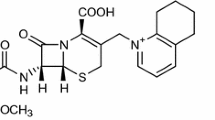
Abstract
Clinical use of the polymyxin antibiotics began approximately 10 years after their discovery in the late 1940s. Their concentrations in biological fluids were measured using microbiological methods. These methods were reasonably accurate for measuring the active polymyxin base, such as polymyxin B and colistin (polymyxin E), but were used inappropriately for measuring the concentrations of “colistin” in humans or animals following the administration of colistimethate, also known as colistin methanesulphonate (CMS). The use of polymyxins for systemic infections waned in the 1970s because of their toxicity and the preference for other antibiotics, but their value for treating infections caused by several important Gram-negative pathogens becoming resistant to other antibiotics was realized in the mid-1990s. The lack of adequate pharmacokinetic and pharmacodynamic knowledge spurred the development of methods more specific for measuring polymyxin B and colistin after their administrations as sulphate salts, and of colistin and CMS after the administration of CMS sodium. These methods have been based on high-performance liquid chromatography, detection and quantification of fluorescent derivatives of the polymyxin bases, or of the bases themselves with detection and quantification by mass spectrometry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
The two polymyxins used in clinical practice are colistin (polymyxin E) and polymyxin B . Their historical use, chemistry and antimicrobial activity have been reviewed in Chap. 3. Briefly, the two polymyxin bases are used as a mixture primarily of colistin A and B (or polymyxin E1 and E2) or as a mixture primarily of polymyxin B1 and B2. The two polymyxin forms denoted as 1 and 2 differ only in their alkanoic acid moiety by a methylene group. Colistin and polymyxin B differ by only one amino acid in the cyclic peptide; colistin contains D-Leu while polymyxin B contains D-Phe.
Colistin for parenteral use has been administered most commonly as its methanesulphonate derivative. This is formed by derivatizing the five available amino groups of the L-diaminobutyric acid moieties with methanesulphonic acid. Therapeutic use commenced in the mid-1950s as the sulphate salts of the bases, and as the sodium salt of the methanesulphonate of colistin (CMS or colistin methanesulphonate; also known as colistimethate ) for systemic administration in the United States in the 1960s. When administered, CMS may not be fully derivatized with methanesulphonate; it may be present in a dosage form as a mix of full and partial derivatives. Likewise, as will become apparent from the chapters covering pharmacokinetics and pharmacodynamics, there will be a complex mix of full and partial derivatives in samples of biological fluids or other aqueous media from experiments evaluating the fate or antimicrobial effectiveness of CMS in vivo or in vitro . This is because of a gradual loss of the methanesulphonate groups over time. Measuring the individual derivatives in such fluids or media has not been achieved. Doing so would be extremely complex, and of questionable value, given that colistin alone is deemed to possess antimicrobial activity [1]. Therefore, a more recent approach has been to perform two measurements on a sample [2, 3]. Firstly, the concentration of “total colistin” is measured, it being the sum of all methanesulphonate derivatives converted to colistin during processing of a sample plus pre-existing colistin in the sample; and, secondly, a measure of the concentration of colistin. When measuring the latter, one should be mindful of the instability of the methanesulphonate derivatives, ensuring appropriate storage and processing of samples under conditions which minimize conversion of any derivatives to colistin [1, 4]. Therefore, researchers should assure themselves that there is no conversion of methanesulphonate derivatives to colistin once a sample has been collected; for example, while separating plasma from a sample of blood, while stored pending analysis, after thawing, during repeated thawing and freezing, during processing and while awaiting chromatographic analysis. The difference between the two concentrations represents the concentration of all methanesulphonate derivatives (designated as CMS) in the sample. In addition, polymyxins are highly surface active, and their adsorption from aqueous solutions onto the surfaces of apparatus used during collection and processing of samples may have an impact on recovery and sensitivity. Generally, this has been minimized by including a cosolvent in stock solutions and either a cosolvent, protein (such as drug-free human plasma) or surfactant is added during the processing of samples of urine or bacterial broth [4,5,6].
This chapter will review the range of methods that have been used for measuring the concentrations of polymyxins in different biological fluids, and will do so in a chronological order that reflects the gradual advances in techniques that have enabled improvements in sensitivity and, more importantly, in specificity and the ease with which they are performed. It will describe methods used for pretreatment of samples, including the important issues raised above regarding stability and adsorption to surfaces, along with the methods for quantifying the concentration of polymyxin in the sample.
6.2 Microbiological Methods
The first method reported for measuring polymyxins in biological fluids appears to have been a microbiological assay for polymyxin (identified later as polymyxin D; [7]) in blood and urine based on its activity towards Brucella bronchiseptica. There was no apparent interference from other components of blood from the mouse, dog and human, and the lowest concentration on the calibration curve using 0.02 mL of blood was 0.25 mg/L [8, 9]. The author described measurement of the concentrations of polymyxin in pooled samples of blood after a single dose of polymyxin hydrochloride to mice (1 mg/kg, s.c.) and a dog (5 mg/kg, i.v.). Replicate analyses of a sample from the dog [8] produced coefficients of variation ranging from 7% to 12% (no concentration was provided). While the method potentially lacks specificity in the presence of other antimicrobial agents co-administered during in vivo studies, be they in animals or humans, it is a measure of antimicrobially-active polymyxin in a biological fluid such as blood to a level of sensitivity comparable to that achieved with HPLC of fluorescent derivatives of colistin (vide infra).
The limitations of nephro- and neuro-toxicity during clinical use in the early 1970s and prior led to an investigation of the binding of polymyxins to tissues following the systemic administration of polymyxin B and CMS to rabbits [10]. Concentrations were measured via their inhibition of B. bronchiseptica using the respective compounds as calibration standards. While the measurements for polymyxin B are likely to be reasonable estimates of its concentrations, those for “CMS” will be complicated by the issues of instability mentioned above and discussed subsequently in this chapter.
