Abstract
Sugarcane (Saccharum officinarum L.) is an important source of sugar, biofuels and bioenergy, helping in meeting requirements of the world from food energy to fuel energy. This chapter covers, in brief, the sugar milling processes from sugarcane crushing to sugar manufacturing, by-products release, biofuels production, and bioelectricity cogeneration. Technological aspects of various forms of bioenergy production including bioethanol, electricity, and biogas, at the sugar industry, are detailed in this chapter. Industrial process and mechanism of molasses fermentation into bioethanol have been described; additionally, the chapter illustrates the approaches for second-generation ethanol production and pretreatment methods for the same. Moreover, the chapter also narrates cogeneration of electricity from sugarcane and cogeneration efficiency enhancement systems like Centrale Thermique de Belle Vue (CTBV) and condensation-extraction steam turbine (CEST). The use of other resources of sugarcane, viz., trash and straw, for obtaining the energy year-round has also been described. Finally, a brief on production of biogas from sugarcane pressmud, bagasse, and vinasse has been presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

Keywords
Sugarcane is an important source of bioenergy. It has been widely grown for sugar production since long, however recently it has emerged as a promising bioenergy engenderment tool. Sugarcane can be exploited for producing ethanol from sucrose (first-generation biofuels), as well as from biomass (second-generation biofuels). It has great energy potential as it is an efficient crop in terms of fixing energy, and a huge biomass producer (Khan et al. 2017a). Moreover, sugarcane can also be utilized for bioelectricity production through cogeneration. Hence, sugarcane sucrose, bagasse, molasses, and sugarcane trash (collected from sugarcane field) can all be used for bioenergy fructification in one form or the other (Leal et al. 2013). This section details the use of various by-products of sugar industry for bioenergy production either in the form of ethanol as fuel for vehicles, heat for the industry, or biogas and bioelectricity for domestic and industrial use.
1 The Sugar Milling Process
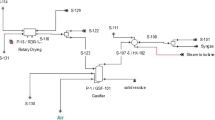
The sugar production in a sugar mill goes through several steps including crushing of sugarcane, squeezing and separation of raw juice from bagasse, and juice clarification through heating and chemical reactants addition. Then, the juice is concentrated by heat inputs that boil it to evaporate moisture leading to the stage of crystallization. Finally, the crystallized sugar is separated from molasses through the process of centrifugation. Several by-products obtained during the manufacturing of crystal sugar are either disposed of into the environment endangering the ecosystem or utilized judiciously to make valuable products with less toxic waste materials. The major by-products of sugar industry are bagasse, molasses, pressmud, vinasse, furnace ash, and steam released during juice evaporation and condensation. Figure 16.1 shows a simplified scheme of the sugar mills process (Colombo et al. 2014; Toasa 2009).
All the steps carried out in a sugar factory require heat, electrical, and mechanical energy. Most of the mill processes’ energy requirements are met out from its own energy source of bagasse acting as feedstock of energy units. The boilers are developed to burn bagasse at about 50% moisture for energy production that is used in the mill in different forms:
-
(i)
The addition of hot water to shredded cane facilitates up to 96% sucrose extraction ravaging only 4% in bagasse during milling.
-
(ii)
The juice is collected in tanks for liming/sulphitation, kept at proper temperature through heat exchange system, and then clarified from settled mud through filtration. This process involves precipitation of insoluble calcium phosphates of variable composition in hot melt liquor by adding phosphoric acid, followed by addition of calcium hydroxide slurry water (milk of lime) with a final pH of 7.2–7.4.
-
(iii)
The clarified juice is evaporated in the evaporator tandem to concentrate the juice, whereas steam is evolved in sufficient quantities for use in various other stages of the process.
-
(iv)
The concentrated juice is boiled in vacuum pans where low pressure and continuous provision of heat makes the juice supersaturated. This saturated syrup is termed as massecuite that comprises of crystals of sugar and molasses.
-
(v)
Massecuite is passed through centrifugation process at centrifuge mills that separates sugar crystals from molasses.
-
(vi)
Molasses are either used for the production of ethanol in distillery units or sent to the storage tanks for sale.
-
(vii)
During sugar production, a lot of surplus heat energy is produced that is either dissipated or used for electricity production.
The general parameters of thermal energy usage in a typical cane sugar factory (Ensinas et al. 2006) can be characterized as follows:
-
(i)
Raw juice leaves extraction system at 35 °C.
-
(ii)
Juice clarification takes place by heating up to 103 °C yielding 14–16% Brix.
-
(iii)
Treated juice enters the first stage of multi-effect evaporation system at 97 °C concentrating it from 15 to 65° Brix while reaching the fifth effect evaporation system. Absolute pressures of the evaporation stages are 1.69, 1.34, 0.98, 0.51, and 0.16 bars, respectively.
-
(iv)
Syrup is continuously heated at 80 °C using steam from the first effect of evaporation station for treatment.
-
(v)
Sugar syrup boiling into vacuum pans with steam from multi-effect evaporation station and concentrates to 91–93% Brix, which is termed as “massecuite.”
-
(vi)
Finally, centrifugation and separating of sugar from molasses take place.
-
(vii)
Overall about 5% of steam is lost in the process.
-
(viii)
Process steam is used at 2.1 bar pressure for sugar drying.
1.1 Bagasse
Bagasse is a fibrous residue generated by sugar industries after sugary juice extraction from sugarcane (Daud et al. 2007; Sun et al. 2004). Bagasse is a product of paramount importance in sugar mills’ operations as a source of ethanol and bioelectricity. On an average, 280 kg bagasse is yielded on crushing of one ton sugarcane, although it varies from 220 to 360 per ton, primarily determined by its fiber, juice, and trash content. In a sugar factory, one ton of refined sugar corresponds to about two tons of bagasse production. Fresh bagasse is also called mill wet bagasse with 48–52% moisture, 48% fiber, and 2–4% sugar and other elements (Lois-Correa et al. 2010; White 2009). The energy value of bagasse basically depends on its fiber content that comprises 30–50% cellulose, 28–35% hemicellulose, 20–25% lignin, 5% sugars, and about 2% minerals on dry weight basis. At 50% moisture, the gross heating value (GHV) of mill wet bagasse is 9361.4 kJ kg−1 and of dry bagasse is 19,498 kJ kg−1 (Abdalla et al. 2018).
Major uses of bagasse at the sugar mills are enlisted as follows:
-
Bagasse can be used for second-generation ethanol production at the sugar mills, which have great importance because of the fact that such kind of ethanol engenderment does not compete with sugar production (Khan et al. 2017b).
-
Bagasse is used to fuel the sugar mill (Antaresti et al. 2002; Charles and Shuichi 2003). In cogeneration system of sugar mills, bagasse is burnt to produce heat and steam for the mill functioning and electricity generation (Ensinas et al. 2006).
-
Enzymatic hydrolysis of sugarcane bagasse can result in glucose, xylose, ethanol, and methane production (Bommarius et al. 2008; Guilherme et al. 2015; Rezende et al. 2011).
-
Sugarcane bagasse and molasses can also find applications in producing fungal invertase (β-fructofuranosidase), a key catalytic enzyme in food industry (Veana et al. 2014). This enzyme is used to prepare artificial sweeteners (Aranda et al. 2006; Ashokkumar et al. 2001). The fructosyltransferase activity of the enzyme helps synthesize fructo-oligosaccharide compounds that improve intestinal microflora and has health benefits (Khandekar et al. 2014; Linde et al. 2009).
-
It is also used to make paper by virtue of its high cellulose content (Daud et al. 2007).
-
It is also employed as animal feed as it contains sugar and fiber.
-
Sugarcane bagasse ash may be partly used in ceramic floor tile due to high SiO2 contents (85.5%); moreover, it can also find applications as a source of fertilizer (Faria 2011).
2 Bioethanol Production at Sugar Industries
Fossil fuels, although played a discrete role in industrialization of emerging economies, yet, they gave rise to environmental and economic concerns (Colombo et al. 2014; International Energy Agency [IEA] 2015; O’Sullivan and Sheffrin 2003). An increasing awareness regarding these issues inculcated engineering to new research areas to evade fossil-dependent economies to an endurable form of growth using renewable green energy sources (Colombo et al. 2014; National Academic Press [NRC] 2000). Traditionally, sugar mills can produce bioethanol for fuel blending and generate their own energy from bagasse and other sugarcane feedstocks for their operations.
Biofuels like ethanol and biodiesel are among the rapidly developing sources of energy. Global production of biofuels amounted to 133 billion liters in 2015 distributing as 62% bioethanol and 24% biodiesel (Kummamuro 2016). Global bioethanol production tripled from its 2000 level and reached 52 billion liters in 2007 and to 99 billion liters in 2013. Brazilian ethanol from sugarcane and American ethanol from maize are by far leading the ethanol production. In 2015 the United States’ corn and Brazil’s sugarcane accounted for about 87% of the world ethanol production (Kummamuro 2016).
Bioethanol is a high-octane fuel which is used mainly as a gasoline stabilizer and replacement of fuel additive methyl tertiary butyl ether (MTBE) —an environmental inimical and contaminant to groundwater. High ethanol blends also help to control surge in prices for petroleum-based fuels (United Nations Development Programme [UNDP] 2009). Moreover, GHG release can be alleviated by increase in ethanol production with the hydrolysis of lignocellulosic cane residues (surplus bagasse and trash) in addition to molasses and sucrose (Seabra and Macedo 2011; Seabra et al. 2014).
Bioethanol originates from carbohydrates like sugar, starch, and celluloses by fermenting them with yeast or other microorganisms. The theoretical yield of ethanol is 617 L ton−1 of sucrose (Rein 2004) with the possible real recovery of 533.7 L ton−1 of sucrose (Table 16.1). Similarly, one ton of molasses yields about 263 L of ethanol. About 27.8 billion liters of bioethanol have been blended with fossil fuels in Brazil for motor vehicles, accounting for 26.3% of total Brazilian fuel consumption in 2017 (Barros and Berk 2018). Ethanol production from sugarcane is economically viable in many of the sugarcane-producing countries because of the drop in sugar support prices and advantages associated with the division of sugarcane production for multiple products. The cost of ethanol production in Brazil is in the range of US$2.50 to 5.70 daL−1 (Galvao et al. 2016; Walter and Dolzan 2009). Likewise, other cane-producing countries could have multipurpose factories for economic advantages as well as for partial replacement of fossil fuels with the ethanol.
2.1 Sugarcane as an Ethanol Source
Feedstocks for ethanol production comprise C3 plants (wheat, barley, and sugar beet) and C4 plants (sugarcane and corn):
-
1.
Corn: Two processes are used in the United States to obtain ethanol from corn, i.e., wet and dry milling process. Dry milling covers about 79% of ethanol production, while wet milling refers to only 21% of ethanol synthesis. Corn needs pre-hydrolysis before fermentation (United States Department of Agriculture [USDA] 2006).
-
2.
Sugar beet: Processing plants convert sugar beet into refined sugar and release molasses and beet pulp as by-products leading to ethanol production.
-
3.
Sugarcane: Sugar mills convert sugarcane to raw and refined sugars along with molasses and bagasse. Molasses and sugars are used in the production of alcohol for beverages and fuel. Raw or refined sugars and molasses do not need pre-hydrolysis before fermentation as in the case of corn or other feedstocks.
-
4.
Other feedstocks: Wheat, barley, and grain sorghum. The conversion efficiency of different feedstocks is listed in Table 16.1.
-
5.
Cellulosic biomass: Sugarcane bagasse and trash, wheat straw, wheat husk, rice straw, etc. need pretreatments before alcoholic fermentation.
