Abstract
There is an unmet need in the treatment of cerebral ischemic stroke to enhance post-stroke functional recovery. Intranasal delivery of therapeutic peptides has been emerging as an important strategy to improve stroke recovery. In this chapter, we introduce the definition and mechanisms of intranasal delivery of therapeutic peptides. We also discuss its advantages and disadvantages in the treatment of stroke. A variety of peptides and the administration regimens that have been tested in stroke animal models are listed. We believe that further investigation in this regard can deepen our understanding of intranasal delivery and may promote its clinical translation in the pursuit of better stroke recovery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Stroke is the second leading cause of death and third main reason for severe disabilities worldwide [1, 2]. Over 80% of stroke cases present as ischemic stroke [3]. Targeting the highly dynamic events that occur during ischemic stroke in the relatively inaccessible brain microenvironment remains a challenge.
After decades of research, a variety of peptides, including erythropoietin (EPO ), interleukin 4 (IL-4), and transforming growth factor (TGF)-β1, have emerged as effective therapeutic agents to treat brain diseases, such as neurodegeneration, pain, psychiatric disorders and stroke [4]. However, some peptides cannot pass the blood brain barrier due to their high molecular weight, thereby requiring some form of invasive delivery to access the brain [5]. Recently, intranasal delivery has gained attention as a novel non-invasive drug delivery route. In this chapter, we will define intranasal delivery, describe its possible mechanisms, and discuss its advantages and disadvantages as a delivery method of therapeutic peptides. Some peptides that have shown protection against stroke through intranasal delivery along with the regimens that have been used, will also be covered in this chapter.
6.2 Definition and Mechanisms of Intranasal Delivery of Therapeutic Peptides
Intranasal delivery is an alternative drug delivery strategy used to treat brain injuries, such as stroke [6]. Intranasal administration circumvents the blood brain barrier (BBB ) and provides a direct and rapid route for drugs to enter the brain and cerebrospinal fluid (CSF ) through the olfactory epithelium [7]. Because of the large absorptive surface area of the nasal cavity (~160 cm2) [8], along with its high vascularization and porous epithelium, drugs or treatment agents delivered through the intranasal route can enter the brain within minutes [9]. Chemicals, peptides, genes, and cells have been successfully delivered to the brain by intranasal delivery to achieve neuroprotection [10].
After intranasal administration of [125I]-labeled proteins to rats and monkeys, radiolabeling has been shown to extend from the olfactory and trigeminal nerve components in the nasal epithelium to the olfactory bulb and brainstem, respectively. The signals from these [125I]-labeled proteins can spread to other central nervous system (CNS ) areas from these initial sites [11, 12]. The olfactory region is adjacent to the CSF flow tracts around the olfactory lobe [13]. Therefore, intranasal administration could lead to direct delivery into the CSF.
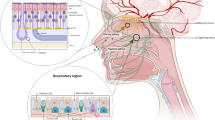
Two possible mechanistic routes have been suggested to underlie direct brain delivery after intranasal administration: (1) extracellular routes and (2) intracellular routes [7]. The extracellular route is a rapid pathway by which the drug is absorbed across olfactory epithelial cells, either by transcellular or paracellular mechanisms. Then the agent can be taken up into the CNS directly [7], which explains why the delivery of intranasal drugs to the brain occurs within minutes [14]. On the other hand, during the intracellular route, drugs are internalized into primary neurons of the olfactory epithelium by endocytosis, which includes pinocytotic mechanisms, followed by intracellular axonal transport to the olfactory bulb [7]. This intracellular pathway may take hours for drugs to be dispersed within the brain [15]. The delivery route and mechanisms of intranasal drug absorption is illustrated in Fig. 6.1.
