Abstract
Pericytes have long been known to contribute indirectly to tumour growth by regulating angiogenesis. Thus, remodelling tumour blood vessels to maintain blood supply is critical for continued tumour growth. A role for pericytes in restricting leakage of tumour cells through blood vessels has also become evident given that adequate pericyte coverage of these blood vessels is critical for maintaining vascular permeability. Interestingly, the relocation of pericytes from blood vessels to the tumour microenvironment results in the emergence of different properties in these cells that actively promote tumour growth and metastasis—functions not associated with their well-studied role in vascular stability and permeability. These form the focus of this review.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cancer
- Cancer cell invasion
- Cancer stem cells
- Epithelial-mesenchymal interactions
- Mesenchymal stem cells
- Metastasis
- Ovarian cancer
- Pericytes
- Tumour microenvironment
- Vascular permeability
- Tumour vasculature
Introduction
The role of the tumour microenvironment (TME) in promoting tumour growth and metastasis is widely recognised and consists of a variety of cells including cancer-associated fibroblasts (CAFs), bone marrow-derived mesenchymal stem cells (BM-MSC), endothelial cells, pericytes and immune cells and the growth factors and proteins they produce. Studies of various types of cancer including ovarian, colorectal, pancreatic and breast demonstrate that stromal signatures predict relapse and recurrence in patients lending strong support to the notion that the TME is a strong contributor to malignant progression (Tothill et al. 2008; Finak et al. 2008; Tsujino et al. 2007; Fujita et al. 2010; Calon et al. 2015). It is possible that not all elements of the TME are pro-tumourigenic—indeed, many tumours are encapsulated by fibroblasts perhaps limiting metastatic spread. It has been suggested that the TME has a role in contributing to resistance against anti-cancer therapeutics (Frame and Serrels 2015). It is therefore important that we gain a better understanding of the contributions of specific subsets of cells found in the TME to cancer progression. This discussion focuses on the role of pericytes given the relatively recent discovery that they have angiogenesis-independent roles in promoting malignant cancer.
Difficulties in Distinguishing Pericytes from CAFs in the TME
CAFs are defined as fibroblasts that universally promote tumour growth, invasiveness and metastasis compared to normal “fibroblasts” (Olumi et al. 1999; Cunha et al. 2003; Kalluri and Zeisberg 2006; Pietras and Ostman 2010). CAFs can originate from diverse sources including tissue-resident myofibroblasts, activated adipocytes and distal bone marrow-derived MSCs/BM-MSCs (Kalluri and Zeisberg 2006; Cirri and Chiarugi 2011), and are mostly identified retrospectively using equivocal “CAF markers”, such as α-SMA (smooth muscle actin) which is activated in all mesenchymal cell types in conditions of stress. Thus, α-SMA is expressed in cultured or activated fibroblasts, myofibroblasts, pericytes and most BM-MSCs (Kalluri and Zeisberg 2006; Cirri and Chiarugi 2011). Lineage-marking studies in animals show that GFP-tagged BM-MSCs home to developing tumours inducing increased metastases (Karnoub et al. 2007; Hung et al. 2005; Mishra et al. 2008; Studeny et al. 2002; Quante et al. 2011), making up about ~20–50% of CAFs. Thus, 50–80% of CAFs are not BM derived and may originate from local fibroblasts or other MSC-like populations such as pericytes. Indeed, our lab has shown that pericytes accelerate tumour growth rates and promote metastatic spread in a xenograft model of ovarian cancer (Sinha et al. 2016). Moreover, we showed that pericytes recruited BM-MSCs to developing tumours, suggesting that they may act upstream of BM-MSCs.
