Abstract
Polymyxin-induced nephrotoxicity is the major dose-limiting factor and can occur in up to 60% of patients after intravenous administration. This chapter reviews the latest literature on the mechanisms of polymyxin-induced nephrotoxicity and its amelioration. After filtration by glomeruli, polymyxins substantially accumulate in renal proximal tubules via receptor-mediated endocytosis mainly by megalin and PEPT2. It is believed that subsequently, a cascade of interconnected events occur, including the activation of death receptor and mitochondrial apoptotic pathways, mitochondrial damage, endoplasmic reticulum stress, oxidative stress and cell cycle arrest. The current literature shows that oxidative stress plays a key role in polymyxin-induced kidney damage. Use of antioxidants have a potential in the attenuation of polymyxin-induced nephrotoxicity, thereby widening the therapeutic window. Mechanistic findings on polymyxin-induced nephrotoxicity are critical for the optimization of their use in the clinic and the discovery of safer polymyxin-like antibiotics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
As reviewed in Chap. 17, the incidence of polymyxin-associated nephrotoxicity is up to 60% in patients with the currently recommended dosage regimens [1,2,3,4,5,6,7]. Recent pharmacological studies have indicated that polymyxin-associated nephrotoxicity is the major dose-limiting adverse effect after parenteral administration [8,9,10,11,12,13,14,15,16] (also Chap. 15). The key features of polymyxin-associated nephrotoxicity include acute tubular damage, decreased creatinine clearance (CrCL), and increased serum urea and creatinine concentrations [9] (also Chap. 17). This chapter focuses on the latest progress in understanding the mechanisms of polymyxin-associated nephrotoxicity.
18.1 Renal Disposition of Polymyxins
18.1.1 Differential Renal Handling of Colistin, Polymyxin B and CMS
Although both colistin and polymyxin B are available for clinical use , they differ in their forms for parenteral administration. Polymyxin B is available as the sulfate salt, whereas colistin is available as the prodrug colistimethate sodium (CMS). After intravenous administration of colistin (sulfate) in rats, the urinary recovery of colistin was less than 1% of the administered dose [17, 18]. In comparison to its anticipated clearance by glomerular filtration (2.3 mL/min/kg), the much lower renal clearance of colistin (0.010 ± 0.008 mL/min/kg) indicates extensive tubular reabsorption in rats [17]. In contrast, the urinary recovery of CMS (as CMS and formed colistin in the kidney and urinary tract) after intravenous administration was approximately 60–70% in rats [19,20,21] and humans [22,23,24]. The greater renal clearance of CMS compared to its anticipated clearance by glomerular filtration indicates net tubular secretion into the urine [19]. As the major structural difference between colistin and CMS is due to the modification of the primary amines of colistin with negatively charged methanesulfonate groups in CMS, the significantly different renal handling and urinary recovery of colistin and CMS are due to the different charges of the two chemical entities. Similar to colistin, very low urinary recovery of polymyxin B following intravenous administration also suggests that non-renal elimination predominates in both rodents [22,23,24] and humans [18, 25,26,27]. Indeed, it has been suggested that polymyxin B undergoes very extensive tubular reabsorption in patients [27, 28].
The very different renal disposition of colistin/polymyxin B and CMS is illustrated in Fig. 18.1. The extensive reabsorption of colistin and polymyxin B from glomerular filtrate to peritubular capillaries would expose tubular cells to high concentrations of these molecules. The net tubular secretion [19] of CMS from peritubular capillaries into the tubular lumen through the epithelial tubular cells may result in intracellular conversion of CMS to colistin [19]. This may enhance the exposure of tubular cells to colistin [29]. In summary, the difference in renal excretion mechanisms of CMS and formed colistin versus polymyxin B is an important factor to modulate the exposure of renal tubular cells to polymyxins and the degree of nephrotoxicity following intravenous polymyxin treatments.
Schematic representation of the renal disposition of (a) CMS and formed colistin and (b) colistin/polymyxin B. Thickness of the arrows corresponds to the magnitude of the processes involved in the renal deposition. (Figure adapted from Zavascki et al. [29]. Permission obtained from the American Society of Microbiology [ASM])
18.1.2 Significant Accumulation of Polymyxins in Renal Tubular Cells
Several recent studies have revealed significant renal accumulation of polymyxins using immunostaining, mass spectrometry imaging, fluorescence microscopy and X-ray fluorescence microscopy (XFM) [30,31,32,33,34,35,36]. As CMS is not stable and is a very complex mixture of numerous methanesulfonated derivatives [37,38,39,40], its disposition in renal tubular cells has not been examined and the studies in the literature employed colistin, polymyxin B or novel polymyxin analogues. In a mouse study, the distribution of polymyxin B in the kidney tissue was examined after intravenous administration using immunostaining with a polymyxin-specific monoclonal antibody [32]. Predominant accumulation of polymyxin B was evident in the renal cortex, in particular the renal proximal tubular cells, but much less in the distal tubular cells (Fig. 18.2) [30, 32, 41]. Furthermore, matrix-assisted laser desorption/ionizing mass spectroscopy (MALDI-MS) imaging revealed that following subcutaneous administration polymyxins largely accumulated in the renal cortex (Fig. 18.2), but not in the lungs, liver or heart [41].
(a) Immunostaining demonstrates the distribution of polymyxin B within mouse kidneys following subcutaneous administration (Insert: 10× magnification of the cortex and medulla) [32]. (b) Targeted MALDI-MS imaging for detecting polymyxin B1 and B2 as Na+ adduct in the kidneys of mice treated with polymyxin B [41]. (Permission obtained from Oxford University Press)
Abdelraouf et al. employed a commercial product, boron-dipyrromethene (BODIPY)-polymyxin B to examine the uptake of polymyxin B by mammalian renal tubular cells (LLC-PK1) [42]. Saturable uptake of polymyxin B into LLC-PK1 cells suggested transporter-mediated uptake of polymyxin B. However, it is important to note that commercially available fluorescent polymyxin probes (e.g. dansyl-polymyxin B and BODIPY-polymyxin B) are devoid of the pharmacological activities of native polymyxins, due to the attachment of relatively large BODIPY or dansyl moieties on the amine groups of the five Dab residues in the polymyxin structure [30, 43,44,45]. The structure-activity relationship (SAR) of polymyxins should be considered when using polymyxin probes for pharmacological research.
