web structure and functioning by increasing the rate of photosynthesis (Haase et al. 2008; Stevnback et al. 2012; Drigo et al. 2013). The world is also witnessing changing weather pattern, for example, change in precipitation level with time is also reported to have significant influence on soil microbial population (Sheik et al. 2011; Castro et al. 2010). Singh et al. (2010) also observed that climate change induced alterations in natural ecosystems and microbial population will have similar changes in the biogeochemical cycles mediated by these microbes. They also reported that there could be addition of new processes to ecosystem due to altered microbial activities which is beneficial or detrimental to plants.
Forchetti et al. (2007) reported the altered plant-associated communities as a result of drought stress. They observed the different subpopulations of endophytes colonizing sunflower grown under drought conditions. Interestingly they could isolate endophytic bacteria with more plant growth-promoting ability in sunflower cultivated under drought than the cultivar grown with sufficient irrigation. Different PGPRs, ecto- or endomycorrhizal taxa, however, are also reported to respond differently to droughts in terms of their patterns of abundance. Examples are from Mediterranean shrubs such as Pinus muricata, Pinus oaxacana, etc. where drought significantly decreased the microbial colonization process (Compant et al. 2010a, b).
In view of these, proper exploitation of agricultural land and associated beneficial microbes remains as a best choice for climate change resilience farming systems as it supports the proper management of soil, water, biodiversity and local resource usage (Sharma et al. 2014).
14.4 Rhythm in Plants and Its Influence on Plant Processes
Intestines of the animals resemble rhizosphere of the plants in many aspects. Several host functions are regulated by microbes inhabiting these zones. Recently, in animals, feeding and diet of the host were reported to alter intestinal microbiota of humans (Leone et al. 2015) and mice (Liang et al. 2015; Zarrinpar et al. 2014) due to diurnal oscillations. It is also proven to silence the host molecular clock genes leading to gut dysbiosis (Thaiss et al. 2014). Harmer (2009) reported the plant innate ability to estimate time within 24 h period to synchronize biological events via circadian clock. Photosynthetic pattern and other physiological activities of the plant may also alter the rhizosphere microbiome similar to animals.
CR in plants regulates central metabolic pathways of carbon (Kolling et al. 2015), expression of genes, stomatal function and photoperiodism associated with seasonal reproduction (Michael et al. 2003; Yanovsky and Kay 2001). This clock shows variation in response to natural variation both between and within species leading to individual plant performance and fitness (Sulpice et al. 2014; Konmonth-Schultz et al. 2013; Yerushalmi et al. 2011) (Fig. 14.2). It also enhances the adaptations of plant to different environments by regulating physiological and developmental states periodically (Graf et al. 2010; Harmer 2009). Even plant pathogens regulate life cycle in response to diurnally regulated host plant metabolism. On the other hand, plant innate immune response for its fitness is regulated by CR through cellular metabolism (Seo and Mas 2015; Roden and Ingle 2009). Hence it serves as a fascinating adaptive force of life on earth. Obviously, it is endogenous helping in keeping the time of day and night for all living organisms. Photosynthetic organisms record such activity in response to different wavelengths of light as they use light as a source of energy. It is compulsory for them to adapt to daily and seasonal fluctuations of light which serves as a selective force to determine time in a circadian manner (Jarillo et al. 2003).
The list of plant processes regulated by CR is increasing; it is playing a vital role in expression of genes, cytosolic ion concentration, phosphorylation of proteins, movement of chloroplast, stomatal regulation, elongation of hypocotyl, movement of leaf and cotyledon, production of hormones, fitness and responsiveness. Its role in synchronizing developmental processes such as flowering time is well documented. Any change in the clock-associated genes was also reported to alter the photoperiodic control of flowering. Over the years even stem elongation, root pressure, cell membrane potential and CO2 exchange are also included in the list (Hubbard et al. 2017). Activity of the plants regulated by CR is tabulated in Table 14.1, and it highlights the need of understanding the phenomenon in other plants too.
