Abstract
Sudden cardiac death (SCD) is among the leading causes of death worldwide, and it remains a public health problem, as it involves young subjects. Current guideline-directed risk stratification for primary prevention is largely based on left ventricular (LV) ejection fraction (LVEF), and preventive strategies such as implantation of a cardiac defibrillator (ICD) are justified only for documented low LVEF (i.e., ≤ 35%). Unfortunately, only a small percentage of primary prevention ICDs, implanted on the basis of a low LVEF, will deliver life-saving therapies on an annual basis. On the other hand, the vast majority of patients that experience SCD have LVEF > 35%, which is clamoring for better understanding of the underlying mechanisms. It is mandatory that additional variables be considered, both independently and in combination with the EF, to improve SCD risk prediction. LV hypertrophy (LVH) is a strong independent risk factor for SCD regardless of the etiology and the severity of symptoms. Concentric and eccentric LV hypertrophy, and even earlier concentric remodeling without hypertrophy, are all associated with increased risk of SCD. In this paper, we summarize the physiology and physiopathology of LVH, review the epidemiological evidence supporting the association between LVH and SCD, briefly discuss the mechanisms linking LVH with SCD, and emphasize the need to evaluate LV geometry as a potential risk stratification tool regardless of the LVEF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization (WHO), sudden cardiac death (SCD) is an unexpected death either within 1 h of symptom onset (event witnessed) or within 24 h of having been observed alive and symptom free (unwitnessed) [1]. Sudden cardiac death is among the leading causes of death worldwide, and it remains a public health problem, as it involves young subjects. Current guideline-directed risk stratification for primary prevention is largely based on left ventricular (LV) ejection fraction (LVEF), and preventive strategies, such as implantation of a cardiac defibrillator (ICD), are justified only for documented low LVEF (i.e., ≤ 35%) [2]. However, contemporary real-world data indicate that no more than 3 to 5% of primary prevention ICDs, implanted on the basis of low LVEF, deliver life-saving therapies on an annual basis [3]. Moreover, in nearly two-thirds of the cases, SCD is the first clinical manifestation of an underlying disease in previously asymptomatic, apparently “healthy” subjects, or in the setting of known cardiac disease in the absence of risk predictor. In those cases, autopsy represents the first, and only, opportunity to establish and register an accurate cause of death [4]. On the other hand, the vast majority (≥ 70%) of patients that experience SCD will have an LVEF > 35% which is clamoring for better understanding of the underlying mechanisms and new ways of identifying patients at increased SCD risk [5].
Several studies have shown that LV hypertrophy (LVH) is a strong independent risk factor for SCD regardless of the etiology and the severity of the symptoms [6,7,8]. Concentric and eccentric LV hypertrophy, and even earlier concentric remodeling without hypertrophy, are all associated with increased risk of SCD. In this paper, we summarize the physiology and physiopathology of LVH, review the evidence supporting the association between LVH and SCD, briefly discuss the mechanisms linking LVH with SCD, and emphasize the need to consider the type and severity of LVH when assessing the risk for SCD.
Left ventricular hypertrophy
Definition
Left ventricular hypertrophy is defined as an increase in LV mass, which can be secondary to an increase in wall thickness, an increase in cavity size, or both. The electrocardiogram (ECG) is moderately accurate in diagnosing LVH; however, it may unmask an abnormal electrophysiological substrate associated with increased mortality [9]. The estimation of LV mass is commonly derived from LV measurements obtained by two-dimensional (2D) echocardiography. According to the American Society of Echocardiography and the European Association of Echocardiography, the criteria for LVH, using wall thickness and cavity dimensions, are an estimated LV mass (indexed for body surface area) of > 102 g/m2 for men and > 88 g/m2 for women [10]. The corresponding criteria for LVH using cardiovascular magnetic resonance (CMR) are > 85 g/m2 and > 68 g/m2, respectively [11].
According to the Association for European Cardiovascular Pathology, a post-mortem diagnosis of LVH is usually based on measurements of (a) total heart weight, assessed against tables of normal weights by age, gender, and body weight/height, and (b) wall thickness, measured at the LV mid-cavity free wall and the interventricular septum (excluding trabeculae), assessed against tables of normal thickness by age, gender, and body weight [4]. For example, in a 57-year-old male, the expected heart weight is 315–327 g, with an upper limit of normal 431 g, whereas both the interventricular septum and posterior wall should measure < 12 mm.
Classification
LVH is classified as physiological or pathological, each form being regulated by distinct cellular signaling pathways [12].
Physiological LVH is characterized by a mild (10–20%) increase in cardiac mass and individual cardiomyocyte growth in both length and width (Fig. 1). Myocardial thickening is part of normal aging [13] and may also be found in athletes usually associated with an increase in cardiac dimensions (“athlete’s heart”) [14]. Sex significantly affects cardiac remodeling in athletes, with females maintaining normal LV geometry, with relative larger increase of cavity dimensions compared with men [15]. Type of sport has a relevant impact, with endurance athletes exhibiting the greatest degree of right ventricular (RV) and LV dimensional remodeling. Physiological LVH is not associated with loss of cardiomyocytes (apoptosis and necrosis), increased interstitial fibrosis, and reactivation of fetal cardiomyocyte gene program [16]. Nevertheless, fibrosis limited to the inferior insertion point has been observed in up to 20–30% of the athletes, irrespective of age, and has been correlated with a cumulative training load and training intensity [17]. The presence of fibrosis extending beyond the insertion points in the interventricular septum or fibrosis elsewhere in the myocardium regardless of its pattern should prompt further evaluation, especially in the younger athlete age group [17].

Reproduced with permission from Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018 Jul;15(7):387–407
Overview of physiological and pathological hypertrophy. The heart undergoes physiological or pathological enlargement of cardiac mass, termed hypertrophy, to decrease ventricular wall stress in response to various stimuli according to Laplace’s law. Physiological hypertrophy can occur during pregnancy and endurance training and is mainly identified by a mild (10–20%) increase in ventricular volume with a coordinated increase in wall thickness (eccentric hypertrophy) and individual cardiomyocyte growth in both length and width. After relief of the stimulus, physiological hypertrophy is reversed and the heart returns to its original dimensions. Conversely, pathological hypertrophy is observed in patients with myocardial infarction, valvular diseases, and metabolic syndrome and is initially identified by a reduction in ventricular chamber dimension with increased wall thickness (concentric hypertrophy), where cardiomyocytes typically increase in thickness more than in length. Pathological hypertrophy leads to ventricular chamber dilatation (eccentric hypertrophy) with impaired contractile function (maladaptive remodeling), with lengthening of individual cardiomyocytes. Pathological hypertrophy often results in heart failure with either preserved or reduced ejection fraction. Solid arrows indicate proven pathways; dashed arrows indicate hypothetical or controversial pathways.