Intranasal delivery pathways across the olfactory epithelium are depicted. Potential transport routes are shown in red. Some agents may be delivered via an intracellular pathway from the olfactory epithelium to the olfactory bulb by olfactory sensory neurons by endocytic, including pinocytotic, mechanisms, followed by intracellular axonal transport to the olfactory bulb. Others agents may pass the olfactory epithelial barrier by paracellular or transcellular transport to reach the lamina propria, and then distribute to the brain in multiple ways: (1) via entry into olfactory blood vessels and the systemic circulation; (2) by absorption into olfactory lymphatic vessels, which leads to the deep cervical lymph nodes of the neck; (3) by diffusion to the subarachnoid space with CSF , or. (4) entry into channels created by olfactory enshreathing cells surrounding the olfactory nerves, where they can access the olfactory bulbs. Subsequently, intranasally delivered drugs are transported from the olfactory bulb to the CSF surrounding the whole brain
6.3 Advantages of Intranasal Delivery of Therapeutic Peptides
Compared to conventional delivery methods, intranasal delivery of therapeutic peptides possesses the following advantages; (1) Safe and less invasive [16] compared to local delivery into the brain, which usually requires intraventricular and parenchymal injections [17, 18]; (2) easier passage into the brain [16]; (3) less requirement for the high diffusibility of therapeutic agents [16]; (4) lower dosage can be used with more reliable pharmacological profiles due to less drug metabolism and degradation along the delivery routes [19, 20]; (5) faster onset of action [21]; (6) less systemic side effects due to the absence of intestinal absorption and degradation by the enterohepatic cycle as well as general distribution [9, 22]; (7) large molecules can gain entry into the brain which is not easily attainable via the oral route [23]; and (8) less requirement for sterile preparation [24].
6.4 Peptides that Have Been Administered Intranasally to Treat Stroke
Recently, there has been increased focus on specific peptides as neuroprotective agents in experimental models of cerebral ischemic stroke. For example, numerous studies have shown that EPO administered subcutaneously, intraperitoneally, or through intra-cerebroventricular (ICV ) injection, is effective against ischemic stroke [25,26,27]. Subsequently, intranasal administration of EPO, as well as other peptides, has shown efficacy in experimental models of ischemic stroke. The protective effects of these peptides mainly fall into four categories: (1) inhibition of neuronal apoptosis, as observed with EPO treatment [28]; (2) amelioration of inflammatory responses, as observed with Tat-NEMO-binding domain (Tat-NBD ), a stable-mutant form of plasminogen activator inhibitor-I (CAPI) [20, 29]; (3) promotion of progenitor cell proliferation and brain repair mechanisms, as detected with insulin-like growth factor-I (IGF-1 ), TGF-β1, and TGF-α; [30,31,32,33] and (4) improvement in white matter function by preventing demyelination and promoting axon sprouting, as observed with tissue plasminogen activator (tPA ) and Apo-transferrin (aTf ) [31, 34]. Table 6.1 summarizes the neuroprotective effects of intranasal administration of these peptides against cerebral ischemic stroke .
In addition to the above-mentioned peptides, there are numerous peptides that have shown to be effective regulators of ischemic brain injury through ICV or subcutaneous administration, such as IL-4 and IL-33 [41, 42]. The efficacy of intranasal delivery of these peptides against ischemic stroke has not been examined. However, they are promising candidates for this new route of administration . Thus further studies investigating the efficacy of intranasal delivery of these two peptides against ischemic stroke is warranted.
6.5 Administration Regimens of Intranasal Delivery in the Treatment of Stroke
One of the advantages of intranasal delivery is that it reduces the effective dosage compared to systemic administration. However, there are still tremendous differences regarding the dosages reported by different studies for different peptides. In addition, some studies also report multiple intranasal peptide administrations or combination of different peptides. In Table 6.2, we summarize the intranasal infusion protocols used to administer various peptides and their corresponding experimental ischemic animal model.
6.6 Disadvantages and Limitations of Intranasal Delivery of Therapeutic Drugs
Despite the well-known advantages of intranasal drug delivery as described above, intranasal delivery of therapeutic drugs also carries some disadvantages: (1) Potentially rapid degradation of the drug due to active mucociliary clearance and the presence of nasal cytochrome P450/peptidases/proteases. This disadvantage can be overcome by covalently binding the drug to polyethylene glycol (PEG ), also known as PEGylation [48]. PEGylation has been used to enhance drug delivery of BDNF into the brain, where it reduced systemic BDNF clearance and facilitated neuroprotection [49, 50]. Intranasal delivery of PEGylated-TGF-α induced similar effects to intracranial delivery of TGF-α, such as inducing neural stem cells in the ependymal layer and progeny in the subventricular zone to proliferate, migrate to the lesion area, and differentiate into context-appropriate neurons [51]. However, intranasal infusion of un-PEGylated TGF-α failed to produce similar effects, which may be attributed to its vulnerability to degradation [33]. (2) Although intranasal delivery can bypass the BBB , the major disadvantage of this route is the limited absorption across the nasal epithelium. This has restricted its application to particularly potent substances. Hyaluronidase, an enzyme that binds to hyaluronic acid, can reduce the viscosity of hyaluronic acid or tissue [52]. Therefore, application of hyaluronidase has become a routine procedure used to facilitate intranasal drug delivery. Using 100 U hyaluronidase 30 min before apelin-13 administration increased its tissue permeability. It helped to reduce microglia activation, decreased the levels of inflammatory cytokines or chemokines and inhibited neuronal apoptosis [35]. However, there is also evidence that without hyaluronidase, apelin-13 alone can produce similar protective effects [53,54,55]. Therefore, further studies should be carried out to determine whether there is actual benefit to applying hyaluronidase. (3) Because of the size of the nasal cavity, there is a limit to the drug volume that can be delivered, which ranges from 25 to 200 μL. Indeed, in order to avoid discomfort, 2 μL drops are typically infused into one nasal cavity at a time, alternating between nostrils every 2 min, which takes over 20 min to complete drug administration.
