Abstract
Atrial fibrillation ablation is the last frontier of ablation using a non-fluoroscopic approach; it is however the procedure with the highest radiation exposure patients and health personnel. Combining 3-D mapping systems and nonionizing imaging modalities such as intracardiac echocardiography (ICE) allows safe and effective completion of the ablation procedure without fluoroscopy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Catheter ablation for atrial fibrillation (AF) is increasingly the treatment of choice for patients with paroxysmal and persistent AF [1]. Due to the complexity of the procedure and the need for transseptal puncture, AF ablation is traditionally associated with long procedure times and high radiation exposure [2, 3]. There has been growing interest in reducing radiation use during these procedures [4]. In addition, there is concern regarding the musculoskeletal affects of wearing lead apparel for prolonged periods [5].
Non-fluoroscopic catheter ablation of AF has been demonstrated in multiple studies and shown to be feasible and safe [6,7,8,9]. The use of electroanatomic mapping systems (EAMS) , contact force technology, and intracardiac echocardiography (ICE) imaging has allowed the procedure to be performed with zero or near-zero fluoroscopic radiation [4, 10, 11].
In this chapter, we will describe the procedural aspects of non-fluoroscopic AF ablation and specifically describe the transseptal procedure using ICE imaging only. Please refer also to the catheter placement chapter for details on how to achieve fluoroscopy free access to the venous vasculature and to the right atrium.
Relevant Anatomy
Transseptal Puncture
The transseptal puncture is typically performed at the fossa ovalis by dropping down the transseptal apparatus from the superior vena cava (SVC) to engage the fossa. Fluoroscopic imaging is usually used to safely advance the apparatus into the SVC prior to the drop. While pulling back the apparatus, fluoroscopy is used to observe for a sudden drop from the SVC-right atrium (RA) junction onto the fossa ovalis. Multiple fluoroscopic views are used to confirm exact positioning, with a typical right anterior oblique (RAO) view showing the apparatus positioned in the middle between the posterior wall of the atrium and tricuspid annulus, and a typical left anterior oblique (LAO) view showing the apparatus facing the left side. At this point a needle is advanced through the fossa into the left atrium (LA), which is followed by the rest of the apparatus (dilator and sheath). In many centers, either transesophageal echocardiography (TEE) or ICE imaging is used to complement fluoroscopy and ensure engagement of the fossa prior to performing the puncture.
Left Atrial Anatomy
Typically there are four pulmonary veins (PVs) that drain blood from the lungs into the LA. However in some patients there is variation in anatomy, with the most common being a common ostium for the left PVs. In addition, many patients have a right middle PV present [12].
The standard of care for AF ablation is wide-antral pulmonary vein isolation (PVI) by creating ablation lesions (either by radiofrequency ablation or by cryoablation) around the PV ostia [1]. Multiple anatomical considerations need to be taken into account while performing the ablation. The esophagus runs in close proximity to the posterior wall of the LA, and can be present either in the middle of the posterior wall, closer to the right PVs or closer to the left PVs [13]. The variable location and close proximity factor in the risk of atrio-esophageal fistula formation post procedure. In addition, the right phrenic nerve usually courses anterior to the right PV ostia, and ablation in that area can lead to phrenic nerve paralysis [14].
Finally, the risk of PV stenosis p ost procedure is higher when ablation lesions are placed closer to the PV ostia, and can be avoided by placing ablation lesions away from the PVs. Hence, wide-area PVI has become the standard of care for AF ablation [1].
Non-fluoroscopic Ablation Approach
Patient Preparation
Patients are all maintained on oral anticoagulation for a minimum of 4 weeks prior to the procedure. Those at particularly high risk of left atrial appendage (LAA) thrombus undergo TEE within 48 h of the procedure to ensure the absence of LAA thrombus. These procedures are commonly performed under general anesthesia or deep conscious sedation.