Pathological LVH is characterized by a moderate (20–40%) to severe (> 40%) increase in cardiac mass and individual cardiomyocyte growth (Fig. 1). In contrast to physiological hypertrophy, it is associated with loss of cardiomyocytes (apoptosis and necrosis), increased interstitial fibrosis, and reactivation of fetal cardiomyocyte gene program. Pathological LVH is usually divided in concentric and eccentric [18]. Concentric hypertrophy represents an increase in the ratio of the wall thickness to the chamber dimension, is due to in parallel addition of sarcomeres causing thickening of the cardiomyocytes, and may be observed in both acquired conditions (e.g., conditions with pressure overload such as hypertension or aortic stenosis) and primary cardiomyopathic processes (e.g., hypertrophic cardiomyopathy [HCM] variant) [18, 19]. There is often increased interstitial and perivascular fibrous tissue deposition, particularly within the subendocardial third of the LV myocardium. Eccentric hypertrophy is characterized by an increase in LV mass with a simultaneous increase in LV dimensions and is due to serial addition of sarcomeres causing elongation of the cardiomyocytes [18, 19]. Eccentric LVH may also be observed in both acquired conditions (e.g., conditions with volume overload such as mitral, aortic regurgitation, and endurance training) and primary cardiomyopathic processes (e.g., dilated cardiomyopathy [DCM]).
Special cases of pathologic LVH exist, besides the aforementioned. Asymmetric LVH, specifically septal one, is typically seen in patients with HCM, among those with prior myocardial infarction and in some patients with arterial hypertension [20]. Idiopathic LVH is defined as LVH in the absence of myocyte disarray or secondary causes. It appears to be a distinct disease entity from HCM and is associated with fatal arrhythmias in individuals (Fig. 2) [21, 22]. Cardiomyopathy not otherwise specified (NOS) exhibits histological evidence of significant cardiomyocyte hypertrophy along with degenerative changes, interstitial fibrous tissue deposition, and even occasionally small foci of myofiber disarray/architectural disarray of muscle bundles, yet the heart may be in the normal size range or only modestly enlarged [18, 23]. Finally, obesity-associated LVH usually includes eccentric LVH, although a concentric pattern is frequently seen [24]. Hypertension, neurohormonal overactivity, and metabolic abnormalities contribute to the development of LVH in obese individuals.

Reproduced with permission from Whyte G, Sheppard M, George K, Shave R, Wilson M, Prasad S, O’Hanlon R, and Sharma S. Post-mortem evidence of idiopathic left ventricular hypertrophy and idiopathic interstitial myocardial fibrosis: is exercise the cause? Br J Sports Med 2008; 42: 304–305
Idiopathic left ventricular hypertrophy. Gross morphology (upper panel) and histological examination (lower panel) of the heart of 57-year-old, highly trained, male runner who died while running a marathon. There was circumferential left ventricular hypertrophy with a heart weight of 480 g. The lateral and anterior free wall measured 20 mm at the midventricular level. Both the interventricular septum and posterior wall measured 19 mm in thickness. There was also hypertrophy of trabeculae and both papillary muscles. The diameter of the left ventricle chamber was reduced to 10 mm. The histology slide of the left ventricular myocardium following sirius red FB3 staining showed idiopathic interstitial myocardial fibrosis.
LVH as a risk factor for sudden cardiac death
There is compelling epidemiological evidence that increased LV mass is associated with increased risk for SCD, both in the general population and in diverse patient groups, such as those with hypertension, coronary artery disease (CAD), aortic stenosis (AS), HCM, secondary cardiomyopathies, and heart failure (HF).
General population
The presence of LVH in the ECG can help identify patients at increased risk of SCD. In the Framingham Heart Study, LVH on an ECG was associated with a 2- to fivefold increased risk of SCD in men but was only a modest risk factor in women [25]. The Mini-Finland Health Survey, a large Finnish general population cohort, was used for the development of an ECG-based risk score using five ECG abnormalities independently associated with SCD risk (heart rate > 80 beats per minute, PR duration > 220 ms, QRS duration > 110 ms, LVH, and T-wave inversion) to identify subjects at high 10-year risk for SCD [26]. This score was subsequently validated in a separate large Finnish general population cohort. Subjects with ≥ 3 ECG abnormalities had a hazard ratio (HR) of 10.23 (95% CI 5.29–19.80) for SCD compared with those without abnormalities. The risk score similarly predicted SCD risk in the validation cohort, in which subjects with ≥ 3 ECG abnormalities had an HR of 10.82 (95% CI 3.23–36.2) for SCD compared with those without abnormalities.
The relation between echocardiographically determined LV mass to the incidence of SCD was also investigated in normal subjects (n = 3,661, ≥ 40 years of age) enrolled in the Framingham Heart Study [8]. The incidence of SCD during an up to 14-year follow-up was 1.64%, whereas the prevalence of LVH was 21.5%. The presence of LVH was associated with a more than two fold higher risk for SCD. This risk increased by 45% for each 50-g/m increment in LV mass [8]. Echocardiograms were also obtained on 2383 participants from the Atherosclerosis Risk in Communities (ARIC) Study (100% African American) and 5366 participants from the Cardiovascular Health Study (CHS) [27]. During a median follow-up of 7.3 years and 13.1 years, respectively, 1.85% ARIC Study and 5.12% CHS participants had SCD. In the meta-analyzed results, independent predictors of SCD were reduced LVEF, mitral annular calcification, mitral E/A > 1.5 or mitral E/A < 0.7 LVH, and left atrial (LA) enlargement. Laukkanen and colleagues echocardiographically assessed LV mass in 905 middle-aged men representative of the general population [28]. During a follow-up period of 20 years, there were a total of 63 (6.96%) SCDs. In a comparison of the top vs. the bottom quartile of LV mass adjusted by body surface area, the multivariable adjusted HR was 2.57 (95% CI 1.24–5.31; P = 0.010). Interestingly, further adjustment for LV function only modestly attenuated the risk of SCD among men with LV mass of > 120 g/m2.
Hypertension
Several studies have shown the association of LVH and SCD in hypertensive patients. Verdecchia and colleagues studied a cohort of 3242 untreated hypertensive patients, without evidence of coronary or cerebrovascular heart disease at entry, for an average of 10.3 years. Prevalence of LVH at ECG was 13.9%. During follow-up, SCD occurred at a rate of 0.10 per 100 patient-years. The rate of SCD was 0.07 and 0.30 per 100 patient-years, respectively, in the cohort of patients without and with ECG LVH (P < 0.01). In a multivariable Cox model, LVH almost tripled the risk of SCD. For each 10 mmHg increase in 24-h ambulatory pulse pressure, the risk of SCD increased by 35% [29].
Hypertension and LVH are established major risk factors for the development of atrial fibrillation (AF) which in turn increases the risk for SCD. The relationship of new-onset AF and SCD was evaluated in 8831 hypertensive patients with electrocardiographic LVH, no history of AF, in sinus rhythm on their baseline ECG, randomly assigned to losartan or atenolol [30]. During a mean follow-up of 4.7 years, new-onset AF occurred in 7.9% patients and SCD in 1.7% patients. In adjusted multivariate Cox analyses, new-onset AF was associated with a > threefold higher risk of SCD.