Classic Functions of Pericytes in Cancer: Stabilising Tumour Blood Supply and Limiting Hypoxia
In cancer, pericytes have been widely studied in the context of their well-known capacity to stabilise blood vessel structure and permeability. Dual targeting of vascular endothelial cells and pericytes using kinase inhibitors or anti-VEGF and anti-PDGFβ antibodies, has a synergistic anti-angiogenic/anti-tumour effect, resulting in increased tumour cell killing in animal models attributed to destabilisation of the tumour microvasculature (Bergers et al. 2003; Druker 2002; Erber et al. 2004; Kuhnert et al. 2008; Maciag et al. 2008). Other studies claiming that tumour growth was unaffected after pericyte removal are equivocal given that a maximal 50% pericyte knockdown (KD) was achieved using AX102, an inhibitor of PDGFB signalling (Sennino et al. 2007), or in PDGFret/ret mice that harbour a mutation in the PDGFB retention motif (Nisancioglu et al. 2010; Lindblom et al. 2003). On the other hand, complete KD of pericytes with NG2 promoter driven thymidine kinase, caused tumour hypoxia which led to epithelial-mesenchymal transition and metastases to the lungs in mouse models of breast/renal cell carcinoma and melanoma (Cooke et al. 2012). These data support the idea that pericytes limit metastatic spread through otherwise leaky tumour blood vessels (Xian et al. 2006).
Tumour Blood Vessel Remodelling Leads to Displacement of Pericytes from Their Vascular Niche
Blood vessel remodelling during tissue repair is a dynamic process requiring the initial detachment of pericytes from endothelial cells from pre-existing vessels resulting in the removal of paracrine signalling between the two cell types that keep vessels in a homeostatic state. Pericyte detachment permits endothelial cell sprouting and proliferation, and subsequent re-association of the two cell types in the newly extended blood vessels – processes driven by angiopoietin-1/2 and Tie2 (Ang/Tie2), transforming growth factor-β (TGF-β), and platelet-derived growth factor-B (PDGFB) and its receptor PDGFR-β (Lindblom et al. 2003; Stapor et al. 2014). Similar mechanisms underlie tumour vessel remodelling although tumour vasculature is typically disorganised with torturous vessels, excessive branching and altered gene expression resulting in impaired vascular structure and increased vessel leakiness (Ruoslahti 2002). Notably, pericytes are more loosely attached to endothelial cells with cytoplasmic projections invading the tumour stroma (Morikawa et al. 2002). It has been shown that detachment of perivascular cells (and subsequent endothelial cell sprouting) is mediated by angiopoietin-2 secreted by activated endothelial cells (Scharpfenecker et al. 2005). Similarly, it has been shown that tumour cells overexpressing PDGFBB xenografted onto mice, induced dissociation of pericytes from tumour blood vessels in a dose-dependent manner increasing vascular permeability leading to vascular impairment (Hosaka et al. 2013). Notably, continued exposure of pericytes to PDGFBB led to down-regulation of PDGFβR that in turn decreased the expression of the α1β1 integrin receptor from the cell surface of pericytes, abrogating their adhesion to extracellular matrix proteins in the blood vessel walls resulting in their detachment from them (Hosaka et al. 2013). Thus, paracrine signalling between pericytes and cancer cells provides an important mechanism by which pericytes can be persuaded to leave their normal microenvironment within microvessels and associate more closely with tumour cells. Given the reports that the precise location of pericytes can alter their functionality , it is clear that pericytes may be able to act directly on tumour cells as part of their mesenchymal microenvironment.
Mesenchymal Stem Cell Properties of Pericytes: Similarities and Distinctions
Given that many tissues are well vascularised, it has been speculated that perivascular cells throughout the body serve as a reservoir of multipotent mesenchymal stem cells that can be recruited upon tissue injury. Certainly, the phenotypic and functional similarities between pericytes and mesenchymal stem cells have been widely reported, serving to underpin the idea that the two cell types are in fact one and the same. Thus, both pericytes and BM-MSCs are CD45−CD31−αSMA+CD146+NG2+PDGFRB+CD73+CD90+, located in a perivascular niche and can differentiate into fat, bone, cartilage, muscle, and neuronal cells (Crisan et al. 2008; Caplan 2008; Paquet-Fifield et al. 2009). However, important distinctions exist between pericytes and MSCs in that an immunosuppressive role has been described for MSCs (Shi et al. 2018) whereas the indications are that in animal studies pericytes or perivascular cells are pro-inflammatory (Mills et al. 2015; Dulauroy et al. 2012) and likely to contribute to delayed healing and fibrosis.