Johnsson (2007) observed that the rhythmic transpiration reflects rhythmic cellular control by guard and subsidiary cells which regulates assimilation of CO2 and transpires water vapour by stomatal openings. In 1979, Raschke expressed the need of a model system to understand the regulation of water system in plant in association with photosynthesis and CO2 transport through stomata. Even before this in 1729, French astronomer De Mairan reported his observation of persistent leaf movements of Mimosa pudica for several days even after the plants were placed in darkness. This laid a foundation for plants’ accurate timing mechanism to synchronize their physiology with daily environmental fluctuations. It was Bunning (1931) who first identified the plant clock which monitor the duration of day and night. He proved its importance by inducing a mutation in a bean gene involved in clock regulation. Recent studies proved beyond doubt that CR increases ability of plants to anticipate and prepare for changes in the environment that occur during day and night.
14.5 Mechanism of CR in Plants in Brief
Mechanism of CR regulated by circadian clock is well established in Arabidopsis; the clock was reported to consist of a series of transcriptionally and post-transcriptionally regulated intertwined feedback loops (Harmer 2009). Even though it is proved in this plant, its existence in other plant species needs to be evaluated (Song et al. 2010). The circadian clock has been found to influence a variety of metabolic functions in the plant including chlorophyll biosynthesis, transport photosystems, starch synthesis and degradation and nitrogen and sulphur assimilation. The clock timing was found to be altered to different concentrations of several metabolites such as glutamate, nitrate, glutamine and sucrose (Gutierrez et al. 2008; Knight et al. 2008). However, due to differences in methodology, these results are sometimes inconsistent across studies, highlighting a need to consider photoperiod duration and the time of sample collection when describing results.
Advances in the identification and characterization of components of the plant circadian system have been made largely through genetic studies in Arabidopsis. The number of genes regulating Arabidopsis circadian clock is approximately 20, in contrast to smaller number of genes regulating the circadian clock of insects, mammals and fungi. As in the mammalian circadian clock, several clock-associated genes from Arabidopsis have overlapping functions. The complexity of phototransduction pathways in plants may contribute to the large number of genes implicated in clock function (Jarillo et al. 2003).
As in other organisms, the circadian system in plants consists of input pathways that provide temporal information from the environment to the clock, the central oscillator mechanism itself and a set of pathways through which the temporal information provided by the clock is used to generate overt rhythms in several processes. During the course of evolution, photoreceptors of plant have developed capability to detect light over a large range of wavelengths and transduce the signal-specific genes regulating the clock. There are three main classes: the phytochromes, having the ability to absorb the red and far-red region of electromagnetic spectrum, and the cryptochromes and phototropins which absorb blue and UV A region of spectrum (Jarillo et al. 2003).
14.6 Plant Rhythm and Its Influence on Rhizosphere Microflora
Waldon et al. proved that the rhizobacteria respond and adapt to increased temperature which in turn regulates the CR. They could isolate rhizobia from nodules of desert woody legume Prosopis glandulosa which is better adapted to 36 °C compared to other strains grown in normal conditions. This proves that the bacteria colonizing distinct soil sites respond differently to certain environmental conditions. Increase in temperature from 10 to 30 °C will decrease the ability of an endophyte Burkholderia phytofirmans to colonize tomato rhizosphere (Pillay and Nowak 1997). It is also reported that bacterial endophytic populations, which colonize plant internal tissues such as stems, roots, leaves, shoots as well as flowers, fruits and seeds, may be affected in a similar manner (Compant et al. 2005, 2008, 2010a, b; Hallmann 2001). Even mycorrhizal hypha reduces its growth in response to elevated CO2 concentrations (Madhu and Hatfield 2013).
Composition and abundance of rhizosphere populations associated with strawberry, potato and oil seed was reported to change over the field season, and this alteration could be because of alternation in time.
Daniel et al. (2004) assessed cycling dynamics in A. thaliana diel cycle associated with exposure to dark and light periods, and they involved study associated with acyclic Arabidopsis line having cca1 gene ectopically overexpressed and also another plant Brachypodium distachyon to prove any alterations in the rhizosphere community among species, wild types and mutants. The data obtained by them completely disproved the observations of Bulgarelli et al. (2012) and suggested that rhizosphere microflora is highly dynamic and are influenced by biotic and abiotic factors along with circadian clocks. This served as clear-cut evidence that CR plays a vital role in deciding both composition of root exudates and also the diversity of rhizosphere microbial community. Even recent reports involving next-generation sequencing of the 16S rRNA gene, soil organic matter composition in the rhizosphere characterized by high-resolution mass spectrometry and 21T Fourier transform ion cyclotron resonance mass spectrometry support this observation (Staley et al. 2017).
