Abstract
Noncompaction cardiomyopathy (NCCM) was first described almost a half century ago, it initially began as an unusual autopsy finding. NCCM has a highly variable clinical presentation and is usually diagnosed when the condition becomes symptomatic or when complications occur in patients. These complications are end-stage heart failure, lethal arrhythmias and thromboembolic events [8–10]. Sudden cardiac death (SCD) is its most striking and visible consequence. The mortality in patients with NCCM has been reported in 18% of adults and 0–13% in children. Most SCD within the NCCM population are due to arrhythmias caused by ventricular tachycardia (VTs) and ventricular fibrillation. The implantable cardioverter defibrillator (ICD) is the single most effective therapy to prevent SCD. However, appropriate risk stratification in these patients are not yet established.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
Noncompaction cardiomyopathy (NCCM) was first described almost a half century ago, it initially began as an unusual autopsy finding [1, 2]. Over the past three decades increased interest and dedicated research have given us more insight about this relatively uncommon and new clinicopathologic entity. However, questions regarding the appropriate diagnosis, management, and prognosis remain unanswered. NCCM is now recognized as a distinct primary genetic cardiomyopathy by the American Heart Association (AHA) and as unclassified cardiomyopathy by the European Society of Cardiology (ESC) [3, 4]. This cardiomyopathy could have poor prognosis in certain adult patients [5, 6]. In childhood the consequences of this disease can be more severe and is often associated with other congenital anomalies [7]. NCCM has a highly variable clinical presentation and is usually diagnosed when the condition becomes symptomatic or when complications occur in patients. These complications are chronic heart failure, lethal arrhythmias and thromboembolic events [8,9,10]. But, Sudden cardiac death (SCD) is the most devastation outcome. The mortality in patients with NCCM has been reported in 18% of adults and 0–13% in children [11].
SCD is defined as death from cardiac causes with an abrupt loss of consciousness less than 1 h after the onset of the symptoms. In the usual clinical practice, coronary artery disease is often the culprit of sudden cardiac death, approximately 65–70%; and with almost 10% of deaths in patients with non-ischemic cardiomyopathies [12]. Non-ischemic cardiomyopathies include hypertrophic cardiomyopathy, dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, takotsubo cardiomyopathy, cardiac amyloidosis, cardiac sarcoidosis and also NCCM. Most sudden cardiac deaths within the NCCM population are due to malignant ventricular arrhythmias, specifically caused by ventricular tachycardia (VT), ventricular fibrillation (VF), with some due to asystole [13, 14]. As an unexpected event, it has devastating effect for the patients and families.
The implantable cardioverter defibrillator (ICD) is the single most effective therapy to prevent sudden death in patients resuscitated from sudden cardiac arrest or after an episode of sustained VT [15]. In patients with ischemic heart disease several large, randomized, multicenter trials have shown the superiority of the ICD over drug (anti-arrhythmic) therapy for both primary and secondary prevention of sudden death [16, 17]. Studies about the role of ICD’s in the prevention of SCD however, are limited in NCCM patients [18]. Furthurmore, the application of ICD therapy in relatively young patients with NCCM for primary prevention has only recently become a focus [19]. Although there is a general consensus that NCCM patients who survive cardiac arrest with VF should be offered ICD for secondary prevention, those patients represent a small portion of the at-risk population.
Epidemiological Overview
In the general population, NCCM is reported in infants at a frequency of 0.80 per 100,000 individuals per year, in children, the incidence is found to be 0.12 per 100,000 individuals per year and in adults 0.05% [6, 23, 30]. In patients with heart failure, the prevalence of NCCM was up to 3-4% [22, 23]. Age at which NCCM is recognized is highly variable, ranging from early infancy to late adulthood [24]. The true prevalence of NCCM may be even higher, because asymptomatic individuals may go unnoticed in studies. Due to increased awareness of NCCM, as wel as the improvement of modern imaging modalities, including cardiac magnetic resonance imaging and CT-scan, the incidence of NCCM will probably increase in the future. Albeith SCD is the most striking complication of NCCM, it is usually believed to occur in relatively small percentage of the population. A large pediatric study in NCCM reports the risk of sudden death to be 6% [20]. However, recent studies have shown sudden death ranging from 13 to 18% in adults and 0–13% in the children with NCCM [11]. The children who did poorly often showed rhythm abnormalities related to sudden death as a predominant sign [20]. The variable prevalence of SCD can be probably best explained by the diagnostic criteria applied for NCCM, length of follow-up and the studied population [6, 20,21,22,23,24,25,26]. There is currently no consensus on absolute diagnostic criteria, which limits the strength of the conclusion regarding exact prevalence and incidence of sudden death in NCCM. Furthermore, epidemiological research in NCCM is often based on retrospective studies of patients referred for echocardiography [10, 27, 28].