Pericytes and Fibrosis
Pericytes are involved in various fibrosis -related pathologies in the kidneys, liver, and skin, acting as progenitors to myofibroblasts, which are the main mediators for extracellular matrix deposition, leading to fibrogenesis and ultimately fibrosis during the healing process (Greenhalgh et al. 2015; Kramann and Humphreys 2014). In a transgenic reporter mouse model, coll1α1-GFP-expressing pericytes were shown to be the main source of myofibroblasts leading to kidney fibrosis (Lin et al. 2008). Similar studies in the liver, where pericytes are known as hepatic stellate cells, also showed that they were the main source of myofibroblasts, and a major player in liver fibrosis (Greenhalgh et al. 2015). Consistent with this, studies with a Cre-transgenic mouse model which labelled hepatic stellate cells in various models of liver injury, demonstrated that they accounted for 82–96% of the myofibroblast pool, which contributes to liver fibrosis (Mederacke et al. 2013). Pericytes have a similar role in skin fibrosis and scar formation as illustrated by genetic fate mapping, revealing that the majority of collagen producing myofibroblasts originate from ADAM12 expressing cells, derived from PDGFRB+ NG2+ perivascular cells or pericytes (Dulauroy et al. 2012). Notably, ablation or knockdown of ADAM12+ cells, was sufficient to limit collagen production in the healing site of injury and reduce fibrosis. These studies demonstrate the ability for pericytes to differentiate into myofibroblasts thereby contributing to fibrosis. They also illustrate the ability of pericytes to contribute to the remodelling of tissue stroma to achieve wound repair thus pointing to ways in which these cells can affect biological processes in an angiogenesis-independent manner.
Pericytes in Cancer and Metastasis
It has been variously postulated that pericytes affect tumour growth and metastasis both positively and negatively. Many of the tumour growth promoting effects are related to establishing a stable vascular network thus ensuring delivery of nutrients to rapidly growing tumour cells and preventing tumour cell dissemination through blood vessels by maintaining vascularity permeability. Both these aspects require adequate pericyte investment on the abluminal surface of tumour blood vessels—experimental depletion of pericytes does indeed result in tumour regression (Bergers et al. 2003) but also leads to hypoxia-induced epithelial-mesenchymal transition increasing metastasis (Cooke et al. 2012). Consistent with this, normalising tumour vasculature by abrogating RGS5 expression (a cell surface protein that is abnormally expressed in tumour vessels), makes tumours more susceptible to chemotherapeutic agents (Hamzah et al. 2008). Moreover, the context in which pericyte dissociation from tumour vessels occurs also affects the response to chemotherapeutic agents as shown for variable levels of PDGFBB expression by various tumours (Hosaka et al. 2013).
Angiogenesis-Independent Mechanisms by Which Pericytes Promote Metastasis
A more direct role for pericytes in promoting cancer growth and metastasis without impact on angiogenesis has recently emerged from several laboratories including our own. It has become increasingly evident that pericytes are potent mesenchymal stem cell-like cells with an ability to promote organ repair and regeneration and multiple mesenchymal lineage differentiation capacity (Crisan et al. 2008; Sa da Bandeira et al. 2017). In the haemopoietic system, pericytes are an integral part of the haemopoietic stem cell niche regulating their maintenance and quiescence through paracrine effects (Sacchetti et al. 2007) supporting haemopoiesis both in vitro and in vivo (Birbrair and Frenette 2016; Morrison and Scadden 2014), as reviewed in Sa da Bandeira et al. (2017). In the process of studying the cellular microenvironment of epithelial renewal in human skin, we discovered a novel, paracrine role for pericytes in influencing skin tissue regeneration in 3D organotypic cultures completely lacking any blood vessels (Paquet-Fifield et al. 2009).