Later work described use of the same bacterial test organism to measure polymyxin B in bovine fluids and tissues [11, 12] following parenteral administration of polymyxin B sulphate. Two years previous, this group published values for the concentrations of polymyxin B, colistin and CMS in serum from calves administered daily doses of polymyxin B sulphate, colistin sulphate, or CMS, respectively, via the intramuscular route for three successive days [13]. The microbiological assay used by this group reported similar values of maximal serum concentration (Cmax) and terminal half-life for polymyxin and colistin. However, greater and lesser values, respectively, were obtained for “CMS” after the administration of CMS. As will become apparent from Chap. 7 describing the pharmacokinetics of colistin in rats administered colistin sulphate and of colistin and CMS after dosing with CMS, the values for “CMS” after administration of CMS are likely to be composites derived from measuring a mix of colistin and CMS in ratios that change over time from the dose; an observation possible only when concentrations in plasma are measured more specifically by chromatographic methods (vide infra).
Even more recently, a microbiological method was described for measuring CMS in serum (and urine) from humans, seemingly with the intention of correlating levels of CMS in blood with toxicity; the method was advocated because it avoided more complex and instrumentally-demanding methods based on liquid chromatography [14]. This method, along with the other microbiological methods discussed above for measuring “CMS”, measures antimicrobial activity that can only be attributed to colistin (assuming there are no other antimicrobially-active interferences). However, there are important issues that should be appreciated: (1) activity was relative to calibration standards prepared using CMS, which has minimal if any inherent antimicrobial activity [15]; (2) it is likely that CMS would degrade during the assay to antimicrobially-active colistin via partially sulphomethylated intermediates [15, 16], and the relative proportions of sulphomethylated colistin to colistin in the calibration standards exposed to the test organism during the assay may be quite different from those in the biological samples. Another report described the pharmacokinetics of “colistin” in a patient using concentrations measured in serum against Acinetobacter baumannii [17]. Further reports have appeared describing microbiological methods for measuring “colistin” following the administration of CMS to a patient [18] and in a study of the pharmacokinetics and dynamics of CMS in a pneumonia model with mice [19]; the same limitations apply to these reports. An extensive pharmacokinetic/pharmacodynamic study in mice used a microbiological method to measure “colistin” in plasma from neutropenic mice administered “colistin”, but it was not clear whether the sulphate salt or CMS was administered and measured [20].
Nevertheless, microbiological assays for polymyxins are legitimate for any analysis of exposure when the polymyxin itself (as a salt of the base) rather than any prodrug (such as CMS) is being administered and the polymyxin is being used as a reference for the preparation of calibration standards and quality controls. Published examples since 2000 include assessment of the exposure to colistin after administration of colistin sulphate to piglets [21], and to polymyxin B following its intravenous administration (presumably as the sulphate) to a young male [22]. The former reported a “detection” range from 0.005 to 3 mg/L [21] and, while the lower value in this range compares favourably with limits of quantification achieved using chromatographic methods (vide infra), no validation was provided for a limit of quantification. Also, no details were provided by the latter report [22]. The majority of methods beyond 2000 have employed chromatographic separation and quantification of fluorescent derivatives or chromatography with quantification by mass spectrometry.
The clinical use of colistin, as CMS, began to increase in the mid-1990s in response to an increasing incidence of infections in patients with cystic fibrosis caused by bacteria resistant to the usually available antibiotics. The authors of one important study at this time concluded that intravenous colistin (as CMS) may be valuable therapy for acute respiratory exacerbations and that the risks of renal toxicity could be minimized with careful monitoring [23]. This group measured the concentrations of “colistin” in blood at steady-state by microbiological assay. The increased use of CMS in response to this and other reports of its use occurred at a time when it was recognized that previous systemic doses may have been excessive. However, the increase was at a time when there was very little information available on its pharmacokinetics and pharmacodynamics that might guide the selection of appropriate doses [24].
6.3 Chromatographic Methods
Given the limitations of microbiological methods for measuring “CMS” and “colistin” in plasma after the administration of CMS, reports began to appear of chromatographic methods being developed that were selective for colistin and CMS (the latter including partially sulphomethylated forms of colistin), with detection appropriate for the required levels of quantification of both these and polymyxin B. These methods have now been established as the most suitable for measuring the concentrations of polymyxin B and colistin in biological fluids following administration of their sulphate salts to animals or humans, or of colistin and CMS after administration of the sodium salt of CMS. The required levels of sensitivity have been achieved by detecting and quantifying fluorescent derivatives of the polymyxin base or by using mass spectrometry; fluorescent derivatives because of a lack of native ultraviolet absorbance sufficient for quantifying clinically relevant concentrations. The concentration of CMS in a sample is determined after hydrolysis of CMS to colistin and quantification of the latter as “total colistin” (from CMS and pre-existing colistin) and, after accounting for differences in molecular weight, subtracting values for the concentration of colistin measured separately from the “total”. When measuring the colistin alone, one must be careful to minimize hydrolysis of CMS.
Le Brun was one of the first to report a liquid chromatographic method for measuring “colistin” in biological fluids from humans; serum, urine and sputum [25]. They adapted a method described 3 years previously for measuring residual colistin in bovine tissues [26]. The latter researchers formed a fluorescent derivative (λEx 340 nm, λEm 440 nm) from reaction of the primary amines of colistin with o-phthalaldehyde. They were measuring colistin in farmed animals most likely exposed to colistin salts, using colistin sulphate and the summed responses from colistin A and B as a reference. However, Le Brun et al. used the method to measure “colistin” in patients who had inhaled CMS, seemingly using CMS as a calibration reference. This group mixed serum or sputum with methanol/trichloroacetic acid to precipitate protein, followed by reaction with o-phthalaldehyde and chromatographic analysis. They did not assess whether processing of samples with such an acidic mix may have facilitated partial or complete conversion of CMS to colistin, but later work (vide infra) indicated that complete conversion is unlikely [3]. The risk of conversion in vitro of methanesulphonate derivatives to colistin during processing of samples containing CMS and colistin will apply to any method [27] that purports to measure the concentrations of “colistin” in samples from humans or animals administered CMS. There is no problem when the biological samples being measured are from subjects (be they animals or humans) administered colistin sulphate or polymyxin B sulphate and calibration standards are prepared using those same substances as reference standards. For example, the formation of a derivative with o-phthalaldehyde was used to measure colistin in plasma (0.5 mL) and in the gastrointestinal contents (1.0 g samples extending from the duodenum to ileum) of pigs following the oral administration of colistin sulphate [28]. Samples were treated with trichloroacetic acid to precipitate protein prior to solid-phase extraction and formation of the derivative. The limit of quantification was 0.25 mg/L and 0.50 mg/kg, respectively.