Advancing the ICE Catheter to the Heart (Fig. 11.1)
After gaining femoral access (please refer to the chapter on catheter placement for further details), which can be guided by vascular ultrasound, the ICE catheter is then advanced into the RA. In our institution, ICE is performed using an 8-French phased-array AcuNav probe at 7.5 MHz (Siemens Medical Solutions distributed by Biosense Webster, Diamond Bar, California). The ICE monitor is connected to the dedicated monitor boom.
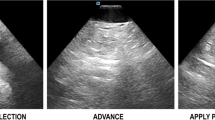
(Panel a) Arrow s howing lumen in the path of the ICE catheter, which allows safe advancement forwards towards the right atrium. (Panel b) Arrow showing soft tissue in the path of the ICE catheter. In this situation, clockwise or counterclockwise torque is needed to show lumen prior to advancing the ICE catheter
In order to place the ICE catheter in the heart safely without fluoroscopy, the probe is advanced from the femoral vein to the RA while ensuring that lumen is present in the path of the probe. This is done by visualizing “black lumen” on the top right of the ICE display (i.e., in front of the probe). If tissue is encountered, the probe can be gently withdrawn and a clockwise or counterclockwise torque can be applied to show lumen, and the probe can be advanced again.
In some instances, a branching point is identified, and the probe can be anteriorly-flexed towards the main branch of the vein to allow it to pass towards the heart. Care should be taken not to advance the probe against resistance.
Baseline ICE Study (Fig. 11.2)
The ICE probe is positioned in the RA, right ventricle (RV), and SVC where visualization of the cardiac structures is performed. Pre-ablation, ICE imaging is used to document baseline ventricular function, valvular function, PV flows, and the absence of a baseline pericardial effusion. In the presence of a cardiac implantable electronic device (CIED) , ICE is used to visualize lead position and assess the insertion sites. Following this, further intra-procedural imaging is performed to monitor catheter position, catheter contact, and monitor for complications.
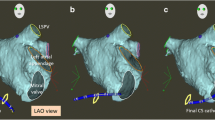
ICE views from the right atrium (RA): clockwise torque is applied to the ICE catheter to move from Panel a through f. (Panel a) RA view showing the aortic root (Ao). (Panel b) RA view showing the tricuspid valve (TV) and right ventricle (RV). (Panel c) Coronary sinus ostium (arrow), mitral valve (MV), and left atrial appendage (LAA). (Panel d) Further clockwise torque showing the left atrium (LA). (Panel e) The fossa ovalis (arrow) where the transseptal puncture would be performed, across from the left pulmonary veins (LPVs) common ostium. (Panel f) Right inferior pulmonary vein (RIPV) ostium shown
Coronary Sinus Cannulation (Fig. 11.3)
Once the ICE probe is positioned in the RA, the coronary sinus ostium can be visualized just posterior to the tricuspid valve by applying clockwise torque on the ICE probe. Following this, a decapolar catheter is advanced into the RA and, by using the EAMS and intracardiac electrograms, advanced into the coronary sinus.
In the case of difficult coronary sinus cannulation, multiple steps are taken in sequence. These include electroanatomic mapping of the coronary sinus using the mapping/ablation catheter and long sheath (thus providing an anatomical “road map” for the decapolar catheter), and utilizing a long pre-shaped Swartz SL3 sheath (St. Jude Medical, St Paul, Minnesota). The SL3 sheath is advanced into the RA utilizing the same technique used to advance the steerable sheath into the SVC prior to transseptal access (see below).
Transseptal Access (Fig. 11.4)
The ICE catheter is advanced into the RA, following which a posterior and right-sided tilt of the ICE catheter is applied to visualize the SVC.