Coronary artery disease
In patients with stable CAD, increased LV mass index is independently associated with all-cause mortality and SCD, even in subjects with normal LVEF. In the Heart and Soul Study, the association of LV mass index with all-cause mortality and SCD was evaluated in 1016 patients with stable CAD using transthoracic echocardiography. During a mean follow-up of 3.55 years, there were 146 deaths and 34 SCD. Total mortality was higher in subjects with LVH (25% vs. 11%, P < 0.001), as was mortality from SCD (6.7% vs. 2.2%, P = 0.001). LVH was associated with both all-cause mortality (HR, 2.0; P < 0.001) and SCD (HR, 3.1; P = 0.003). Findings were similar in the subgroup with LVEF ≥ 55%. Analyzed as a continuous variable, every 20-unit increase in LV mass index increased the adjusted HR of death by 22% and adjusted HR of SCD by 40% [31].
The additive effect of LVH on survival related to the extent of CAD and LVEF was evaluated in a study that included 1089 consecutive black patients who underwent both coronary angiography and M-mode echocardiography and were followed-up for 5 years [32]. The corresponding attributable risk fractions for single-vessel disease, multivessel disease, LV dysfunction, and LVH were 1%, 22%, 9%, and 37%, respectively, meaning that for every 100 deaths in this cohort, LVH independently accounted for 37.
Aortic stenosis
AS is a progressive disease affecting around 3% of adults aged ≥ 75 years in the western world [33]. SCD is a significant concern in these patients, with a reported incidence ranging from 0.3% per year to 3.1% per year, depending on AS severity as well as a variety of non-valve-related factors [34, 35]. The incidence and potential risk factors of SCD in asymptomatic patients with AS were assessed the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study [36]. A total of 1849 patients with mild to moderate AS and available clinical, echocardiographic, and follow-up data were analyzed. During an overall follow-up of 46.1 ± 14.6 months, SCD occurred in 27 asymptomatic patients (1.5%). Cox regression analysis identified age, increased LV mass index, and lower BMI as independent risk factors of SCD.
Hypertrophic cardiomyopathy
In HCM, the magnitude of LVH is directly related to the risk of SCD and is a strong and independent predictor of prognosis. Young patients with extreme hypertrophy, even those with few or no symptoms, appear to be at substantial long-term risk and deserve consideration for interventions to prevent SCD. Spirito et al. assessed the relation between LVH and mortality in 480 consecutive patients with HCM. Over a mean follow-up of 6.5 years, 65 of the 480 patients died, 23 suddenly, 15 of HF, and 27 of non-cardiac causes or stroke. The risk of SCD increased progressively and in direct relation to wall thickness. Twenty years after the initial evaluation, patients with a wall thickness ≤ 19 mm had a cumulative risk close to 0%, whereas those with a wall thickness ≥ 30 mm had a risk of close to 40%. The highest rate of SCD was observed in the youngest patients with a wall thickness of ≥ 30 mm [37].
In an international multi-center observational cohort study, 572 phenotype-positive young patients (< 18 years) with isolated HCM at diagnosis were included [38]. The 5-year proportion of SCD events was 9.1%, and 2 out of the 8 parameters independently associated with SCD were measures of LVH. More specifically, risk predictors included age at diagnosis, documented non-sustained ventricular tachycardia, unexplained syncope, septal diameter z-score, LV posterior wall diameter z-score, LA diameter z-score, peak LV outflow tract (LVOT) gradient, and presence of a pathogenic variant.
Secondary cardiomyopathies
Sudden cardiac death is frequent in other secondary cardiomyopathies, such as in cardiac amyloidosis (CA) and in Fabry disease (FD).
-
(i)
Cardiac amyloidosis has an incidence of 18 per 100,000 person-years, and is an underestimated cause of HF and cardiac arrhythmias [39]. Data linking CA with potentially life-threatening arrhythmias are scarce and mainly based on evidence derived from single-center studies or population registries dealing with epidemiological features of the patients [39, 40]. Τhe exact mechanism of these arrhythmias is less well defined and likely multi-factorial. Bradyarrhythmias are more common in the transthyretin form of amyloidosis, while the risk for ventricular arrhythmias is higher in the AL form [41, 42]. Patients with CA have frequent dysfunction in the His-Purkinje system which is demonstrated with HV prolongation, a condition that should be noted because ventricular arrhythmias in CA are caused by PVC arising from the distal Purkinje system [43, 44]. Since bradyarrhythmias are common in this infiltrative cardiomyopathy, there is general consensus that early intervention with cardiac pacing is often beneficial. The role of implantable defibrillators is more contentious as sustained ventricular arrhythmia is less frequent particularly in wild type ATTR [45].
-
(ii)
Fabry disease, also known as Anderson-Fabry disease, is a rare genetic disease, inherited in an X-linked manner that can affect many parts of the body including the heart. It is caused by mutations of the GLA gene that result in a deficiency of the enzymatic activity of α-galactosidase A and consequent accumulation of glycosphingolipids, [46]. A systematic review of the literature estimated the occurrence of VT in 15.3% of the patients and the proposed mechanism of sustained VT was re-entry related to myocardial fibrosis [47]. ICD implantation is recommended in patients who suffered sudden cardiac arrest due to VT/ventricular fibrillation or sustained VT causing syncope or hemodynamic compromise and have a life expectancy of > 1 year. Evidence is lacking to guide ICD implantation in primary prevention in this patient population [48]. As in CA, precise SCD risk prediction in early stages of FD remains a challenge.
Heart failure
Very similarly to hypertension, AF, CAD, and AS, LVH in patients with HF is also associated with a higher risk of SCD. The Studies of Left Ventricular Dysfunction (SOLVD) trial reported a strong association between LV mass and mortality, both in patients with a reduced and in those with a preserved LVEF. After adjustment for LVEF, a 1-SD increase in LV mass had a risk ratio of 1.3 (P = 0.012). Patients with LVEF > 35% and LV mass > 298 g had equivalent risk for SCD compared to those with LVEF < 35% and LV mass < 298 g, indicating that LVH in patients with preserved LVEF was as powerful a predictor as low LVEF in patients with normal LV mass [49].
The Oregon Sudden Unexpected Death Study compared LVEF with LV mass as predictors of SCD in 191 SCD patients with ante-mortem echocardiograms unrelated to the SCD event and compared these to 203 controls with CAD. LVH was significantly more prevalent in SCD patients compared to controls, even in patients with LVEF > 35% (42% in cases vs. 21% in controls; P < 0.0001). After adjusting for other significant risk factors, the odds ratio (OR) for SCD in patients with a LVEF ≤ 35% but no LVH was 1.9, and 1.8 in patients with LVH but no reduced LVEF. If both conditions were present the OR increased to 3.5, demonstrating additive effects of LVH and LVEF ≤ 35% on SCD risk [50]. In a subsequent analysis of the same study, adult SCD cases were compared with geographic controls with no prior history of SCD. Archived echocardiograms performed closest and prior to the SCD event were reviewed. LV geometry was defined as normal, concentric remodeling (normal LV mass index and increased relative wall thickness), concentric hypertrophy, or eccentric hypertrophy. Analysis was restricted to those with LVEF ≤ 40%. A total of 246 subjects were included in the analysis. SCD cases, compared to controls, had lower LVEF (29.4 ± 7.9% vs 30.8 ± 6.3%, P = 0.021). Fewer cases presented with normal LV geometry (30.2% vs 43.2%, P = 0.048) and more with eccentric hypertrophy (40.7% vs 25.7%, P = 0.025). In a multivariate model, eccentric hypertrophy was independently predictive of SCD [51].