These reports suggest the possible role of circadian clock on the rhizosphere community. The timing of bacterial cycling in relation to that of Arabidopsis further suggests that diurnal dynamics influence microbial association with plant carbon metabolism and exchange. In view of this, Grayston et al. (2001), Staley et al. (2017) and Dunfield and Germida (2003) suggest that previous studies done without relevance to time of day may need to be reevaluated with regard to the impact of diurnal cycles on the rhizosphere microbial community. Along with this, they also suggest that caution should be taken when conclusions are drawn about root-associated microbial community structure based on the results of a single time point.
14.7 Conclusions and Outlook
Plant-rhizosphere microbiome interactions are highly relevant because rhizosphere microflora is reported to strongly influence plant fitness and biomass which in turn inform evolutionary studies of adaptation, agronomic practices and conservation much needed for sustainable agriculture. Climate change and global warming are the major threats to living organisms, resulting in alterations of normal process of evolution. It is a forced artificial evolution; inevitably all the organisms have to respond and adopt. Especially elevated CO2 and pattern of light radiation are affecting several natural phenomena including plant-microbe interactions in rhizosphere. If this harmony is not understood and integrated with the bioinoculant performance in the field, the desired effect of bioinoculants on plant growth is naturally affected. It is the right time to evaluate the efficacy of all bioinoculants with special reference to individual plant CR responses.
Genes regulating CR are highly sensitive and regulated by several environmental parameters. Alterations in CR are reported to alter the rhizosphere community structure due to changing pattern of diurnal fluxes of carbon, water or nutrients from plant roots. Clock misfunction would bring in differences in this structure in general and alterations in rare taxa in particular leading to differences in community function required for plant performances. It is the right time to understand the clock genes associated with plants, after which rhizosphere engineering or suitable microbial consortia or bioinoculants can be developed to increase the plant processes associated with plant health, growth and yield.
References
Abbott L, Murphy D (2003) Soil biology fertility: a key to sustainable land use in agriculture. Kluwer Academic, Dordrecht, pp 187–203
Adeola AJ, Apapa AN, Adeyemo AI, Alaye SA, Ogunjobi JA (2014) Seasonal variation in plants consumption pattern by foraging Olive baboons (Papio anubis. Lesson, 1827) inside Kainji lake national park, Nigeria. J Appl Sci Environ Manage 18:481–484
Barber DA, Martin JK (1976) The release of organic substances by cereal roots into soil. New Phytol 76:69–80
Baudoin E, Benizri E, Guckert AV (2002) Impact of growth stage on the bacterial community structure along maize roots, as determined by metabolic and genetic fingerprinting. Appl Soil Ecol 19:135–145
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Blasing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17:3257–3281
Bressan M, Roncato MA, Bellvert F, Comte G, Haichar EZF, Achouak W, Berge O (2009) Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. Int Soc Microb Ecol J 3:1243–1257
Bulgarelli D, Rott M, Schlaeppi K, Van Themaat EV, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root inhabiting bacterial microbiota. Nature 488:91–95
Bunning E (1931) Untersuchungen uber die autonomen tagesperiodischen Bewungen der Primarblatter von Phaseolus multiflorus. Jahrb Wiss Bot 75:439–480
Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW (2010) Soil microbial community response to multiple experimental climate change drivers. Appl Environ Microbiol 76:999–1007
Cieslinski G, van Rees KCJ, Huang PM (1997) Low molecular weight organic acids released from roots of durum wheat and flax into sterile nutrient solutions. J Plant Nutr 20:753–764