Using synchrotron X-ray fluorescence (XFM), fluorescence, and scanning electron microscopy, a recent correlative microscopic study discovered the extraordinary accumulation of polymyxins in rat (NRK-52E) and human (HK-2) kidney proximal tubular cells [30]. Based upon the polymyxin SAR model [45], a novel dual-module fluorescent probe, FADDI-096, was designed, consisting of a dansyl group in the N-terminus and an iodine fluorophore at position 6 D-Phe of polymyxin B (Fig. 18.3). Unlike the commercially available fluorescent polymyxin probes BODIPY-polymyxin B and dansyl-polymyxin B, FADDI-096 has the structural features required for the biological activity of natural polymyxins. For example, similar to polymyxin B, FADDI-096 displayed antibacterial activity (MIC 8 mg/L against P. aeruginosa ATCC 27853) and the ability to induce oxidative stress in both NRK-52E and HK-2 cells [30]. Therefore, it is a valid probe to investigate the nephrotoxicity of polymyxins.
Structures of (a) polymyxin B1, (b) FADDI-096, and the molecular model of FADDI-096 with Escherichia coli Kdo2-Lipid A [30]. (Permission obtained from ACS Publications)
Quantitative mapping of polymyxin distributions in single rat (NRK-52E) and human (HK-2) kidney tubular cells revealed that the remarkable intracellular accumulation of FADDI-096 was both concentration- and time-dependent (Fig. 18.4). With the extracellular concentrations of 5 and 50 μM, intracellular concentrations of FADDI-096 were approximately 1,930- to 4,760-fold higher in NRK-52E cells at 1 and 4 h, respectively. Consistent with the XFM imaging results, the significant intracellular accumulation of FADDI-096 was also observed in the same cells using fluorescence microscopy (Fig. 18.4). These correlative microscopy results demonstrate the overlap of the dansyl and iodine signals from FADDI-096 itself. While FADDI-096 concentrations in the bathing solution increased tenfold (i.e. 5 vs 50 μM), its intracellular concentrations (23.8 ± 6.63 mM vs 110 ± 28.2 mM, respectively) only increased approximately 4.62-fold in NRK-52E cells. This finding indicated that the significant accumulation of polymyxins by NRK-52E cells was saturable and likely carrier-mediated [46]. In HK-2 cells, the intracellular concentration of FADDI-096 (31.0 ± 5.69 mM) was approximately 3,100-fold higher than the concentration in the bathing solution (10 μM for 4 h). Interestingly, the XFM results also revealed a significant increase in the intracellular calcium concentration, which is a potential stimulus to trigger apoptosis [47]. No correlation was observed between the localization of FADDI-096 and other elements including phosphorus and sodium [30].
Single-cell correlative microscopy results demonstrate the accumulation of FADDI-096 in NRK-52E and HK-2 cells [30]. (a) Fluorescence images of NRK-52E cells (i) without treatment, (ii) treated with 5 μM FADDI-096 for 4 h, (iii) treated with 50 μM FADDI-096 for 1 h, (iv) treated with 50 μM FADDI-096 for 4 h; and HK-2 cells (v) without treatment, (vi) treated with 10 μM FADDI-096 for 4 h. (b) Iodine distribution within the same NRK-52E and HK-2 cells as shown in panel A; iodine concentrations (μg/cm2) are shown using a linear scale from zero to the maximum value; the yellow numbers note the maximum iodine concentration in each sample. (c) SEM images of the same NRK-52E and HK-2 cells identified in panel A. (d) Correlation of signals from fluorescence microscopy (i: Green), XFM (ii: Blue to red), and surface morphology from SEM (iii: Grey); and their superposition. (e) Accumulation of FADDI-096 in single NRK-52E and HK-2 cells measured via iodine content using XFM as shown in panel A (mean ± SD; n = 10). Scale bar: 10 μm. (Permission obtained from ACS Publications)
Collectively, the immunostaining, mass spectrometry imaging and XFM results all demonstrate the very substantial uptake of polymyxins by renal tubular cells and the potential involvement of transporters; these results are consistent with the pharmacokinetic findings from rats and humans [17, 28, 29]. Further investigations are required to elucidate the detail mechanisms of polymyxin accumulation in renal tubular cells.
18.1.3 Roles of Transporters in the Uptake of Polymyxins by Kidney Tubular Cells
The significant accumulation of polymyxins in renal tubular cells indicates that transporters play an important role in the uptake of colistin and polymyxin B in kidneys [30, 48, 49]. Different transport mechanisms exist in the elimination of drugs, toxins, and endogenous compounds by kidney tubular cells [50,51,52,53,54,55,56,57]. Megalin is a key endocytic receptor for reabsorption of the proteins and small bioactive molecules present in the glomerular filtrate [58], and has been demonstrated to mediate the significant reabsorption of polymyxins by renal tubular cells [46, 59, 60]. Moreover, colistin displays competitive inhibition for binding to megalin with cytochrome c (a known substrate for megalin) [46]. In megalin-shed rats, decreased accumulation of colistin in the kidneys and increased excretion in urine suggest that megalin is important for the reabsorption of colistin by tubular cells [46]. Co-administration of colistin with cytochrome c or fragment of albumin (FRALB) caused a decreased urinary excretion of N-acetyl-β-D-glucosaminidase (NAG), a marker of tubular damage; this suggested the prevention of colistin-induced tubular damage by blocking megalin-mediated uptake [46]. The key role of megalin in the reabsorption of polymyxins is also supported by the finding that co-administration of colistin with succinylated bovine gelatin polypeptides (known competitive inhibitors of the reabsorption of peptide and protein substrates of megalin) decreased both the accumulation of colistin in kidney tissue and also its nephrotoxic effect in a murine model [49]. It has been shown in both in vitro and in vivo models that inhibition of megalin supressed the colistin-induced damage to renal tubular cells [60].