The formation of other fluorescent derivatives for the chromatographic quantification of colistin in biological fluids has been published. Colistin A (polymyxin E1) was extracted from plasma of the rat and dog using a 96-well C8 disk extraction plate prior to reacting it with dansyl chloride. The product was described as a penta-dansyl derivative (λEx 344 nm, λEm 518 nm) that was “confirmed” with mass spectrometry [29], but the conditions for formation of the confirmed product were not identical to those for its formation during preparation of the biological samples for chromatography. The investigators intended using the method as part of the development of a single component of colistin (colistin A) as a potential therapeutic agent, and used colistin A to prepare calibration standards. The limit of quantification (in 0.2 mL plasma) was 0.05 mg/L.
In seeking to gain a better understanding of the pharmacokinetics and pharmacodynamics of colistin and CMS, separate and more specific methods were developed for measuring colistin in plasma from rats after the administration of colistin sulphate, and for measuring colistin and CMS after the administration of CMS [2, 3]. The methods relied on forming a fluorescent derivative from reaction of the amine of the L-diaminobutyric acid residues of colistin with 9-fluorenylmethyl chloroformate (FMOC). After adding trichloroacetic acid/methanol to samples of plasma, colistin in the supernatant was retained under basic conditions on a C18 solid-phase extraction cartridge. Extraneous substances were eluted and the reaction initiated by adding a small volume of a concentrated solution of FMOC into the cartridge. The derivatives of colistins A and B were eluted, chromatographed on a C18 analytical column, and detected and quantified from their fluorescence at 315 nm following excitation at 260 nm (Fig. 6.1).
Typical chromatograms obtained from fluorescence detection for drug-free human plasma (left) and drug-free plasma spiked with colistin sulphate (1 mg/L) (right). The fluorescent derivative of colistin A was eluted at about 26.5 min, of colistin B at about 22 min and of netilmicin, the internal standard, at about 18.5 min. [2]
Concentrations were calculated from a calibration curve of the ratio of the summed areas of the two chromatographic peaks for colistins A and B to an internal standard against the concentration of colistin sulphate. In samples spiked freshly with a “high” concentration of CMS (10 mg/L), there was no quantifiable conversion of CMS to colistin when measured for colistin only [3]. A separate sample was treated with sulphuric acid to convert CMS and partial methanesulphonate derivatives formed in vivo to colistin, and then added to a cartridge for derivatizing with FMOC. The limit of quantification for colistin in plasma was 0.10 mg/L (from a 0.25 mL volume of sample) and for CMS it was 0.20 mg/L (0.15 mL) [30]. The two methods were developed for measuring both substances after administering CMS to rats (Fig. 6.2) [31] and to patients with cystic fibrosis [30]. Since then, they have been adapted/modified over the subsequent decade for measuring both substances in a more recent study of patients with cystic fibrosis [32], and after administering CMS to patients receiving continuous ambulatory peritoneal dialysis [33], and to critically-ill patients [34]; for studies with mice [35]; for measuring colistin and CMS in bronchoalveolar lavage fluid from rats after intratracheal administration of CMS [36], and for measuring both substances after administering four different brands of CMS to rats [37]. The method for colistin alone has been used also for measuring colistin in broth culture after adding colistin sulphate [38]; colistin in mouse brain after administration of colistin sulphate [39, 40]; and, for measuring colistin in plasma, urine and kidney tissue from rats administered colistin sulphate [41, 42]. Minor modifications to the volume of sample used, and the chromatographic mobile phase, and a change from trichloroacetic acid/methanol to acetonitrile for precipitating protein, have been made since initial publication of the two methods. Concentrations of CMS in plasma after administration of CMS in vivo (or in other media during experiments conducted, for example, with CMS in vitro) were calculated from the difference between “total colistin” (i.e. colistin plus methanesulphonate derivatives converted to colistin during incubation with sulphuric acid [3]), and colistin measured separately [2].
Mean (± S.D.) concentrations of CMS and colistin in the plasma of rats following an intravenous dose of colistin methanesulphonate (Sigma, St. Louis, MO, USA; 15 mg/kg). Modified from Li et al. [31]
Later, the method for colistin [2] was applied to measuring the concentrations of polymyxin B in plasma from humans administered polymyxin B sulphate [43] and of a congener of polymyxin B, NAB 7061 (one of the amino acids in polymyxin B replaced with another) in plasma and urine of rats [44]. The limit of quantification was 0.125 mg/L with 0.10 mL of plasma; identical to the original method [2]. The method was applied subsequently for measuring polymyxin B in critically ill patients administered intravenous polymyxin B sulphate [45,46,47], some of whom were receiving continuous renal replacement or intermittent haemodialysis.
Subsequent reports from other research groups have applied the two methods [2, 3], with or without slight modifications, for measuring colistin in serum after the intravenous administration of CMS to critically ill patients [48]; colistin in serum and cerebrospinal fluid after the administration of CMS [49, 50]; colistin in plasma and bronchoalveolar lavage fluid from humans after administration of CMS [51, 52]; colistin in plasma and tissues (liver, muscle and kidney) from ducks administered colistin sulphate intramuscularly or in their feed [53]; and, colistin in plasma from pigs administered colistin sulphate [54].