A variety of arrhythmias has been identified in association with NCCM, including bundle branch reentry, idiopathic VT, right ventricular outflow tract (RVOT) origin, left bundle branch and right bundle branch morphologies, bidirectional, fascicular VT, polymorphic and VF [27,28,29,30,31,32]. But malignant ventricular tachyarrhythmia’s (VA’s), including cardiac arrest due to VT/VF, have been considered as the hallmark [29]. Ventricular arrhythmias are reported in 38–47% in adult NCCM population and 0–38% in children with NCCM [11, 22]. In our series, 14 out of 84 patients (16.7%) presented primarily with SCD/VA’s [34].
Pathophysiology of SCD/VA in the NCCM population
Substantial data from several electrocardiograms and Holter’s show that sudden death events in NCCM are caused by sustained ventricular tachyarrhythmias, like rapid ventricular tachycardia (VT) and/or ventricular fibrillation [13, 44, 45].
However, the pathogenesis of arrhythmia's in NCCM patients is still poorly understood. It has been hypothesized that an arrest in the embryogenic development of the heart, results in disturbed compaction process of the myocardium. Normal myocardium gradually compacts from the epicardium inward and capillaries are formed by the compressed intertrabecular recesses [8]. Concurrent inappropriate maturation of the primary cardiac conduction system could cause a more pronounced manifestation of rhythm disturbances [21, 47, 51]. Histological examination shows myocardium around deep intertrabecular recesses that may serve as slow conducting zones with reentry. Impaired flow reserve, causing intermittent ischemia, may play a role [51]. Subendocardial ischemia and coronary microcirculation disturbances could cause VT and VF [47]. It has been also hypothesized that abnormalities of the cardiac conduction system or intramyocardial fibrosis and scar formation could be a trigger. Recently, we described a mismatch between the origin of premature ventricular complexes (PVC’s) and the noncompacted segments in NCCM. The PVC’s on the surface electrocardiograms of NCCM patients looked mainly originating from the conduction system and related myocardium [41]. In another case series of 9 patients by Muser et al. VA substrate typically localized in the mid-apical LV segments, whereas focal PVCs often arisen from LV basal–septal regions and/or papillary muscles [42]. In a case report by Casella et al., electro-anatomic mapping in a 43-year old man, ventricular noncompaction is characterized by electrical abnormalities including low voltage and scar areas, mainly related to the presence and extent of myocardial fibrosis rather than noncompacted myocardium [43].
According to current guidelines frequent PVCs and runs of NSVT in subjects with a prior myocardial infarction have been associated with an increased risk of death [52]. In contrast, in patients with non-ischemic cardiomyopathy, PVCs do not appear to be associated with a worse prognosis although data are limited [53]. In a recent study no statistically significant correlation could be found between the origin of PVCs and the occurrence of a previous spontaneous VT. Also the data suggest that PVCs in NCCM originate mainly from areas that are not affected echocardiographically by NCCM [54].
Also, prior theories about ventricular arrhythmias in NCCM involve microreentry in the trabeculated myocardium, epicardial coronary hypoperfusion, and concurrent developmental arrest of the conduction system [55]. However, electrophysiological mapping in NCCM patients with sustained VT did not reveal the anatomical substrate. Another relevant finding in recent years, is a high prevalence of early repolarization (ER) being reported in patients presenting with cardiac arrest or sudden cardiac death (Fig. 5.1). Previous studies showed a high prevalence of ER in NCCM patients, especially in those patients presenting with malignant ventricular arrhythmias (75%). Interestingly, early repolarization was also frequently observed (31%) in NCCM patients not presenting with ventricular arrhythmias [13, 29].