Many antibiotics are organic acids or bases and, depending on their pKa values, are present as anions or cations in the physiological environment. Recently, carrier-mediated renal tubular reabsorption of colistin has been suggested from studies conducted ex vivo [61]. Using isolated perfused rat kidney, Ma et al. examined the renal disposition and the potential role of kidney transporters in the disposition of colistin [61]. A considerable amount of colistin (administered as colistin sulfate) was removed from the perfusate, but only a relatively low proportion (<10%) was ultimately excreted into the urine, indicating the accumulation of colistin in the kidney tissue [61]. The extensive reabsorption of colistin was inhibited by tetraethylammonium (TEA, a typical substrate of rat OCTN1 [62]), glycine-glycine (Gly-Gly), and hydrochloric acid, suggesting that the renal reabsorption of colistin was mediated by organic transporters and peptide transporters (e.g. OCTN1 and OCTN2) and might be sensitive to the pH of urine [61]. Since colistin is a peptide and the di-peptide Gly-Gly is a typical substrate/inhibitor for PEPT [63], the isolated perfused rat kidney results suggest that colistin might undergo reabsorption via polypeptide transporters (PEPT1 and PEPT2) in the renal tubular cells [61].
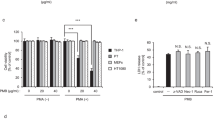
A recent study systematically investigated the inhibitory effects of colistin and polymyxin B on the substrate uptake mediated through 15 essential solute carrier transporters (SLCs) in over-expressing HEK293 cells [64]. Both polymyxins had no or only very mild inhibitory effect on the transport activity of the SLCs examined, except human peptide transporter 2 (PEPT2). The concentrations of colistin and polymyxin B required to inhibit 50% uptake (IC50) of the specific human PEPT2 substrate [3H]glycyl-sarcosine were 11.4 ± 3.1 and 18.3 ± 4.2 μM, respectively (Fig. 18.5). PEPT2 is a key SLC expressed particularly in the kidneys and brain [64]. It is a low-capacity high-affinity proton-coupled cotransporter, mainly involved in the renal reabsorption of peptides and peptide-like substrates (including drugs) to maintain systemic nitrogen homeostasis [65]. [3H]Polymyxin B1 and a fluorescent polymyxin probe MIPS-9541 were also employed as a complementary approach to examine the cellular uptake by PEPT2. The results revealed a significant inhibition of PEPT2-mediated uptake by glycyl-sarcosine, colistin or polymyxin B [64]. Collectively, it is very likely that PEPT2 also plays a critical role in the renal tubular accumulation of polymyxins.
Inhibitory effect of polymyxins on PEPT2-mediated uptake of [3H]Gly-Sar [64]. Cellular uptake of [3H]Gly-Sar was measured in the absence or presence of (a) colistin and (b) polymyxin B. (c) Inhibition of MIPS-9541 uptake by Gly-Sar, colistin or polymyxin B in PEPT2 transfected HEK293 cells. (Permission obtained from the Oxford University Press)
18.1.4 Localisation of Polymyxins in Renal Tubular Cells
There is limited information on the co-localisation of polymyxins with different organelles in renal tubular cells. By incorporating a single dansyl fluorophore in the hydrophobic regions of the polymyxin core structure, we designed, synthesised, and evaluated four novel regioselectively labeled monodansylated polymyxin B probes (MIPS-9541, MIPS-9542, MIPS-9543, and MIPS-9544) for intracellular localisation studies [31]. We examined their antimicrobial activities, cellular uptake, and apoptotic effects on NRK-52E cells. It became evident that incorporation of a dansyl group at position 6 or 7 (e.g. MIPS-9543 and MIPS-9544) of polymyxins is appropriate for generating fluorescent polymyxin probes for intracellular imaging and mechanistic studies. Confocal fluorescence imaging experiments conducted with MIPS-9543 and MIPS-9544 reveal partial co-localisation of polymyxins with both endoplasmic reticulum and mitochondria in NRK-52E cells. Super-resolution imaging is required to elucidate the intracellular localisation of polymyxins in renal tubular cells and the toxic effect on subcellular organelles [31].
In summary, the accumulation, intra-cellular trafficking and localisation of polymyxins in renal tubular cells have not been fully elucidated, and the mechanistic findings may lead to novel approaches to attenuate polymyxin-induced nephrotoxicity.
18.2 Effects of Polymyxins on Renal Tubular Cells
The execution of renal tubular cell death is usually highly orchestrated and interconnected between cell cycle , apoptosis , necrosis and autophagy [66,67,68]. Depending on the insult and stimulus, tubular cell death can simultaneously trigger multiple pathways and lead to the activation of common downstream cascades [69, 70]. The current literature shows that polymyxin treatment can cause cell cycle arrest, apoptosis and autophagy in renal tubular cells in vitro and in vivo .