In a separate analysis of the Oregon Sudden Unexpected Death Study, SCD cases with moderately reduced or preserved LVEF and geographical controls did not differ in age, sex, or LVEF, but increased LV mass was more common in SCD cases. A total of 29% of SCD cases presented with normal LV geometry, 35% had concentric remodeling, 25% concentric LVH, and 11% eccentric LVH. In multivariate model, concentric remodeling, concentric LVH, and eccentric LVH were associated with increased risk of SCD [52].
Predictors of sudden death (SD)/aborted cardiac arrest (ACA) in HF patients with LVEF ≥ 45% (n = 1,767) were evaluated in the Americas region of the Aldosterone Antagonist Therapy for Adults With Heart Failure and Preserved Systolic Function (TOPCAT) trial. During a median 3.0-year follow-up, 77 patients experienced SD/ACA, and 312 experienced non-SD/ACA. ECG LVH was more frequent in the SD/ACA group than in the non-SD/ACA group (13% vs. 8.3%, respectively; P = 0.21) [53].
Heart failure with preserved ejection fraction
Despite the increasing prevalence and economic burden of HFpEF, little is known about specific modes of death in this patient population. Inadequate understanding of the specific CV and non-CV mechanisms driving terminal events renders therapeutic development difficult and has contributed to the lack of success of drug and device therapy to alter the disease trajectory. The majority of deaths in HFpEF are CV deaths, comprising 51–60% of deaths in epidemiological studies and ~ 70% in clinical trials [54]. Among CV deaths, SCD and HF death are the leading cardiac modes of death in HFpEF clinical trials. Non-CV mortality, however, represents an important competing risk. Vaduganathan et al. conducted a systematic review of 1608 published HFpEF papers with mode-of-death data [55]. Although sudden death accounted for 25 to 30% of deaths in trials, its definition was nonspecific and it remained unclear what proportion represented arrhythmic deaths. In this rigorous and comprehensive systematic review, only 8 major RCTs and 24 epidemiological studies collected information on mode of death in HFpEF over a 30-year period, highlighting 4 major points: (1) the majority of patients with HFpEF die of CV-related causes, but there is significant variability between RCTs and epidemiological studies; (2) specific CV causes of death are infrequently adjudicated and poorly defined; (3) SCD accounts for up to 30 to 40% of CV-related deaths in HFpEF; and (4) non-CV modes of death may be an important competing risk, accounting for up to 20 to 30% of deaths in HFpEF studies. In a very recent prospective Japanese study, Kitai et al. examined the differences in the incidence and mechanisms of death among patients with HFrEF, HFmrEF, and HFpEF, after discharge from the index hospitalization [56]. The authors reported that the mode of death was similar among the HF subtypes. During a median follow-up period of 470 days, all-cause death occurred in 21.5% in the HFrEF group, 22.5% in the HFmrEF group, and 24.0% in the HFpEF group. CV deaths occurred in 14.7%, 13.8%, and 13.7%, respectively. Finally, SCD occurred in 2.6% of all patients (3.2% in the HFrEF group, 2.0% in the HFmrEF group, and 2.5% in the HFpEF group). Given the non-negligible incidence of SCD in patients with HFpEF and HFmrEF found in this study, further studies appear to be warranted to identify a high-risk subset in this population. A longitudinal multicenter, global registry of patients with HFpEF is urgently needed to map its natural history. Importantly, reporting and definitions of modes of death must be standardized and tailored to the HFpEF population. More frequent systematic autopsies and long-term rhythm monitoring may help clarify the underlying pathology and mechanisms driving mortal events in this patient population.
LVH in aytopsy studies of sudden cardiac death
LVH is a frequent finding in autopsy studies of SCD. In a single center, retrospective study investigating sudden death cases, the five most common findings among victims (mean age, 37 years; range 0–62 years; 75% males) who died unexplained due to suspected or confirmed cardiac disease were LVH and/or enlarged heart (35%), coronary atherosclerosis (31%), myocardial fibrosis (19%), dilated chambers (7%), and myocardial inflammation (5%) [57].
A retrospective case–control study compared cardiac findings of male firefighters aged 18 to 65 years who died on duty of cardiac-related causes with those who died of non-cardiac trauma–related causes [58]. Among cardiac cases, the most prevalent (82%) underlying pathologic substrate was comorbid CAD and cardiomegaly/LVH. Cardiac cases had a higher prevalence of cardiomegaly (heart weight > 450 g), LVH (LV wall thickness ≥ 12 mm), and severe coronary artery stenosis (≥ 75%) than trauma controls (all P < 0.001). In multivariate analyses, heart weight > 450 g, coronary artery stenosis ≥ 75%, and evidence of a prior myocardial infarction were strong independent predictors of SCD.
Recently, 19,740 autopsies conducted in both children (age 7–17 years) and adults (age 18–65 years) at a forensic medicine facility in Australia between 2006 and 2015 were reviewed [59]. There were 201 sports-related adult deaths at an incidence rate of 0.76–1.49 per 100,000 participant-years. Structural abnormalities were common in adult cardiac deaths; 38% had cardiac weight ≥ 500 g, and 55% had LV wall thickness > 15 mm. Of the 15 child deaths, 33% were arrhythmogenic or presumed arrhythmic, and 5 33% were inherited cardiomyopathies (2 HCM, 3 arrhythmogenic right ventricular cardiomyopathy).
LVH as a bystander in sudden cardiac death
There is often inability to legitimately deduce a cause-and-effect relationship between LVH and SCD on the basis of an observed association between them. The example of sudden death due to acute aortic dissection (AAD) with LVH as a bystander is the easiest to understand.
Although AAD is relatively uncommon, it ranks high among necropsy-confirmed causes of out-of-hospital sudden death in the general population [60]. In a study that included 338 autopsy cases with AAD (86% type A and 14% type B), cardiomegaly with LVH (heart weight 606 ± 156 g) was found in 85%, and was the most common preexisting clinical risk factor identified [61]. Remarkably similar is the case with CAD being the primary cause of SCD and LVH simply co-existing. Seventy-one SCD victims (age 49.5 ± 7 years, 86% male) without an initially apparent cause of death were evaluated at the Hennepin County Medical Examiner’s office from August 2001 to July 2004 [62]. On autopsy, 80% of the subjects had high-grade coronary artery stenoses, whereas acute coronary lesions and previous silent MI were found in 27% and 34%, respectively. LVH and cardiomegaly were found in 58% and 59% of the cases, respectively.
A study of 525 WHO-defined autopsied SCDs examined the prevalence of the subset of sudden arrhythmic cardiac death (SACD), defined as death for which no identifiable non-arrhythmic cause of death was found (e.g., acute cerebrovascular accident, viscus perforation, tamponade, vascular rupture, pulmonary embolism, hemorrhage, lethal toxicology/occult overdose, acute HF with pulmonary edema) without exclusion of underlying or associated cardiac disease [23]. SACD was present in only 55.8% of overall, 65% of witnessed, and 53% of unwitnessed WHO-defined SCDs. Leading causes of death were CAD (32%), occult overdose (13.5%), cardiomyopathy (10%), LVH (8%), and neurological (5.5%). In the same study, a history of hypertension was present in 60% of the cases and one-third of autopsy defined SACDs were attributable to non-ischemic cardiomyopathies and LVH without histological evidence of HCM [23].