The pathophysiology of ER and associated arrhythmias in NCCM remains unclear. Increased regional trabeculation, with deep intramyocardial invaginations carrying the Purkinje system deeper into the mid-myocardium, as in NCCM, may cause inhomogeneous depolarization and repolarization. This transmural heterogeneity may result in the development of (malignant) ventricular arrhythmias [29]. This looks be in line with the recent findings that show, that normal LV twist is absent in patients with NCCM, which is probably due to also an immature endocardial helical system. Further studies are needed to identify the mechanism of the arrhythmias in NCCM to adopt an appropriate therapeutic approach for this distinct patient group.
Case Report
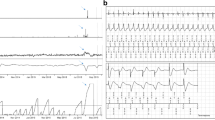
A 35-year old woman was admitted to the cardiology department because of palpitations. A 24-h Holter monitor and an echocardiogram were ordered after routine control. The 24h-Holter showed frequent PVCs and nonsustained VTs (Fig. 5.2a, b) and the echocardiogram showed characteristics of “asymmetrical hypertrophic cardiomyopathy”. The patient received ACE inhibitors, she didn’t continue the beta-blockers, probably because of intolerance. After her discharge from the hospital, the patient remained a couple years asymptomatic. Unfortunately, 3 years after the initial presentation, she was again admitted but now because of an out-of-hospital cardiac arrest. She had collapsed suddenly in a grocery store and was resuscitated by bystanders. Ambulance had arrived after 6 min and the ECG monitor showed ventricular fibrillation. She was successfully resuscitated. During her hospitalization a new echocardiogram revealed moderate LV dysfunction (LVEF 35%) and heavy trabeculations were noted at the posterior and apical walls (Fig. 5.3a, b). Considering the NCCM with decreased LVEF and VF as complication, an ICD device according to current guidelines was successfully implanted for secondary prophylaxes. The patient received drug therapy and she was discharged without complications. After the implantation of the ICD, she had several appropriate ICD shocks with need of adjustment of the medical therapy (Fig. 5.3). Last years, the patient remains stable, NYHA class I, with also stable moderate LV dysfunction.
Clinical Presentation
NCCM is a heterogeneous and unforeseeable disease with respect to its natural history and clinical expression. A significant number of affected individuals go unrecognized or only have intermittent symptoms. The symptomatic NCCM patients may have (atypical) chest pain, dyspnea (with or without exertion), palpitations, edema, syncope, embolic ischemic stroke, myocardial infarction, pulmonary embolism, or sudden cardiac death [10]. Patients, whether adults or pediatric, should undergo a careful history taking and physical examination, with specific attention to cardiac symptoms such as unexplained syncope, palpitations and discomfort on the chest. Also, a thorough family history of CMPs and SCD should be obtained. Major clinical manifestations of NCCM are: heart failure, supraventricular of ventricular arrhythmias, and/or thrombo-embolic events [40]. In this chapter we will focus on the presentation, diagnosis and managment of malignant ventricular arrhythmias and sudden cardiac death.
Patients with NCCM are often accompanied by electrocardiographic abnormalities. The frequency of these abnormalities is high, approximately 90% in adults and in pediatric patients [11]. However, these ECG findings were thought to be not specific [56]. Although, conduction delay and QTc prolongation were correlated with reduced systolic function and LV hypertrophy was significantly associated with thromboembolism. Against that, patients with normal ECGs at presentation had often a preserved left ventricular ejection fractions (LVEF) [29].