The ethanol yields of corn are lower than sugarcane and sugar beet. In sugarcane or sugar beet allied fermentation units, the sugarcane or sugar beet extract, sugars, and molasses are used to make ethanol. Almost 25 L of sugarcane molasses is produced with each 100 kg raw sugar production (USDA 2006). Using raw sugar recovery factor of 12.26% and molasses sucrose contents of 49.2% for a sugarcane factory, one ton of sugarcane yields about 126.8 kg sucrose enabling to produce 73.8 L ethanol (Table 16.1). Sugar recovery is somewhat more in sugar beet compared against that of the sugarcane. Relatively more beet sugar recovery (15.5%) and molasses sucrose contents of 50% (molasses are 4% by weight of sugar beet) enable it to produce 93.9 L ethanol ton−1 of sugar beet. One ton of molasses would yield 262.7 L of ethanol while raw and refined sugars would produce 512.5 and 533.7 L ethanol, respectively. However, per hectare yield of sugarcane is extremely high as compared to sugar beet making it an ideal candidate for finding applications as a biofuels source to meet huge demands of the same.
2.2 Technological Aspects of Ethanol Production from Sugarcane
The prerequisites for ethanol production are:
-
Uninterrupted accessibility of feedstock in larger quantities
-
Escalation in production efficiency to make the process sparingly suitable
-
Environmentally and instrumentally safe process
Steps involved in bioethanol production process are feedstock collection, feedstock preparation, washing/separation/hydrolysis, fermentation using yeast, distillation, dehydration, and removal of solid residues and CO2 (Barriga 2003). Major determining step, however, is fermentation that involves microbial activities under specific conditions. Different species of bacteria and yeasts responsible for alcoholic fermentation have been investigated by a number of workers (Behera et al. 2012; Bangrak et al. 2011; Cazetta et al. 2007; Gasmalla et al. 2012; Morias et al. 2007). Current industrial ethanol fermentation is largely achieved through Saccharomyces cerevisiae that is tolerant to low pH and high ethanol concentrations; moreover, Zymomonas mobilis bacterium is also employed for ethanol production from glucose and sucrose (Yang et al. 2007).
Molasses appears to be the cheapest source of ethanol production that can be purified to make absolute and rectified spirit (Sam 2012). The chemicals required during fermentation process are sulfuric acid, phosphoric acid, and ammonium sulfate. The important chemical reactions incurred in the process are:
-
C12H22O11 + H2O → 2C6H12O6 yeast/enzyme invertase (Sam 2012)
-
C6H12O6 → 2C2H5OH + 2CO2 yeast/enzyme zymase (Morias et al. 2007)
-
2C6H12O6 + H2O → ROH + RCHO (high molecular weight alcohols and aldehydes produced as side reaction, Sam 2012)
Molasses from sugar industries are stored in large-volume storage tanks for continuous operation of distilleries. Molasses, at first, are diluted with water to the level of 15–20% sugar concentration (Gasmalla et al. 2012). Then acids are added to adjust pH between 4.5 and 5 for the growth of yeast to break up the sucrose in the diluter equipment. A yeast culture tank containing ammonium and magnesium phosphate is used for yeast propagation that produces invertase and zymase type of catalytic enzymes. A schematic diagram is given for molasses fermentation in Fig. 16.2.
The treated molasses and the yeast are then supplied to the fermentation chamber at a ratio of about 20 to 1 and powered with heating coils or jackets to maintain temperature of 20–35 °C in the tanks. The fermentation process is carried out for 30–70 hours considering temperature, sugar concentration, and yeast count. During this process carbon dioxide is also produced by microbial activity. Henceforth the mixture is strained to remove solid and slurry material leaving alcohol and water behind; where alcohol concentration is around 10% that is fed to the distillation unit for refining.
Distillation and dehydration of alcohol mixture are carried out in distillation unit for purifying the ethanol. In distillation, ethanol and water are separated by considering their different boiling points. The series of distillations lead to 95% pure ethanol leaving behind some intermolecular spaced water in it. This intermolecular spaced water is escaped through dehydration, which is done either by azeotropic distillation using entrainer (benzene or cyclohexane) or by molecular sieves like zeolite with pore size under 0.4 nm—preferably 0.3 nm (Angstrom)—that trap 0.44 nm ethanol molecules and drain out water molecules having 0.28 nm diameter (Carmo and Gubulin 1997; Díaz et al. 2010).
2.3 Ethanol Production from Lignocellulosic Biomass: Bagasse, Pressmud, Trash, and Others
Apart from sugar, and first-generation ethanol, sugarcane is also a great source of lignocellulosic biomass which can be subjected to second-generation ethanol production. High biomass yields of sugarcane make it an excellent source of the same in this regard. Sugarcane trash, straw, and bagasse, all can be employed for alcoholic fermentation. Bagasse , the fibrous residue obtained after extracting the juice from sugarcane during the sugar production process, has great potential as substrate for second-generation ethanol as it is found in large quantities in the sugarcane-growing countries. Currently, bagasse is either stored in a stockpile or burned for cogeneration, hence the growing interest in developing technologies for its conversion not only to ethanol but other petroleum-based chemicals like polymers, resins, and organic acids.
Plant cell wall is composed of cellulose, hemicellulose, and lignin. Sugar or molasses fermentation involves plain pretreatment steps as they do not need saccharification. Contrarily, lignocellulosic biomass needs another pretreatment to solubilize cellulose. There are about 73.9 million tons of dry wasted crops and about 1.5 billion tons of dry lignocellulosic biomass that need proper utilization annually (Kim and Dale 2005). The lignocellulosic biomass fermentation is of significance regarding food vs. fuel issues as these feedstocks does not impact sugar production. The pretreatment for lignocellulosic biomass is prerequisite to hydrolyze and delignify the material for enzymatic effectiveness (Gould 1985). Such pretreatments include acid treatment with sulfuric acid (Esteghlalian et al. 1997), alkaline treatment by aqueous ammonia to take out 70–85% lignin and solubilize 40–60% hemicelluloses (Kim et al. 2003) without affecting other components (McMillan 1997), and thermo-acid treatment. The steam (hydrothermal) treatment (150–230 °C) breaks down the plant cell wall through hydrolysis to ease enzymatic biodegradations (Boussarsar et al. 2009; Shaibani et al. 2011; Stenberg et al. 1998). Delignification in sugarcane bagasse with dilute acid-sodium hydroxide yields 96 and 85% of hemicellulose and lignin fractions, respectively, increasing the cellulose conversion from 22.0% in untreated to 72.4% in treated bagasse (Rezende et al. 2011).
The pretreatment of cellulosic biomass facilitates enzymatic hydrolysis to produce glucose and xylose as depicted in Table 16.2 (Guilherme et al. 2015; Rezende et al. 2011; Shaibani et al. 2011), followed by fermentation for ethanol (Bommarius et al. 2008; Sun and Cheng 2002). Another method called immobilized cell system provides high density in the reactor that allows elevated flow rate to squeeze the process time. It works through attachment of yeast to a surface, entrapment in a porous matrix, and containment behind an obstacle and self-agitation (Verbelen et al. 2006). The entrapped yeast in porous matrix produces 11.5 times more ethanol than the free yeast cells (Nigam 2000).
Cellulose conversion technologies are just emerging, technically unsound yet and commercially immature, hence will only allow utilization of lignocellulosic parts of sugarcane after having a dynamic research in the area (Rezende et al. 2011).
2.3.1 Biomass Composition
Lignocellulosic biomass of cane crop, such as sugarcane bagasse and energy cane bagasse, are composed of cellulose (30–43% DW, a linear polymer of glucose units linked by a β-glucoside bond), hemicellulose (23–27% DW, a branched heteropolymer with xylan as the major constituent, which is a heteropolysaccharide with varying proportions of xylose, arabinose, galactose, and mannose and with other groups such as glucuronic acid or acetic acid attached to its backbone), lignin (25–27% DW, a complex, heterogeneous, and branched polymer of phenolic and enolic compounds), and other components, e.g., protein, ash, and extractives (Aita and Kim 2011; Oladi and Aita 2017; Oladi and Aita 2018). The association and complexity of the lignin-polysaccharides complex make enzymatic accessibility a challenge, which is the main obstacle in the bioconversion of fermentable sugars to second-generation ethanol (Aita and Kim 2011).
2.3.2 Biomass Pretreatment
Once the biomass is harvested, collected, and transported to the processing plant, the next step is to convert the biomass into ethanol. This can be accomplished by depolymerizing the lignin-polysaccharides matrix into their respective monomers. US DOE has reported that pretreatment is the second largest production cost following the cost of feedstock and has predicted that this would still be the case in future commercial-scale facilities (Humbirt and Aden 2008). The high costs are related to the need for reactors capable of operating under high temperatures and pressures, the use of corrosive catalysts and the need for their recovery (Stephen et al. 2012). Several technologies have been developed for the conversion of lignocellulosic biomass into ethanol and are grouped into two categories, biochemical and thermochemical conversion technologies. In biochemical conversion, the cellulose and hemicellulose present in the lignocellulosic biomass are broken down to their monomeric sugars (glucose, xylose, arabinose, mannose, and galactose) and then fermented into ethanol. The lignin which cannot be fermented into ethanol is fed into a boiler for energy production. In thermochemical conversion, the lignocellulosic biomass is gasified to produce syngas (a mixture of carbon monoxide, hydrogen, carbon dioxide, methane, and nitrogen) and then fermented or catalytically converted to ethanol.
2.3.3 Biochemical Conversion
The overall target of biochemical conversion is to provide enzymes better accessibility to the polymeric sugars, thus enhancing the bio-digestibility of lignocellulosic biomass. The biochemical conversion of bagasse to ethanol can be accomplished by the following steps: pretreatment, detoxification, hydrolysis, fermentation, and distillation. A successful biochemical conversion method should improve enzymatic accessibility by having minimal fermentable sugar losses, reducing the formation of toxic or inhibitory compounds (i.e., organic acids, furan derivatives, phenolic compounds), generating minimum waste products, having low capital and energy costs, and being suitable for a wide range of lignocellulosic biomass materials (Sun and Cheng 2002). The toxic compounds generated from the degradation of cellulose, hemicellulose, and lignin during pretreatment have shown inhibitory effects on downstream processes (i.e., enzymatic hydrolysis, microbial fermentation) thus the need for their removal (Alvira et al. 2010). Biochemical conversion methods can be classified as physical (e.g., grinding, extrusion, irradiation), chemical (e.g., acid, alkaline, liquid hot water, ionic liquids), physicochemical (i.e., steam explosion, AFEX), biological, or a combination of these methods. Although dilute acid, alkaline, and steam pretreatments are the technologies most commonly used, a variety of biochemical pretreatments have been used for sugarcane and energy cane bagasse, each having inherent advantages and disadvantages (Table 16.3).
2.3.4 Detoxification
Hydrolysates that result from the biochemical pretreatment of lignocellulosic biomass may contain by-products other than sugars, including organic acids, furans, and phenolic compounds. These inhibitory compounds can negatively affect downstream processes such as enzymatic hydrolysis and fermentation. The nature and concentration of the generated inhibitory compounds are directly related to pretreatment conditions and biomass composition (Jönsson and Martín 2016).
Detoxification methods can be categorized into physical (i.e., evaporation), physicochemical (i.e., liquid-liquid extraction, ion-exchange resins, overliming, activated carbon, flocculation), microbial (i.e., Issatchenkia spp., Trichoderma spp.), and enzymatic (i.e., laccases, peroxides) (Canilha et al. 2012; Deng et al. 2018; Deng and Aita 2018; Palmqvist and Hahn-Hägerdal 2000). There might be a need to combine several detoxification strategies to reach the target concentration level for inhibitors as each of these strategies have some inherent shortcomings (Ranjan et al. 2009). Sugar losses while applying detoxification strategies to pretreated biomass hydrolysates should be negligible (Mussatto and Roberto 2004). According to Sivers et al. (1994), the cost of a hydrolysate detoxification process can be up to 22% of the total cost of ethanol production.