A subsequent pharmacokinetic study applied the method above [2] to examine the pharmacokinetics of “colistin” in humans after inhalation of CMS [55]. These investigators claimed to have measured colistin A (polymyxin E1) but it was not clear which substance had indeed been used for preparation of the calibration standards: colistin A, colistin sulphate, or CMS. From chromatographic analysis of the calibration standards, only the peak for colistin A was used to construct a calibration curve. The sulphate salts of colistins A and B account for more than 85% of colistin sulphate and the ratios of the two can differ considerably between batches of the raw material [26] and, hence, between batches of CMS manufactured from colistin. It is important to include the peak responses for colistins A and B when constructing a calibration curve, plus the responses from the two species in biological fluids following administration of CMS. The ratios of the two components can be established by direct chromatographic analysis of the raw material, and quantified by either UV absorption [2] or mass spectrometry [4]. The validity of these two methods for assessing relative content of the components has been confirmed by their quantification in chromatographic eluate using evaporative light scattering [56].
Greater access to mass spectrometry for detection and quantification has produced a number of reports of well-described methods for measuring CMS and colistin after a dose of CMS, of colistin after colistin sulphate, and of polymyxin B after dosing with polymyxin B sulphate.
A method developed for measuring colistin in perfusate and urine collected from experiments examining the fate of colistin in the isolated perfused rat kidney also described measuring the substance in human plasma and urine [57]. Protein precipitation was achieved by mixing the samples (0.2 mL) with trichloroacetic acid/methanol followed by further clean-up using solid-phase extraction, with a portion of the eluate subjected to LC-MS/MS. Extraction was deemed necessary to maintain sufficient and consistent sensitivity. Summed intensities of the product ions from the two transitions each for colistin A and colistin B relative to an internal standard, polymyxin B1, were used to construct calibration curves. Prior to this, the proportions of colistin A and B in the reference material were established. Limits of quantification were 0.028–0.056 mg/L for colistin A and 0.016–0.032 for colistin B, depending on the biological fluid. Interestingly, this level of sensitivity was not able to be achieved when similar methods were used for preparing samples of bovine milk and tissue [58] for chromatography, despite the larger sample sizes and a more sensitive model of mass spectrometer. It is likely that the lower limits claimed by Ma et al. [57] could be extended with a more sensitive mass spectrometer. The method [57] is suitable for measuring colistin after the administration of colistin sulphate and could also be adapted for measuring polymyxin after polymyxin sulphate. However, the authors did not establish its suitability for measuring colistin in the presence of CMS.
A well-described method for measuring colistin A and B plus the concentrations of their respective methanesulphonate prodrugs in human plasma (0.10 mL) used only one step, precipitation of protein with 0.1% trifluoroacetic acid in acetonitrile, prior to chromatography connected to tandem mass spectrometry [5]. It described chilling of collected blood, separating plasma from red blood cells soon afterwards, thawing of previously frozen and stored samples of plasma in an ice bath, rapid processing of them in small batches, and storage of the supernatant at 4 °C prior to chromatography. The limits of quantification for colistin A and B were 0.019 and 0.010 mg/L, respectively.. The concentrations of CMS were calculated by difference [2, 3]. Unfortunately, the authors did not present data that validated their method for measuring CMS [5]. The method has been used by these Swedish and Greek collaborators for measuring colistin and CMS in a number of studies with intensive care patients administered CMS [59,60,61]. The precautions they describe in the preparation of samples, or variations of them, are not exclusive to these reports, but are necessary to minimize conversion of methanesulphonate derivatives to colistin when measuring colistin alone (see further discussion on stability below). In some instances, while the procedures described would appear to minimize conversion, it has not always been proven unequivocally [62].
Likewise, three subsequent methods based on liquid chromatography-mass spectrometry achieved comparable limits of quantification with the same (0.1 mL) [56, 63] or double the volume of plasma [64], but they also lack data validating the methods for measuring CMS in spiked samples of plasma. Data were provided with respect to the stability of colistin in plasma kept at room temperature for up to 12 h [63], but none could be identified that demonstrated lack of conversion of CMS to colistin during processing of samples. The first and third methods [56, 64] improved efficiency and accuracy by automated processing; samples of plasma thawed to 4 °C for measuring colistin were added directly into 96-well solid-phase extraction plates. With this procedure, it is quite likely that there was minimal conversion of CMS to colistin during processing of the samples, but no data in either publication [56, 64] could be identified to confirm this.
A method for measuring polymyxin B1 and B2 and colistin A and B (as well as vancomycin) in 0.5 mL of plasma from humans claimed an advantage of not requiring “a long and expensive procedure of SPE” (solid-phase extraction). However, while the authors [65] used polymyxin B sulphate as a reference for preparing calibration standards of polymyxin B1 and B2, they appear to have used colistin methanesulphonate (incorrectly) as reference standards for polymyxin E1 and E2. In contrast, another method published in the same year is comprehensive [4]. It describes limits of quantification similar to those described previously for colistin A and B [5], albeit using 2.5-times the volume of plasma, but also provides validated limits for CMS A (0.029 mg/L) and B (0.01 mg/L). The method described the processing of calibration standards and quality controls containing CMS in plasma by solid-phase extraction after conversion of the prodrug to colistin with sulphuric acid [3]. For measuring colistin alone in samples from patients administered CMS, conversion of CMS to colistin was minimized by processing previously frozen samples within 1 h of being thawed and simply diluting them with water prior to solid-phase extraction, rather than adding acetonitrile/acid to precipitate protein prior to extraction [5]. Figure 6.3 demonstrates application of the method for measuring concentrations of CMS and colistin in a subject administered CMS [4].