Supraventricular arrhythmias and conduction abnormalities are relatively common in NCCM patients. They occur in up to one-quarter of the patients, including atrial fibrillation, atrial flutter, paroxysmal supraventricular tachycardia, or complete atrioventricular block [21,22,23]. The symptoms may manifest as palpitations, (near) syncope or heart failure, with potiential tachycardiomyopathy. Atrial fibrillation occurs in 5–29% of adult patients with NCCM but has not been described in pediatric patients. Wolff-Parkinson-White syndrome found often in patients with NCCM [11]. Wolff-Parkinson-White syndrome is one of the best known preexcitation syndromes. It is characterized by the presence of an accessory pathway which predisposes tachyarrhythmias and sudden death. Wolff-Parkinson-White syndrome was found often in pediatric patients 13–15% and in 0–3% of adult patients with NCCM [11, 57, 58] . Also, sinus node dysfunction can be a clinical manifestation of NCCM. Recently, association with ion channel gene HCN4 mutation is described linked to sinus bradycardia and NCCM.
Ventricular arrhythmias are since its initial description prevalent in patients with NCCM in both adults as in children. In a study of 17 adult patients with NCCM, VT was observed in almost the half of the patients during a follow-up of 30 months. Five out of these 8 patients died during the follow up. An impaired left ventricular systolic function was seen in 82% of the patients [23]. Electric instability can lead to short episodes of VT, also termed as non-sustained VT. If sustained, it can be life threatening and could lead to hemodynamic compromise. Between VT in NCCM and impaired systolic function there seems to be a correlation, but it can’t be concluded that a normal systolic function excludes the risk of VT [13]. In our study, about the indications for an implantable cardioverter defibrillator therapy and the outcomes in 77 adult NCCM patients, 44 of them had such a device according to the guidelines for non-ischaemic cardiomyopathy. During a follow-up (mean) of 33 months, eight patients presented with appropriate defibrillator shocks as a result of sustained ventricular tachycardia. Most of patients implanted with an ICD for secondary prophylaxis has minimal LV dysfunction and no HF. This suggests that patients with NCCM could be at high risk for sudden cardiac death regardless of the presence of HF and/or significant LV dysfunction [19].
Diagnosis and Risk Stratification
Early diagnosis of noncompaction cardiomyopathy can be challenging, given the low prevalence in general practice [23, 25, 60]. Another important factor is the phenotypic heterogeneity of the population [29, 61]. Clinical presentation can be variable from asymptomatic patients to end-stage heart failure, or supraventricular, ventricular arrhythmias, including ventricular tachycardia, ventricular fibrillation and sudden cardiac death. Moreover, no consensus has been reached yet regarding the diagnostic criteria and the best diagnostic approach. Therefore, pathomorphological findings currently appear to be the gold standard for diagnosing NCCM with Jenni criteria most useful for the daily clinical practice [27]. The debate on the true incidence and prevalence of malignant ventricular arrhythmias in NCCM patients continues, because of the lack of large-scale controlled, randomized trials. Thus, definitive risk factors for SCD remains speculative.
The annual incidence of SCD in the general population is 0.1–0.2%, specific subgroups of patients like coronary artery disease and reduced LV function, dilated cardiomyopathy (DCM), arrhytmogenic cardiomyopathy (ARVC), hypertrophic cardiomyopathy (HCM), Brugada syndrome, long QT syndrome, and NCCM are at higher risk. The only way for appropriate risk stratification is by collecting relevant clinical data in a multi-center, prospective registry and follow-up studies. This is however lacking in most nonischemic cardiomyopathies, with no exception for the new disease entity of NCCM. Despite lack of definitive risk factors, it is still important to evaluate each patient for potential risk factors for SCD. The risk factors for SCD that are most commonly cited include: increased LV size, decreased LV systolic function, and the presence of ventricular arrhythmias [6, 20, 25]. Possible other high-risk features are symptomatic heart failure (NYHA class: II to IV) and atrial fibrillation in adults and repolarization abnormalities (ST changes and T wave inversion) in pediatric patients may also indicate a poor outcome [26, 29]. Therefore, periodic echocardiogram and Holter recordings are recommended [20]. Risk of SCD seems greatest in pediatric patients, less than 1 year of age [6, 20, 25]. Subsequent studies showed that sex, localization, and degree of (non)compaction did not seem to be risk factors. Inducible arrhythmias during electrophysiology (EP) testing has been suggested to be useful in NCCM, however it has not been shown to be a reliable predictor of SCD in NCCM patients. But, it must be said that data regarding EP is limited. The usefulness of EP for risk stratification in NCCM remains to be determined [9]. In the following section, we describe out current clinical approach in the view of the scarce contemporary evidence, but with a our more than decade experience with a broad spectrum of NCCM patients.