18.2.1 Polymyxins Induce Cell Cycle Arrest
Eadon et al. reported that cell cycle arrest is associated with colistin-induced nephrotoxicity in a murine model using microarray [71]. C57/BL6 mice were intraperitoneally administered with saline or 16 mg/kg/day colistin (in two divided doses), and kidneys were collected after 3 and 15 days. Gene expression microarray analysis of kidney tissues identified 21 differentially expressed genes during the colistin treatment. Up-regulation of the differentially expressed genes from both microarray and RT-PCR results suggested that the cellular injury induced by colistin was mediated through p53 pathway to inhibit cell cycle progression. Up-regulation of CCNB1, CDC2 and the indifferent expression of CDK2, CCND, CCNE genes following colistin treatment indicated G2/M as the point of arrest in the cell cycle. Moreover, translocation of cyclin B1 to the nucleus is another indicator of cell cycle arrest at the G2/M phase induced by colistin [71]. It was also demonstrated that the expression of galectin-3 was up-regulated, supporting the cell cycle arrest through G1/S and G2/M [71]. The up-regulation of galectin-3 is potentially an early marker of the colistin-induced kidney injury. The detection of the proliferating cell nuclear antigen following exposure to colistin for 3 days indicates the emergence of subclinical kidney injury through the blockade of DNA replication at S phase, and subtle pathogenic injury was also observed. Cell cycle arrest may represent a protective mechanism for recovering from colistin-induced nephrotoxicity. However, activation of p53 and galectin-3 can also lead to the apoptotic cell death if the cellular damage is non-recoverable [72, 73]. We examined polymyxin-induced cell death in HK-2 cells and a mouse model using biochemical and molecular approaches. Interestingly, our results indicate the association of DNA damage with polymyxin B induced nephrotoxicity, leading to chromosome mis-segregation and genome instability [74, 75]. There is still much to be learned on polymyxin-induced nephrotoxicity and systems investigations are required to elucidate the complex interplay of major biochemical pathways in polymyxin-induced toxicity in renal tubular cells .
18.2.2 Polymyxins Induce Apoptosis and Oxidative Stress In Vitro and In Vivo
Recent studies revealed that colistin-induced renal tubular apoptosis in vitro and in animals [48, 76, 77]. After colistin treatment (cumulative dose of 20.5 mg/kg over 5 days) in rats, Yousef et al., discovered in the kidneys increased TUNEL positive nuclei (%) and fragmentation of DNA, a biochemical hallmark of apoptosis (Fig. 18.6) [48]. Similar results were observed in rat proximal tubular cells (NRK-52E) treated with colistin (0.1 mM for 24 h). Dai et al. revealed the involvement of the death receptor, mitochondrial and endoplasmic reticular pathways in colistin-induced apoptosis in mouse kidney tissues [78]. Colistin was intravenously administered to mice (7.5 or 15 mg of colistin/kg/day in two doses) for 7 days. After 7 days, a significant decrease of Bcl-2 and a concomitant increase of Cytc, AIF, cleaved caspase-9 and cleaved caspase-3 were observed. These findings confirmed that both mitochondria-dependent and -independent pathways are involved in colistin-induced apoptosis in mouse kidneys [78]. Furthermore, significantly increased expression of Fas, FasL, and FADD, and cleavage of caspase-8 were also revealed in the colistin-treated mouse kidneys, demonstrating the involvement of death receptor mediated pathway in colistin-induced apoptosis [78]. Interestingly, the increased expression of tBid indicated the cross-talk between the death receptor and mitochondria apoptotic pathways. In addition, significantly increased concentrations of Grp78/Bip, cleaved ATF6, GADD153/CHOP and caspase-12 were observed in mice following colistin treatment, suggesting that the endoplasmic reticulum pathway is also involved in colistin-induced apoptosis. To date, it is still unknown how each apoptosis pathway is triggered and the interplay among them.
TUNEL positive nulcei (black arrows) after immunohistochemical staining in kidney sections of rats treated for 5 days with (a) saline and (b) colistin (cumulative dose of 20.5 mg/kg). (Figure modified from Yousef et al. [48] and permission obtained from Oxford University Press)
Using cell culture, the activation of caspase-3/8/9, DNA damage and translocation of membrane phosphatidylserine following polymyxin B treatment has been demonstrated in rat (NRK-52E) kidney tubular cells (Fig. 18.7) [79]. In NRK-52E cells treated with polymyxin B (1.0 mM for 24 h), positive labelling with the caspase substrate Red-VAD-FMK showed the presence of activated caspase-3, 8 and 9. Polymyxin-induced apoptosis in NRK-52E cells was also confirmed by positive labelling TUNEL assay and annexin V-PI double staining. Polymyxin-induced apoptosis was both concentration- and time-dependent in NRK-52E and HK-2 cells. Interestingly, HK-2 cells displayed higher sensitivity to polymyxin B induced toxicity than NRK-52E cells [79]. Mingeot-Leclercq et al. and Vaara et al. also demonstrated dose-dependent cytotoxic activity of polymyxins in porcine renal proximal tubular cells (LLC-PK1) and HK-2 cells, respectively [7, 80].
Double staining with annexin V and PI in NRK-52E cells [79]. (a) Control cells. (b) Cells treated with 1.25 mM polymyxin B for 24 h. (c) Cells treated with 1.0 μM staurosporine. In each panel, the upper left quadrant represents cells stained by annexin V (early-apoptotic cells), the bottom right quadrant represents cells stained by PI (necrotic cells), the upper right quadrant represents cells stained by both annexin V and PI (late-apoptotic cells), and the bottom left quadrant represents cells not stained by annexin V or PI (viable cells). (d–f) Viability data for panels A to C. (d) Control cells. (e) Cells treated with 1.25 mM polymyxin B. (f) Cells treated with 1.0 μM staurosporine. The error bars represent SD. (Permission obtained from the American Society of Microbiology [ASM])
The relative toxic effect of polymyxin B1, polymyxin B2, colistin A and colistin B were examined in HK-2 cells and mice [81]. Comparable nephrotoxicity was observed in mice with mild to moderate histological damage; however, polymyxin B1 and colistin A showed >3-fold higher in vitro apoptotic effect on HK-2 cells than polymyxin B2 and colistin B, respectively. As there is only one carbon difference in the N-terminal fatty acyl group between the two major components of polymyxin B and colistin (Fig. 1.6), these results indicate that the hydrophobicity of the N-terminal fatty acyl group of polymyxins plays an important role in polymyxin-induced apoptosis. As shown in Fig. 1.6, the only difference between polymyxin B1 and colistin A (also polymyxin B2 and colistin B) is position 6 (i.e. D-Phe versus D-Leu); therefore, the hydrophobicity at position 6 is also important to the toxicity on renal tubular cells [81]. The lack of differences in their in vivo nephrotoxicity may be due to the sensitivity of the mouse model or the slightly different PK of the two major components of both polymyxins [17, 18].