6.7 Future Directions and Concluding Remarks
As a non-invasive approach , intranasal delivery of therapeutic peptides has numerous advantages, including easier passage to the brain, reduced dosage, and faster onset of action. Thus it is emerging as a promising alternative route for drug administration in the treatment of cerebral ischemic stroke. Multiple therapeutic peptides, including EPO , tPA , TGF-β1 have been shown to protect against stroke when administered intranasally. However, there are a lot of unknowns pertaining to the delivery details, such as the concentration that is needed, the number of injections required, and the times at which the drug should be delivered. Further investigation into intranasal delivery of therapeutic peptides as a neuroprotective strategy may lead to an easily accessible stroke treatment strategy, and hence better recovery after stroke.
References
Chen R, et al. Decreased percentage of peripheral naive T cells is independently associated with ischemic stroke in patients on hemodialysis. Int Urol Nephrol. 2017;49(11):2051–60.
Whiteford HA, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–86.
Mozaffarian D, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322.
Strand FL. Neuropeptides: general characteristics and neuropharmaceutical potential in treating CNS disorders. Prog Drug Res. 2003;61:1–37.
Lalatsa A, Schatzlein AG, Uchegbu IF. Strategies to deliver peptide drugs to the brain. Mol Pharm. 2014;11(4):1081–93.
Ma M, et al. Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci. 2008;9:117.
Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17.
Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol. 2006;34(3):252–69.
Fortuna A, et al. Intranasal delivery of systemic-acting drugs: small-molecules and biomacromolecules. Eur J Pharm Biopharm. 2014;88(1):8–27.
Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614–28.
Thorne RG, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–96.
Thorne RG, et al. Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience. 2008;152(3):785–97.
Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72.
Thorne RG, Frey WH 2nd. Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40(12):907–46.
Shipley MT. Transport of molecules from nose to brain: transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat germ agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res Bull. 1985;15(2):129–42.
Rhim T, Lee DY, Lee M. Drug delivery systems for the treatment of ischemic stroke. Pharm Res. 2013;30(10):2429–44.
Wang Y, et al. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials. 2012;33(9):2681–92.
Chen MY, et al. Surface properties, more than size, limiting convective distribution of virus-sized particles and viruses in the central nervous system. J Neurosurg. 2005;103(2):311–9.
Thompson BJ, Ronaldson PT. Drug delivery to the ischemic brain. Adv Pharmacol. 2014;71:165–202.
Yang D, et al. Intranasal delivery of cell-penetrating anti-NF-kappaB peptides (tat-NBD) alleviates infection-sensitized hypoxic-ischemic brain injury. Exp Neurol. 2013;247:447–55.
Saver JL, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480–8.
Pires A, et al. Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci. 2009;12(3):288–311.
Ozsoy Y, Gungor S, Cevher E. Nasal delivery of high molecular weight drugs. Molecules. 2009;14(9):3754–79.
Costantino HR, et al. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337(1–2):1–24.
Iwai M, et al. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38(10):2795–803.
Iwai M, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41(5):1032–7.
Zhang F, et al. Enhanced delivery of erythropoietin across the blood-brain barrier for neuroprotection against ischemic neuronal injury. Transl Stroke Res. 2010;1(2):113–21.