Management
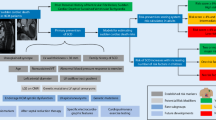
ICD therapy is an effective therapy to prevent sudden cardiac death. The ICD therapy can be applied for primary and secondary prevention. Primary prevention means that individuals are at high risk for, but did not yet have an episode of sustained VT, VF or resuscitated cardiac arrest, especially in the presence of spontaneous ventricular unrest in the form of frequent premature ventricular complexes and/or non-sustained VT. ICD therapy is applied for secondary prevention in patients who have been resuscitated from cardiac arrest or present with documented, sustained ventricular tachyarrhythmia, or unexplained (near)-syncope. Current guidelines recommend implantation of an ICD in patients with impaired left ventricular (LV) function (LVEF ≤35%) caused by coronary artery disease or cardiomyopathy [15, 63]. Ventricular tachyarrhytmias, including cardiac arrest due to VT/VF, are reported in 38–47% and sudden death in 13–18% of adult patients with NCCM [6, 64]. Therefore, implantation of an ICD in these patients is a valid option, although in previous trials no known NCCM patients were included. However, no specific risk factors for SCD in NCCM patients have yet been identified [13]. In our center, our approach for risk stratification of SCD in NCCM patients, whether symptomatic or asymptomatic, is described in the flowchart, (Fig. 5.4). For secondary prevention of sudden cardiac death, an ICD is always advised. If there is a significant dysfunction (i.e. LVEF ≤35%), especially in the setting of symptoms or signs of systolic heart failure, a prophylactic ICD for primary prevention is advised. In other patients empiric individualized risk stratification should be applied, after through anamnesis, physical examination, ECG, an exercise test, and a 48h-Holter. If spontaneous ventricular unrest is found, i.e. nonsustained VT’s, especially in the setting of systolic LV dysfunction (LVEF <50%), familial history of premature SCD <50 years, early repolarization and/or fragmented QRS on the 12-leads ECG, a prophylactic ICD should be considered after through extensive counseling and shared-decision making. With this empiric approach, no episode of SCD, sustained VT or syncope had been encountered in our national referral center for NCCM in the Netherlands with > 200 outpatient patients and up to 15 years of follow-up.
Information on the long-term outcome after ICD therapy in this population remains however limited. Caliskan et al. investigated the indications and outcomes of ICDs in 77 adult patients with NCCM, of whom 44 had a device implanted on the basis of current guidelines for non-ischaemic cardiomyopathy. During a mean follow up of 34 months, eight patients presented with appropriate ICD shocks because of sustained VT after a median of 6 months. The relatively high percentage of appropriate shocks for sustained VT in our population confirms that these NCCM patients are at high risk for SCD and that implanting ICD in this population is an appropriate approach.
It is an interesting finding to see that all the appropriate ICD interventions in our population were due to (fast) VTs, although it isn’t known whether the initial rhythm from our SCD/VF patients was also started with a VT.
Conclusion
In conclusion, in patients with NCCM, malignant ventricular arrhythmia’s and sudden cardiac death are frequently encountered. In selected patients, an ICD implantation in these patients is a valid option and highly effective. Until we have reliable prospective data, it remains reasonable to use the current guidelines for management of patients with VA and the prevention of SCD in non-ischemic cardiomyopathy patients.
References
Feldt RH, Rahimtoola SH, Davis GD, Swan HJ, Titus JL. Anomalous ventricular myocardial patterns in a child with complex congenital heart disease. Am J Cardiol. 1969;23:732–4.
Finsterer J, Zarrouk-Mahjoub S. Grant et al. 1926 did not provide the first description of left ventricular hypertrabeculation/noncompaction. Int J Cardiol. 2013;169:e51–2.
Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB, American Heart Association, Council on Clinical Cardiology, Heart Failure and Transplantation Committee, Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups, Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–16.
Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–6.