Finally, of 352 incident SCDs in the San Francisco Postmortem Systematic Investigation of Sudden Cardiac Death Study, 95% underwent systematic evaluation including full autopsy [63]. Of these, 5.4% were sudden neurologic deaths, which accounted for 14.9% of the non-cardiac sudden deaths. A history of epilepsy was identified in 4.5% of sudden deaths. Of these patients, 3 had nonspecific cardiac findings (minor thickening of septal and posterior left ventricle and interstitial fibrosis), two cardiac hypertrophy, two cardiomyopathy, and one HF.
Taken together LVH may be directly involved in the acute lethal event or may simply be a bystander (Fig. 3).
Schematic illustration of the relationship between left ventricular hypertrophy (LVH) and sudden cardiac death (SCD). Solid arrows indicate causal relationship, whereas dotted arrows indirect relationship (LVH may be a bystander). For example, LVH is a common finding in SCD due to acute aortic dissection (AAD) because both LVH and AAD are frequently the result of hypertension
Risk stratification scores for SCD: feasibility and problems
The ability to predict risk allows healthcare providers to propose which patients might benefit most from certain therapies, and is relevant to payers’ demands to justify clinical and economic value. A number of risk prediction models have been published to statistically predict the risk of future outcomes associated with HF. The Seattle Heart Failure Model (SHFM), a validated prediction model for total mortality in HF [64], may provide information about the likely mode of death among ambulatory HF patients. Mozaffarian et al. used the SHFM to assess the mode of death in 10,538 ambulatory HF patients with predominantly systolic dysfunction [65]. Compared with a SHFM score of 0, patients with a score of 1 had a 50% higher risk of sudden death, patients with a score of 2 had a nearly threefold higher risk, and patients with a score of 3 or 4 had a nearly sevenfold higher risk (P < 0.001 for all comparisons). Using commonly available baseline clinical and demographic variables, Shadman et al. developed a novel multivariate regression model, the Seattle Proportional Risk Model (SPRM), to identify variables associated with a disproportionate risk of sudden death in 9885 patients with HF without ICDs [66]. Lower ejection fraction and better functional class were associated with a greater proportion of mortality due to sudden death. Younger age, male sex, and higher body mass index were independently associated with a greater proportional risk of sudden death, while diabetes mellitus, hyper/hypotension, higher creatinine level, and hyponatremia were associated with a disproportionately lower risk of sudden death. In a recent prospective study, Fukuoka et al. evaluated the incidence of SCD within a multicenter Japanese registry of 2240 HF patients hospitalized for acute decompensation, and externally validated the SPRM [67]. During the 2-year follow-up, the cumulative incidence of SCD was 3.4%, the SPRM performed reasonably well, and helped improving SCD prediction and ICD implantation. However, there are several limitations and problems in the implementation of risk prediction models in clinical practice. Though it may be easy to develop a risk model to stratify patients who did or did not have an event within a cohort, where the outcomes are already known, it is far more difficult to validate the risk model in another cohort and, more importantly, to apply the model to assist in making decisions in individual patients [68]. Therefore, when applied to diverse population cohorts, a particular risk model may significantly overestimate or underestimate the absolute risk. Weather risk prediction models will help to better identify the HF patient at high-risk for SCD remains a subject of future research.
The role of myocardial biopsy in the differential diagnosis and in risk stratification
Due to its invasive nature, routine use of myocardial biopsy in everyday clinical practice remains quite limited. Missing standardization in biopsy performance, the bioptic sampling error, missing therapeutic and prognostic consequences, subjectivity in the judgment and interpretation of the findings, and potential complications in performing myocardial biopsies are further limitations of the method [69].
The role of imaging modalities for the risk stratification in SCD
Cardiac imaging has developed several indices beyond LVEF that permit identification of patients at high risk for SCD. Echocardiography remains the imaging technique of first choice to assess left and right ventricular function. Modern methods like strain imaging have provided the ability to discover diseased areas of the myocardium, potentially arrhythmogenic, that were previously indistinguishable. Apart from echocardiography, cardiac magnetic resonance (CMR) is currently considered the reference standard for the measurement of the cardiac chamber dimensions and function and provides the unique opportunity to identify the presence and extent of myocardial edema/inflammation, generalized or focal, which can be a substrate for arrhythmia [70].
Ventricular arrhythmias as a mechanism of sudden cardiac death in LVH
An important mechanism contributing to the high mortality and sudden death in LVH is ventricular arrhythmia.
Ventricular arrhythmia
In most cases in which LVH is the presumed cause of SCD, the underlying mechanism is the development of lethal arrhythmias. An important proof of connection of LVH with arrhythmias was the systematic research of Chatterjee et al. including 27,141 hypertensive patients. The incidence of ventricular arrhythmias in the presence of LVH was 5.5% compared with 1.2% in patients without LVH (P < 0.001). The occurrence of ventricular tachycardia (VT) or ventricular fibrillation (VF) was 2.8-fold greater in the presence of LVH (OR 2.83; 95% CI 1.78–4.51), and there was no significant heterogeneity (I2 = 9%) [71]. It should be noted, however, that the progression of non-sustained to sustained ventricular arrhythmias in LVH also depends on the presence or absence of coexisting morbidities. This is especially true for hypertensive LVH and less so in well-known pro-arrhythmic conditions such as HCM and myocardial infarction (Fig. 4) [72].
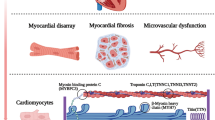
Arrhythmogenic substrate
LVH is arrhythmogenic, especially when associated with depressed LVEF (Fig. 5) [73]. Cellular hypertrophy, stretch, electrolyte abnormalities, and interstitial fibrosis can change the electrical properties of cardiomyocytes (see below) [74]. Moreover, LVH is commonly associated with subendocardial ischemia. The coronary arteries fail to grow at a rate sufficient to compensate for the muscular hypertrophy and the myocardium becomes relatively underperfused [73, 75]. Thus, LVH is linked with impaired coronary vasodilator reserve, decreased high energy phosphate content, impaired fatty acid oxidation, and reduced myocardial glucose transport into the myocardial cell. As a result, an increased ischemic zone on top of a reduced repolarization reserve is created, which initially causes diastolic dysfunction, and hence there is an increased risk for VF and SCD [6].

Reproduced with permission from Stevens, S.M.; K. Reinier; and S.S. Chugh, Increased left ventricular mass as a predictor of sudden cardiac death: is it time to put it to the test? Circ Arrhythm Electrophysiol 2013;6(1):212–7
Arrhythmogenic mechanisms of sudden cardiac death (SCD) associated with left ventricular (LV) hypertrophy (LVH) and severely reduced LV ejection fraction. These mechanisms are likely to have additive effects in increasing risk of SCD.