Compant S, Reiter B, Sessitsch A, Nowak J, Clément C, Ait Barka E (2005) Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol 71:1685–1693
Compant S, Kaplan H, Sessitsch A, Nowak J, Ait Barka E, Clément C (2008) Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: from the rhizosphere to inflorescence tissues. FEMS Microbiol Ecol 63:84–93
Compant S, Clément C, Sessitsch A (2010a) Colonization of plant growth-promoting bacteria in the rhizo- and endosphere of plants: importance, mechanisms involved and future prospects. Soil Biol Biochem 42:669–678
Compant S, Marcel GA, Heijden VD, Sessitsch A (2010b) Climate change effects on beneficial plant-microorganisms interaction. FEMS Microbiol Ecol 66:197–214
Daniel X, Sugano S, Tobin EM (2004) CK2 phosphorylation of CCA1 is necessary for its circadian oscillator function in Arabidopsis. Proc Natl Acad Sci 101:3293–3297
Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hiberd JM, Millar AJ, Webb AA (2005) Plant circadian clocks increase photosynthesis, growth, survival and competitive advantage. Science 309:630–633
Dowson-Day MJ, Millar AJ (1999) Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J 17:63–71
Drigo B, Kowalchuk GA, van Veen JA (2008) Climate change goes underground: effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biol Fertil Soils 44:667–679
Drigo B, Kowalchuk GA, Knapp BA, Pijl AS, Boschker HT, Veen JA (2013) Impacts of 3 years of elevated atmospheric CO2 on rhizosphere carbon flow and microbial community dynamics. Glob Chang Biol 19:621–636
Dunfield KE, Germida JJ (2003) Seasonal changes in the rhizosphere microbial communities associated with field grown genetically modified Canola (Brassica napus). Appl Environ Microbiol 69:7310–7318
Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96:271–290
Engelmann W, Johnsson A (1998) Rhythms in organ movement. In: Lumsden PJ, Millar AJ (eds) Biological rhythms and photoperiodism in plants. BIOS Scientific, Oxford, pp 35–50
Fejes E, Nagy F (1998) Molecular analysis of circadian clock regulated gene expression in plants: features of the ‘output’ pathways. In: Lumsden PJ, Millar AJ (eds) Biological rhythms and photoperiodism in plants. BIOS Scientific, Oxford, pp 99–118
Finlayson SA, Lee IJ, Mullet JE, Morgan PW (1999) The mechanism of rhythmic ethylene production in sorghum. The role of phytochrome B and simulated shading. Plant Physiol 119:1083–1089
Forchetti G, Masciarelli O, Alemano S, Alvarez D, Abdala G (2007) Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl Microbiol Biotechnol 76:1145–1152
Foster KR, Morgan PW (1995) Genetic regulation of development in Sorghum bicolor (IX. The ma3 R allele disrupts diurnal control of gibberellin biosynthesis). Plant Physiol 108:337–343
Fowler SG, Cook D, Tomashow MF (2005) Low temperature induction of Arabidopsis CBF1, 2 and 3 is gated by the circadian clock. Plant Physiol 137:961–968
Gomes NCH, Heuer H, Schonfeld J, Costa R, Hagler-Mendoca L, Smalla K (2001) Bacterial diversity of the rhizosphere of maize (Zea mays) grown in tropical soil studies by temperature gradient gel electrophoresis. Plant Soil 233:167–180
Gomez LA, Simon E (1995) Circadian rhythm of Robinia pseudoacacia leaflets movements: role of calcium and phytochrome. Photochem Photobiol 61:210–215
Graf A, Schlereth A, Stitt M, Smith AM (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci 107:9458–9463
Grayston SJ, Griffith GS, Mawdsley JL, Campbell CD, Bardgett RD (2001) Accounting for variability in soil microbial communities for temperate upland grassland ecosystems. Soil Biol Biochem 33:533–551
Green RM, Tingay S, Wang ZY, Tobin EM (2002) Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol 129:576–584
Greenham K, McClung CR (2015) Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet 16:598–610
Guadagno CR, Ewers BE, Weinig C (2018) Circadian rhythms and redox state in plants. Front Plant Sci 9:247–256