Mitochondrial stress occurred during polymyxin-induced apoptosis in NRK-52E cells (Fig. 18.8) [82]. In healthy rat kidney tubular cells NRK-52E, mitochondria predominantly were filamentous, whereas in cells undergoing apoptotic cell death mitochondria became fragmented. Concentration- and time-dependent transitions of the mitochondrial morphology from the filamentous (regular) to fragment (stressed) were observed in NRK-52E cells following polymyxin B treatment (1.0 and 2.0 mM up to 24 h) [82]. A concentration-dependent perturbation of mitochondrial morphology was associated with the loss of mitochondrial membrane potential (Δψ). Furthermore, it was also evident that polymyxin B induced toxicity was associated with the generation of reactive oxygen species (ROS). Our recent metabolomic study discovered the perturbation of taurine-hypotaurine pathway in polymyxin-treated kidney HK-2 and NRK-52E cells, indicating a loss of cellular capacity to scavenge ROS [41].
(a) Loss of mitochondrial membrane potential measured by fluorescence microscopy using tetramethylrhodamine ethyl ester in NRK-52E cells treated with polymyxin B (0.25, 0.5 and 1.0 mM for 24 h). (b–c) Polymyxin B treatments caused concentration- and time-dependent production of mitochondrial superoxide in NRK-52E cells [82]. (Permission obtained from the American Society of Microbiology [ASM])
Collectively, a working model (Fig. 18.9) was proposed based on the recent literature to understand the complex mechanism of polymyxin-induced apoptosis in renal tubular cells [78]. The precise mechanisms of polymyxin-induced nephrotoxicity remain unknown and require further studies.
Schematic diagram of the proposed mechanisms of polymyxin-induced apoptosis [78]. (Permission obtained from the American Society of Microbiology [ASM])
18.3 Amelioration of Polymyxin-Induced Nephrotoxicity
Current efforts to minimise the incidence and impact of polymyxin-associated nephrotoxicity in patients rely on monitoring of renal function and electrolyte balance, avoidance of concurrent nephrotoxic agents (if feasible) and optimization of the polymyxin dose [24]. These have been discussed in Chap. 17 and in the literature [29]. Significant efforts have been made over the last decade to attenuate polymyxin-induced nephrotoxicity using different approaches, including decreasing the uptake by renal tubular cells, attenuating polymyxin-induced oxidative stress , and modifying the polymyxin structure (Chap. 20) [45, 48, 49, 61, 76, 77, 83, 84].
A number of animal studies investigated the potential role of co-administered agents to ameliorate polymyxin-induced nephrotoxicity; the majority of these studies involved antioxidants. Ozyilmaz et al., demonstrated that N-acetylcysteine (NAC) ameliorated polymyxin-induced oxidative stress and nephrotoxicity in rats [76]. Yousef et al., reported decreased excretion of urinary NAG and less histopathological damage in rat kidneys following co-administration of ascorbic acid (50 or 200 mg/kg) with colistin (cumulative dose, 36.5 mg/kg), compared to rats treated with colistin or ascorbic acid alone [48]. Similar results have been reported with the co-administration of melatonin, polyaspartic acid, grape seed extract, and methionine [77, 83,84,85]. Methionine (100 or 400 mg/kg co-administered) protected against polymyxin-induced kidney damage in mice (polymyxin B 35 mg/kg, twice daily over 3.5 days) and significantly attenuated mitochondrial oxidative stress in NRK-52E cells [84]. Interestingly, the pharmacokinetics of polymyxin B in rats were not affected by co-administration of methionine [84]. Ozkan et al., also reported that colistin-induced oxidative stress and apoptosis in rat kidney tissues were attenuated by co-administration of grape seed proanthocyanidin extract, using kidney function estimates from blood urea nitrogen (BUN), creatinine plasma levels and renal histopathological scores [83]. Similarly, protection against colistin-induced apoptosis by proanthocyanidin extract was observed by measuring apoptotic index, caspase-1, caspase-3, and calpain-1 in the kidney tissues [83]. It should be noted that considering animal scaling, a relatively low dose of CMS (300,000 IU/kg/day by intraperitoneal administration, equal to 9 mg colistin base activity/kg/day) was used in the study [83]. Whereas the above co-administered agents probably rely on their antioxidant effects for nephroprotection, the ameliorating effect of co-administered succinylated bovine gelatin polypeptides (Gelofusine) appears to rely on the ability of these peptides to decrease accumulation of polymyxins in renal tissue [49].
Thus far, there is little information on the protection from polymyxin-associated nephrotoxicity in patients. A preliminary randomized controlled study was conducted in 28 patients to investigate the potential nephroprotective effect of intravenous ascorbic acid (2 g every 12 h) against colistin-associated nephrotoxicity in patients requiring intravenous colistin [86]. The RIFLE classification system was employed in this small clinical study and urinary neutrophil gelatinase-associated lipocalin (NGAL) and NAG were measured as markers of renal damage. The plasma colistin concentrations and clinical outcomes in both groups were not significantly different. The lack of nephroprotective effect by ascorbic acid in this clinical study might be due to the small patient number, insufficient dose, and/or the failure of animal models to mimic clinical disease [86]. On the contrary, Dalfino et al. showed the protective effect of intravenous ascorbic acid against nephrotoxicity of colistin (CMS) in critically-ill patients [87]. Acute kidney injury (AKI) was observed in 30% of patients treating with concurrent ascorbic acid, whereas the rate of AKI was about 67% in patients who did not receive ascorbic acid [87]. Furthermore, this observation was statistically significant (P < 0.05, adjusted odds ratio, 0.27 [95% confidence interval, 0.13–0.57]). However, it is important to consider the potential limitations of this study, particularly the small, non-randomized nature and the lack of characterization of patients between the groups. It is also critical to consider the possible effect of ascorbic acid on polymyxin pharmacokinetics/pharmacodynamics in patients [48]. Nevertheless, well-designed clinical studies are warranted to develop novel approaches to attenuate polymyxin-induced nephrotoxicity.