Sympathetic nervous system and renin–angiotensin–aldosterone axis overactivity are two important factors [74]. Increased sympathetic tone is a potential trigger for arrhythmias and activation of the renin–angiotensin–aldosterone axis promotes vasoconstriction, ischemia, apoptosis, and fibrosis [76]. Raised angiotensin II levels are associated with reduction of intracellular resistance and conduction velocity and shortening of the refractory period in cardiac myocytes. In addition, raised aldosterone stimulates perivascular and interstitial fibrosis of the coronary and systemic circulation. Cardiac fibroblasts have a high affinity for aldosterone, leading to accumulation of arrhythmological islets of collagen [6, 77, 78].
Increased fibrosis is a well-recognized factor accounting for non-homogeneous propagation of electrical impulses throughout the myocardium [73, 75]. As a consequence, the irregular hypertrophy pattern impedes the homogeneous propagation of the electric impulse throughout the myocardium. Any disturbance of impulse propagation can give rise to reentry mechanisms and thereby lead to ectopic impulse formation and ventricular arrhythmias [75].
Excessive fluctuation in arterial pressure, as occurs in labile hypertension, may be arrhythmogenic because it continuously changes the loading conditions of the LV, causing intermittent myocardial ischemia. The stretch-activated channel in the cytoplasmic membrane detects external physical stimuli (e.g., in the form of pressure changes), giving rise to a sequence of intracellular ionic events that affect the electrical stability of the cell promoting potentially malignant arrhythmias [6, 73, 79].
Myocardial disarray (defined as bundles of myocytes oriented perpendicularly or obliquely to each other or interspersed in different directions), which is generally seen in patients with HCM but may also appear to a lesser extent in patients with hypertensive LVH, may also contribute to arrhythmia generation [80].
Electrophysiological abnormalities
Metabolic disarrangements and abnormal redox state due to various heart diseases causing LVH lead to arrhythmological events through direct or indirect modulation of cardiac ion channel/transporter function, with the main disorder being intracellular calcium growth and induced early afterdepolarizations (EADs) and delayed afterdepolarizations (DADs) [81].
LVH is associated with a reduction in connexin (Cx) 43 expression that slows cell to cell conduction with adverse effects on electrical signal transmission [73]. There are also abnormalities of repolarization (prolonged QTc interval) and depolarization (prolonged QRS interval), both of which facilitate the phenomenon of re-entry [73]. Moreover, the density of Na+ and K+ pumps decreases, which in turn leads to a decrease in intracellular K+ concentration and prolonged repolarization (proarrhythmic factor). ATP-sensitive K+ channels are more likely to remain open during ischemia in hypertrophied hearts as compared to normal myocytes, which further prolongs repolarization and facilitates delayed afterdepolarization and triggered activity, causing sustained arrhythmias [73, 76]. Ischemia also leads to an intracellular increase of H+ which is exchangeable with Na+. The intracellular increase of Na+ eventually leads to an increase in cytosolic Ca2+[82].
Molecular mechanisms
Signaling pathways leading to pathologic hypertrophy may also lead to arrhythmological events and SCD. For example, the binding of the peptide hormones angiotensin II and endothelin to their receptors leads to intracellular Ca2+ increase through inositol trisphosphate (IP3) [83] from the endoplasmic and sarcoplasmic reticulum which then activates the Ca2+–calmodulin complex and calcineurin. Through them protein kinase Ca (PKCa) is activated and inducing contractile dysfunction and arrhythmogenesis [84, 85]. Catecholamines bind to their receptors and activate adenylyl cyclase leading cyclic AMP (cAMP) formation which promotes an increase in cytosolic Ca2+ levels leading to fibrosis, hypertrophy, impaired contractility, and arrhythmogenesis [86]. Upregulation of exchange proteins directly activated by cAMP (EPACs) resulting from the same signaling pathway contributes to pathological hypertrophy and to β-adrenergic-receptor-induced arrhythmias [87,88,89]. Likewise, the overexpression of transient receptor potential channels (TRPCs) in response to pressure overload [90] and stromal interaction molecule 1 (STIMI 1) induced activation of nuclear factor of activated T-cells (NFAT) and calcium/calmodulin-dependent protein kinase II (CaMKII) promote pathologic hypertrophy and arrhythmias [91].
Genomic mechanisms
Hemodynamic stress and neuroendocrine signals induced by conditions such as myocardial infarction, aortic stenosis, hypertension, and valvular disease lead to a pathological response/remodeling through activation of intracellular signaling pathways. Some of these pathways lead to cell growth and functional change through gene expression of new proteins [92].
MicroRNAs (miRNAs) are endogenous, short (~ 22 nucleotide), evolutionarily conserved, non-coding RNAs that regulate gene expression at the post-transcriptional level. Binding of miRNA to mRNA causes a decrease in protein expression. Interestingly, miRNAs are differentially expressed in the failing myocardium and play an important role in the development of HF by targeting genes that affect various functions in cardiac remodeling process including myocyte hypertrophy, excitation–contraction coupling, increased myocyte loss, and myocardial fibrosis [93].
The change in ribonucleic acid level is the very beginning of remodeling. Thum and colleagues were the first to link miRNA to HF in 2007 and pointed out that in injured myocardium, there is a start of a genetic program that leads to cell fall and death [93]. Since then, a wealth of research on miRNAs, either in the human genome or in experimental animals, has identified the association between different miRNAs with either cardiac hypertrophy or HF [92].
Kakimoto et al. presented a study comparing patients with LVH and controls without LVH who had all died of various causes of HF and underwent an autopsy. Overexpression of miRNA221 was observed in LVH deaths. Human miRNA221 are located X chromosome and inhibits autophagy and promotes HF. Cardiac mitochondrial autophagy plays an important role against pressure overload and its inhibition promotes age related LVH and HF. They also found that miRNA221 was significantly higher in patients with LVH and SCD than in patients with LVH alone, estimating that as an indicator it reflects not only the presence of LVH but also impending mortality [94]. Topkara and colleagues established an association between miRNA1 downregulation and LVH development. Most importantly, this was associated with the induction of arrhythmias in both normal and post-MI myocardium [95, 96]. Downregulation of miRNA-1 and miRNA-133 allows for the increased expression (release) of growth-related genes that are responsible for hypertrophy/HF-associated arrhythmogenesis [97].
Clinical implications
With an annual incidence of 250,000 to 300,000 in the USA, SCD is among the leading causes of death worldwide and it remains a public health problem, as it involves young subjects or patients [98]. Many different clinical scenarios, from simple hypertension to CAD, HF, HCM, or asymptomatic valvulopathies have been associated with higher risk of SCD. In the era of medications such as beta-blockers, mineralocorticoid antagonists and angiotensin receptor neprilysin inhibitors or interventions such as ICD, that can all either individually or in combination substantially decrease the incidence of arrhythmogenic SCD, identifying the high-risk patient, who will potentially benefit from such interventions, are of outmost importance.
Current guideline-directed risk stratification for primary prevention is largely based on LVEF, and strategies such as ICD implantation are justified only for documented low LVEFs (i.e., ≤ 35%). This approach, however, likely misses most cases of SCD and patients with LVH could account for a proportion of that burden. Ideally ICD therapy would prevent more SCD. If LVH determined from the same echo that assesses the LVEF is included in the risk-stratification algorithm, it is likely that more high-risk patients would be identified. These would include some patients who have increased cumulative risk (LVH plus low LVEF) as well as a subgroup with LVH and preserved LVEF.