Gutierrez RA, Stokes TL, Thum K, Xu X, Obertello M, Katari MS, Tanurdzic M, DeanA NDC, McClung CR, Coruzzi GM (2008) Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc Natl Acad Sci 105:4939–4944
Haase S, Philippot L, Neumann G, Marhan S, Kandeler E (2008) Local response of bacterial densities and enzyme activities to elevated atmospheric CO2 and different N supply in the rhizosphere of Phaseolus vulgaris L. Soil Biol Biochem 40:1225–1234
Halberg F, Halberg E, Barnum CP, Bittner JJ (1959) Physiologic 24-hour periodicity in human beings and mice, the lighting regimen and daily routine. In: Withrow RB (ed) Photoperiodism and related phenomenon in plants and animals, vol 55. Education Publishing, Washington, DC, pp 803–878
Halberg F, Carandente F, Cornelissen G, Katinas GS (1977) Glossary of chronobiology. For Chron 4:1–189
Hallmann J (2001) Plant interactions with endophytic bacteria. In: Jeger MJ, Spence NJ (eds) Biotic interactions in plant–pathogen associations. CABI Publishing, Wallingford, pp 87–119
Harmer SL (2009) The circadian system in higher plants. Annu Rev Plant Biol 60:357–377
Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA, Zhu T, Wang X (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290:2110–2113
Hennessey TL, Field CB (1991) Oscillations in carbon assimilation and stomatal conductance under constant condition. Plant Physiol 96:831–836
Hillman WS (1956) Injury of tomato plants by continuous light and unfavorable photoperiodic cycles. Am J Bot 43:89–96
Hubbard CJ, Brock MT, Dipen LTAV, Maignien L, Ewers BE, Weining C (2017) The plant circadian clock influences rhizosphere community structure. Int Soc Microb Ecol J 12:400–410
Igiehon NO, Babalola OO (2018) Rhizosphere microbiome modulators: contributions of nitrogen fixing bacteria towards sustainable agriculture. Int J Environ Res Public Health 15:576–610
IPCC Climate Change Reports (2007) Impacts, adaptations and vulnerability. Cambridge University Press, Cambridge, pp 79–89
Jarillo JA, Capel J, Cashmore AR (2003) Physiological and molecular characteristics of plant circadian clocks. In: Sehgal A (ed) Molecular biology of circadian rhythms. Wiley, Hoboken, NJ, pp 185–209
Johnsson A (2007) Oscillations in plant transpiration. In: Mancuso S, Shabala S (eds) Rhythms in plants: phenomenology, mechanisms and adaptive significance. Springer, Berlin, pp 225–230
Jones TL, Ort DR (1997) Circadian regulation of sucrose phosphate synthase activity in tomato by protein phosphatase activity. Plant Physiol 113:1167–1175
Jouve L, Greppin H, Agosti RD (1998) Arabidopsis thaliana floral stem elongation: evidence for an endogenous circadian rhythm. Plant Physiol Biochem 36:469–472
Kamilova F, Kravchenko LV, Shaposhinkov AI, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars and L-tryptophan in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant-Microbe Interact 19:250–256
Kandeler E, Mosier AR, Morgan JA, Milchunas DG, King JY, Rudolph S, Tscherko D (2006) Response of soil microbial biomass and enzyme activities to the transient elevation of carbon dioxide in a semi-arid grassland. Soil Biol Biochem 38:2448–2460
Kellmann JW, Hoffrogge R, Piechulla B (1999) Transcriptional regulation of oscillating steady-state Lhc mRNA levels: Characterization of two Lhca promoter fragments in transgenic tobacco plants. Biol Rhythm Res 30:264–271
Kim HY, Cote GG, Crain RC (1993) Potassium channels in Samanea saman protoplasts controlled by phytochrome and the biological clock. Science 260:960–962
Kloppstech K (1985) Diurnal and circadian rhythmicity in the expression of light induced nuclear messenger RNAs. Plant 165:502–506
Knight H, Thomson AJW, McWatters HG (2008) Sensitive to freezing integrates cellular and environmental inputs to the plant circadian clock. Plant Physiol 148:293–303
Kolling K, Thalmann M, Muller A, Jenny C, Zeeman SC (2015) Carbon partitioning in Arabidopsis thaliana is a dynamic process controlled by the plant metabolic status and its circadian clock. Cell Environ 38:1965–1979