18.4 Conclusions
Significant progress has been made over the last two decades in understanding the mechanism of polymyxin-induced nephrotoxicity. It is clear that polymyxins are substantially accumulated in renal tubular cells, causes oxidative stress and apoptosis via the activation of the death receptor, mitochondria and endoplasmic reticulum mediated pathways. However, the complex interplay of multiple pathways remains undefined in polymyxin-induced nephrotoxicity, and systems investigations on the mechanisms of polymyxin-induced nephrotoxicity are required. The mechanistic findings will provide key pharmacological information for the development of novel interventions to minimise polymyxin-induced nephrotoxicity in patients, as well as important chemical biology knowledge for the discovery of new-generation polymyxins.
References
Kubin CJ, Ellman TM, Phadke V, Haynes LJ, Calfee DP, Yin MT (2012) Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J Infect 65:80–87
Falagas ME, Kasiakou SK (2006) Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10:R27
Dezoti Fonseca C, Watanabe M, Vattimo MF (2012) Role of heme oxygenase-1 in polymyxin B-induced nephrotoxicity in rats. Antimicrob Agents Chemother 56:5082–5087
Elias LS, Konzen D, Krebs JM, Zavascki AP (2010) The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J Antimicrob Chemother 65:2231–2237
Kvitko CH, Rigatto MH, Moro AL, Zavascki AP (2011) Polymyxin B versus other antimicrobials for the treatment of Pseudomonas aeruginosa bacteraemia. J Antimicrob Chemother 66:175–179
Pastewski AA, Caruso P, Parris AR, Dizon R, Kopec R, Sharma S, Mayer S, Ghitan M, Chapnick EK (2008) Parenteral polymyxin B use in patients with multidrug-resistant gram-negative bacteremia and urinary tract infections: a retrospective case series. Ann Pharmacother 42:1177–1187
Mingeot-Leclercq MP, Tulkens PM, Denamur S, Vaara T, Vaara M (2012) Novel polymyxin derivatives are less cytotoxic than polymyxin B to renal proximal tubular cells. Peptides 35:248–252
Evans ME, Feola DJ, Rapp RP (1999) Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother 33:960–967
Falagas ME, Kasiakou SK (2005) Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 40:1333–1341
Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL (2011) Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294
Crass RL, Rutter WC, Burgess DR, Martin CA, Burgess DS (2017) Nephrotoxicity in patients with or without cystic fibrosis treated with polymyxin B compared to colistin. Antimicrob Agents Chemother 61:e02329-02316
Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K (2005) Evaluation of colistin as an agent against multi-resistant in Gram-negative bacteria. Int J Antimicrob Agents 25:11–25
Phe K, Shields RK, Tverdek FP, Aitken SL, Guervil DJ, Lam WYM, Musgrove RJ, Luce AM, Tam VH (2016) Predicting the risk of nephrotoxicity in patients receiving colistimethate sodium: a multicentre, retrospective, cohort study. J Antimicrob Chemother 71:3585–3587
John JF, Falci DR, Rigatto MH, Oliveira RD, Kremer TG, Zavascki AP (2018) Severe infusion-related adverse events and renal failure in patients receiving high-dose intravenous polymyxin B. Antimicrob Agents Chemother 62:e01617-01617
Benattar YD, Omar M, Zusman O, Yahav D, Zak-Doron Y, Altunin S, Elbaz M, Daitch V, Granot M, Leibovici L, Paul M (2016) The effectiveness and safety of high-dose colistin: prospective cohort study. Clin Infect Dis 63:1605–1612
Babic JT, Manchandani P, Ledesma KR, Tam VH (2017) Evaluation of urinary KIM-1 for prediction of polymyxin B-induced nephrotoxicity. Antimicrob Agents Chemother 61:e01735-01717
Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K (2003) Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother 47:1766–1770
Sivanesan S, Roberts K, Wang JP, Chea SE, Thompson PE, Li J, Nation RL, Velkov T (2017) Pharmacokinetics of the individual major components of polymyxin B and colistin in rats. J Nat Prod 80:225–229
Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K (2004) Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother 53:837–840
Marchand S, Lamarche I, Gobin P, Couet W (2010) Dose-ranging pharmacokinetics of colistin methanesulphonate (CMS) and colistin in rats following single intravenous CMS doses. J Antimicrob Chemother 65:1753–1758
He H, Li JC, Nation RL, Jacob J, Chen G, Lee HJ, Tsuji BT, Thompson PE, Roberts K, Velkov T, Li J (2013) Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother 68:2311–2317
Zhao M, Wu XJ, Fan YX, Zhang YY, Guo BN, Yu JC, Cao GY, Chen YC, Wu JF, Shi YG, Li J, Zhang J (2018) Pharmacokinetics of colistin methanesulfonate (CMS) in healthy Chinese subjects after single and multiple intravenous doses. Int J Antimicrob Agents 51:714–720
Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O (2011) Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther 89:875–879
Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G (2009) Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 48:1724–1728
Abdelraouf K, He J, Ledesma KR, Hu M, Tam VH (2012) Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob Agents Chemother 56:5724–5727
Manchandani P, Dubrovskaya Y, Gao S, Tam VH (2016) Comparative pharmacokinetic profiling of different polymyxin B components. Antimicrob Agents Chemother 60:6980–6982