2.3.5 Hydrolysis Technologies
Hydrolysis is the process that comes after biochemical pretreatment and detoxification, and it involves breaking down the polymeric sugars into their monomeric forms. This process is often catalyzed by an acid or enzymes, and it is critical in the production of ethanol since the quality of the hydrolysate will affect the subsequent fermentation process. This strategy not only offers the possibility of substrate specificity but reduces processing time from weeks to hours without the need of carbohydrate consumption as seen with biological pretreatment methods (Moreno et al. 2015). Hydrolysis represents 25–30% of the operational costs (Valdivia et al. 2016). Acid hydrolysis involves the use of dilute or concentrated acids, and it is only applicable to lignocellulosic biomass that has been pretreated with dilute acid technologies. Dilute acid (0.4%) at 215 °C with 3 min residence time are employed for converting cellulose to glucose (Hamelinck et al. 2005). A drawback is the recovery of the acid in high yields. However, two US companies, BlueFire Renewables and Virdia, claim to have overcome this challenge at pilot scales using concentrated sulfuric acid (Arkenol process) and hydrochloric acid (cold acid solvent extraction process), respectively (Hayes 2016).
Compared to acid hydrolysis processes, enzymatic hydrolysis is the preferred method due to its effectiveness, mild pH (4–5) and temperature (45–55 °C), and non-corrosive properties (Aditiya et al. 2016; Mohapatra et al. 2017). The highest glucose yields that can be achieved with untreated biomass using excessive amounts of enzymes will not exceed 20% (Mosier et al. 2005). Despite the improvement in the digestibility of lignocellulosic material after pretreatment, the complex structure of lignocellulosic biomass still requires the use of enzymes, e.g., cellulase, to yield maximum carbohydrate conversions. Enzymatic hydrolysis strongly depends on microbial species, biomass chemical composition, pretreatment method, and enzyme mode of action (Pothiraj et al. 2014). Enzyme loadings, the use of accessory enzymes (xylanase, feruloyl esterase, pectinase, laccase), and the presence of inhibitory compounds (phenolic compounds, furan derivatives, organic acids) can also affect carbohydrate conversion yields during enzymatic hydrolysis (Bussamra et al. 2015). Enzymes used for lignocellulosic biomass hydrolysis can be produced by both bacteria such as Clostridium cellulovorans and fungi such as Trichoderma reesei, Aspergillus niger, and Pycnoporus spp. (Mohapatra et al. 2017; Talebnia et al. 2010).
The availability of cost-effective commercial enzymes to produce second-generation ethanol remains a challenge, and innovative bioprocesses to produce a new generation of enzymes are still needed. Current commercially available enzymes include Accellerase®1500 (DuPont), a mixture of exoglucanase, endoglucanase, xylanase, and β-glucosidase. Accellerase® XP (DuPont), Accellerase® XC (DuPont), and Accellerase® BG (DuPont) are accessory enzymes with cellulose and hemicellulose activities which can be used in combination with Accellerase®1500. Cellic® CTec2 (Novozymes®) and HTec2 (Novozymes®) contain cellulase, β-glucosidase, xylanase, and endoxylanase, respectively. Celluclast® 1.5 L (Novozymes®) has cellulase activity.
2.3.6 Fermentation
The hexose (mostly glucose) and pentose (mostly xylose) sugars released during the hydrolysis of bagasse or lignocellulosic biomass are subsequently converted into ethanol via anaerobic or aerobic fermentation by a variety of microorganisms. The yeast Saccharomyces cerevisiae has been historically used for the fermentation of glucose into ethanol. However, this organism cannot ferment pentose sugars, a significant limitation since pentose sugars account for at least 25% of the mass balance of many lignocellulosic biomass materials (Hayes and Hayes 2009). For lignocellulosic ethanol to be economical, fermentation of both hexose and pentose sugars must result in high yields. Theoretically, each kg of glucose and xylose can produce 0.45 kg carbon dioxide and 0.51 kg ethanol (Hamelinck et al. 2005). A way to overcome this obstacle is to use microbial genetic engineering tools. Common targeted organisms include Zymomonas mobilis, Escherichia coli, and Saccharomyces cerevisiae (Lawford and Rousseau 1991). However, in some cases, the genetically modified strains of these conventional fermentative microorganisms are not sufficiently robust to function in large-scale industrial environments (Hahn-Hagerdal et al. 2007). Several integrated technologies have been proposed to increase the efficacy of ethanol production such as separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF), simultaneous saccharification and cofermentation (SSCF), and consolidated bioprocessing (CBP).
2.3.6.1 Separate Hydrolysis and Fermentation (SHF)
SHF is a classic two-step process configuration where lignocellulosic biomass hydrolysis and fermentation are carried out separately thus allowing each step to be performed at its optimal operating conditions (pH and temperature) and at relatively shorter times (Sarris and Papanikolaou 2016). Substrate concentration at 10% (w/w) solid loadings are defined as the most adequate considering arising mixing difficulties and the accumulation of sugars which inhibit enzyme activity (end-product inhibition), thus ultimately affecting ethanol yields (Jambo et al. 2016; Sánchez and Cardona 2008). The following ethanol concentrations have been reported with steam pretreated sugarcane bagasse (26 g L−1), liquid hot water pretreated sugarcane bagasse (25 g L−1), phosphoric acid pretreated sugarcane bagasse (25 g L−1), sulfuric acid pretreated sugarcane bagasse (20 g L−1), dilute ammonia pretreated sugarcane bagasse (20 g L−1) or energy cane bagasse (23 g L−1), and ionic liquid (1-ethyl-3-methylimidazolium acetate) pretreated energy cane bagasse (18 g L−1) (Aita et al. 2011; Bezerra and Ragauskas 2016; Cao and Aita 2013; Neves et al. 2016; Qiu et al. 2014; Torres da Silva et al. 2016).
2.3.6.2 Simultaneous Saccharification and Fermentation (SSF)
Hydrolysis and fermentation are combined in a single reactor so that glucose is fermented (separately of pentoses) or cofermented to ethanol as soon as the sugars are released by enzymes, thus overcoming the accumulation of hydrolytic end products (glucose and cellobiose) which are inhibitory to cellulolytic enzymes (Ferreira et al. 2010). Benefits of this process include ease of operation, low equipment requirement than SHF, and the presence of ethanol in the medium which reduces the contamination risk of external microflora (Vohra et al. 2014). SSF also allows for high solid loadings (30% w/w) (Mohapatra et al. 2017). Major drawbacks include difficulty in optimizing process parameters for both enzymes and microorganisms, such as incompatible hydrolysis (45–50 °C) and fermentation (28–30 °C) temperatures, ethanol toxicity to microorganisms, and enzyme inhibition by ethanol (Rastogi and Shrivastava 2017). The use of protein engineering to lower the optimum temperatures of enzymes would be a challenge so the alternative is to use thermotolerant strains that could grow well and produce ethanol at higher temperatures (Hasunuma and Kondo 2012). SSF still requires the microorganism to be grown in a separate reactor where 9% of the sugars from hydrolysates are used to grow cellular mass (Hayes 2016). A study conducted with 10% sugarcane bagasse pretreated with sodium hydroxide/hydrogen peroxide combination resulted in 25 g L−1 ethanol using Kluyveromyces maxianus DW08 as the ethanol-fermenting yeast (Cheng et al. 2008). Other ethanol concentrations reported include liquid hot water pretreated sugarcane bagasse (19 g L−1), sulfuric acid pretreated sugarcane bagasse (18 g L−1), and phosphoric acid pretreated sugarcane bagasse (17 g L−1) (Bezerra and Ragauskas 2016; Neves et al. 2016; Torres da Silva et al. 2016).
2.3.6.3 Simultaneous Saccharification and Cofermentation (SSCF)
A simplified process where the pretreated lignocellulosic biomass material is combined with different enzymes and microorganisms in the same reactor with the purpose of hydrolyzing both the hexose and pentose sugars and fermenting them into ethanol (Lynd 1996). However, SSCF has been slow to develop commercially because optimal conditions required for hydrolysis and fermentation are different, and improved microbial strains are needed for the cofermentation of all sugars (Chandrakant and Bisaria 1998; Koppram et al. 2013). Saccharomyces cerevisiae TMB3400, a xylose-fermenting recombinant strain, and P. stipitis CBS6054, a naturally xylose-fermenting strain, were compared in SSF of non-detoxified hydrolysate from steam pretreated sugarcane bagasse previously impregnated with sulfate (Rudolph et al. 2007). The highest ethanol concentration (26.7 g L−1) was obtained with S. cerevisiae TMB3400, whereas P. stipitis CBS6054 resulted in 19.5 g L−1 under aerated conditions.
2.3.6.4 Consolidated Bioprocessing (CBP)
CBP represents the ultimate simplification of the enzymatic hydrolysis and microbial fermentation process where a fungus (i.e., Trichoderma reesei, Fusarium oxysporum, Neurospora crassa, Aspergillus spp., Rhizopus spp., Paecilomyces spp.), a yeast (i.e., Candida shehatae, Pachysolen tannophilus, Saccharomyces cerevisiae, Pichia stipitis), or a bacterium (i.e., Clostridium thermocellum, Escherichia coli, Klebsiella oxytoca, Zymomonas mobilis) is capable of both hydrolyzing the polysaccharides and fermenting them into ethanol in a single reactor (Aditiya et al. 2016; Taherzadeh et al. 2000). CBP has the potential to offer the lowest production cost for ethanol but current limitations include low yields and long periods for fermentation by up to 12 days (Koutinas et al. 2014). Thermophilic microorganisms, e.g., Clostridium spp., have an advantage over conventional yeast in that they can withstand high temperatures, but a major obstacle for their industrial application is their low ethanol tolerance (<2% v/v) (Rastogi and Shrivastava 2017). No microorganisms or compatible combinations of microorganisms are available that exhibit the whole combination of features required for the development of CBP (Kazi et al. 2010). The success of this approach relies heavily on genetic and metabolic engineering for the development of CBP-enabling microorganisms for the industrial production of second-generation ethanol.
2.3.7 Thermochemical Pretreatment
Gasification involves the thermal (600–1000 °C) decomposition of both lignin and polysaccharides into a syngas in the presence of an oxidizing agent (oxygen or steam) (Kumar et al. 2009). Syngas is mainly a mixture of carbon monoxide, carbon dioxide, hydrogen, water, and short-chain hydrocarbons which can then be converted into fuels and chemicals (e.g., ethanol, methanol, higher alcohols, gasoline) by either fermentative microorganisms or metal catalysts (Sutton et al. 2001). The quality of syngas depends on the type of biomass (i.e., chemical composition, moisture, particle size, tar content), design of the gasifier (i.e., updraft or downdraft fixed bed reactors, bubbling or recirculating fluidized bed reactors), and operational conditions of the reactor (Kennes et al. 2016). A challenge of current gasification technology is the presence of large amounts of tar (a mixture of unconverted organic materials and ash) in the syngas, which results in plugging of downstream equipment (compressors and gas engines) (Watson et al. 2018). Other limitations facing this technology include separation of gaseous products and the poisoning of catalysts by gasification products (hydrogen sulfide, thiophene, carbonyl sulfide) (Kochermann et al. 2015; Yin et al. 2016).
Anaerobic bacteria (i.e., Clostridium spp.) can ferment syngas into ethanol; however, the composition of syngas should be optimized so that it has reduced impurities including tar, ash, nitrogen oxides, and hydrogen sulfide and it mainly contains carbon monoxide, carbon dioxide, and hydrogen (Brown 2005). Advantages of the biological route over the use of catalysts include the use of lower processing temperatures and pressures, and higher ethanol yields, thus reducing the energy and capital cost of conversion (Farzad et al. 2016). Limitations involve providing optimum growth conditions (levels of nutrients and impurities) for microorganisms and microbial sensitivity to both impurities and high concentrations of end products (Mohammadi et al. 2011). In catalyst-based ethanol production, syngas is mixed with water and methanol to improve yields of higher alcohols, and the mixture is passed through a catalyst to obtain not just ethanol but methanol, higher alcohols, and other hydrocarbon products (Dwivedi et al. 2009). Natural catalysts (dolomite, hematite, trona) and transition metal catalysts (Ni-Mg-Al, Ni, NiO) are preferred due to their ease of recovery at the end of gasification (Guan et al. 2009; Wu et al. 2006). Inexpensive, large-scale biomass gasifiers have yet to be demonstrated as well as the successful commercial-scale production of fuels from syngas.