Konmonth-Schultz HA, Golembeski GS, Imaizumi T (2013) Circadian clock regulated physiological outputs: dynamic responses in nature. Semin Cell Dev Biol 24:407–413
Landry LG, Chappel CCS, Last RL (1995) Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 109:1159–1166
Leone V, Gibbons SM, Martinez K, Hutchinson AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli HN, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB (2015) Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17:681–689
Li J, Ou-Lee TM, Raba R, Amundson RG, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5:171–179
Liang X, Bushman FD, FitzGerald GA (2015) Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci 112:10479–10484
Linnaeus C (1770) Systema natvrae oer regna tria natvrae, secvndvm classes, ordines, genera, species cvm characteribvs, et differentiis. Tomvs III, pp 1–236
Liu Z, Taub CC, McClung CR (1996) Identification of an Arabidopsis Rubisco Activase (RCA) minimal promoter regulated by phytochrome and the circadian clock. Plant Physiol 112:43–51
Madhu M, Hatfield JL (2013) Dynamics of plant root growth under increased atmospheric carbon dioxide. Agron J 105:657–669
Mairan D (1729) Observation botanique. Hist Acad Roy Sci:35–36
McClung CR, Kay SA (1994) Circadian rhythms in Arabidopsis thaliana. In: Meyweowitz EM, Somerville CR (eds) Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 615–637
Merbach W, Mirus E, Knof G, Remus R, Ruppel S, Russow R, Gransee A, Schulze J (1999) Release of carbon and nitrogen compounds by plant roots and their possible ecological importance. J Plant Nutr Soil Sci 162:373–383
Michael TP, McClung CR (2003) Enhancer trapping reveals widespread circadian clock transcriptional control in Arabidopsis thaliana. Plant Physiol 132:629–639
Michael TP, Salome PA, Yu HJ, Spencer TR, Sharp EL, McPeek MA, Alonso JM, Ecker JR, McClung CR (2003) Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302:1049–1053
Miethling R, Wieland G, Backhaus H, Tebbe CC (2000) Variation of microbial rhizosphere communities in response to crop species, soil origin and inoculation with Sinorhizobium meliloti L33. Microb Ecol 40:43–56
Millar AJ, Kay SA (1991) Circadian control of cab gene transcription and mRNA accumulation in Arabidopsis. Plant Cell 3:541–550
Millar AJ, Short SR, Chua NH, Kay SA (1992) A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4:1075–1087
Muller LM, Korff MV, Davis SJ (2014) Connections between circadian clocks and carbon metabolism reveal species-specific effects on growth control. J Exp Bot 65:2915–2923
Nagy F, Kay SA, Chua N-H (1988) A circadian clock regulates transcription of the wheat Cab-1 gene. Genes Dev 2:376–382
Nimmo HG (2000) The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci 5:75–80
Piechulla B (1999) Circadian expression of the light harvesting complex protein genes in plants. Chronobiol Int 16:115–128
Pilgrim ML, Caspar T, Quail PH, McClung CR (1993) Circadian and light regulated expression of nitrate reductase in Arabidopsis. Plant Mol Biol 23:349–364
Pillay VK, Nowak J (1997) Inoculum density, temperature, and genotype effects on in vitro growth promotion and epiphytic and endophytic colonization of tomato (Lycopersicon esculentum L.) seedlings inoculated with a pseudomonad bacterium. Can J Microbiol 43:54–361
Raja P, Uma S, Gopal H, Govindarajan K (2006) Impact of bioinoculants consortium on rice root exudates, biological nitrogen fixation and plant growth. J Biol Sci 6(5):815–823
Rangaswami G (1988) Soil plant microbe interrelationships. Indian Phytopathol 41:165–172
Raschke K (1979) Movements of stomata. In: Haupt W, Feinleib ME (eds) Physiology of movements, encyclopedia of plant physiology, vol 7. Springer, Berlin, pp 383–441
Roden LC, Ingle RA (2009) Lights, rhythms, infection: the role of light and the circadian clock in determining the outcome of plant pathogen interactions. Plant Cell 21:2546–2552
Samach A, Coupland G (2000) Time measurement and the control of flowering in plants. Bioassays 22:38–47