Zavascki AP, Goldani LZ, Cao GY, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J (2008) Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 47:1298–1304
Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP (2013) Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531
Zavascki AP, Nation RL (2017) Nephrotoxicity of Polymyxins: is there any difference between Colistimethate and Polymyxin B? Antimicrob Agents Chemother 61:e02319-02316
Azad MA, Roberts KD, Yu HH, Liu B, Schofield AV, James SA, Howard DL, Nation RL, Rogers K, de Jonge MD, Thompson PE, Fu J, Velkov T, Li J (2015) Significant accumulation of polymyxin in single renal tubular cells: a medicinal chemistry and triple correlative microscopy approach. Anal Chem 87:1590–1595
Yun B, Azad MAK, Nowell CJ, Nation RL, Thompson PE, Roberts KD, Velkov T, Li J (2015) Cellular uptake and localization of polymyxins in renal tubular cells using rationally designed fluorescent probes. Antimicrob Agents Chemother 59:7489–7496
Yun B, Azad MA, Wang J, Nation RL, Thompson PE, Roberts KD, Velkov T, Li J (2014) Imaging the distribution of polymyxins in the kidney. J Antimicrob Chemother 70:827–829
Nilsson A, Goodwin RJA, Swales JG, Gallagher R, Shankaran H, Sathe A, Pradeepan S, Xue AX, Keirstead N, Sasaki JC, Andren PE, Gupta A (2015) Investigating nephrotoxicity of polymyxin derivatives by mapping renal distribution using mass spectrometry imaging. Chem Res Toxicol 28:1823–1830
Manchandani P, Zhou J, Ledesma KR, Truong LD, Chow DS, Eriksen JL, Tam VH (2016) Characterization of polymyxin B biodistribution and disposition in an animal model. Antimicrob Agents Chemother 60:1029–1034
Velkov T, Yun B, Schneider EK, Azad MA, Dolezal O, Morris FC, Nation RL, Wang J, Chen K, Yu HH, Wang L, Thompson PE, Roberts KD, Li J (2016) A novel chemical biology approach for mapping of polymyxin lipopeptide antibody binding epitopes. ACS Infect Dis 2:341–351
Vattimo Mde F, Watanabe M, da Fonseca CD, Neiva LB, Pessoa EA, Borges FT (2016) Polymyxin B nephrotoxicity: from organ to cell damage. PLoS One 11:e0161057
Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K (2003) Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother 47:1364–1370
Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD (2015) Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234
Metcalf AP, Hardaker LEA, Hatley RHM (2017) A simple method for assaying colistimethate sodium in pharmaceutical aerosol samples using high performance liquid chromatography. J Pharm Biomed Anal 142:15–18
British Pharmacopoeia (2018) Monographs for colistimethate sodium. In: British Pharmacopoeia 2018. Stationery Office, London, pp I-675–I-678
Azad MAK, Cheah SE, Yun B, Maifiah MHM, Johnson MD, Wang J, Boughton BA, Chapman R, Gould J, Hertzog P, Velkov T, Creek DJ, Li J (2015) Understanding polymyxin-induced nephrotoxicity: combination of transcriptomics, metabolomics and mass spectrometry imaging. Interscience Conference of Antimicrobial Agents and Chemotherapy/International Congress of Chemotherapy, San Diego, CA, USA, A-942: Poster
Abdelraouf K, Chang KT, Yin T, Hu M, Tam VH (2014) Uptake of polymyxin B into renal cells. Antimicrob Agents Chemother 58:4200–4202
Azad MA, Yun B, Roberts KD, Nation RL, Thompson PE, Velkov T, Li J (2014) Measuring polymyxin uptake by renal tubular cells: is BODIPY-polymyxin B an appropriate probe? Antimicrob Agents Chemother 58:6337–6338
Velkov T, Roberts KD, Nation RL, Thompson PE, Li J (2013) Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol 8:711–724
Velkov T, Thompson PE, Nation RL, Li J (2010) Structure-activity relationships of polymyxin antibiotics. J Med Chem 53:1898–1916
Suzuki T, Yamaguchi H, Ogura J, Kobayashi M, Yamada T, Iseki K (2013) Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother 57:6319–6324
Mattson MP, Chan SL (2003) Calcium orchestrates apoptosis. Nat Cell Biol 5:1041–1043
Yousef JM, Chen G, Hill PA, Nation RL, Li J (2012) Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother 67:452–459
Sivanesan S, Azad MAK, Schneider EK, Ahmed MU, Huang J, Wang J, Li J, Nation RL, Velkov T (2017) Gelofusine ameliorates colistin-induced nephrotoxicity. Antimicrob Agents Chemother 61:e00985-17
Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ (1994) Expression cloning of a rat liver Na(+)-independent organic anion transporter. Proc Natl Acad Sci U S A 91:133–137
Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, Ganapathy V (1998) Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem 273:15971–15979
Busch AE, Quester S, Ulzheimer JC, Waldegger S, Gorboulev V, Arndt P, Lang F, Koepsell H (1996) Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J Biol Chem 271:32599–32604
Okuda M, Saito H, Urakami Y, Takano M, Inui K (1996) cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem Biophys Res Commun 224:500–507
Nigam SK (2015) What do drug transporters really do? Nat Rev Drug Discov 14:29–44
Petzinger E, Geyer J (2006) Drug transporters in pharmacokinetics. Naunyn Schmiedeberg’s Arch Pharmacol 372:465–475
Hua WJ, Hua WX, Jian Z, Wei PH, Ni LY, Hua LY, Wen CD, Ying Z, Li C (2016) The role of drug transporters in the pharmacokinetics of antibiotics. Curr Drug Metab 17:799–805
International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236
Eshbach ML, Weisz OA (2017) Receptor-mediated endocytosis in the proximal tubule. Annu Rev Physiol 79:425–448