2.3.8 Distillation
One of the main issues associated with ethanol production from lignocellulosic biomass (biochemical and thermochemical conversion platforms) relies on the cost-effective recovery of ethanol from the hydrolysates or fermentation broths. Ethanol recovery through common distillation methods is not technically challenging but it requires significant amounts of energy to yield concentrations of up to 85 wt% ethanol (Huang et al. 2008). The energy required to separate ethanol from water by distillation methods amounts to 10% of the energetic content of the recovered ethanol, with an exponential increase for ethanol concentrations below 10% (Vane 2008). Only anhydrous ethanol (>99 wt% ethanol) can be blended with gasoline and be used in conventional gasoline-burning engines. Ethanol and water form an azeotrope at 95 wt% ethanol thus making it impossible to recover pure ethanol through simple distillation; hence, the need for a special dehydration process to recover anhydrous ethanol (Haelssig et al. 2012). The most commonly used techniques for ethanol dehydration include adsorption distillation, azeotropic distillation, chemical dehydration, diffusion distillation, extractive distillation, and membrane distillation (Aditiya et al. 2016). Liquid (water, unutilized fermentable sugars, process chemicals) and solid (mostly lignin) wastes are generated at the end of the ethanol process and are collectively known as stillage, which can neither be sent to the sewer system nor be discharged into a water body or soil (Sheehan and Greenfield 1980). Several stillage utilization techniques have been developed to recover energy (heat and power generation) and process water, as well as its potential use as animal feed, fertilizers, and road-building materials (Baral et al. 2017).
2.4 Stress Management During Fermentation
The stress factors for yeast which affect fermentation efficiency include concentrations of sugar and ethanol, bacterial infection, temperature, nutrient levels, and mycotoxins (Bleoanca and Bahrim 2013). Yeast can normally tolerate one stress at a time to some extent. However, simultaneous two or more stresses appear to be deleterious for yeast. Lactic and acetic acids are the spin-off produced by the contaminating bacteria (lactobacilli, acetobacter, and gluconobacter), which may hinder the fermentation process at higher concentrations.
Before rectification, some aldehydes produced in the process of fermentation are detached at aldehyde column. The stream in columns accumulates aldehydes at the top, fuselol-containing ethanol in the middle, and water at the bottom. The middle stream is fed to rectification column to relent 95% pure ethanol.
Process of ethanol production by molasses fermentation (Sam 2012)
2.5 Uses of Ethanol as Biofuel
-
Gasoline is blended with ethanol for petrol engine vehicles for environmental benefits. Routine fuel additives like tetraethyl lead is environmentally unsafe, MTBE is water pollutant while toluene and benzene are also deleterious to health. Ethanol, due to structural oxygen, lessens the release of damaging GHGs, like unburnt hydrocarbons and carbon monoxide.
-
Gasoline can be blended with anhydrous ethanol from 100 to 5% or less. In Brazil the flex-fuel vehicles (FFV) can use all the blends of ethanol. In countries, like Sweden, a maximum of 85% ethanol blend (E85) is used while in some countries E10 is used.
-
Ethanol is also used to make ethyl tertiary butyl ether (ETBE) retaining 44% ethanol, an oxygenated octane used as gasoline blend.
-
Diesel engines are also being tested for ethanol use.
2.6 Cost of Ethanol Production
The cost of first-generation ethanol production using different feedstocks is given in Fig. 16.3. The cost estimates are based only on estimated costs of feedstock, sugarcane market prices, and processing cost but exclude capital and transportation costs. The data indicates that cost of production varies with change in feedstock. The total cost of converting sugarcane into ethanol in Brazil appears the lowest as it has been estimated approximately US$2.14 daL−1 (per decaliter), while maximum cost incurred on refined sugar conversion into ethanol is in the United States (US$10.49 daL−1).
Estimated ethanol production cost with different feed stocoks in different countries ∗USA, ∗∗Brazil, ∗∗∗E.U. (USDA 2006)
Some feedstocks are cheaper than the processing costs while the others are costly. Hence refine and raw sugars are dearer feedstocks compared to sugarcane, corn, and molasses feedstocks. The cost of converting sugar beets into ethanol was estimated at US$5.07 daL−1 in the European Union (EU) that is the maximum cost among all the feedstocks. Molasses obtained from sugarcane or sugar beets as well as corn appear to be cost-competitive feedstock for ethanol production. Hence, different feedstocks have variable cost-effectiveness for ethanol production in different countries.
3 Bioelectricity Production at Sugar Mills Through Cogeneration
Cogeneration, meaning a joint heat and power production, is the concurrent creation of electricity, heat, and/or cooling with a single source of fuel at or near the sink. The most common fuels for cogeneration are natural gas, coal, plant materials (bagasse, rice husk, sugarcane trash, etc.), and gas from sludge or landfill material, liquid fuels, and renewable gases. Bagasse can be fired against coal, oil, or natural gas in a power plant to heat the boilers. The steam produced in the boilers can be used as a heat source for industrial (process heat) and domestic purposes. It can also be used in steam turbines for bioelectricity generation.
Bagasse cogeneration was initiated in Mauritius and Hawaii where about 26 and 10% of grid electricity were obtained from sugar mills in 1926–1927, respectively (International Sugar Organization [ISO] 2009). Cogeneration improved efficiency of the sugar plants to the tune of about 50% than separate electricity and power production. Traditional sugar mills generate their own heat and power but with low steam and temperature installation systems, whereas in high-efficiency cogeneration systems effective boilers permit spare electricity and economical heat basis for sugar processing. Traditional sugar mills generate 10–20 kW electrical energy per ton of cane for internal use, while modern sugar mills can have the efficient cogeneration systems of up to 115–120 kW per ton of cane (Kamate and Gangavati 2009).
Many countries have inadequate renewable energy resources and oil, gas, or coal reserves and hydropower supplies. Sugarcane bagasse signifying 30% of cane is commonly used incompetently to fulfil the factory’s energy requirement for cane processing (Deepchand 2005), the competency of which can further be used to create surplus energy. In Mauritius, more than 90% sugar factories export 318 Gigawatt bagasse-made bioelectricity (40% of total) to the national grid in crushing season (Deepchand 2005). In the late 1980s, an annual increase in electricity use and demand in Mauritius was estimated to be 11 and 9.5%, respectively. Although Mauritius offers a successful demonstration of bagasse energy for other countries, it produces 60 kW of electricity per ton of cane that is less than 125 kW ton−1 made through Centrale Thermique de Belle Vue (CTBV) operated with 2 × 35 MW power plants at about 82 bars (Deepchand 2005).
Bagasse is burnt in furnaces to make steam for power production. Its value as a fuel depends mainly on its calorific value that is sequentially affected by its water content. A good milling process occurs at 45% bagasse moisture content, whereas the milling efficiency is reduced at 52% moisture. Mostly the boilers are designed to work at about 50% bagasse moisture content. Cogeneration with bagasse is among one of the most successful energy projects and is being established in several sugarcane-producing countries like Mauritius, the United States, India, Brazil, and Pakistan. Simultaneous heat and power generation from sugarcane bagasse presents a renewable energy alternative to uphold sustainable growth, boost sugar industry’s income, and climate resilience through production of carbon-neutral electricity (De Rosa and Salvadori 2007).
3.1 Potential for Cogeneration in Sugarcane-Growing Countries
Table 16.4 shows Food and Agriculture Organization’s statistics (FAOSTAT 2017) regarding the cogeneration potential of different countries. A significant amount of bioelectricity can be exported to the grids using two profitable technologies of steam pressures (44 and 82 bars) from the sugar mills having a minimum cane crushing of 200 to 300 ton per hour. It also emphasizes coupling of less capacity plants into larger units. There are about 107 countries producing sugarcane, whereas 85 countries are producing sugarcane more than 100,000 tons per year (FAOSTAT 2017). This table also summarizes the overall estimates for electricity production from sugarcane in the countries having annual production of 1.5 million tons of cane.
3.2 Mechanism of Cogeneration
The sugar industry entails heat, electric, and mechanical energy to execute the milling process. Generally, the energy production in a factory is designed merely to furnish the sugar plant requirements where a range of machines and processes are taking place (Colombo et al. 2014).
Bagasse is fired in boiler house to generate heat and dispensing water steam. The boilers are large cylindrical chambers containing lower smaller part for burning of bagasse while the upper big part contains water in tubes that is in immediacy to the lower part for receiving heat (Fig. 16.4). Boilers are tied with backpressure or condensing-extraction steam turbines which deliver steam and electrical energy to the system (Khatiwada et al. 2012; Purohit and Michaelowa 2007). The steam is used either for mill processes or at high pressure for revolving turbines of electricity generation system. The general schematic view of bagasse-based power plant is described in Fig. 16.4. A sugar mill crushing ~2000 ton sugarcane per day is able to generate ~14,000 kW of bioelectricity daily including about 6000 kW for the industry’s internal consumption. The major steps involved in production of electricity using bagasse as raw material are as follows:
-
(i)
Bagasse storage in a moisture-free area of sugar mill.
-
(ii)
A railing system transfers bagasse from storage to boilers.
-
(iii)
The water in the tubes passes through economizers to make it warmer, and blazing of bagasse converts water into high-pressure steam that is partly used in sugar manufacturing process.
-
(iv)
The high-pressure steam flows through controlled tubes to the turbines that rotate them to operate connected generators.
-
(v)
Consequently, electricity is generated that is used in sugar industry while excess is provided to the grids.
In a sugar mill’s cogeneration systems, the general parameters of concern have been reported by many researcher (Colombo et al. 2014; Ensinas et al. 2006; Hassuani et al. 2005), which include:
-
Moisture in wet bagasse: 50%
-
Wet bagasse low heat value (LHV): 7500 kJ kg−1
-
Bagasse mean LHV: 7984 kJ kg−1
-
Process mechanical energy demand: 16 kW per ton of cane
-
Process electricity demand: 12 kW per ton of cane
-
Boiler’s thermal efficiency: 85%
-
Steam turbines isentropic efficiency: 80%
-
Boilers and turbines operate at 15–105 bar pressure with analogous temperature of 300–525 °C
-
Pump isentropic efficiency: 80%
-
Electric generator efficiency: 96%
-
Mill electric engines efficiency: 89%
3.3 Cogeneration Efficiency Perfection
Generally low-efficiency cogeneration systems like Rankine steam cycles and old back pressure steam turbines (BPST) have been used for cogeneration, but recently superior cogeneration systems are being focused (Dias et al. 2013; Macedo et al. 2001; Pellegrini et al. 2013), which upshot more surplus electrical energy. As suggested by Banco Nacional de Desenvolvimento Econômico e Social (BNDES) and Centro de Gestão e Estudos Estratégicos (CGEE), surplus energy per ton of sugarcane processing is practicable with higher-pressure condensation-extraction steam turbines (CEST) than with BPST as shown in Table 16.5 (BNDES and CGEE 2008; Khatiwada et al. 2012; Purohit and Michaelowa 2007). Hence, conventional sugar mills are able to generate only 10–20 kW of electrical energy by spending about 500 kg steam and process 1 ton of cane (Deshmukh et al. 2013). Advanced sugar mills having dexterous cogeneration systems, on the other hand, can yield electrical energy of about 120 kW with 1 ton of cane processing (Kamate and Gangavati 2009). A further enhancement in power generation is also attainable by following process steam saving techniques with modifications in sugar mills. Such technology lowers down the steam usage of about 280–300 kg ton−1 of sugarcane processing including ethanol distillation, the surplus of which can be used to enhance the electricity generation. Fractional use of sugarcane trash further promotes surplus power generation (ISO 2009).