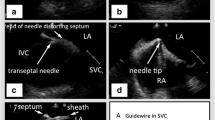
(Panel a) From the right atrial view, posterior and right-ward tilt is applied to the ICE catheter, and the SVC is visualized. (Panel b) A wire (arrow) is advanced into the SVC. (Panel c) A long sheath/dilator assembly (arrow) is advanced over the wire into the SVC. (Panel d) The wire is interchanged for the transseptal needle, and the apparatus is pulled back onto the fossa ovalis. (Panel e) Torque is removed off the ICE catheter to allow for direct visualization of the fossa ovalis and to guide the transseptal puncture. (Panel f) In cases where the dilator/needle assembly crosses the septum but the sheath does not due to resistance at the septum, a wire (arrow) can be advanced into the left pulmonary veins to serve as a “rail” to push the dilator and sheath assembly further into the left atrium in a safe fashion
A long J-wire is passed from the right femoral vein to the SVC, followed by advancement of a long steerable sheath (Agilis NxT, St. Jude Medical, St. Paul, MN) over the wire into the SVC (under ICE visualization). A Brockenbrough (BRK) needle (St. Jude Medical, St. Paul, MN) is advanced into the sheath and the apparatus is then pulled back to the fossa ovalis, following which the transseptal puncture is performed under ICE guidance. At this point, saline contrast can be injected to show LA filling and confirming the LA location of the needle tip. The dilator and sheath are carefully advanced over the needle, and once the sheath is in good position, the needle and dilator are withdrawn.
Challenging Transseptal Access Scenarios
Some challenges can be encountered during non-fluoroscopic transseptal access. One of these scenarios is the inability to advance the wire from the groin into the SVC (due to either coiling in the RA or advancing into the RV). In this case, the J-wire can be advanced into the RA (as confirmed by ICE). Next, the transseptal sheath-dilator assembly is advanced over the wire so that the tip is estimated to be in the mid-IVC region. The dilator and J-wire are then withdrawn, and the ablation catheter is advanced through the sheath. The ablation catheter’s location could be tracked on the EAMS system and then advanced into the SVC as guided by both ICE imaging and the electroanatomical map. Once the ablation catheter is in the SVC, the sheath can be advanced over it into the SVC, and the ablation catheter can be again interchanged for the wire and dilator. At this point, the transseptal drop can be performed in a standard fashion.
Another scenario that can occur after successful transseptal puncture is the inability to advance the sheath over the dilator into the LA due to a thickened septum. One possible solution is to advance a wire through the dilator and into the left PVs. The location of the wire within the left PVs can be confirmed by advancing the ICE probe into the RV outflow tract and clocking the catheter to show the LAA-PV ridge, and the wire can be steered safely into the left PVs and not into the LAA. At this point, the wire can serve as a “rail” to advance the sheath and dilator further into the LA safely.
An additional scenario that can be encountered is the inability to drop the apparatus onto the fossa ovalis. In this situation, the ablation catheter can be inserted through the steerable sheath and be guided onto the fossa ovalis by ICE imaging. The sheath is then advanced over the ablation catheter so it can be in contact with the fossa, and the ablation catheter is removed. Now, the dilator (and BRK needle within it) is advanced through the sheath and directly onto the fossa ovalis, and the transseptal puncture is performed in a standard fashion.
Left Atrial Mapping
Following transseptal puncture, the LA is mapped using an EAMS. A steerable multi-electrode mapping catheter is used to form a map of the LA, particularly focusing on the PVs, LAA, and the mitral valve annulus (Fig. 11.5). Careful attention is paid to the PV ostia, which are identified while positioning the multi-electrode catheter via ICE imaging.
Using ICE imaging to anatomically localize the left atrial appendage (LAA) and pulmonary vein ostia on the electroanatomic mapping system. (Panel a) The multi-electrode catheter is placed at the LAA ostium. (Panel b) The multi-electrode catheter is placed at the right inferior pulmonary vein ostium. (Panel c) The multi-electrode catheter is placed at the left pulmonary veins common ostium. (Panel d) The multi-electrode catheter is placed at the right superior pulmonary vein ostium
Following this, the multi-electrode catheter is interchanged for the ablation catheter and ablation is started. In our institution, a single transseptal puncture is performed during non-fluoroscopic ablation; however, a double transseptal puncture to allow the use of both the ablation and multi-electrode catheter simultaneously is also feasible. The most frequently used ablation strategies for PVI include point-by-point ablation using radiofrequency energy delivered through an irrigated catheter or cryoablation technology.