Schaffer R, Landgraf J, Accerbi M, Simon V, Larson M, Wisman E (2001) Microarray analysis of diurnal and circadian regulated genes in Arabidopsis. Plant Cell 13:113–123
Schilling G, Gransee A, Deubel A, Lezovic G, Ruppel S (1998) Phosphorus availability, root exudates and microbial activity in the rhizosphere. Ucits Pflanzenernahrung Boden 161:465–478
Seo PJ, Mas P (2015) Stressing the role of the plant circadian clock. Trends Plant Sci 20:230–237
Sharma AK, Bhattacharyya PN, Rajkhowa DJ, Jha DK (2014) Impact of global climate change on beneficial plant-microbe association. Annals Biol Res 5:36–37
Sheik CS, Beasley WH, Elshahed MS, Zhou X, Luo Y, Krumholz LR (2011) Effect of warming and drought on grassland microbial communities. Int Soc Microb Ecol J 5:1692–1700
Singh BK, Bardgett RD, Smith P, Reay DS (2010) Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol 8:779–790
Song YH, Ito S, Imaizumi T (2010) Similarities in the circadian clock and photoperiodism in plants. Curr Opin Plant Biol 13:594–603
Staiger D, Apel K (1999) Circadian clock-regulated expression of an RNA binding protein in Arabidopsis: characterization of a minimal promoter element. Mol Gen Genet 261:811–819
Staiger D, Apel K, Trepp G (1999) The Atger3 promoter confers circadian clock-regulated transcription with peak expression at the beginning of night. Plant Mol Biol 40:873–882
Staley C, Ferrieri AP, Tfaily MM, Cui Y, Chu RK, Wang P, Shaw JB, Ansong CK, Brewer H, Norbeck AD, Markillie M, Amaral FD, Tuleski T, Pellizzaro T, Agtuca B, Ferrieri R, Tringe SG, Pasa-Tolic L, Stacey G, Sadowsky MJ (2017) Diurnal cycling of rhizosphere bacterial communities is associated with shifts in carbon metabolism. Microbiome 5:65–78
Stevbak K, Scherber C, Gladbach DJ, Beier C, Mikkelsen TN, Christensen S (2012) Interactions between above and below ground organisms modified in climate change experiments. Nat Clim Chang 2:805–888
Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Mas P, Panda S, Kreps JA, Kay SA (2000) Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289:768–771
Sulpice R, Flis A, Ivakov AA, Apelt F, Krohan N, Encke B et al (2014) Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol Plant 7:137–155
Taiz L, Zeiger E (1998) Plant physiology. Sinauer Associates, Sunderland, MA, pp 543–590
Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abrmson L, Katz MN, Korem T, Zmora N, Kuperman Y, Biton I, Gilad S, Harmelin A, Shapiro H, Halpern Z, Seqal E, Elinay E (2014) Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159:517–529
Vancura V (1964) Root exudates of plants. Analysis of root exudates of barley and wheat in their initial phases of growth. Plant Soil 21:231–234
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132:44–51
Yanovsky MJ, Kay SA (2001) Signaling networks in the plant circadian system. Curr Opin Plant Biol 4:429–435
Yerushalmi S, Yakir E, Green RM (2011) Circadian clocks and adaptation in Arabidopsis. Mol Ecol 20:1155–1165
Zarrinpar A, Chaix A, Yooseph S, Panda S (2014) Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab 20:1006–1017
Zhong HH, McClung CR (1996) The circadian clock gates expression of two Arabidopsis catalase genes to distinct and opposite circadian phases. Mol Gen Genet 251:196–203
Zolla G, Badri DV, Bakker MG, Manter DK, Vivanco JM (2013) Soil microbiomes vary in their ability to confer drought tolerance to Arabidopsis. Appl Soil Ecol 68:1–9
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Maddur Puttaswamy, R. (2019). Circadian Rhythms in Plant-Microbe Interaction: For Better Performance of Bioinoculants in the Agricultural Fields. In: Giri, B., Prasad, R., Wu, QS., Varma, A. (eds) Biofertilizers for Sustainable Agriculture and Environment . Soil Biology, vol 55. Springer, Cham. https://doi.org/10.1007/978-3-030-18933-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-18933-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18932-7
Online ISBN: 978-3-030-18933-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)