3.4 Efficiency of Cogeneration Systems
Ensinas et al. (2006) studied four configurations of cogeneration systems that could be applied in sugarcane factories. Configuration system-I comprised steam cycle with back pressure steam turbine. In this case the sugar process governs the steam formation by the boilers without condensation system. It is the most common cogeneration system in sugar factories operative during the crushing seasons only (Table 16.6). About 470 kg saturated process steam at 2.1 bar pressure per ton of cane is used leaving 9.3% surplus bagasse and producing electricity of 46 kW ton−1 cane for mill processing (LS-1, Case 1). The increase in pressure and temperature of live steam drops the bagasse saving with higher electricity production. Case 2 appeared non-feasible to generate electricity although it saves more bagasse. Configuration-II comprised Rankine cycle with extraction condensation turbine system where condenser has more operation options and flexibility of functioning in crushing and non-crushing times at 0.085 bar condensation pressure. It works best at 2.1 bar process steam using 335 kg steam ton−1 cane with some modifications, like vapor bleeding and heating from first to fourth effect of evaporation, repeated usage of process steam, and addition of sixth effect evaporation station in the factory layout. This configuration consumes maximum bagasse in the cogeneration system with surplus electricity of 70–79.4 kW ton−1 cane.
Configuration-III relied on a gasifier that converts bagasse into syngas to fuel the gas turbine (Ensinas et al. 2006). In a sugar factory, around 593 Nm3 syngas ton−1 cane could be produced. The exhaust gases from the gas turbine generate 2.1 bar steam in a HRSG for sugar process. Configuration-IV was a BIGCC cycle that also worked with a bagasse gasifier as fuel for gas turbine. The steam generated in a HRSG from thermal energy of exhaust gases is used for sugar process at 2.1 bar, and high-pressure steam operates turbine to generate electricity.
Although Configuration-IV generates high electricity of about 185 kW ton−1 cane, configurations III and IV were rated inefficient due to low efficiency of gasification process and requirements of high complementary fuel energy input (150–421 MW) than configurations I and II that operate with steam cycles alone (Ensinas et al. 2006). Hence Configuration-II makes possible the use of all the bagasse for electricity production and offers the possibility of operation of the system during the whole year using a complementary fuel like cane trash (Leal et al. 2013).
In some conformations of biomass gasification, bagasse dryer, gasifier, and gas cleaning system are involved to work for heat recovery steam generator (HRSG) or biomass integrated gasification combined cycle (BIGCC) with exhaust gases to produce steam for the process. With these conformations the HRSG can yield about 140 kW electricity per ton of sugarcane, while a BIGCC-operated plant may provide 200–250 kW surplus energy per ton of sugarcane (Khatiwada et al. 2012; Pellegrini et al. 2013).
Colombo et al. (2014) evolved a special mathematical model to elaborate the energy and material process balances for estimating the effect of different working environments on cogeneration systems for idealizing the process competence. They introduced the term “renewable efficiency” to elucidate the extent of green power that a process plant generates. The new designs may include up to 33 MW of extra bioelectricity to the grids. Hence, sugar plants are being made efficient either by augmenting pressure and temperature of boilers or switching to BIGCC systems (BNDES and CGEE 2008; Khatiwada et al. 2012; Pellegrini et al. 2013). Both plans involve exchange of steam-driven machines with electrical ones. The electrical energy generation with superior turbines let better and easy energy conversion that results in more surplus of electrical energy.
Colombo et al. (2014) proposed two repowering layouts: In the first option superheated Rankine cycle was placed in conjunction with the boiler C scheme that works at 2.7 bar of regeneration and condensing pressure. The scheme involves turbine inlet, regeneration bleeding, process bleeding, turbine outlet, condenser outlet, process condensed water, addition of regeneration vapor, and pump and boiler’s inlet. The second scheme was analogous to first option with medium pressure reheating system. This scheme worked for similar time in a year with immediate access of 295 MW of fuel energy. In Option II, the expansion was divided into two blocks of turbines (VHP inlet and VHP outlet) because of the reheating at first place. These schemes verified 11.0 and 12.7% internal rate of return for Options I and II, respectively.
3.5 Use of Cane Trash as Cogeneration Fuel
Cane trash is the dried leaves in the form of field residue left after harvesting and cleaning of the cane stalk. It could be one of the most interesting complementary fuels for the sugarcane factories. It can be recovered from the cane fields to the quantity of about 125 kg ton−1 sugarcane (Leal et al. 2013; Macedo et al. 2001) and can be used as fuel in addition to natural gas during off-seasons (Khatiwada et al. 2016; Rodrigues et al. 2003). Its LHV is 12.6 MJ kg−1 for which cogeneration plants have been partially shifted on this fuel in some countries.
3.6 Advantages of Cogeneration
Cogeneration’s role is utmost in current climate change scenario where thwart in global warming by mitigating CO2 emissions is a priority in international agenda. It could also be a fascinating source of income for sugarcane factories in future benefitting from the mechanisms represented in Kyoto protocol, like Clean Development Mechanisms. The following are the other benefits of cogeneration system (Table 16.7).
Moreover, cogeneration systems have remarkably superior efficiency as against conventional thermoelectric electricity generation as presented in Table 16.8.
However, apart from benefits of cogeneration, there are also some challenges which need to be tackled:
-
High internal implementation and equipment costs
-
Additional maintenance, repair, and operation (MRO) expenses
-
Unpleasant price for sales of excess power
-
More price of bagasse (Mauritius US$3.70 ton−1, Pakistan US$2.50 ton−1) compared to the price of other electricity fuels
For bagasse energy development, it is essential to have sugarcane processing modernization for efficient bagasse usage, national grid’s transmission lines’ connection with the bagasse/mill power plants, and use of coal, gas, sugarcane trash, or other renewable sources as off-season fuel to ensure regular power export.
4 Biogas from Sugarcane Pressmud, Bagasse, and Vinasse
Domestic and industrial fuel demands are mainly met with oil, coal, natural gas, forest wood, and woody material which are limited and being exploited constantly, whereas a lot of industrial, agricultural, and domestic wastes are thrown as such causing environmental pollution. Biogas generation from the anaerobic digestion has been revealed to be one of the viable technologies to find an appropriate application of these wastes.
Sundaranayagi et al. (2017) described that anaerobic digestion process of solid wastes convolutedly involves several groups of anaerobes. Methane is a major component of biogas (60–65%) followed by carbon dioxide (30–40%) and hydrogen (0–1%). Anaerobic digestion comprises four biochemical mechanisms called hydrolysis, acidogenesis, acetogenesis, and methanogenesis. The anaerobic digestion results in biopolymers’ conversion to monomers, followed by the conversion of soluble monomers into short- and long-chain fatty acids by acidogens. Subsequently, acetic acid production takes place along with small quantities of hydrogen and carbon dioxide (by acetogens), and finally, methane and carbon dioxide are generated through methanogenesis.
Production of biogas from waste and organic residues has been investigated for decades (Marek et al. 2014). General materials for biogas production are lignocellulosic biomasses, organic compounds, animal wastes, industrial water, and municipal solid wastes (Hadiyarto et al. 2017; Brown et al. 2012; Wilawan et al. 2014). A combination of water, sheep dung, and hyacinth in a ratio of 84:12:4 can produce 360 L biogas per kg of substrate (Patil et al. 2014). Oleszek et al. (2014) produced biogas from weeds and grass varieties of walnuts. Similarly, sugarcane residues and sugar industry’s wastes like bagasse, pressmud, trash, and vinasse have been effectively employed for biogas production alone or in combination with other organic materials (Rouf et al. 2010; Sathish and Vivekanandan 2015; Talha et al. 2016).
Bagasse, as discussed earlier, is a lignocellulosic residue of sugar mills comprising mainly cellulose, hemicellulose, and lignin (Maryana et al. 2014; Talha et al. 2016). Cellulose and hemicelluloses are long-chain sugar monomers that can be converted into biogas through pretreatment and hydrolysis (Eshore et al. 2017; Hendriks and Zeeman 2009). Mechanical, thermochemical, alkali, or acid pretreatments convert complex organic molecules of bagasse and other compounds into simple sugars, amino acids, and fatty acids (López González et al. 2013, 2014; Modenbach and Nokes 2014).
Sumardiono et al. (2017) obtained biogas yield of 51.04 L kg−1 with substrate combination of bagasse treated with 2% NaOH for 24 hours and 20% cow’s rumen. Anaerobic codigestion of pressmud and 1 N NaOH treated bagasse resulted in higher cumulative biomethane yield than untreated substrates or the substrates alone (Talha et al. 2016). Further, mixing of pressmud with bagasse at 25:75 ratio (C/N ratio 24.70) yielded the best cumulative biomethane and was considered the efficient method of biogas production. Less C/N ratio (9.86) of pressmud lowers the biomethanation , whereas mixing it with a higher C/N ratio (~27) bagasse-like substrates (for optimum C/N ratio of ~25) elevates biomethanation. Anaerobic digestion of pressmud starts in a short time of 4–5 hours to produce biogas and pressmud with bagasse yields biogas containing 52% methane (Sundaranayagi et al. 2017). In another biomethanation study, maximum biogas yield was obtained as 0.68 m3 m−3 of 1:1 pressmud to water ratio resulting in methane concentration of 67% at 30–35 °C mesophilic conditions (Sathish and Vivekanandan 2015).
Literature reveals that nickel, cobalt, and iron are desired elements for microbial activity, which can further be compensated through addition of deficient nutrients in the substrate for improved biogas yields (Sundaranayagi et al. 2017). Methanation of pressmud along with cow dung inoculum and trace elements (Ni, Co, and Fe) for 30 days revealed that addition of Fe yielded the maximum biogas (520 mL day−1) in the anaerobic digestion process. Sundaranayagi et al. (2017) also suggested that a proper anaerobic digestion needs a balance in nutrition especially carbon, nitrogen, phosphorous, and sulfur. Moreover, too high C:N ratio may also have depressing effect on microbial functioning.
Vinasse is a sugar distillery’s waste that is environmentally unhealthy if disposed of or used untreated in agriculture, due to biological oxygen demand (BOD) of about 25,000 mg L−1 and chemical oxygen demand (COD) of ~48,000 mg L−1 (Baez-Smith 2006). However, it has high potential for biomethanation with a possibility of more than 70% conversion of its COD to methane during anaerobic biodigestion (Rao 1999). An alcohol distillery producing 500 L ethanol day−1 has the ability to produce 73,000 m3 biogas day−1 from vinasse in its allied biodigestion plants that corresponds to about 14.6 L m−3 of vinasse (de Souza et al. 2011; Salomon et al. 2011). The anaerobic biodigestion treatment of vinasse preserves its fertilization potential (phosphorus, potassium, and nitrogen) and decreases its BOD and COD up to 90% and 70%, respectively, to make it safer for agricultural use (Baez-Smith 2006; Salomon et al. 2011).
A biochemical methane potential assay for the energy potential of sugar industry wastes was performed by Janke et al. (2015). It revealed that methane yield varied considerably with the nature of substrate, whereas maximum methane yield was obtained from bagasse and minimum from vinasse on fresh mass basis (Table 16.9). Such results were mainly attributed to the variation in substrate properties and water contents. Hence, the energy-related applications of sugarcane industry not only limit to ethanol and bioelectricity production, but the same industry has great potential to serve for biogas supplies as well.
5 Bioproduct Production at the Sugar Industry
In addition to first- and second-generation ethanol, bioelectricity, and biogas, the generation of value-added products and utilization of processed by-products can help offset the cost associated with sugar and ethanol production. Such is the case with companies like BlueFire Renewables, Virdia (acquired by Stora Enso), Gevo, Amyris, Codexis, LS-9 (acquired by REG Life Sciences, LLC), and Virent who have shifted their research interest from second-generation ethanol to specialty products.
Inhibitory by-products generated during the processing of ethanol from lignocellulosic biomass can be recovered and used as potential platform chemicals to many bio-based products including silage and animal feed preservation, food preservatives, catalysts, and plasticizers from formic acid and levulinic acid and in the production of biodegradable polymers as in the case of acetic acid (Choi et al. 2015; Hietala et al. 2016; Le Berre et al. 2014; Ranjan et al. 2009). HMF can be converted to levulinic acid, dimethylfuran, 2,5-furandicarboxylic acid, and dihydroxymethylfuran, which are building blocks in the manufacture of alternative fuels, polymers, foams, and polyesters (Rosatella et al. 2011). Furfural has several applications as an additive in anti-acids, inks, fungicides, adhesives, and flavoring agents (Bozzell and Petersen 2010: Cai et al. 2014).