Manchandani P, Zhou J, Babic JT, Ledesma KR, Truong LD, Tam VH (2017) Role of renal drug exposure in polymyxin B-induced nephrotoxicity. Antimicrob Agents Chemother 61:e02391-16
Hori Y, Aoki N, Kuwahara S, Hosojima M, Kaseda R, Goto S, Iida T, De S, Kabasawa H, Kaneko R, Aoki H, Tanabe Y, Kagamu H, Narita I, Kikuchi T, Saito A (2017) Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J Am Soc Nephrol 28:1783–1791
Ma Z, Wang J, Nation RL, Li J, Turnidge JD, Coulthard K, Milne RW (2009) Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob Agents Chemother 53:2857–2864
Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, Ganapathy V (2000) Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta 1466:315–327
Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC 3rd (1999) Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Phys 276:F658–F665
Lu XX, Chan T, Xu CH, Zhu L, Zhou QT, Roberts KD, Chan HK, Li J, Zhou FF (2016) Human oligopeptide transporter 2 (PEPT2) mediates cellular uptake of polymyxins. J Antimicrob Chemother 71:403–412
Daniel H, Rubio-Aliaga I (2003) An update on renal peptide transporters. Am J Physiol Renal 284:F885–F892
Chaabane W, User SD, El-Gazzah M, Jaksik R, Sajjadi E, Rzeszowska-Wolny J, Los MJ (2013) Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp 61:43–58
Wang S, Zhang C, Hu L, Yang C (2016) Necroptosis in acute kidney injury: a shedding light. Cell Death Dis 7:e2125
Linkermann A (2016) Nonapoptotic cell death in acute kidney injury and transplantation. Kidney Int 89:46–57
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73:994–1007
Hanigan MH, Devarajan P (2003) Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther 1:47–61
Eadon MT, Hack BK, Alexander JJ, Xu C, Dolan ME, Cunningham PN (2013) Cell cycle arrest in a model of colistin nephrotoxicity. Physiol Genomics 45:877–888
Sutton TA, Hato T, Mai E, Yoshimoto M, Kuehl S, Anderson M, Mang H, Plotkin Z, Chan RJ, Dagher PC (2013) p53 is renoprotective after ischemic kidney injury by reducing inflammation. J Am Soc Nephrol 24:113–124
Chen SC, Kuo PL (2016) The role of galectin-3 in the kidneys. Int J Mol Sci 17:565
Yun B, Zhang T, Azad MAK, Wang J, Nowell CJ, Kalitsis P, Velkov T, Hudson DF, Li J (2016) Targeting the ‘Achilles heel’: investigating the mechanisms of polymyxin-induced nephrotoxicity. ComBio 2016, Brisbane, Australia: Poster-101
Yun B, Zhang T, Azad MAK, Wang J, Nowell CJ, Kalitsis P, Velkov T, Hudson DF, Li J (2018) Polymyxin B causes DNA damage in HK-2 cells and mice. Arch Toxicol 92:2259–2271
Ozyilmaz E, Ebinc FA, Derici U, Gulbahar O, Goktas G, Elmas C, Oguzulgen IK, Sindel S (2011) Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine? Intens Care Med 37:141–146
Yousef JM, Chen G, Hill PA, Nation RL, Li J (2011) Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother 55:4044–4049
Dai C, Li J, Tang S, Li J, Xiao X (2014) Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob Agents Chemother 58:4075–4085
Azad MA, Finnin BA, Poudyal A, Davis K, Li J, Hill PA, Nation RL, Velkov T, Li J (2013) Polymyxin B induces apoptosis in kidney proximal tubular cells. Antimicrob Agents Chemother 57:4329–4335
Vaara M, Vaara T (2013) The novel polymyxin derivative NAB739 is remarkably less cytotoxic than polymyxin B and colistin to human kidney proximal tubular cells. Int J Antimicrob Agents 41:292–293
Roberts KD, Azad MAK, Wang JP, Horne AS, Thompson PE, Nation RL, Velkov T, Li J (2015) Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin B and colistin: last-line antibiotics against multidrug-resistant Gram-negative bacteria. ACS Infect Dis 1:568–575
Azad MA, Akter J, Rogers K, Nation RL, Velkov T, Li J (2015) Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob Agents Chemother 59:2136–2143
Ozkan G, Ulusoy S, Orem A, Alkanat M, Mungan S, Yulug E, Yucesan FB (2013) How does colistin-induced nephropathy develop and can it be treated? Antimicrob Agents Chemother 57:3463–3469
Azad MAK, Sivanesan S, Wang J, Chen K, Nation RL, Thompson PE, Roberts KD, Velkov T, Li J (2017) Methionine ameliorates polymyxin-induced nephrotoxicity by attenuating cellular oxidative stress. Antimicrob Agents Chemother 62:e01254-01217
Azad MAK, Zhu Y, Han ML, Wang J, Creek DJ, Velkov T, Li J (2016) Effect of poly-L-aspartic acid on polymyxin-induced nephrotoxicity: a systems pharmacology approach. ASM Microbe 2017:Poster-217
Sirijatuphat R, Limmahakhun S, Sirivatanauksorn V, Nation RL, Li J, Thamlikitkul V (2015) Preliminary clinical study of the effect of ascorbic acid on colistin-associated nephrotoxicity. Antimicrob Agents Chemother 59:3224–3232
Dalfino L, Puntillo F, Ondok MJM, Mosca A, Monno R, Coppolecchia S, Spada ML, Bruno F, Brienza N (2015) Colistin-associated acute kidney injury in severely ill patients: a step toward a better renal care? A prospective cohort study. Clin Infect Dis 61:1771–1777
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Azad, M.A.K., Nation, R.L., Velkov, T., Li, J. (2019). Mechanisms of Polymyxin-Induced Nephrotoxicity. In: Li, J., Nation, R., Kaye, K. (eds) Polymyxin Antibiotics: From Laboratory Bench to Bedside. Advances in Experimental Medicine and Biology, vol 1145. Springer, Cham. https://doi.org/10.1007/978-3-030-16373-0_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-16373-0_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16371-6
Online ISBN: 978-3-030-16373-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)