Following ablation, the multi-electrode catheter is used to confirm elimination of all PV potentials and, in the presence of ablation lines, persistent bidirectional block as the procedural endpoint. Intravenous adenosine can also be administered to assess dormant conduction, and additional ablation can be applied if this is present.
Results of Non-fluoroscopic Atrial Fibrillation Ablation
Non-fluoroscopic PVI for paroxysmal AF has been reported utilizing ICE imaging, intracardiac electrograms, and electroanatomic mapping. Ferguson et al. demonstrated that non-fluoroscopic ablation of AF was feasible and safe in 21 cases; however they required fluoroscopic rescue for two cases to aid with transseptal puncture [6]. One possible reason for this may be the use of rotational ICE in their study, which differs from standard phased-array ICE used in most electrophysiologic procedures.
Reddy et al., Bulava et al., and Lyan et al. published three case series of non-fluoroscopic ablation in patients with paroxysmal AF [7,8,9]. In the first series [7], which included 20 patients, prolonged procedure times were noted with non-fluoroscopic ablation and there was no comparison to standard fluoroscopic ablation. There were no complications in this series.
In the second series [8], there were 40 patients with paroxysmal AF randomized to a fluoroscopic and non-fluoroscopic approach. In the non-fluoroscopic arm, one patient required fluoroscopic rescue for confirmation of femoral access. All non-fluoroscopic cases were performed safely with no increase in procedure time. There were no serious procedure-related complications, and no difference in arrhythmia-free survival at 12 months between the two groups.
In the third series [9], which included 245 patients with paroxysmal AF randomized to a fluoroscopic and non-fluoroscopic approach, comparable safety profiles and procedure durations to fluoroscopic ablation were observed. Three patients in the non-fluoroscopic ablation group required fluoroscopy to guide pericardial access during drain insertion for iatrogenic effusion, and there were two patients in the fluoroscopic arm that also required drain insertion. AF ablation without fluoroscopy did not affect procedure duration or procedural long-term efficacy.
More recently, at our own institution, we have performed 70 consecutive non-fluoroscopic LA ablation procedures (31 paroxysmal AF, 33 persistent AF and 6 LA flutters), all of which were completed without the need for rescue fluoroscopy [15]. There was an initial increase in procedural time for ablation of LA arrhythmias upon transitioning to non-fluoroscopic ablation. However, after excluding the first 20 cases to allow for operator learning, the transition to non-fluoroscopic ablation was not associated with an increase in mean procedural time. All procedures were completed successfully with no complications.
Finally, a small case series by Razminia et al. showed the feasibility of balloon cryoablation by an ICE-guided non-fluoroscopic approach [16]. Five consecutive patients with paroxysmal AF underwent the procedure. Hemodynamic pressure monitoring was used to confirm venous occlusion by the balloon, and 20 PVs were successfully isolated. There were no major complications during the study.
Limitations of Non-fluoroscopic Atrial Fibrillation Ablation
There are important limitations to performing non-fluoroscopic catheter ablation of AF. Possible limitations include patients with CIEDs. In the case of single or dual chamber devices, the atrial and ventricular leads can be visualized with ICE, avoided with catheters, and checked at the end of the procedure to ensure no dislodgement [15]. However, in patients with coronary sinus leads as part of cardiac resynchronization therapy, although the coronary sinus lead body can be visualized, its course along the coronary sinus venous system and the tip cannot be seen without fluoroscopy. Hence, there is a risk of coronary sinus lead dislodgement if fluoroscopy is not used, and this would likely be identified at device interrogation or radiological imaging post-procedure.