Concentrations of colistins A and B (as the free base) and CMSs A and B (as CMS without the sodium ion) in plasma versus time from a human volunteer administered a single intravenous infusion of 80 mg CMS (Colimycin for injection, Sanofi-Aventis). From Gobin et al. [4]
The method was also applied to measuring colistin and CMS in human urine; 0.2 mL of urine was mixed with half its volume of drug-free plasma “to avoid the loss of colistin by adsorption” to the 5 mL polypropylene tubes used [4]. This procedure was also found necessary by others for urine [64] and haemodiafiltrate [62], although one other group overcame the loss of polymyxin B by adding 0.5% of 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulphonate (CHAPS; a surfactant) to the sample after collection [6]. A shorter chromatographic time than described in a previous method [5] allowed the processing of larger batch sizes of samples for storage at 4 °C in an autosampler prior to analysis by liquid chromatography-mass spectrometry [4].
In 2015, a further improvement on this method was achieved with the same solid-phase extraction material but with a 96-well system and, more importantly, a chromatographic column containing an ethylene-bridged hybrid material with bound amide functional groups [66]. It is apparent from a visual comparison of chromatograms [4, 66] that the improved chromatographic efficiency provided a greater sensitivity and slightly lower limits of quantification; meanwhile using a slightly lesser volume of sample than the earlier method (plasma of 0.18 mL [66] rather than 0.25 mL [4]). However, the method also lacks data validating the measurement of CMS in plasma.
This was followed a year later by a method for measuring polymyxins B1, B2 and B1–1 (an isomer of B1) in human plasma and urine (described for measuring this group of polymyxins in the latter medium for the first time) [6]. The polymyxins were extracted in an automated manner from plasma using reversed-phase C8 HLB sorbent and from urine (to which surfactant had been added, vide supra) with a reversed-phase/weak cation-exchange sorbent (both Oasis®, from Waters). These authors achieved limits for the quantification of all three polymyxins in plasma (0.1 mL) and urine (0.2 mL) of 0.005 mg/L. They are superior to values reported by Thomas et al. [67] of 0.1 mg/L for polymyxins B1 and B2 using 0.25 mL of plasma although, as the authors rightly state, inspection of their chromatograms would suggest an order of magnitude lower could be achievable. Interestingly, the former authors’ measurements of the three polymyxins in plasma and urine, albeit in only one subject, suggests differences in renal clearance between them [6].
Other methods based on liquid chromatography – mass spectrometry suffer from descriptions that are not clear or are incomplete. It is difficult sometimes to ascertain limits of quantification, volumes of sample used, and whether conversion of CMS to colistin in samples has been minimized and/or evaluated after collection of the samples and during their processing for quantifying colistin in studies where CMS has been administered.
For example, an appreciation of the sensitivity of the method for polymyxin B is difficult to ascertain because the volume of sample, detail of the method and quantifiable limits were not provided [68]; a limit of 0.25 mg/L can be construed from data for intra-/inter-day variations (CVs) of less than 8% for concentrations spanning the calibration range of 0.25–10.0 mg/L. Members of the same group subsequently described use of the same method for examining the pharmacokinetics of polymyxin B1, isoleucine-polymyxin B1 and summed polymyxins B2 and B3 (the two were not resolved chromatographically and the concentrations of B3 considered negligible in most samples) and their concentrations in renal tissue and urine after a single intravenous dose of polymyxin B sulphate to rats [69]. This was followed by another report of a study examining the pharmacokinetics and efficacy of polymyxin B sulphate after it had been encapsulated into a liposomal delivery system and administered intravenously to mice [70]. The concentrations of the four components of polymyxin B in 0.20 mL of serum and epithelial lining fluid were determined using UPLC-MS/MS. Trichloroacetic acid in an organic solvent was added to precipitate proteins, and the dried extract from the supernatant following centrifugation reconstituted in mobile phase (formic acid, acetonitrile and water) for chromatography. The authors had separated and purified the four components previously using preparative liquid chromatography [71], and confirmed their identity with mass spectrometry. It is assumed, therefore, that calibration curves for calculating their individual concentrations in the two fluids [72] were prepared using the purified components. The initial work from data on reproducibility for the calibration standards suggests a limit of quantification of 0.25 mg/L from an unknown volume of sample [68]; the final publication reports a limit of 0.006 mg/L for all four polymyxin B compounds (B1, isoleu-B1, B2 and B3) with 0.2 mL of serum or epithelial fluid [70].
6.4 Stability of CMS and Colistin
As noted previously, CMS is converted to colistin in vivo after the administration of CMS. The conversion occurs also in vitro in biological samples collected from studies where CMS has been administered (e.g. a pharmacokinetic study) and in studies assessing antimicrobial activity with CMS. Apparent from Fig. 6.2 are the considerably higher concentrations of CMS compared to colistin in plasma from a pharmacokinetic study in rats after an intravenous dose of CMS [31], and the higher concentrations also in humans [4, 30, 34, 59, 61], especially during the first 4 h after a dose of CMS (Fig. 6.3). Therefore, it is critical for ensuring accurate measurement of the concentrations of colistin in such studies that there is minimal conversion of CMS to colistin in the time between collection of the sample and measuring colistin.
The method for measuring colistin in plasma [2] was used for an extensive assessment of its stability when stored in a range of aqueous media (water, plasma and isotonic phosphate buffer, 0.067 mol/L, pH 7.4) and its formation from CMS stored separately in identical media plus Meuller-Hinton broth. The presence of CMS remaining in water was also examined qualitatively using strong anion exchange chromatography [16]. The levels of colistin A and B in water remained unchanged after storage at 4 °C for 60 days and at 37 °C for 120 h. When stored in the buffer (approximately 1.5 pH units higher than the solution of colistin sulphate in water) and human plasma at 37 °C, its stability was reduced markedly; more so in plasma than in the buffer. After incubation of CMS in water for 12 h at this temperature, there were clear qualitative changes in the chromatogram for CMS, suggesting partial conversion to products derivatized to a lesser degree with methanesulphonate . Between 10% and 15% of CMS in buffer and plasma had degraded to colistin within 2 h, irrespective of the source of CMS raw material. Interestingly, later work found that the stability of CMS was greater at a higher concentration in plasma (30 mg/L vs 2 mg/L); an observation made also in aqueous solutions of CMS for administration to patients [73]. It was attributed to the formation of micelles by CMS, which protected the prodrug from conversion to colistin [74].