With this analysis of physiology and physiopathology of LVH, we suggest that this particular “biomarker” might prove an excellent predictor of impending SCD. It is likely that in the years to come, quantification of LVH will be an additional criterion for more accurate risk stratification and more proper treatment options in this diverse population.
Areas of uncertainty: future research
There are a lot of areas of uncertainty regarding LVH and SCD. First, as discussed previously, we do not know which is the best imaging modality to quantify LVH (echocardiography vs. CMR); CMR seems more accurate but less available, whereas echocardiography is readily available but with higher inter/intra-observer variability. Second, what is the prognostic value of serial LVH measurements? What is the clinical implications of LVH regression? Is it the same in HFrEF and in HFpEF, in young and in elderly patients? What is the incremental predictive value of LVH on top of prognostic risk models? Can prediction of patient outcomes in HF based on routinely collected claims data be improved with machine learning methods and incorporating linked electronic medical records? These are questions that need to be answered in the near future. Future research, should focus on how to improve HF risk models incorporating SCD risk prediction. In this regard, machine learning approaches will inevitably be employed [99]. The added value of augmenting claims-based predictive models with electronic medical record-derived information will most likely be evaluated in the future. Prediction of SCD in the era of “Precision Medicine” (still often used interchangeably with the older term “Personalized Medicine”) is undoubtedly a hot issue for future research.
Conclusion
Concentric and eccentric LV hypertrophy, and even earlier concentric remodeling without hypertrophy, are all associated with increased risk of SCD. These findings suggest the potential utility of evaluating LV geometry as a potential risk stratification tool in combination with or regardless of the LVEF.
References
World Health Organization (1985) Sudden cardiac death : report of a WHO scientific group [meeting held in Geneva from 24 to 27 October 1984]. World Health Organization, Geneva
Al-Khatib SM et al (2018) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 138(13):e210–e271
Kober L et al (2016) Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 375(13):1221–1230
Basso C et al (2017) Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch 471(6):691–705
Stecker EC et al (2006) Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 47(6):1161–1166
Kahan T, Bergfeldt L (2005) Left ventricular hypertrophy in hypertension: its arrhythmogenic potential. Heart (British Cardiac Society) 91(2):250–256
Vakili BA, Okin PM, Devereux RB (2001) Prognostic implications of left ventricular hypertrophy. Am Heart J 141(3):334–341
Haider AW et al (1998) Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 32(5):1454–1459
Ferdinand KC, Maraboto C (2019) Is electrocardiography-left ventricular hypertrophy an obsolete marker for determining heart failure risk with hypertension? J Am Heart Assoc 8(8): p. e012457
Lang RM et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270
Kawel-Boehm N et al (2020) Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson 22(1):87
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15(7):387–407
Triposkiadis F, Xanthopoulos A, Butler J (2019) Cardiovascular aging and heart failure: JACC review topic of the Week. J Am Coll Cardiol 74(6):804–813
Niederseer D et al (2020) Role of echocardiography in screening and evaluation of athletes. Heart
D'Ascenzi F et al (2020) Female athlete's heart: sex effects on electrical and structural remodeling. Circ Cardiovasc Imaging 13(12): p. e011587
Olah A et al (2016) Physiological and pathological left ventricular hypertrophy of comparable degree is associated with characteristic differences of in vivo hemodynamics. Am J Physiol Heart Circ Physiol 310(5):H587–H597
Malek LA, Bucciarelli-Ducci C (2020) Myocardial fibrosis in athletes: additional considerations. Clin Cardiol 43(11):1208
Cunningham KS, Spears DA, Care M (2019) Evaluation of cardiac hypertrophy in the setting of sudden cardiac death. Forensic sciences research 4(3):223–240
Katz AM, Rolett EL (2016) Heart failure: when form fails to follow function. Eur Heart J 37(5):449–454
Kuznetsov VA et al (2010) Asymmetric septal hypertrophy in patients with coronary artery disease. Eur J Echocardiogr 11(8):698–702
Finocchiaro G et al (2020) Diagnostic yield of hypertrophic cardiomyopathy in first-degree relatives of decedents with idiopathic left ventricular hypertrophy. Europace 22(4):632–642
Whyte G et al (2008) Post-mortem evidence of idiopathic left ventricular hypertrophy and idiopathic interstitial myocardial fibrosis: is exercise the cause? Br J Sports Med 42(4):304–305
Tseng ZH et al (2018) Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation 137(25):2689–2700
Aurigemma GP, de Simone G, Fitzgibbons TP (2013) Cardiac remodeling in obesity. Circ Cardiovasc Imaging 6(1):142–152
Kannel WB et al (1998) Sudden coronary death in women. Am Heart J 136(2):205–212
Holkeri A et al (2020) Predicting sudden cardiac death in a general population using an electrocardiographic risk score. Heart 106(6):427–433
Konety SH et al (2016) Echocardiographic predictors of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. Circ Cardiovasc Imaging 9(8)
Laukkanen JA et al (2014) Left ventricular mass and the risk of sudden cardiac death: a population-based study. J Am Heart Assoc 3(6): p. e001285
Verdecchia P et al (2019) Sudden cardiac death in hypertensive patients. Hypertension 73(5):1071–1078
Okin PM et al (2013) Relationship of sudden cardiac death to new-onset atrial fibrillation in hypertensive patients with left ventricular hypertrophy. Circ Arrhythm Electrophysiol 6(2):243–251
Turakhia MP, Schiller NB, Whooley MA (2008) Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study). Am J Cardiol 102(9):1131–1135
Liao Y et al (1995) The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA 273(20):1592–1597
Baumgartner H et al (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38(36):2739–2791
Prejean SP et al (2021) Review of published cases of syncope and sudden death in patients with severe aortic stenosis documented by electrocardiography. Am J Cardiol
Taniguchi T et al (2018) Sudden death in patients with severe aortic stenosis: observations from the current as registry. J Am Heart Assoc 7(11)
Minners J et al (2020) Sudden cardiac death in asymptomatic patients with aortic stenosis. Heart 106(21):1646–1650
Spirito P et al (2000) Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 342(24):1778–1785
Miron A et al (2020) A validated model for sudden cardiac death risk prediction in pediatric hypertrophic cardiomyopathy. Circulation 142(3):217–229
Gilstrap LG et al (2019) Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service medicare beneficiaries in the United States. Circ Heart Fail 12(6): p. e005407
Maurer MS et al (2016) Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 68(2):161–172
John RM (2018) Arrhythmias in cardiac amyloidosis. J Innov Card Rhythm Manag 9(3):3051–3057
Orini M et al (2019) Noninvasive mapping of the electrophysiological substrate in cardiac amyloidosis and its relationship to structural abnormalities. J Am Heart Assoc 8(18):e012097–e012097
Reisinger J et al (1997) Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol 30(4):1046–1051