Castaneda-Arellano R, Feria-Velasco AI, Rivera-Cervantes MC. Activity increase in EpoR and Epo expression by intranasal recombinant human erythropoietin (rhEpo) administration in ischemic hippocampi of adult rats. Neurosci Lett. 2014;583:16–20.
Yang D, et al. Taming neonatal hypoxic-ischemic brain injury by intranasal delivery of plasminogen activator inhibitor-1. Stroke. 2013;44(9):2623–7.
Liu XF, et al. Non-invasive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci Lett. 2001;308(2):91–4.
Guardia Clausi M, et al. Inhalation of growth factors and apo-transferrin to protect and repair the hypoxic-ischemic brain. Pharmacol Res. 2016;109:81–5.
Mishra L, Derynck R, Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310(5745):68–71.
Guerra-Crespo M, et al. Intranasal administration of PEGylated transforming growth factor-alpha improves behavioral deficits in a chronic stroke model. J Stroke Cerebrovasc Dis. 2010;19(1):3–9.
Xia Y, et al. Tissue plasminogen activator promotes white matter integrity and functional recovery in a murine model of traumatic brain injury. Proc Natl Acad Sci U S A. 2018;115(39):E9230–8.
Chen D, et al. Intranasal delivery of apelin-13 is neuroprotective and promotes angiogenesis after ischemic stroke in mice. ASN Neuro. 2015;7(5):1759091415605114. https://doi.org/10.1177/1759091415605114.
Luo Y, et al. CART peptide induces neuroregeneration in stroke rats. J Cereb Blood Flow Metab. 2013;33(2):300–10.
Kim ID, et al. Intranasal delivery of HMGB1-binding heptamer peptide confers a robust neuroprotection in the postischemic brain. Neurosci Lett. 2012;525(2):179–83.
Zhao YZ, et al. Intranasal delivery of bFGF with nanoliposomes enhances in vivo neuroprotection and neural injury recovery in a rodent stroke model. J Control Release. 2016;224:165–75.
Zhang H, et al. Intranasal delivery of exendin-4 confers neuroprotective effect against cerebral ischemia in mice. AAPS J. 2016;18(2):385–94.
Sun BL, et al. Intranasal delivery of granulocyte colony-stimulating factor enhances its neuroprotective effects against ischemic brain injury in rats. Mol Neurobiol. 2016;53(1):320–30.
Zhao X, et al. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci. 2015;35(32):11281–91.
Yang Y, et al. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J Neurosci. 2017;37(18):4692–704.
Yu YP, et al. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387(1):5–10.
Teste IS, et al. Dose effect evaluation and therapeutic window of the neuro-EPO nasal application for the treatment of the focal ischemia model in the Mongolian gerbil. ScientificWorldJournal. 2012;2012:607498.
Chen N, et al. Subacute intranasal administration of tissue plasminogen activator improves stroke recovery by inducing axonal remodeling in mice. Exp Neurol. 2018;304:82–9.
Liu Z, et al. Subacute intranasal administration of tissue plasminogen activator increases functional recovery and axonal remodeling after stroke in rats. Neurobiol Dis. 2012;45(2):804–9.
Fletcher L, et al. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111(1):164–70.
Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97(10):4167–83.
Sakane T, Pardridge WM. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm Res. 1997;14(8):1085–91.
Wu D, Pardridge WM. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc Natl Acad Sci U S A. 1999;96(1):254–9.
Guerra-Crespo M, et al. Transforming growth factor-alpha induces neurogenesis and behavioral improvement in a chronic stroke model. Neuroscience. 2009;160(2):470–83.
Clement WA, et al. The use of hyaluronidase in nasal infiltration: prospective randomized controlled pilot study. J Laryngol Otol. 2003;117(8):614–8.
O’Donnell LA, et al. Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem. 2007;102(6):1905–17.
Zeng XJ, et al. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp Cell Res. 2010;316(11):1773–83.
Yang Y, et al. Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3K/Akt and ERK1/2 signaling pathways. Neurosci Lett. 2014;568:44–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Huang, T., Smith, A., Chen, J., Li, P. (2019). Intranasal Delivery of Therapeutic Peptides for Treatment of Ischemic Brain Injury. In: Chen, J., Wang, J., Wei, L., Zhang, J. (eds) Therapeutic Intranasal Delivery for Stroke and Neurological Disorders. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-030-16715-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-16715-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16713-4
Online ISBN: 978-3-030-16715-8
eBook Packages: MedicineMedicine (R0)