Colistin was reported to be stable in plasma stored at −20 °C and −80 °C for up to 2 months [4]. No data was provided but, from the limits of quantification for CMS and data for the storage of plasma spiked with CMS under the same conditions and period, it can be estimated that there was no more than 1% conversion to colistin. This supports previous data [1]: CMS and colistin in plasma stored at −80 °C were stable for 4 months and 6–8 months, respectively. The “loss by adsorption”, alluded to above and previously [5], was reported later [75] to be significant when dilutions of stock solutions of colistin were made using test-tubes made of soda lime glass, polystyrene and polypropylene. The least degree of loss was from low protein-binding microtubes. Although no quantitative data could be located in support, the usual procedure for minimizing adsorption is to add human plasma to those samples lacking protein prior to processing them for chromatographic analysis [4, 5]. Alternatively, it is evident from a more recent publication that the addition of a surfactant to urine after collection achieves almost 100% recovery of polymyxins B1, B1–1 and B2 [6].
These observations highlight the need for careful handling of biological samples collected from, for example, studies examining the pharmacokinetics of CMS and colistin after the administration of CMS. It is proposed that any method to be used for such studies should have conducted experiments to validate the handling of samples after collection, their storage, and their handling during processing of samples prior to forming a fluorescent derivative for chromatography or during processing prior to direct chromatographic analysis with mass spectrometric detection.
6.5 Conclusions
In summary, there have been three predominant approaches for measuring the concentrations of colistin and polymyxin B, and the prodrug of colistin (CMS), in biological fluids: microbiological assay, liquid chromatography with detection and quantification of fluorescent derivatives, and liquid chromatography with detection and quantification by mass spectrometry. The second and third approaches have facilitated rapid advances in understanding the preclinical and clinical pharmacology of polymyxins (and their relevant prodrugs) over the last 15 years. They are capable of achieving the sensitivity required to measure concentrations in samples from clinical and pharmacokinetic studies in humans, and pharmacokinetic and pharmacodynamic studies in animals , and some of the methods using these approaches have been well validated. Of the three, the most appropriate and convenient for the majority of research laboratories would be liquid chromatography in combination with triple quadrupole mass spectrometry; even a single quadrupole may be sufficient [76] and could be adapted for clinical samples. The processing of samples is generally relatively simple, but one must ensure that there is insignificant conversion of CMS to colistin when quantifying the latter in samples where CMS is present also. The only limitation is access to a mass spectrometer. The formation of fluorescent derivatives has sufficient sensitivity but does require the additional step of forming the derivative during processing of the samples. These two approaches are designed to quantify the polymyxin base. If samples are from subjects or animals administered CMS, the concentrations of the base are determined before and after forced conversion of the prodrug to the base. From these separate determinations, the concentration of prodrug in biological fluid can be calculated. Microbiological methods have, in general, suffered from insufficient validation. Potentially, such methods possess sufficient sensitivity for measuring the concentrations of polymyxin B after therapeutic doses (and of colistin after administering colistin sulphate; available in China), but they are time-consuming. Often, they are described as being used to measure “colistin” in studies where CMS is investigated without taking any account of the presence of its prodrug, the lack of antimicrobial activity of that prodrug, and its potential conversion to colistin in both the samples and calibration standards to differing degrees during incubation. Some chromatography-based methods also suffer from this shortcoming.
References
Dudhani RV, Nation RL, Li J (2010) Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J Antimicrob Chemother 65(7):1412–1415
Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW (2001) A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 761(2):167–175
Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J (2002) Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob Agents Chemother 46(10):3304–3307
Gobin P, Lemaitre F, Marchand S, Couet W, Olivier JC (2010) Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob Agents Chemother 54(5):1941–1948
Jansson B, Karvanen M, Cars O, Plachouras D, Friberg LE (2009) Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J Pharm Biomed Anal 49(3):760–767
Meng M, Wang L, Liu S, Jaber O, Gao L, Chevrette L, Reuschel S (2016) Simultaneous quantitation of polymyxin B1, polymyxin B2 and polymyxin B1-1 in human plasma and treated human urine using solid phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr B 1012–1013:23–36
Brownlee G, Bushby SRM, Short EI (1949) The pharmacology of polymyxin A, B, and D. Ann NY Acad Sci 51(5):952–967
Stansly PG (1949) A simple method for the assay of polymyxin in blood and urine. Ann NY Acad Sci 51:980–981
Stansly PG (1948) Studies on polymyxin: an assay method for blood and urine. Proc Soc Exp Biol Med 68:301–304
Craig WA, Kunin CM (1973) Dynamics of binding and release of the polymyxin antibiotics by tissues. J Pharmacol Exp Ther 184(3):757–765
Ziv G, Schultze WD (1982) Pharmacolunetics of polymyxin B administered via the bovine mammary gland. J Vet Pharmacol Ther 5:123–129
Ziv G, Nouws JF, van Ginneken CA (1982) The pharmacokinetics and tissue levels of polymyxin B, colistin and gentamicin in calves. J Vet Pharmacol Ther 5(1):45–58
Ziv C, Wanner M, Nicolet J (1980) Clinical pharmacology of polymyxin B, colistin and colistimethate in young calves. J Vet Pharmacol Ther 3:87–94