Furthermore, the effective extraction and recovery of lignin-derived phenolic compounds (vanillin, phenol, coumaric acid) and lignin by-products (technical lignins) can generate additional revenues, while the remaining lignin is burned for energy. Lignin-derived phenolic compounds have applications in the food, pharmaceutical, and cosmetic industries (Tejado et al. 2007; Zhang et al. 2013). Technical lignins (complex phenolic polymers) possess antimicrobial, anticarcinogenic, and antioxidant properties with potential applications in food, medicine, polymers, and cosmetics (Espinoza-Acosta et al. 2016). Alternative means of utilizing the hemicellulose sugars could be also explored to produce alternative chemicals such as lactic acid (for use in packaging, prosthetics, and drugs), furfural, and xylitol (for use as a sweetener and preservative and in tooth remineralization) (Machado et al. 2016; Martinez et al. 2013; Naidua et al. 2018).
6 Conclusion
Sugarcane is an important source of food and fuel energy. Its molasses and bagasse have tremendous potential to produce ethanol (through fermentation) and bioelectricity (through cogeneration), respectively. Furthermore, sugar industry can also serve the provision of biogas utilizing surplus of its wastes, pressmud, and bagasse. Hence, recycling and renewability of resources of sugar industry can play an utmost important role in generating various forms of renewable bioenergy, to mitigate the CO2 emissions and contribute toward tackling climate change. Moreover, such applications are also fascinating for sugarcane factories keeping in view the economic benefits. Only a few countries like Brazil are exploiting these resources while others need to adopt similar models to lessen the reliance on fossil fuels and energy.
Abbreviations
- 5-HMF:
-
5-Hydroxymethylfurfural
- AFEX:
-
Ammonia fiber explosion
- BIGCC:
-
Biomass integrated gasification combined cycle
- BOD:
-
Biological oxygen demand
- BPST:
-
Back pressure steam turbines
- CBP:
-
Consolidated bioprocessing
- CEST:
-
Condensation-extraction steam turbines
- COD:
-
Chemical oxygen demand
- CTBV:
-
Centrale Thermique de Belle Vue
- DOE:
-
Department of Energy
- DW:
-
Dry weight
- FAO:
-
Food and Agriculture Organization
- FFV:
-
Flex-fuel vehicles
- GHV:
-
Gross heating value
- HRSG:
-
Heat recovery steam generator
- ISO:
-
International Sugar Organization
- MTBE:
-
Methyl tertiary butyl ether
- SHF:
-
Separate hydrolysis and fermentation
- SSCF:
-
Simultaneous saccharification and cofermentation
- SSF:
-
Simultaneous saccharification and fermentation
- USDA:
-
United States Department of Agriculture
References
Abdalla AM, Hassan TH, Mansour ME (2018) Performance of wet and dry bagasse combustion in Assalaya sugar factory. Sudan Innov Ener Res 7:179–184. https://doi.org/10.4172/2576-1463.1000179
Aditiya HB, Mahlia TMI, Chong WT, Nur H, Sebayang A (2016) Second generation bioethanol production: a critical review. Renew Sust Energy Rev 66:631–653
Aita G, Kim M (2011) Pretreatment technologies for the conversion of lignocellulosic materials to bioethanol. In: Sustainability of the sugar and sugar-ethanol industries. ACS Press, Oaklyn, pp 117–145. https://doi.org/10.1021/bk-2010-105
Aita GA, Salvi DA, Walker MS (2011) Enzyme hydrolysis and ethanol fermentation of dilute ammonia pretreated energy cane. Bioresour Technol 102(6):4444–4448
Alvira P, Tomas-Pejo E, Ballesteros M, Negro M (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Antaresti YS, Setiyadi HW, Yogi YP (2002) The effect of chemical and biopulping process to bagasse pulp. In Proceeding: Symposium of Malaysian Chemical Engineers (SOMChE) and Regional Symposium on Chemical Engineering (RSCE), Petaling Jaya
Aranda C, Robledo A, Loera O et al (2006) Fungal invertase expression in solid-state fermentation. Food Technol Biotechnol 44:229–233
Ashokkumar B, Kayalvizhi N, Gunasekaran P (2001) Optamization of media for β-fructofuranosidase production by Aspergillus niger in submerged and solid state fermentation. Process Biochem 37:331–338
Baez-Smith C (2006) Anaerobic digestion of vinasse for the production of methane in the sugarcane distillery. In: Proceeding SPRI conference on sugar processing, Aguas de São Pedro, 17–20 Sept 2016, p 268–287
BNDES and CGEE (2008) Sugarcane-based bioethanol: energy for sustainable development, 1st edn. Banco Nacional de Desenvolvimento Econômico e Social and Centro de Gestão e Estudos Estratégicos, Rio de Janeiro, p 304
Bangrak P, Limtong S, Phisalaphong M (2011) Continuous ethanol production using immobilized yeast cells entrapped in loofa-reinforced alginate carriers. Braz J Microbiol 41:676–684
Baral NR, Wituszynski DM, Martin J, Shah A (2017) Sustainability assessment of cellulosic biorefinery stillage utilization methods using energy analysis. Energy 109:13–28
Barriga A (2003) Energy system II. University of Calgary/OLADE, Quito
Barros S, Berk C (2018) Brazil biofuels annual, Global Agricultural Information Network (GAIN) Report Number. BR18017, USDA Foreign Agricultural Service, Washington, DC. https://gain.fas.usda.gov/Recent%20GAIN%20Publications/Biofuels%20Annual_Sao%20Paulo%20ATO_Brazil_8-10-2018.pdf. Accessed 11 Dec 2018
Behera S, Rama CM, Ramesh CR (2012) Ethanol fermentation of sugarcane molasses by Zymomonas mobilis MTCC 92 immobilized in Luffa cylindrica L sponge discs and Ca-alginate matrices. Braz J Microbiol 43(4):1499–1507. https://doi.org/10.1590/S1517–83822012000400034
Bezerra TL, Ragauskas AJ (2016) A review of sugarcane bagasse for second generation bioethanol and biopower production. Biofuels Bioprod Biorefin 10:634–647
Bleoanca I, Bahrim G (2013) Overview on brewing yeast stress factors. Rom Biotech Lett 18(5):8559–8572. https://www.rombio.eu/vol18nr5/1%20Bleoanca.pdf
Bommarius AS, Katona A, Cheben SE et al (2008) Cellulose kinetics as a function of cellulose pretreatment. Metab Eng 10:370–381
Boussarsar H, Roge B, Mathlouthi M (2009) Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose. Bioresour Technol 100:6537–6542
Bozzell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem 12:539–554
Brown RC (2005) Biomass refineries based on hybrid thermochemical-biological processing: an overview. In: Kamm B, Gruber VR, Kamm M (eds) Biorefineries industrial processes and products, vol 1. Wiley, Weinheim, pp 227–252
Brown D, Shi J, Li Y (2012) Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour Technol 124:379–386. https://doi.org/10.1016/j.biortech.2012.08.051
Bussamra BC, Freitas S, Da Costa A (2015) Improvement on sugar cane bagasse hydrolysis using enzymatic mixture designed cocktail. Bioresour Technol 187:173–181
Cai CM, Zhang T, Kumar R, Wyman CE (2014) Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J Chem Technol Biotechnol 89(1):2–10
Canilha L, Chandel AK, Milessi S, Antunes FAF, Freitas L, Das Gracas AF, Da Silva SS (2012) Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J Biomed Biotechnol 2012:989572. https://doi.org/10.1155/2012/989572
Cao S, Aita G (2013) Enzymatic hydrolysis and ethanol yields of combined surfactant and dilute ammonia treated sugarcane bagasse. Bioresour Technol 131:357–364
Carmo MJ, Gubulin JC (1997) Ethanol-water adsorption on commercial 3A zeolites: kinetic and thermodynamic data. Braz J Chem Eng 14(3). https://doi.org/10.1590/S0104-66321997000300004
Cazetta ML, Celligoi MAPC, Buzato JB, Scarmino IS (2007) Fermentation of molasses by Zymomonas mobilis: effects of temperature and sugar concentration on ethanol production. Bioresour Technol 98:2824–2828
Chandrakant P, Bisaria VS (1998) Simultaneous bioconversion of cellulose and hemicellulose to ethanol. Crit Rev Biotechnol 18(4):295–331
Charles M, Shuichi F (2003) Electricity from bagasse in Zimbabwe. Biomass Bioenergy 25:197–207
Cheng K, Zhang J, Ping W, Ge J, Zhou Y, Ling H, Xu M (2008) Sugarcane bagasse mild alkaline/oxidative pretreatment for ethanol production by alkaline recycle process. Appl Biochem Biotechnol 151:43–50
Choi S, Song CW, Shin JH, Lee SY (2015) Biorefineries for the production of top building block chemicals and their derivatives. Metab Eng 28:223–239
Colombo G, Ocampo-Duque W, Rinaldi F (2014) Challenges in bioenergy production from sugarcane mills in developing countries: a case study. Energies 7:5874–5898. https://doi.org/10.3390/en7095874
Daud S, Salleh SS, Salleh MN, Kasim FH, Saad SA (2007) Analysis of chemical composition in sugarcane bagasse and rice straw for their suitability using in paper production. In: Proceedings of 1st international conference on sustainable materials engineering (ICoSM), Penang, 9–11 June 2007, p 291–292
De Rosa J, Salvadori M (2007) Advantages and challenges of cogeneration. Frost and Sullivan Market Insight. http://www.frost.com/prod/servlet/market-insight-print.pag?docid=110279425. Accessed 22 Nov 2018
de Souza SNM, Santos RF, Fracaro GPM (2011) Potential for the production of biogas in alcohol and sugarcane plants for use in urban buses in the Brazil. In: Proceeding World Renewable Energy Congress (Bioenergy Technol), Linkoping, 8–13 May 2011, p 418–424
Deepchand K (2005) Sugarcane bagasse energy cogeneration: lessons from Mauritius. Paper presented to the parliamentarian forum on energy legislation and sustainable development, Cape Town, 5–7 October 2005
Deng F, Aita G (2018) Detoxification of dilute ammonia pretreated energy cane bagasse enzymatic hydrolysate by soluble polyelectrolyte floccculants. Ind Crop Prod 112:681–690
Deng F, Cheong D, Aita G (2018) Optimization of activated carbon detoxification of dilute ammonia pretreated energy cane bagasse enzymatic hydrolysate by response surface methodology. Ind Crop Prod 115:166–173
Deshmukh R, Jacobson A, Chamberlin C, Kammen D (2013) Thermal gasification or direct combustion: comparison of advanced cogeneration systems in the sugarcane industry. Biomass Bioenergy 55:163–174
Dias MOS, Junqueira TL, Cavalett O et al (2013) Cogeneration in integrated first and second generation ethanol from sugarcane. Chem Eng Res Des 91:1411–1417
Díaz JC, Gil-Chávez ID, Giraldo L, Moreno-Piraján JC (2010) Separation of ethanol-water mixture using type-A zeolite molecular sieve. J Chem 7:483–495
Dwivedi P, Alavalapati J, Lal P (2009) Cellulosic ethanol production in the United States: conversion technologies, current produciton status, economics, and emerging developments. Energy Sustain Dev 13:174–182
Ensinas AV, Nebra SA, Lozano MA, Serra L (2006) Analysis of cogeneration systems in sugar cane factories: alternatives of steam and combined cycle power plants. In: Proceedings of the ECOS, Aghia Pelagia, 12–14 July 2006, p 1177–1184
Eshore S, Mondal C, Das A (2017) Production of biogas from treated sugarcane bagasse. Int J Eng Sci Technol 6:224–227. https://doi.org/10.5958/2277-1581.2017.00025.0
Espinoza-Acosta JL, Torres-Chávez PI, Ramírez-Wong B, López-Saiz CM, Montaño-Leyva B (2016) Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. Bioresources 11(2):5452–5481
Esteghlalian A, Hashimoto AG, Fenske JJ, Penner MH (1997) Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresour Technol 59:129–136