Kobza R, Jenni R, Erne P, Oechslin E, Duru F. Implantable cardioverter-defibrillators in patients with left ventricular noncompaction. Pacing Clin Electrophysiol. 2008;31:461–7.
Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36:493–500.
Stollberger C, Finsterer J. Unmet needs in the cardiologic and neurologic work-up of left ventricular hypertrabeculation/noncompaction. Expert Rev Cardiovasc Ther. 2016;14:1151–60.
Jenni R, Oechslin EN, van der Loo B. Isolated ventricular non-compaction of the myocardium in adults. Heart. 2007;93:11–5.
Steffel J, Kobza R, Namdar M, Wolber T, Brunckhorst C, Luscher TF, Jenni R, Duru F. Electrophysiological findings in patients with isolated left ventricular non-compaction. Europace. 2009;11:1193–200.
Finsterer J, Stollberger C, Towbin JA. Left ventricular noncompaction cardiomyopathy: cardiac, neuromuscular, and genetic factors. Nat Rev Cardiol. 2017;14:224–37.
Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004;109:2965–71.
Kannel WB, Thomas HE Jr. Sudden coronary death: the Framingham Study. Ann N Y Acad Sci. 1982;382:3–21.
Stollberger C, Finsterer J. Arrhythmias and left ventricular hypertrabeculation /noncompaction. Curr Pharm Des. 2010;16:2880–94.
Soni A, LeLorier P. Sudden death in nondilated cardiomyopathies: pathophysiology and prevention. Curr Heart Fail Rep. 2005;2:118–23.
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association and Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–484.
Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–83.
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83.
Kobza R, Steffel J, Erne P, Schoenenberger AW, Hurlimann D, Luscher TF, Jenni R, Duru F. Implantable cardioverter-defibrillator and cardiac resynchronization therapy in patients with left ventricular noncompaction. Heart Rhythm. 2010;7:1545–9.
Caliskan K, Szili-Torok T, Theuns DA, Kardos A, Geleijnse ML, Balk AH, van Domburg RT, Jordaens L, Simoons ML. Indications and outcome of implantable cardioverter-defibrillators for primary and secondary prophylaxis in patients with noncompaction cardiomyopathy. J Cardiovasc Electrophysiol. 2011;22:898–904.
Brescia ST, Rossano JW, Pignatelli R, Jefferies JL, Price JF, Decker JA, Denfield SW, Dreyer WJ, Smith O, Towbin JA, Kim JJ. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation. 2013;127:2202–8.
Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–13.
Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T, Hamada H, Hirose O, Isobe T, Yamada K, Kurotobi S, Mito H, Miyake T, Murakami Y, Nishi T, Shinohara M, Seguchi M, Tashiro S, Tomimatsu H. Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol. 1999;34:233–40.
Ritter M, Oechslin E, Sutsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72:26–31.
Lofiego C, Biagini E, Pasquale F, Ferlito M, Rocchi G, Perugini E, Bacchi-Reggiani L, Boriani G, Leone O, Caliskan K, ten Cate FJ, Picchio FM, Branzi A, Rapezzi C. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart. 2007;93:65–71.
Aras D, Tufekcioglu O, Ergun K, Ozeke O, Yildiz A, Topaloglu S, Deveci B, Sahin O, Kisacik HL, Korkmaz S. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail. 2006;12:726–33.
Stollberger C, Blazek G, Wegner C, Finsterer J. Heart failure, atrial fibrillation and neuromuscular disorders influence mortality in left ventricular hypertrabeculation/noncompaction. Cardiology. 2011;119:176–82.
Burke A, Mont E, Kutys R, Virmani R. Left ventricular noncompaction: a pathological study of 14 cases. Hum Pathol. 2005;36:403–11.
Hussein A, Karimianpour A, Collier P, Krasuski RA. Isolated noncompaction of the left ventricle in adults. J Am Coll Cardiol. 2015;66:578–85.
Miyake CY, Kim JJ. Arrhythmias in left ventricular noncompaction. Card Electrophysiol Clin. 2015;7:319–30.
Arbustini E, Weidemann F, Hall JL. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol. 2014;64:1840–50.
