Abstract
Purpose
The protective effect of N-acetylcysteine (NAC) on nephrotoxicity due to contrast nephropathy and reperfusion-induced ischemia has been reported in experimental models. However, its efficacy on colistin-induced nephrotoxicity has not been elucidated yet. The primary aim of this study was to evaluate the nephrotoxic effect of colistin and to investigate the possible protective effect of NAC on colistin-induced nephrotoxicity. The secondary aim was to research the systemic effects of nephrotoxicity-induced oxidative stress on the lung.
Methods
Eighteen female Sprague-Dawley rats were randomly assigned and were given (a) 1 ml/kg sterile saline, (b) 300,000 IU/kg/day colistin, and (c) 300,000 IU/kg/day colistin and 150 mg/kg NAC for six consecutive days.
Results
Plasma blood urea nitrogen (BUN), creatinine, urinary creatinine, urinary protein, plasma TNF-alpha levels, renal tissue superoxide dismutase (SOD) and malondialdehyde (MDA) activity and immunocytochemical findings were evaluated. Colistin exerted nephrotoxicity and achieved a significant increase in plasma BUN and creatinine levels and renal tissue SOD levels. NAC exhibited no significant effect on biochemical parameters but reduced renal tissue SOD level and reversed immunocytochemical staining of inducible nitric oxide synthase (i-NOS) and neurotrophin-3. Increased oxidative stress in the lung tissue of the rats treated with colistin has also been documented. Additionally, NAC significantly reduced the immunostaining of endothelial NOS (e-NOS) and i-NOS in the lung tissue.
Conclusions
Colistin-induced renal toxicity may be attributable to oxidative damage. The combined treatment of colistin plus NAC seems to have a beneficial role in restoration of the oxidant injury which may be related to its antioxidant effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colistin is a cyclic cationic polypeptide antibiotic which is widely used after the appearance of gram negative bacteria which are resistant to almost all classes of commercially available antibiotics [1–3]. Nephrotoxicity is the most frequently observed limited side effect and results in either early discontinuation of treatment or worse prognosis [1, 4].
N-Acetylcysteine (NAC) is a thiol-containing compound which plays a key role in detoxification of free radicals [5]. A number of reports indicated the protective effect of NAC on drug-induced nephrotoxicity but the outcome on colistin-induced nephrotoxicity has not been investigated yet [6, 7].
The primary aim of this experimental study was to evaluate the nephrotoxic effect of colistin and to research the possible protective effect of NAC on colistin-induced nephrotoxicity. In order to explore the potential role of oxidative stress in colistin-induced nephrotoxicity, two enzymatic markers which induce nitric oxide (NO) synthesis [endothelial NOS (e-NOS) and inducible NOS (i-NOS)] and neurotrophin-3 (NT-3), which is a well-known marker of tissue injury, were selected for immunocytochemical analysis. Because increased oxidative stress is not restricted locally and may spread by systemic circulation, we planned to investigate the systemic effects of oxidative stress on the lung, which is a main target of oxidant overload, as a secondary aim.
Materials and methods
Drugs
Colistin was obtained from Forest Laboratories (Bexley, UK) and NAC was obtained from Husnu Arsan Pharmaceuticals (Istanbul, Turkey).
Animals
Adult female Sprague-Dawley rats each weighing 225 ± 25 g were obtained from the experimental animals laboratory of our institute and were kept in appropriate conditions. The study protocol was approved by the animal research ethics committee of the institute. All experiments were performed according to the rules of the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1996).
Experimental design
The animals were randomly divided into three groups with six rats each as follows: group 1 was the control group which was administered 1 ml/kg intraperitoneal (i.p.) sterile saline; group 2 was assigned to receive 300,000 IU/kg/day i.p. colistin (colistimethate sodium; 1 million IU/vial); and group 3 was treated with 300,000 IU/kg/day colistin and 150 mg/kg NAC i.p. in each single dose for six consecutive days. On the seventh day, 24 h after the last injection, the animals were killed.
Biochemical assay
Plasma levels of BUN, creatinine, and urine levels of protein and creatinine were measured with standard kits and colorimetric methods. Plasma TNF-alpha levels were investigated by using a standard ELISA kit (KRC3011, BioSource, California). Tissue SOD and MDA levels were measured with colorimetric method by using standard kits (from Cayman, USA, and Oxis Research, Foster City, USA, respectively).
Immunohistochemical examination
For immunohistochemical examination, the Zymed Histostain-Plus Broad Spectrum kit was used (Ref/Cat No. 85-9043, Lot 457501A, Zymed, USA). Slides were examined with a Photo-light microscope (DCM4500 Image Analyze System and QWin Programme, DFC280 Plus Camera, Leica, Germany). A semiquantitative scoring system was used to assess the immunolabeling intensity. The HSCORE was calculated by using the following equation: HSCORE = _Pi(i + 1), where i is the intensity of labeling with a value of 1, 2, or 3 and Pi is the percentage of labeled epithelial and stromal cells for each intensity, varying from 0 to 100% [8].
Statistical analysis
SPSS for Windows software was used for the statistical analysis of the results (SPSS for Windows; Chicago, IL, USA). Results are presented as mean ± SD and percentiles. Paired sample t test, one-way ANOVA analysis of variance, and post hoc multiple comparison test (Bonferonni) were performed on the data of the biochemical variables to examine differences between the groups. A difference was considered statistically significant at p < 0.05.
Results
Colistin caused an elevation of BUN and plasma creatinine levels and a decrease in creatinine clearance (ClCr) (p < 0.05), while urine creatinine and protein levels did not alter (p > 0.05). NAC treatment did not change these parameters (Table 1).