Another limitation is the potential need for fluoroscopy in the case of a complication. For example, if a pericardial effusion develops during the procedure, the use of fluoroscopy can easily confirm the presence of the wire in the epicardial space prior to drain insertion. Although this can also be performed with echocardiography, fluoroscopy does have the advantage of ease of use and efficiency in such a scenario.
Finally, non-fluoroscopic AF ablation relies heavily on the operators’ skill in ICE image guidance and interpretation. Thus, familiarity and experience with ICE imaging is very important, especially to trouble-shoot difficult transseptal access scenarios.
Conclusion
Non-fluoroscopic ablation of AF is safe and feasible. There is a learning curve to consider, and procedural duration does improve with increasing operator experience. Challenges can be overcome with the use of ICE imaging and electroanatomic mapping.
References
Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e21.
Macle L, Weerasooriya R, Jais P, et al. Radiation exposure during radiofrequency catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2003;26:288–91.
Lickfett L, Mahesh M, Vasamreddy C, et al. Radiation exposure during catheter ablation of atrial fibrillation. Circulation. 2004;110:3003–10.
Voskoboinik A, Kalman ES, Savicky Y, et al. Reduction in radiation dose for atrial fibrillation ablation over time: a 12-year single-center experience of 2344 patients. Heart Rhythm. 2017;14:810–6.
Birnie D, Healey JS, Krahn AD, et al. Prevalence and risk factors for cervical and lumbar spondylosis in interventional electrophysiologists. J Cardiovasc Electrophysiol. 2011;22:957–60.
Ferguson JD, Helms A, Mangrum JM, et al. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol. 2009;2:611–9.
Reddy VY, Morales G, Ahmed H, et al. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm. 2010;7:1644–53.
Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38:797–806.
Lyan E, Tsyganov A, Abdrahmanov A, et al. Nonfluoroscopic catheter ablation of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2018;41:611–9.
Sommer P, Bertagnolli L, Kircher S, et al. Safety profile of near-zero fluoroscopy atrial fibrillation ablation with non-fluoroscopic catheter visualization: experience from 1000 consecutive procedures. Europace. 2018;20:1952–8.
Lee G, Hunter RJ, Lovell MJ, et al. Use of a contact force-sensing ablation catheter with advanced catheter location significantly reduces fluoroscopy time and radiation dose in catheter ablation of atrial fibrillation. Europace. 2016;18:211–8.
Wannasopha Y, Oilmungmool N, Euathrongchit J. Anatomical variations of pulmonary venous drainage in Thai people: multidetector CT study. Biomed Imaging Interv J. 2012;8:e4.
Nair GM, Nery PB, Redpath CJ, Lam B-K, Birnie DH. Atrioesophageal fistula in the era of atrial fibrillation ablation: a review. Can J Cardiol. 2014;30:388–95.
Bai R, Patel D, Di Biase L, et al. Phrenic nerve injury after catheter ablation: should we worry about this complication? J Cardiovasc Electrophysiol. 2006;17:944–8.
Sadek MM, Ramirez FD, Nery PB, et al. Completely non-fluoroscopic catheter ablation of left atrial arrhythmias and ventricular tachycardia. J Cardiovasc Electrophysiol. 2018;30:78–88.
Razminia M, Demo H, Arrieta-Garcia C, D’Silva OJ, Wang T, Kehoe RF. Nonfluoroscopic ablation of atrial fibrillation using cryoballoon. J Atr Fibrillation. 2014;7:1093.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sadek, M.M. (2019). Non-fluoroscopic Catheter Ablation of Atrial Fibrillation. In: Proietti, R., Wang, Y., Yao, Y., Zhong, G., Lin Wu, S., Ayala-Paredes, F. (eds) Cardiac Electrophysiology Without Fluoroscopy. Springer, Cham. https://doi.org/10.1007/978-3-030-16992-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-16992-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16991-6
Online ISBN: 978-3-030-16992-3
eBook Packages: MedicineMedicine (R0)