Faria KCP (2011) Master thesis, UENF-LAMAV, Campos dos Goytaczes-RJ, Brazil
Farzad S, Mandegari M, Gorgens J (2016) A critical review on biomass gasification, co-gasification and their environmental assessments. Biofuel Res J 12:483–495
Fatma S, Hameed A, Noman M, Ahmed T, Shahid M, Tariq M, Sohail I, Tabassum R (2018) Lignocellulosic biomass: a sustainable bioenergy source for the future. Protein Pept Lett 25:1–16
Ferreira V, de Oliveira FM, da Silva MS, Pereira JN (2010) Simultaneous saccharification and fermentation process of different cellulosic substrates using a recombinant Saccharomyces cerevisiae harboring the B-glucosidase gene. Electron J Biotechnol 13:2
Food and Agriculture Organization Statistics (2017) Crop production: Sugarcane. http://www.fao.org/faostat/. Accessed 12 Jul 2018
Galvao ACF, Poppe MK, Rocha BB, Durieux L, Nogueira LAH, Macedo IC (2016) Second generation sugarcane bioenergy and biochemicals: advanced low-carbon fuels for transport and industry. Center for Strategic Studies and Management (CGEE), Brasília, p 103
Gasmalla MAA, Yang R, Nikoo M, Man S (2012) Production of ethanol from sudanese sugar cane molasses and evaluation of its quality. J Food Process Technol 3:163. https://doi.org/10.4172/2157-7110.1000163
Gould JM (1985) Studies on the mechanism of alkaline peroxide delignification of agricultural residues. Biotech Bioenergy 27:225–231. https://doi.org/10.1002/bit.260270303
Guan Y, Luo S, Liu S, Xiao B, Cai L (2009) Steam catalytic gasification of municipal solid waste for producing tar-free fuel gas. Int J Hydrog Energy 34(23):9341–9346. https://doi.org/10.1016/j.ijhydene.2009.09.050
Guilherme AA, Dantas PVF, Santos ES, Fernandes FA, Macedo GR (2015) Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugar cane bagasse. Braz J Chem Eng 32:23–33. https://doi.org/10.1590/0104-6632.20150321s00003146
Hadiyarto A, Sumardiono S, Budiyono B, Fofana FF, Fauzi I (2017) The effect of solid-state anaerobic digestion (SS-AD) and liquid anaerobic digestion (L-AD) method in biogas production of rice husk. Waste Technol 1(1):1–5. https://doi.org/10.12777/wastech.5.2.2017.1-15
Haelssig JB, Tremblay AY, Thibault J (2012) A new hybrid membrane separation process for enhanced ethanol recovery: process description and numerical studies. Chem Eng Sci 68:492–505
Hahn-Hagerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund M (2007) Towards industrial pentose-fermenting yeast strains. Appl Environ Microbiol 74:5031–5037
Hamelinck C, Hooijdonk G, Faaij A (2005) Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 28:384–410
Hassuani SJ, Leal MRLV, Macedo IC (eds) (2005) Biomass power generation: sugar cane bagasse and trash, 1st edn. PNUD – CTC, Piracicaba, p 216
Hasunuma T, Kondo A (2012) Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process Biochem 47:1287–1294
Hayes D (2016) Second generation fuels: why are they taking so long? In: Lund P, Byrne J, Berndes G, Vasalos I (eds) Advances in bioenergy: the sustainability challenge, 1st edn. Wiley, Chichester, p 163
Hayes DJ, Hayes MHB (2009) The role that lignocellulosic feedstocks and various biorefining technologies can play in meeting Ireland’s biofuels targets. Biofuels Bioprod Biorefin 3:500–520
Hendriks AT, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18. https://doi.org/10.1016/j.biortech.2008.05.027
Hietala J, Vuori A, Johnsson P, Pollari I, Reutemann W, Kieczka H (2016) Formic acid. Ullmann’s encyclopedia of industrial chemistry. Wiley, Hoboken, pp 1–22
Huang HJ, Ramaswamy S, Tschirner UW, Ramarao BV (2008) A review of separation technologies in current and future biorefineries. Sep Purif Technol 62:1–21
Humbirt D, Aden A (2008) Biochemical production of ethanol from corn stover: 2008 state of technology model. Technical report No.: NREL/TP-510-46214. National Renewable Energy Laboratory, Golden
International Energy Agency (2015) World energy outlook 2015. IEA. Organisation for Economic Co-operation and Development, Paris, p 718. https://www.iea.org/publications
International Sugar Organization (2009) Cogeneration opportunities in the world sugar industry. In: Bulletin MECAS Studies—International Sugar Organization 5(9). http://www.isosugar.org
Jambo SA, Abdulla A, Azhar SHM, Marbawi H, Gansau JA, Ravindra P (2016) A review on third generation bioethanol feedstock. Renew Sust Energ Rev 65:756–769. https://doi.org/10.1016/j.rser.2016.07.064
Janke L, Leite A, Nikolausz M, Schmidt T, Liebetrau J, Nelles M, Stinner W (2015) Biogas production from sugarcane waste: assessment on kinetic challenges for process designing. Int J Mol Sci 16:20685–20703. https://doi.org/10.3390/ijms160920685
Jӧnsson LJ, Martin C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Kamate CS, Gangavati BP (2009) Cogeneration in sugar industries: technology options and performance parameters-a review. Cogen Distrib Gener J 24:6–33. https://doi.org/10.1080/15453660909595148
Kazi FK, Fortman JA, Anex RP, Hsu DD, Aden A, Dutta A, Kothandaraman G (2010) Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 89:S20–S28
Kennes D, Abubackar HN, Diaz M, Veiga M, Kennes C (2016) Bioethanol production from biomass: carbohydrate vs syngas fermentation. J Chem Technol Biotechnol 91:304–317
Khan MT, Seema N, Khan IA, Yasmine S (2017a) Applications and potential of sugarcane as an energy crop. In: Gorawala P, Mandhatri S (eds) Agricultural research updates, vol 16. Nova Science Publishers, New York, pp 1–24
Khan MT, Seema N, Khan IA, Yasmine S (2017b) The green fuels: evaluation, perspectives, and potential of sugarcane as an energy source. Environ Res J 10(4)
Khandekar DC, Palai T, Agarwal A, Bhattacharya PK (2014) Kinetics of sucrose conversion to fructo-oligosaccharides using enzyme (invertase) under free condition. Bioprocess Biosyst Eng 37(12):2529–2537. https://doi.org/10.1007/s00449-014-1230-5
Khatiwada D, Seabra J, Silveira S, Walter A (2012) Power generation from sugarcane: biomass-a complementary option to hydroelectricity in Nepal and Brazil. Energy 48:241–254
Khatiwada D, Leduc S, Silveira S, McCallum I (2016) Optimizing ethanol and bioelectricity production in sugarcane biorefineries in Brazil. Renew Energy 85:371–386
Kim S, Dale BE (2005) Life cycle assessment of various cropping systems utilized for producing biofuels: bioethanol and biodiesel. Biomass Bioenergy 29:426–439
Kim TH, Kim JS, Sunwoo C, Lee YY (2003) Pretreatment of corn stover by aqueous ammonia. Bioresour Technol 90:39–47. https://doi.org/10.1016/S0960-8524(03)00097-X
Köchermann J, Schneider J, Matthischke S, Rönsch S (2015) Sorptive H2S removal by impregnated activated carbons for the production of SNG. Fuel Process Technol 138:37–41. https://doi.org/10.1016/j.fuproc.2015.05.004
Koppram R, Nielsen F, Albers E, Lambert A, Wannstrom S, Welin L (2013) Simultaneous saccharification and co-fermentation for bioethanol production using corncobs at lab, PDU and demo scales. Biotechnol Biofuels 6:2
Koutinas AA, Vlysidis A, Pleissner D, Kopsahelis N (2014) Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem Soc Rev 43:2587–2627
Kumar A, Jones D, Hanna M (2009) Thermochemical biomass gasification: a review of the current status of the technology. Energies 2:556–581
Kummamuro B (2016) WBA global bioenergy statistics. World Bioenergy Association, Stockholm
Lawford HG, Rousseau JD (1991) Fuel ethanol from hardwood hemicellulose hydrolysate by genetically engineered Escherichia coli B carrying genes from Zymomonas mobilis. Biotechnol Lett 13:191–196
Le Berre C, Serp P, Kalck P, Torrence GP (2014) Acetic acid. Ullmann’s encyclopedia of industrial chemistry. Wiley, Hoboken, pp 1–34
Leal MRLV, Galdos MV, Scarpare FV, Seabra JE, Walter A (2013) Sugarcane straw availability, quality, recovery and energy use: a review. Biomass Bioenergy 53:11–19
Linde D, Macias I, Fernández-Arrojo L, Plou FJ, Jiménez A, Fernández-Lobato M (2009) Molecular and biochemical characterization of a beta-fructofuranosidase from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 75:1065–1073
Lois-Correa J, Flores-Vela A, Ortega-Grimaldo D, Berman-Delgado J (2010) Experimental evaluation of sugar cane bagasse storage in bales system. J Appl Res Technol 8:365–377
López González LM, Vervaeren H, Reyes IP, Dumoulin A, Romero OR, Dewulf J (2013) Thermo-chemical pre-treatment to solubilize and improve anaerobic biodegradability of press mud. Bioresour Technol 131:250–257
López González LM, Reyes IP, Dewulf J, Budde J, Heiermann M, Vervaeren H (2014) Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour Technol 169:284–290
Lynd LR, Elamder RT, Wyman CE (1996) Likely features and costs of mature biomass ethanol technology. Appl Biochem Biotechnol 57–58(1):741–761
Macedo IC, Leal MRLV, Hassuani SJ (2001) Sugar cane residues for power generation in the sugar/ ethanol mills in Brazil. Energy Sustain Dev 5(1):77–82
Machado G, Leon S, Santos F, Lourega R, Dullius J, Mollmann ME, Eichler P (2016) Literature review on furfural production from lignocellulosic biomass. Nat Resour J 7(03):115
Marek M, Bialobrzewski I, Zielinski M, Dębowski M, Krzemieniewski M (2014) Optimizing low-temperature biogas production from biomass by anaerobic digestion. Renew Energy 69:219–225
Martinez FAC, Balciunas EM, Salgado JM, González JMD, Converti A, de Souza Oliveira RP (2013) Lactic acid properties, applications and production: a review. Trends Food Sci Technol 30(1):70–83
Maryana R, Ma’rifatun D, Wheni AI, Satriyo KW, Rizal WA (2014) Alkaline pretreatment on sugarcane bagasse for bioethanol production. Energy Procedia 47:250–254
McMillan JD (1997) Bioethanol production: status and prospects. Renew Energy 10:295–302
Modenbach AA, Nokes S (2014) Effects of sodium hydroxide pretreatment on structural components of biomass. Am Soc Agric Biol Eng 57:1187–1198
Mohammadi M, Najafpour GD, Younesi H, Lahijani P, Uzir MH, Mohamed AR (2011) Bioconversion of synthesis gas to second generation biofuels: a review. J Renew Sustain Ener 15(9):4255–4273
Mohapatra S, Mishra C, Behera S, Thatoi H (2017) Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from grass biomass: a review. Renew Sust Energ Rev 78:1007–1032
Moreno AD, Ibarra D, Alvira P, Tomás-Pejó E, Ballesteros M (2015) A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit Rev Biotechnol 35(3):342–354
Morias PB, Rosa CA, Linardi VR, Carazza F, Nonato EA (2007) Production of fuel alcohol by saccharomyces starins from tropical habitats. Biotechnol Lett 18:1351–1356
Mosier N, Hendrickson R, Ho N, Sedlak M, Ladisch MR (2005) Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour Technol 96:1986–1993
Mussatto SI, Roberto IC (2004) Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol 93:1–10
Naidua D, Hlangothib S, John M (2018) Bio-based products from xylan: a review. Carbohydr Polym 179:28–41
National Academic Press (2000) Biobased industrial products: priorities for research and commercialization, Washington, DC, p 162. https://doi.org/10.17226/5295