Wootton M, Holt HA, MacGowan AP (2005) Development of a novel assay method for colistin sulphomethate. Clin Microbiol Infect 11:243–244
Bergen PJ, Li J, Rayner CR, Nation RL (2006) Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50(6):1953–1958
Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K (2003) Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother 47(4):1364–1370
Healy DP, Sombun AD, Gardner JC, Good K, Durkee PJ, Toner L, Rieman MT, Neely AN, Kagan RJ (2011) Pharmacokinetics of colistin in an adolescent boy with extensive burn injury. J Burn Care Res 32(1):e7–e11
Jimenez-Mejias ME, Pichardo-Guerrero C, Marquez-Rivas FJ, Martin-Lozano D, Prados T, Pachon J (2002) Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant acinetobacter baumannii meningitis. Eur J Clin Microbiol Infect Dis 21(3):212–214
Aoki N, Tateda K, Kikuchi Y, Kimura S, Miyazaki C, AIshii Y, Tanabe Y, Gejyo F, Yamaguchi K (2009) Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J Antimicrob Chemother 63(3):534–542
Hengzhuang W, Wu H, Ciofu O, Song Z, Hoiby N (2012) In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob Agents Chemother 56(5):2683–2690
Lin B, Zhang C, Xiao X (2005) Toxicity, bioavailability and pharmacokinetics of a newly formulated colistin sulfate solution. J Vet Pharmacol Ther 28(4):349–354
Sarria JC, Angulo-Pernett F, Kimbrough RC, McVay CS, Vidal AM (2004) Use of intravenous polymyxin B during continuous venovenous hemodialysis. Eur J Clin Microbiol Infect Dis 23:340–341
Conway SP, Pond MN, Watson A, Etherington C, Robey HL, Goldman MH (1997) Intravenous colistin sulphomethate in acute respiratory exacerbations in adult patients with cystic fibrosis. Thorax 52:987–993
Hermsen ED, Sullican CJ, Rotschafer JC (2003) Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications. Infect Dis Clin North Am 17:545–562
Le Brun PP, de Graaf AI, Vinks AA (2000) High-performance liquid chromatographic method for the determination of colistin in serum. Ther Drug Monit 22(5):589–593
Decolin D, Leroy P, Nicolas A, Archimbault P (1997) Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J Chromatogr Sci 35(12):557–564
Reed MD, Stern RC, O’Riordan MA, Blumer JL (2001) The pharmacokinetics of colistin in patients with cystic fibrosis. J Clin Pharmacol 41(6):645–654
Guyonnet J, Manco B, Baduel L, Kaltsatos V, Aliabadi MH, Lees P (2010) Determination of a dosage regimen of colistin by pharmacokinetic/pharmacodynamic integration and modeling for treatment of G.I.T. disease in pigs. Res Vet Sci 88(2):307–314
Gmur DJ, Bredl CR, Steele SJ, Cai S, VanDevanter DR, Nardella PA (2003) Determination of polymyxin E1 in rat plasma by high-performance liquid chromatography. J Chromatogr B 789(2):365–372
Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J (2003) Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother 52(6):987–992
Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K (2004) Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother 53(5):837–840
Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ, Nation RL, McIntosh MP (2014) Pulmonary and systemic pharmacokinetics of Inhaled and Intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother 58(8):2570–2579
Koomanachai P, Landersdorfer CB, Chen G, Lee HJ, Jitmuang A, Wasuwattakul S, Sritippayawan S, Li J, Nation RL, Thamlikitkula V (2014) Pharmacokinetics of colistin methanesulfonate and formed colistin in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother 58(1):440–446
Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL (2011) Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55(7):3284–3294
Dudhani RV, Turnidge J, Coulthard K, Milne R, Rayner CR, Li J, Nation RL (2010) Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother 54(3):1117–1124
Yapa SWS, Li J, Porter CJ, Nation RL, Patel K, McIntosh MP (2013) Population pharmacokinetics of colistin methanesulfonate in rats: achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrob Agents Chemother 57(10):5087–5095
He H, Li JC, Nation RL, Jacob J, Chen G, Lee HJ, Tsuji BT, Thompson PE, Roberts KD, Velkov T, Li J (2013) Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother 68(10):2311–2317
Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW (2008) Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother 61(3):636–642
Jin L, Li J, Nation RL, Nicolazzo JA (2009) Brain penetration of colistin in mice assessed by a novel high-performance liquid chromatographic technique. Antimicrob Agents Chemother 53(10):4247–4251
Jin L, Li J, Nation RL, Nicolazzo JA (2012) Effect of systemic infection induced by Pseudomonas aeruginosa on the brain uptake of colistin in mice. Antimicrob Agents Chemother 56(10):5240–5246
Yousef JM, Chen G, Hill PA, Nation RL, Li J (2012) Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother 67(2):452–459
Yousef JM, Chen G, Hill PA, Nation RL, Li J (2011) Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother 55(9):4044–4049
Cao G, Ali F, Chiu F, Alexandre P, Zavascki A, Nation R, Li J (2008) Development and validation of a reversed-phase high-performance liquid chromatography assay for polymyxin B in human plasma. J Antimicrob Chemother 62(5):1009–1014
Ali FE, Cao G, Poudyal A, Vaara T, Nation RL, Vaara M, Li J (2009) Pharmacokinetics of novel antimicrobial cationic peptides NAB 7061 and NAB 739 in rats following intravenous administration. J Antimicrob Chemother 64(5):1067–1070
Zavascki AP, Li J, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL (2008) Pharmacokinetics of intravenous polymyxin B in critically-ill patients. Clin Infect Dis 47:1298–1304
Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP (2013) Population pharmacokinetics of intravenous polymyxin B in critically ill patients: Implications for selection of dosage regimens. Clin Infect Dis 57:524–531
Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Saitovitch D, Wang J, Forrest A, Nation RL, Zavascki AP, Li J (2013) Pharmacokinetics of polymyxin B in patients on continuous venovenous haemodialysis. J Antimicrob Chemother 68(3):674–677
Markou N, Markantonis SI, Dimitrakis E, Panidis D, Boutzouka E, Karatzas S, Rafailidis P, Apostolakos H, Baltopoulos G (2008) Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: A prospective, open-label, uncontrolled study. Clin Ther 30(1):143–151