In view of the fact that pericytes are MSC-like and that MSCs had been reported to promote breast cancer metastasis (Karnoub et al. 2007) and ovarian cancer growth (McLean et al. 2011), we sought to establish whether pericytes may be a critical element of the TME with a more direct role in cancer progression. Recognising that the stromal signature of serous ovarian cancer patients reported by the Australian Ovarian Cancer Study Group (AOCS) (Tothill et al. 2008) had markers of both fibroblasts and pericytes, we used the molecular signature previously generated by us for both individual cell types (Paquet-Fifield et al. 2009) to interrogate the AOCS patient dataset annotated for patient outcomes (Sinha et al. 2016). Remarkably, the pericyte signature outperformed the ovarian cancer stromal signature at predicting early relapse revealing that those serous ovarian cancer patients carrying a high pericyte score (evidenced by a set of 146 genes co-expressed by both pericytes and ovarian cancer stromal cells), relapsed significantly earlier with a mean progression-free survival/PFS time of 9 months (vs. 29 months in those with a low pericyte score; n = 215), despite similar treatment (p = 0.00067 vs. p = 0.0011 from Tothill et al. 2008). Notably, the fibroblast signature was relatively poorer at predicting relapse (p = 0.01). Subsequently, we used a xenograft model to demonstrate that pericytes could act as CAFs when co-injected with ovarian cancer cell lines and that critically the tumour vasculature derived entirely from host murine cells remained unaffected with respect to the number of blood vessels or pericyte investment . Thus, co-injection of human pericytes with OVCAR-5 or OVCAR-8 cells, accelerated tumour growth rates and caused rapid dissemination to local tissues increasing metastases in a dose-dependent manner, typical of ovarian cancer spread clinically (Sinha et al. 2016). Notably, the human pericytes remained in the tumour stroma not associating with the tumour vasculature presumably due to species-specific incompatibility of signals that might otherwise result in their incorporation into tumour microvessels. This study provided the first clear evidence uncoupling the pro-angiogenic versus pro-metastatic function of pericytes in cancer. This suggests to us that when pericytes are dissociated from blood vessels they promote metastasis—a novel site of pericyte action, whereas their normal location in blood vessels restricts metastasis (Fig. 5.1). Consistent with a paracrine role for pericytes in promoting tumour cell metastasis, transwell co-culture experiments showed that pericytes increased ovarian cancer cell migration and invasion through matrigel; in other experiments we were able to demonstrate increased ovarian cancer cell proliferation with pericyte co-culture (Sinha et al. 2016). Interestingly, recent work with a variety of epithelial cancers has shown that pericytes contribute to cancer progression by giving rise to CAFs when dissociated from tumour blood vessels (Hosaka et al. 2013).
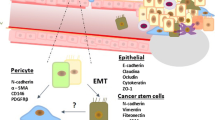
Schematic model of the role of pericytes in promoting malignant cancer progression. Localisation of pericytes in intimate association with blood vessels results in a tightly encapsulated ovarian cancer tumour (left) whilst placing pericytes directly within the tumour microenvironment leads to increased tumour cell proliferation, increased recruitment of αSMA+ stromal cells including cells expressing markers of BM-MSCs, induction of EMT and invasion, and metastatic spread to distant organs. Based on Sinha et al. (2016)
It is also likely that pericytes promote aggressive tumour growth by affecting the cancer stem cell compartment within tumours preferentially by secreting paracrine regulators. In support of this, it has been reported that human cancer-associated MSCs found in the TME of ovarian cancer increased the incidence of ALDH+CD133+ cancer stem cells via BMP-2 (McLean et al. 2011). In recent work from our laboratory, we have shown that pericytes can dictate the orientation of cell divisions within the skin’s proliferative compartment, i.e. the basal layer, increasing planar presumed symmetric cell divisions delaying differentiation thereby maintaining epidermal cells in a more primitive state via BMP-2 (Zhuang et al. 2018). These data and work from lower organisms such as Drosophila demonstrating a role for BMP signalling in maintaining “stem-ness ” of neighbouring cells (Kawase et al. 2004; Song et al. 2004) points to a conserved mechanism for the stem cell niche in regulating the fate of adjacent cells.
Future Trends and Directions
The full spectrum of functional capabilities of pericytes are only just starting to emerge and the closer investigators look beyond their classic role in vascular stability and permeability, the more seems to be uncovered (Ribeiro and Okamoto 2015). For instance, their ability to influence the inflammatory response by acting as a physical barrier to extravasation of immune cells and secreting a vast array of cytokines and extracellular matrix molecules are only recently being appreciated (reviewed in Navarro et al. 2016). Pericytes have been implicated in metastasis by increasing tumour cell intravasation at distal sites through endosialin (Viski et al. 2016), by regulating the metastatic niche via KLF4 (Paiva et al. 2018) and suppressing the immune response to brain tumours (Sena et al. 2018). The exact nature of molecular crosstalk between pericytes and cancer cells needs to be studied—one possibility is that they may contribute to tumour growth by differentiating into fat cells, which act as a source of energy driving cancer cell growth and metastasis (Huang et al. 2018). Another an exciting prospect is that exosomes secreted by cancer cells signal pericytes to become CAFs (Ning et al. 2018). Thus, a clear driver of future work has to be the identification of specific subsets of pericytes with cutting-edge technologies such as single cell RNA seq as reported recently for murine brain vascular cells including pericytes (Vanlandewijck et al. 2018). Moreover, the commonalities and distinctions in functional gene expression related to specific anatomical sites and organs needs to be addressed urgently to broaden our understanding of how these cells contribute to tissue renewal, wound repair, cancer, and ageing. A thorough understanding of pericyte cellular and molecular biology and their immense impact on neighbouring cells is essential to devise improved stem cell and regenerative medicine and interventions in cancer progression. An underlying concept is that just as we acknowledge that there are cancer stem cells within tumours that can drive cancer progression, a similar recognition of subsets of “cancer stromal stem cells” is much overdue.