Pignatelli RH, McMahon CJ, Dreyer WJ, Denfield SW, Price J, Belmont JW, Craigen WJ, Wu J, El Said H, Bezold LI, Clunie S, Fernbach S, Bowles NE, Towbin JA. Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation. 2003;108:2672–8.
Nugent AW, Daubeney PE, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG, National Australian Childhood Cardiomyopathy Study. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112:1332–8.
Kovacevic-Preradovic T, Jenni R, Oechslin EN, Noll G, Seifert B, Attenhofer Jost CH. Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience. Cardiology. 2009;112:158–64.
Patrianakos AP, Parthenakis FI, Nyktari EG, Vardas PE. Noncompaction myocardium imaging with multiple echocardiographic modalities. Echocardiography. 2008;25:898–900.
Goud A, Padmanabhan S. A rare form of cardiomyopathy: left ventricular non-compaction cardiomyopathy. J Community Hosp Intern Med Perspect. 2016;6:29888.
Stollberger C, Finsterer J, Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol. 2002;90:899–902.
Jefferies JL, Wilkinson JD, Sleeper LA, Colan SD, Lu M, Pahl E, Kantor PF, Everitt MD, Webber SA, Kaufman BD, Lamour JM, Canter CE, Hsu DT, Addonizio LJ, Lipshultz SE, Towbin JA, Pediatric Cardiomyopathy Registry Investigators. Cardiomyopathy phenotypes and outcomes for children with left ventricular myocardial noncompaction: results from the pediatric cardiomyopathy registry. J Card Fail. 2015;21:877–84.
Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, Sharma S, Elliott PM. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J. 2008;29:89–95.
Peters F, Khandheria BK, dos Santos C, Matioda H, Maharaj N, Libhaber E, Mamdoo F, Essop MR. Isolated left ventricular noncompaction in sub-Saharan Africa: a clinical and echocardiographic perspective. Circ Cardiovasc Imaging. 2012;5:187–93.
Towbin JA, Jefferies JL. Cardiomyopathies due to left ventricular noncompaction, mitochondrial and storage diseases, and inborn errors of metabolism. Circ Res. 2017;121:838–54.
Lauer RM, Fink HP, Petry EL, Dunn MI, Diehl AM. Angiographic demonstration of intramyocardial sinusoids in pulmonary-valve atresia with intact ventricular septum and hypoplastic right ventricle. N Engl J Med. 1964;271:68–72.
Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–37.
Towbin JA, Lorts A, Jefferies JL. Left ventricular non-compaction cardiomyopathy. Lancet. 2015;386:813–25.
Fazio G, Corrado G, Zachara E, Rapezzi C, Sulafa AK, Sutera L, Pizzuto C, Stollberger C, Sormani L, Finsterer J, Benatar A, Di Gesaro G, Cascio C, Cangemi D, Cavusoglu Y, Baumhakel M, Drago F, Carerj S, Pipitone S, Novo S. Ventricular tachycardia in non-compaction of left ventricle: is this a frequent complication? Pacing Clin Electrophysiol. 2007;30:544–6.
Sato Y, Matsumoto N, Takahashi H, Imai S, Yoda S, Kasamaki Y, Takayama T, Kunimoto S, Koyama Y, Saito S, Uchiyama T. Cardioverter defibrillator implantation in an adult with isolated noncompaction of the ventricular myocardium. Int J Cardiol. 2006;110:417–9.
Guvenc TS, Ilhan E, Alper AT, Eren M. Exercise-induced right ventricular outflow tract tachycardia in a patient with isolated left ventricular noncompaction. ISRN Cardiol. 2011;2011:729040.
Derval N, Jais P, O’Neill MD, Haissaguerre M. Apparent idiopathic ventricular tachycardia associated with isolated ventricular noncompaction. Heart Rhythm. 2009;6:385–8.
Barra S, Moreno N, Providencia R, Goncalves H, Primo JJ. Incessant slow bundle branch reentrant ventricular tachycardia in a young patient with left ventricular noncompaction. Rev Port Cardiol. 2013;32:523–9.