Markantonis SL, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, Dimopoulou E, Baltopoulos G (2009) Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother 53(11):4907–4910
Ziaka M, Markantonis SL, Fousteri M, Zygoulis P, Panidis D, Karvouniaris M, Makris D, Zakynthinos E (2013) Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob Agents Chemother 57(4):1938–1940
Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M (2010) Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 138(6):1333–1339
Athanassa ZE, Markantonis SL, Fousteri MZ, Myrianthefs PM, Boutzouka EG, Tsakris A, Baltopoulos GJ (2012) Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 38(11):1779–1786
Zeng Z, Wu J, Yang G, Chen Z, Huang X, Ding H (2010) Study of colistin depletion in duck tissues after intramuscular and oral administration. J Vet Pharmacol Ther 33(4):408–410
He J, Tang S, Li L, Zhang C, Li X, Xia X, Xiao X (2011) Pharmacokinetics of a novel amoxicillin/colistin suspension after intramuscular administration in pigs. J Vet Pharmacol Ther 34(1):42–50
Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H (2006) Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother 57(2):306–311
Dotsikas Y, Markopoulou CK, Koundourellis JE, Loukas YL (2011) Validation of a novel LC-MS/MS method for the quantitation of colistin A and B in human plasma. J Sep Sci 34(1):37–45
Ma Z, Wang J, Gerber JP, Milne RW (2008) Determination of colistin in human plasma, urine and other biological samples using LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 862(1–2):205–212
Sin DW, Ho C, Wong Y, Ho S, Ip AC (2005) Analysis of major components of residual bacitracin and colistin in food samples by liquid chromatography tandem mass spectrometry. Anal Chim Acta 535(1–2):23–31. https://doi.org/10.1016/j.aca.2004.11.063
Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H (2009) Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 53(8):3430–3436
Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE (2012) Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 56(8):4241–4249
Karvanen M, Plachouras D, Friberg LE, Paramythiotou E, Papadomichelakis E, Karaiskos I, Tsangaris I, Armaganidis A, Cars O, Giamarellou H (2013) Colistin methanesulfonate and colistin pharmacokinetics in critically ill patients receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 57(1):668–671
Leporati M, Ornella Bua RO, Mariano F, Carignano P, Stella M, Biancone L, Marco Vincenti M (2014) Determination by LC–MS/MS of Colistins A and B in plasma and ultrafiltrate from critically ill patients undergoing continuous venovenous hemodiafiltration. Ther Drug Monit 36:182–191
Gikas E, Bazoti FN, Katsimardou M, Anagnostopoulos D, Papanikolaou K, Inglezos I, Skoutelis A, Daikos GL, Tsarbopoulos A (2013) Determination of colistin A and colistin B in human plasma by UPLC–ESI high resolution tandem MS: application to a pharmacokinetic study. J Pharm Biomed Anal 83:228–236
Zhao M, Wu XJ, Fan YX, Guo BN, Zhang J (2016) Development and validation of a UHPLC–MS/MS assay for colistin methanesulphonate (CMS) and colistin in human plasma and urine using weak-cation exchange solid-phase extraction. J Pharm Biomed Anal 124:303–308
Cheng C, Liu S, Xiao D, Hollembaek J, Yao L, Lin J, Hansel S (2010) LC–MS/MS method development and validation for the determination of polymyxins and vancomycin in rat plasma. J Chromatogr B 878(28):2831–2838
Mercier T, Tissot F, Gardiol C, Corti N, Wehrli S, Guidi M, Csajka C, Buclin T, Couet W, Marchetti O, Decosterd LA (2015, 1369) High-throughput hydrophilic interaction chromatography coupled to tandem mass spectrometry for the optimized quantification of the anti-Gram-negatives antibiotic colistin A/B and its pro-drug colistimethate. J Chromatogr A:52–63
Thomas TA, Broun EC, Abildskov KM, Kubin CJ, Horan J, Yin MT, Cremers S (2012) High performance liquid chromatography–mass spectrometry assay for polymyxin B1 and B2 in human plasma. Ther Drug Monit 34:398–405
Kwa AL, Lim TP, Low JG, Hou J, Kurup A, Prince RA, Tam VH (2008) Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn Microbiol Infect Dis 60(2):163–167
Abdelraouf K, He J, Ledesma KR, Hu M, Tam VH (2012) Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob Agents Chemother 56(11):5724–5727
He J, Abdelraouf K, Ledesma KR, Chow DS-L, Tam VH (2013) Pharmacokinetics and efficacy of liposomal polymyxin B in a murine pneumonia model. Int J Antimicrob Agents 42(6):559–564
Tam VH, Cao H, Ledesma KR, Hu M (2011) In vitro potency of various polymyxin B components. Antimicrob Agents Chemother 55(9):4490–4491
He J, Gao S, Hu M, Chow DS-L, Tam VH (2013) A validated ultra-performance liquid chromatography–tandem mass spectrometry method for the quantification of polymyxin B in mouse serum and epithelial lining fluid: application to pharmacokinetic studies. J Antimicrob Chemother 68:1104–1110
Wallace SJ, Li J, Rayner CR, Coulthard K, Nation RL (2008) Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob Agents Chemother 52(9):3047–3051
Wallace SJ, Li J, Nation RL, Prankerd RJ, Velkov T, Boyd BJ (2010) Self-assembly behavior of colistin and its prodrug colistin methanesulfonate: implications for solution stability and solubilization. J Phys Chem B Condens Matter Mater Surf Interfaces Biophysical 114(14):4836–4840
Karvanen M (2013) Optimization of colistin dosage in the treatment of multiresistant gram-negative infections. Doctoral thesis, Uppsala Universitet, Uppsala
Cheah S-E, Jurgen B, Bulitta JB, Li J, Nation RL (2014) Development and validation of a liquid chromatography–mass spectrometry assay for polymyxin B in bacterial growth media. J Pharm Biomed Anal 92:177–182
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Milne, R.W. (2019). Bioanalysis and Stability of Polymyxins. In: Li, J., Nation, R., Kaye, K. (eds) Polymyxin Antibiotics: From Laboratory Bench to Bedside. Advances in Experimental Medicine and Biology, vol 1145. Springer, Cham. https://doi.org/10.1007/978-3-030-16373-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-16373-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16371-6
Online ISBN: 978-3-030-16373-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)