References
Bergers, G., et al. (2003). Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. The Journal of Clinical Investigation, 111(9), 1287–1295.
Birbrair, A., & Frenette, P. S. (2016). Niche heterogeneity in the bone marrow. Annals of the New York Academy of Sciences, 1370(1), 82–96.
Calon, A., et al. (2015). Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nature Genetics, 47(4), 320–329.
Caplan, A. I. (2008). All MSCs are pericytes? Cell Stem Cell, 3(3), 229–230.
Cirri, P., & Chiarugi, P. (2011). Cancer associated fibroblasts: the dark side of the coin. American Journal of Cancer Research, 1(4), 482–497.
Cooke, V. G., et al. (2012). Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell, 21(1), 66–81.
Crisan, M., et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell, 3(3), 301–313.
Cunha, G. R., et al. (2003). Role of the stromal microenvironment in carcinogenesis of the prostate. International Journal of Cancer, 107(1), 1–10.
Druker, B. J. (2002). STI571 (Gleevec) as a paradigm for cancer therapy. Trends in Molecular Medicine, 8(4 Suppl), S14–S18.
Dulauroy, S., et al. (2012). Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nature Medicine, 18(8), 1262–1270.
Erber, R., et al. (2004). Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. The FASEB Journal, 18(2), 338–340.
Finak, G., et al. (2008). Stromal gene expression predicts clinical outcome in breast cancer. Nature Medicine, 14(5), 518–527.
Frame, M. C., & Serrels, A. (2015). FAK to the rescue: activated stroma promotes a “safe haven” for BRAF-mutant melanoma cells by inducing FAK signaling. Cancer Cell, 27(4), 429–431.
Fujita, H., et al. (2010). alpha-Smooth muscle actin expressing stroma promotes an aggressive tumor biology in pancreatic ductal adenocarcinoma. Pancreas, 39, 1254–1262.
Greenhalgh, S. N., Conroy, K. P., & Henderson, N. C. (2015). Healing scars: targeting pericytes to treat fibrosis. QJM: An International Journal of Medicine, 108(1), 3–7.
Hamzah, J., et al. (2008). Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature, 453(7193), 410–414.
Hosaka, K., et al. (2013). Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nature Communications, 4, 2129.
Huang, J., et al. (2018). Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell, 33(4), 770–784 e6.
Hung, S. C., et al. (2005). Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clinical Cancer Research, 11(21), 7749–7756.
Kalluri, R., & Zeisberg, M. (2006). Fibroblasts in cancer. Nature Reviews. Cancer, 6(5), 392–401.
Karnoub, A. E., et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature, 449(7162), 557–563.
Kawase, E., et al. (2004). Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development, 131(6), 1365–1375.
Kramann, R., & Humphreys, B. D. (2014). Kidney pericytes: Roles in regeneration and fibrosis. Seminars in Nephrology, 34(4), 374–383.
Kuhnert, F., et al. (2008). Soluble receptor-mediated selective inhibition of VEGFR and PDGFRbeta signaling during physiologic and tumor angiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 105(29), 10185–10190.
Lin, S.-L., et al. (2008). Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. The American Journal of Pathology, 173(6), 1617–1627.
Lindblom, P., et al. (2003). Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes & Development, 17(15), 1835–1840.
Maciag, P. C., et al. (2008). Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Research, 68(19), 8066–8075.
McLean, K., et al. (2011). Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. The Journal of Clinical Investigation, 121(8), 3206–3219.
Mederacke, I., et al. (2013). Fate-tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its etiology. Nature Communications, 4, 2823–2823.