Santoro F, Manuppelli V, Brunetti ND. Multiple morphology ventricular tachycardia in non-compaction cardiomyopathy: multi-modal imaging. Europace. 2013;15:304.
Seres L, Lopez J, Larrousse E, Moya A, Pereferrer D, Valle V. Isolated noncompaction left ventricular myocardium and polymorphic ventricular tachycardia. Clin Cardiol. 2003;26:46–8.
Junga G, Kneifel S, Von Smekal A, Steinert H, Bauersfeld U. Myocardial ischaemia in children with isolated ventricular non-compaction. Eur Heart J. 1999;20:910–6.
Ruberman W, Weinblatt E, Goldberg JD, Frank CW, Shapiro S. Ventricular premature beats and mortality after myocardial infarction. N Engl J Med. 1977;297:750–7.
Packer M. Lack of relation between ventricular arrhythmias and sudden death in patients with chronic heart failure. Circulation. 1992;85:I50–6.
Van Malderen S, Wijchers S, Akca F, Caliskan K, Szili-Torok T. Mismatch between the origin of premature ventricular complexes and the noncompacted myocardium in patients with noncompaction cardiomyopathy patients: involvement of the conduction system? Ann Noninvasive Electrocardiol. 2017;22:e12394.
Engberding R, Bender F. Identification of a rare congenital anomaly of the myocardium by two-dimensional echocardiography: persistence of isolated myocardial sinusoids. Am J Cardiol. 1984;53:1733–4.
Steffel J, Duru F. Rhythm disorders in isolated left ventricular noncompaction. Ann Med. 2012;44:101–8.
Caliskan K, Balk AH, Jordaens L, Szili-Torok T. Bradycardiomyopathy: the case for a causative relationship between severe sinus bradycardia and heart failure. J Cardiovasc Electrophysiol. 2010;21(7):822–4.
Milano A, Vermeer AM, Lodder EM, Barc J, Verkerk AO, Postma AV, van der Bilt IA, Baars MJ, van Haelst PL, Caliskan K, Hoedemaekers YM, Le Scouarnec S, Redon R, Pinto YM, Christiaans I, Wilde AA, Bezzina CR. HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. Coll Cardiol. 2014;64(8):745–56.
Paparella G, Capulzini L, de Asmundis C, Francesconi A, Sarkozy A, Chierchia G, Brugada P. Electro-anatomical mapping in a patient with isolated left ventricular non-compaction and left ventricular tachycardia. Europace. 2009;11:1227–9.
Sandhu R, Finkelhor RS, Gunawardena DR, Bahler RC. Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction. Echocardiography. 2008;25:8–12.
Paterick TE, Gerber TC, Pradhan SR, Lindor NM, Tajik AJ. Left ventricular noncompaction cardiomyopathy: what do we know? Rev Cardiovasc Med. 2010;11:92–9.
Jenni R, Wyss CA, Oechslin EN, Kaufmann PA. Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J Am Coll Cardiol. 2002;39:450–4.
Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292:2874–9.
Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–71.
Narang R, Cleland JG, Erhardt L, Ball SG, Coats AJ, Cowley AJ, Dargie HJ, Hall AS, Hampton JR, Poole-Wilson PA. Mode of death in chronic heart failure. A request and proposition for more accurate classification. Eur Heart J. 1996;17:1390–403.
Murphy RT, Thaman R, Blanes JG, Ward D, Sevdalis E, Papra E, Kiotsekoglou A, Tome MT, Pellerin D, McKenna WJ, Elliott PM. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26:187–92.
Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med. 1993;119:1187–97.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kaya, E., Otten, M., Yap, SC., Szili-Torok, T., Caliskan, K. (2019). Malignant Arrhythmias and Sudden Cardiac Death in Patients with Noncompaction Cardiomyopathy: Prevalence, Prevention, and Use of Implantable Cardiac Defibrillators. In: Caliskan, K., Soliman, O., ten Cate, F. (eds) Noncompaction Cardiomyopathy. Springer, Cham. https://doi.org/10.1007/978-3-030-17720-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-17720-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17719-5
Online ISBN: 978-3-030-17720-1
eBook Packages: MedicineMedicine (R0)