Mlcochova H et al (2006) Catheter ablation of ventricular fibrillation storm in patients with infiltrative amyloidosis of the heart. J Cardiovasc Electrophysiol 17(4):426–430
John RM, Stern DL (2020) Use of implantable electronic devices in patients with cardiac amyloidosis. Can J Cardiol 36(3):408–415
Azevedo O et al (2021) Fabry disease and the heart: a comprehensive review. Int J Mol Sci 22(9):4434
Higashi H et al (2011) Endocardial and epicardial substrates of ventricular tachycardia in a patient with Fabry disease. Heart Rhythm 8(1):133–136
Linhart A et al (2020) An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur J Heart Fail 22(7):1076–1096
Quiñones MA et al (2000) Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 35(5): p. 1237–44
Reinier K et al (2011) Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm 8(8):1177–1182
Phan D et al (2016) Left ventricular geometry and risk of sudden cardiac arrest in patients with severely reduced ejection fraction. J Am Heart Assoc 5(8)
Aro AL et al (2017) Left-ventricular geometry and risk of sudden cardiac arrest in patients with preserved or moderately reduced left-ventricular ejection fraction. Europace 19(7):1146–1152
Vaduganathan M et al (2018) Sudden death in heart failure with preserved ejection fraction: a competing risks analysis from the TOPCAT trial. JACC Heart Fail 6(8):653–661
Chan MM, Lam CS (2013) How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail 15(6):604–613
Vaduganathan M et al (2017) Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 69(5):556–569
Kitai T et al (2020) Mode of death among Japanese adults with heart failure with preserved, midrange, and reduced ejection fraction. JAMA Netw Open 3(5): p. e204296
Yazdanfard PD et al (2020) Non-diagnostic autopsy findings in sudden unexplained death victims. BMC Cardiovasc Disord 20(1):58
Smith DL et al (2018) Pathoanatomic findings associated with duty-related cardiac death in US firefighters: a case-control study. J Am Heart Assoc 7(18): p. e009446
Dennis M et al (2018) A 10-year review of sudden death during sporting activities. Heart Rhythm 15(10):1477–1483
Manfredini R et al (1996) Out-of-hospital sudden death referring to an emergency department. J Clin Epidemiol 49(8):865–868
Huynh N et al (2019) Clinical and pathologic findings of aortic dissection at autopsy: review of 336 cases over nearly 6 decades. Am Heart J 209:108–115
Adabag AS et al (2010) Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J 159(1):33–39
Kim AS et al (2016) Sudden neurologic death masquerading as out-of-hospital sudden cardiac death. Neurology 87(16):1669–1673
Levy WC et al (2006) The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 113(11):1424–1433
Mozaffarian D et al (2007) Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation 116(4):392–398
Shadman R et al (2015) A novel method to predict the proportional risk of sudden cardiac death in heart failure: derivation of the Seattle Proportional Risk Model. Heart Rhythm 12(10):2069–2077
Fukuoka R et al (2020) Prediction of sudden cardiac death in Japanese heart failure patients: international validation of the Seattle Proportional Risk Model. Europace 22(4):588–597
Levy WC, Anand IS (2014) Heart failure risk prediction models: what have we learned? JACC Heart Fail 2(5):437–439
Kuhn H, Lawrenz T, Beer G (2005) Indication for myocardial biopsy in myocarditis and dilated cardiomyopathy. Med Klin (Munich) 100(9):553–561
Delgado V, Bucciarelli-Ducci C, Bax JJ (2016) Diagnostic and prognostic roles of echocardiography and cardiac magnetic resonance. J Nucl Cardiol 23(6):1399–1410
Chatterjee S et al (2014) Meta-analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol 114(7):1049–1052
Nadarajah R, Patel PA, Tayebjee MH (2021) Is hypertensive left ventricular hypertrophy a cause of sustained ventricular arrhythmias in humans? J Hum Hypertens
Stevens SM, Reinier K, Chugh SS (2013) Increased left ventricular mass as a predictor of sudden cardiac death: is it time to put it to the test? Circ Arrhythm Electrophysiol 6(1):212–217
Winslow RD, Mehta D, Fuster V (2005) Sudden cardiac death: mechanisms, therapies and challenges. Nat Clin Pract Cardiovasc Med 2(7):352–360
Messerli FH (1999) Hypertension and sudden cardiac death. Am J Hypertens 12(12 Pt 3):181s–188s
Shenasa M, Shenasa H (2017) Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int J Cardiol 237:60–63
Tin LL, Beevers DG, Lip GY (2002) Hypertension, left ventricular hypertrophy, and sudden death. Curr Cardiol Rep 4(6):449–457
Weber KT et al (1993) Myocardial fibrosis: role of angiotensin II and aldosterone. Basic Res Cardiol 88(Suppl 1):107–124
Sideris DA et al (1989) Arrhythmogenic effect of high blood pressure: some observations on its mechanism. Cardiovasc Res 23(11):983–992
Stroumpoulis KI, Pantazopoulos IN, Xanthos TT (2010) Hypertrophic cardiomyopathy and sudden cardiac death. World J Cardiol 2(9):289–298
Yang KC et al (2015) Mechanisms of sudden cardiac death: oxidants and metabolism. Circ Res 116(12):1937–1955
Rubart M, Zipes DP (2005) Mechanisms of sudden cardiac death. J Clin Invest 115(9):2305–2315
Zhang L et al (2013) Phospholipase Cε hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell 153(1):216–227
Braz JC et al (2004) PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med 10(3):248–254
Newton AC, Antal CE, Steinberg SF (2016) Protein kinase C mechanisms that contribute to cardiac remodelling. Clin Sci (Lond) 130(17):1499–1510
Sato PY et al (2015) The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol Rev 95(2):377–404
Métrich M et al (2008) Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res 102(8):959–965
Morel E et al (2005) cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res 97(12):1296–1304
Pereira L et al (2015) Novel Epac fluorescent ligand reveals distinct Epac1 vs. Epac2 distribution and function in cardiomyocytes. Proc Natl Acad Sci USA 112(13): p. 3991–6
Eder P, Molkentin JD (2011) TRPC channels as effectors of cardiac hypertrophy. Circ Res 108(2):265–272
Troupes CD et al (2017) Role of STIM1 (Stromal Interaction Molecule 1) in hypertrophy-related contractile dysfunction. Circ Res 121(2):125–136
van Rooij E et al (2006) A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A 103(48):18255–18260
Topkara VK, Mann DL (2011) Role of microRNAs in cardiac remodeling and heart failure. Cardiovasc Drugs Ther 25(2):171–182
Kakimoto Y et al (2018) Overexpression of miR-221 in sudden death with cardiac hypertrophy patients. Heliyon 4(6): p. e00639
Topkara VK, Mann DL (2010) Clinical applications of miRNAs in cardiac remodeling and heart failure. Per Med 7(5):531–548
Yang B et al (2007) The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med 13(4):486–491
Divakaran V, Mann DL (2008) The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res 103(10):1072–1083
Fishman GI et al (2010) Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 122(22):2335–2348
Desai RJ et al (2020) Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA Netw Open 3(1): p. e1918962
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G.G. No disclosures, A.D. No disclosures, AX Honoraria from Novartis, J.S. No disclosures, F.T. Research support and honoraria from Amgen, Bayer, Boehringer Ingelheim, Elpen, Lilly, Menarini, Merck, Novartis, Sanofi, Servier, Vianex and WinMedica.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giamouzis, G., Dimos, A., Xanthopoulos, A. et al. Left ventricular hypertrophy and sudden cardiac death. Heart Fail Rev 27, 711–724 (2022). https://doi.org/10.1007/s10741-021-10134-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10134-5