Mills, S. J., et al. (2015). Effects of human pericytes in a murine excision model of wound healing. Experimental Dermatology, 24(11), 881–882.
Mishra, P. J., et al. (2008). Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Research, 68(11), 4331–4339.
Morikawa, S., et al. (2002). Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. The American Journal of Pathology, 160(3), 985–1000.
Morrison, S. J., & Scadden, D. T. (2014). The bone marrow niche for haematopoietic stem cells. Nature, 505(7483), 327–334.
Navarro, R., et al. (2016). Immune regulation by pericytes: Modulating innate and adaptive immunity. Frontiers in Immunology, 7, 480.
Ning, X., et al. (2018). Exosomes released by gastric cancer cells induce transition of pericytes into cancer-associated fibroblasts. Medical Science Monitor, 24, 2350–2359.
Nisancioglu, M. H., Betsholtz, C., & Genove, G. (2010). The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-A blockade. Cancer Research, 70(12), 5109–5115.
Olumi, A. F., et al. (1999). Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Research, 59(19), 5002–5011.
Paiva, A. E., et al. (2018). Pericytes in the premetastatic niche. Cancer Research, 78(11), 2779–2786.
Paquet-Fifield, S., et al. (2009). A role for pericytes as microenvironmental regulators of human skin tissue regeneration. The Journal of Clinical Investigation, 119(9), 2795–2806.
Pietras, K., & Ostman, A. (2010). Hallmarks of cancer: interactions with the tumor stroma. Experimental Cell Research, 316(8), 1324–1331.
Quante, M., et al. (2011). Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell, 19(2), 257–272.
Ribeiro, A. L., & Okamoto, O. K. (2015). Combined effects of pericytes in the tumor microenvironment. Stem Cells International, 2015, 868475.
Ruoslahti, E. (2002). Specialization of tumour vasculature. Nature Reviews. Cancer, 2(2), 83–90.
Sa da Bandeira, D., Casamitjana, J., & Crisan, M. (2017). Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacology & Therapeutics, 171, 104–113.
Sacchetti, B., et al. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell, 131(2), 324–336.
Scharpfenecker, M., et al. (2005). The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. Journal of Cell Science, 118(Pt 4), 771–780.
Sena, I. F. G., et al. (2018). Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Medicine, 7(4), 1232–1239.
Sennino, B., et al. (2007). Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Research, 67(15), 7358–7367.
Shi, Y., et al. (2018). Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nature Reviews Nephrology, 14, 493–507.
Sinha, D., et al. (2016). Pericytes promote malignant ovarian cancer progression in mice and predict poor prognosis in serous ovarian cancer patients. Clinical Cancer Research, 22(7), 1813–1824.
Song, X., et al. (2004). Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development, 131(6), 1353–1364.
Stapor, P. C., et al. (2014). Pericyte dynamics during angiogenesis: new insights from new identities. Journal of Vascular Research, 51(3), 163–174.
Studeny, M., et al. (2002). Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Research, 62(13), 3603–3608.
Tothill, R. W., et al. (2008). Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clinical Cancer Research, 14(16), 5198–5208.
Tsujino, T., et al. (2007). Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clinical Cancer Research, 13(7), 2082–2090.
Vanlandewijck, M., et al. (2018). A molecular atlas of cell types and zonation in the brain vasculature. Nature, 554(7693), 475–480.
Viski, C., et al. (2016). Endosialin-expressing pericytes promote metastatic dissemination. Cancer Research, 76(18), 5313–5325.
Xian, X., et al. (2006). Pericytes limit tumor cell metastasis. The Journal of Clinical Investigation, 116(3), 642–651.
Zhuang, L., Lawlor, K. T., Schlueter, H., Pieterse, Z., Yu, Y., & Kaur, P. (2018). Pericytes promote skin regeneration by inducing epidermal cell polarity and planar cell divisions. Life Science Alliance, 1(4), e201700009.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pieterse, Z., Sinha, D., Kaur, P. (2019). Pericytes in Metastasis. In: Birbrair, A. (eds) Pericyte Biology in Disease. Advances in Experimental Medicine and Biology, vol 1147. Springer, Cham. https://doi.org/10.1007/978-3-030-16908-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-16908-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16907-7
Online ISBN: 978-3-030-16908-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)