Neves PV, Pitarelo AP, Ramos LP (2016) Production of cellulosic ethanol from sugarcane bagasse by steam explosion: effect of extractives content, acid catalysis and different fermentation technologies. Bioresour Technol 208:184–194
Nigam JN (2000) Continuous ethanol production from pineapple cannery waste using immobilized yeast cell. J Biotechnol 80:189–193
O’Sullivan A, Sheffrin S (2003) Economics: principle and tools, 2nd edn. Prentice Hall, Upper Saddle River
Oladi S, Aita G (2017) Optimization of liquid ammonia pretreatment variables for maximum enzymatic hydrolysis yield of energy cane bagasse. Ind Crop Prod 103:122–132
Oladi S, Aita G (2018) Interactive effect of enzymes and surfactant on the cellulose digestibility of un-washed and washed dilute ammonia pretreated energy cane bagasse. Biomass Bioenergy 109:221–230
Oleszek M, Król A, Tys J, Matyka M, Kulik M (2014) Comparison of biogas production from wild and cultivated varieties of reed canary grass. Bioresour Technol 156:303–306. https://doi.org/10.1016/j.biortech.2014.01.055
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74(1):17–24
Patil HJ, AntonyRaj MA, Shankar BB, Shetty MK, Kumar BP (2014) Anaerobic co-digestion of water hyacinth and sheep waste. Energy Procedia 52:572–578
Pellegrini LF, de Oliveira JS, Burbano JC (2013) Supercritical steam cycles and biomass integrated gasification combined cycles for sugarcane mills. Energy 35:1172–1180
Pothiraj C, Arumugam R, Gobinath M (2014) Sustaining ethanol production from lime pretreated water hyacinth biomass using mono and co-cultures of isolated fungal strains with Pichia stipitis. Bioresour Bioprocess 1:27
Purohit P, Michaelowa A (2007) CDM potential of bagasse cogeneration in India. Energy Policy 35:4779–4798
Qiu Z, Aita G, Mahalaxmi S (2014) Optimization by response surface methodology of processing conditions for the ionic liquid pretreatment of energy cane bagasse. J Chem Technol Biotechnol 89(5):682–689
Ranjan R, Thust S, Gounaris CE, Woo M, Floudas CA, Von Keitz M, Valentas KJ, Wei J, Tsapatsis M (2009) Adsorption of fermentation inhibitors from lignocellulosic biomass hydrolyzates for improved ethanol yield and value-added product recovery. Microporous Mesoporous Mater 122:143–148
Rao PJM (1999) An overview of the co-products industries in India. In: Proceedings of the XXIII International Society of Sugar Cane Technologists, 22–26 Feb 1999, New Delhi
Rastogi M, Shrivastava S (2017) Recent advances in second geenration bioethanol production: an insight to pretreatment, saccharification and fermentation processes. Renew Sust Energ Rev 80:330–340
Rein P (2004) Feasibility study on the production of fuel alcohol from Louisiana Molasses. Sugar Bull 82(12):13–17
Rezende CA, de Lima MA, Maziero P, Ribeiro deAzevedo E, Garcia W, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:54–71. https://doi.org/10.1186/1754-6834-4-54
Rodrigues M, Walter A, Faaij A (2003) Co-firing natural gas in biomass integrated gasification/ combined cycle system. Energy 28:1115–1131
Rosatella AA, Simeonov SP, Frade RF, Afonso CA (2011) 5-Hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications. Green Chem 13(4):754–793
Rosillo-Calle F, De Groot P, Hemstock SL, Woods J (2015) The biomass assessment handbook: energy for a sustainable environment, 2nd edn. Routledge/Taylor & Francis Group, New York
Rouf MA, Bajpai PK, Jotshi CK (2010) Optimization of biogas generation from press mud in batch reactor. Bangladesh J Sci Ind Res 45:371–376
Rudolph A, Baudel H, Zacchi G, Hahn-Hagerdal B, Liden G (2007) Simultaneous saccharification and fermentation of steam-pretreated bagasse using Saccharomyces cerevisiae TMB3400 and Pichia stipitis CBS6054. Biotechnol Bioeng 99:783–790
Salomon KR, Lora EES, Rocha MH, Almazán OO (2011) Cost calculations for biogas from vinasse biodigestion and its energy utilization. Sugar Ind 136(4):217–223
Sam KK (2012) Ethyl alcohol or ethanol production from molasses by fermentation. http://www.inclusive-science-engineering.com/ethyl-alcohol-or-ethanol-production-from-molasses-by-fermentation. Accessed 4 June 2018
Sánchez OJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99(13):5270–5295
Sarris D, Papanikolaou S (2016) Biotechnological production of ethanol: biochemistry, process and technologies. Eng Life Sci 16:307–329
Sathish S, Vivekanandan S (2015) Experimental investigation on biogas production using industrial waste (press mud) to generate renewable energy. Int J Innov Res Sci Eng Technol 4:388–392
Seabra JEA, Macedo IC (2011) Comparative analysis for power generation and ethanol production from sugarcane residual biomass in Brazil. Energy Policy 39:421–428. https://doi.org/10.1016/j.enpol.2010.10.019
Seabra JEA, Macedo IC, Leal MRLV (2014) Greenhouse gases emissions related to sugarcane ethanol. In: Luis Augusto Barbosa Cortez (Coord) sugarcane bioethanol-R&D for productivity and sustainability, Editora Edgard Blücher, São Paulo. https://doi.org/10.5151/BlucherOA-Sugarcane-SUGARCANEBIOETHANOL_29. p 291–300
Shaibani N, Ghazvini S, Andalibi MR, Yaghmaei S (2011) Ethanol production from sugarcane bagasse by means of enzymes produced by solid state fermentation method. Int J Chem Mol Eng 5(11):966–969
Sheehan GJ, Greenfield PF (1980) Utilization, treatment and disposal of distillery waste water. Water Res 14(3):257–277
Sivers MV, Zacchi G, Olsson L, Hahn-Hugerdal B (1994) Cost analysis of ethanol production from willow using recombinant E. coli. Biotechnol Prog 10:555–560
Stenberg K, Tenborg C, Galbe M, Zacchi G (1998) Optimization of steam pretreatment of SO2- impregnated mixed softwoods for ethanol production. J Chem Technol Biotechnol 71:299–308
Stephen JD, Mabee W, Saddler WE, Saddler JN (2012) Will second generation ethanol be able to compete with first generation ethanol? Opportunities for cost reduction. Biofuels Bioprod Biorefin 6:159–176
Sumardiono S, Riyanta AB, Matin HH, Kusworo TD, Jos B (2017) Increasing biogas production from sugar cane baggase by anaerobic co-digestion with animal manure. In: Iskandar I, Ismadji S, Agustina TE, Yani I, Komariah LN, Hasyim S (eds) Proceedings MATEC Web of Conferences: Sriwijaya International Conference on Engineering, Science and Technology (SICEST), Bangka Island, Nov 9–10, 2016, vol. 101. https://doi.org/10.1051/matecconf/201710102014
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. https://doi.org/10.1016/S0960-8524(01)00212-7
Sun JX, Sun XF, Sun RC, Su YQ (2004) Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr Polym 56:195–204
Sundaranayagi S, Sirajunnisa A, Ramasamy V (2017) Production of biogas by anaerobic digestion of press mud using iron, cobalt and nickel. Indian J Sci Res 14(1):374–377
Sutton D, Kelleher BP, Ross JRH (2001) Review of literature on catalysts for biomass gasification. Fuel Process Technol 73:155–173
Taherzadeh MJ, Gustafsson L, Niklasson C, Liden G (2000) Physiological effects of 5-hydroxymethylfurfural on Saccharomyces cerevisiae. Appl Microbiol Biotechnol 53(6):701–708
Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101(13):4744–4753
Talha Z, Ding W, Mehryar E, Hassan M, Bi J (2016) Alkaline pretreatment of sugarcane bagasse and filter mud codigested to improve biomethane production. Biomed Res Int:10. https://doi.org/10.1155/2016/8650597
Tejado A, Pena C, Labidi J, Echeverria JM, Mondragon I (2007) Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour Technol 98:1655–1663
Toasa J (2009) Colombia: a new ethanol producer on the rise? USDA Economic Research Service, Washington, DC. http://www.ers.usda.gov. Accessed 10 Nov 2018
Torres da Silva G, Chiarello LM, Lima EM, Ramos LP (2016) Sono-assisted alkaline pretreated of sugarcane bagasse for cellulosic ethanol production. Catal Today 269:21–28
UNDP (2009) Preliminary assessment of bioenergy production in the Caribbean. United Nations Development Programme, Barbados and the OECS
United States Department of Agriculture (2006) The economic feasibility of ethanol production from sugar in the United States. https://www.usda.gov/oce/reports/energy/EthanolSugarFeasibilityReport3.pdf. Accessed 20 Nov 2018
Valdivia M, Galan JL, Laffarga J, Ramos JL (2016) Biofuels 2020: biorefineries based on lignocellulosic materials. Microb Biotechnol 9(5):585–594
Vane LM (2008) Separation technologies for the recovery and dehydration of alcohols from fermentation broths. Biofuels Bioprod Biorefin 2:553–588
Veana F, Martínez-Hernández JL, Aguilar CN, Rodríguez-Herrera R, Michelena G (2014) Utilization of molasses and sugar cane bagasse for production of fungal invertase in solid state fermentation using Aspergillus niger GH1. Braz J Microbiol 45:373–377
Verbelen PJ, De Schutter DP, Delvaux F, Verstrepen KJ, Delvaux FR (2006) Immobilized yeast cell systems for continuous fermentation applications. Biotechnol Lett 28(19):1515–1525
Vohra M, Manwar J, Manmode R, Padgilwar S, Patil S (2014) Bioethanol production: feedstock and current technologies. J Environ Chem Eng 2:573–584
Walter A, Dolzan P (2009) Country report: Brazil, IEA bioenergy task 40, sustainable bio-energy trade securing supply and demand. UNICAMP, Campinas, p 66
Watson J, Zhang Y, Si B, Chen WT, de Souza R (2018) Gasification of biowaste: a critical review and outlooks. Renew Sust Energ Rev 83:1–17
White JE (2009) Investigation into the hydrothermal treatment of sugarcane bagasse under near and super critical conditions. Doctoral Dissertation, Louisiana State University. https://digitalcommons.lsu.edu/gradschool_dissertations/1164
Wilawan W, Pholchan P, Aggarangsi P (2014) Biogas production from co-digestion of Pennisetum pururem cv. Pakchong 1 grass and layer chicken manure using completely stirred tank. Energy Procedia 52:216–222
Wu W, Kawamoto K, Kuramochi H (2006) Hydrogen-rich synthesis gas production from waste wood via gasification and reforming technology for fuel cell application. J Mater Cycles Waste Manage 8(1):70–77. https://doi.org/10.1007/s10163-005-0138-1
Yang ST, Liu X, Zhang Y (2007) Metabolic engineering – applications, methods and challenges. In: Yang ST (ed) Bioprocessing for value added products from renewable resources. Elsevier Publishers, Amsterdam, pp 73–118
Yin H, Lee T, Choi J, Yip ACK (2016) On the zeolitic imidazolate framework-8 (ZIF-8) membrane for hydrogen separation from simulated biomass-derived syngas. Microporous Mesoporous Mater 233:70–77. https://doi.org/10.1016/j.micromeso.2015.10.033
Zhang AP, Liu CF, Sun RC, Xie J (2013) Extraction, purification, and characterization of lignin fractions from sugarcane bagasse. BioResources 8:1604–1614
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ahmad, S., Ali, M.A., Aita, G.M., Khan, M.T., Khan, I.A. (2019). Source-Sink Relationship of Sugarcane Energy Production at the Sugar Mills. In: Khan, M., Khan, I. (eds) Sugarcane Biofuels. Springer, Cham. https://doi.org/10.1007/978-3-030-18597-8_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-18597-8_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18596-1
Online ISBN: 978-3-030-18597-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)