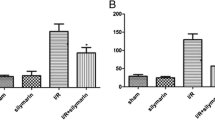
Plasma TNF-alpha and tissue MDA levels were not changed by colistin or NAC treatment (p > 0.05). However, colistin resulted in an increase in tissue SOD levels in kidney and this increase was prevented with NAC use (p < 0.05) [Fig. 1 of the electronic supplementary material (ESM)].
Kidney histopathology
Light microscopy findings were comparable among all three groups. Immunohistochemical evaluation of the control group displayed negative to light expression of NT-3 and i-NOS in proximal tubules, distal tubules, and medullary collecting tubules of the kidney. e-NOS staining was prominent in the glomerules and tubular capillary. In the colistin group, high expression of NT-3 and i-NOS was observed in glomerular capillary, distal tubuli, intertubular capillary, and collecting tubules, while e-NOS staining was reduced in these segments (p < 0.05). In the colistin + NAC group, the expression of e-NOS was lower in the glomeruli and higher in the distal and collecting tubules. Also, i-NOS activity was decreased in the glomeruli, distal, and collecting tubules. Although immunostaining of NT-3 was clearly weaker than the colistin groups, it was still a bit stronger than the controls (Table 2) (e-NOS results are presented as Fig. 2 of the ESM). i-NOS and NT-3 results are presented in Figs. 1 and 2.
i-NOS immunocytochemical staining of kidney tissue control group (I) , colistin group (II), colistin + NAC group (III). a ×100, thick arrows glomerular capillary, thin arrows tubular capillary, P proximal tubule, D distal tubule. b ×400, CT collecting tubule, asterisks capillary between collecting tubules. c ×400
NT-3 immunocytochemical staining of kidney tissue control group (I), colistin group (II), colistin + NAC group (III). a ×100, thick arrows glomerular capillary, thin arrows tubular capillary, P proximal tubule, D distal tubule. b ×400, CT collecting tubule, asterisks capillary between collecting tubules. c ×400
Lung histopathology
Under light microscopy examination, the control group revealed normal histology of the lung and NT-3 expression was found to be moderate to severe. In the colistin group normal alveolar structure was rarely observed. Alveolar saccus and ductus were enlarged and interalveolar septum was thickened. NT-3 immunoreactivity and i-NOS were found to be higher while e-NOS staining was lower than the controls (p < 0.05). In the colistin + NAC group, the general characteristics were broadly similar to the control group. The immunoreactivity of e-NOS and i-NOS were lower than in the colistin group, whereas NT-3 staining was comparable (Table 2) (figure not shown).
Discussion
The present study indicated that colistin caused nephrotoxicity in rats and although concomitant use of NAC did not significantly improve biochemical parameters and ClCr, it reversed the increased levels of SOD and improved the immunohistochemical expression of NT-3 and i-NOS which was predominantly observed in the collecting tubules of the colistin group. In addition, increased e-NOS and i-NOS staining, which has also been documented in the lung tissue of the colistin group, was recovered with NAC use.
Colistin is a valuable therapeutic option in clinical practice which is increasingly used for multidrug resistant gram negative pathogens [1–4, 9–12]. Renal toxicity due to colistin is reported to be dose dependent and mainly present as acute tubular necrosis [10]. Although the exact mechanism is still a matter of debate, d-amino acid and fatty acid components are proposed to be the causes of nephrotoxicity due to colistin [10].
NAC is an inexpensive, easy-to-use drug with no significant toxicity. NAC exhibits its antioxidant property in several ways including interaction with the electrophilic groups of reactive oxygen species (ROSs) and being a glutathione (GSH) precursor [13]. The pathogenic role of oxidative stress and the protective effect of NAC have been documented in sepsis, reperfusion-induced ischemia, and drug-induced nephrotoxicity due to gentamicin and cisplatin [14–17]. Nevertheless the possible effect on colistin-induced nephrotoxicity has not been evaluated yet.
The results of the present study are in accordance with the previous reports which verified nephrotoxicity due to colistin use [2, 9]. Although light microscopy revealed comparable results, increased renal function tests indicate acute tubular necrosis in the study group. The cellular sources of NO in the renal tissue are mainly proximal and distal tubules [18]. We have demonstrated the augmentation of e-NOS, i-NOS, and NT-3 expression due to colistin use in the tubular region. In addition, the expression was significantly decreased due to concomitant use of NAC in the tubular zone. The reversed immunocytochemical staining of i-NOS activity, rather than e-NOS may indicate i-NOS as the underlying cause of nephrotoxicity related to colistin-induced oxidative damage. This idea can also be supported with the reversed i-NOS staining at the tubular zone which is the main affected region in colistin-induced nephrotoxicity. Reduction of the elevated SOD levels and reversal of the increase in the tubular immunohistochemical expression of NT-3 and i-NOS with the use of NAC indicate that oxidative stress may be the underlying mechanism of nephrotoxicity due to colistin. NAC may have a beneficial role in restoring the tissue, although not reversing all alterations. More interestingly, the improvement of the immunocytochemical staining of the lung tissue due to NAC use revealed that the oxidative stress induced by colistin use is not restricted to the kidney. Excessively produced oxidants could migrate to the lung through systemic circulation and result in the demonstrated damage. Systemic effects of oxidant overload due to drug nephrotoxicity should be highlighted with further investigations.
There may be several reasons for the reduced SOD rank and reversed immunocytochemical findings without any alteration in BUN and creatinine levels. At first, the dosage used or the duration of the treatment may be inadequate. Second, NAC was administered simultaneously with colistin in this experiment. Early administration which is suggested for contrast nephrotoxicity may augment the protective effect [7]. Third, the rats were not further hydrated during the experiment which is a widely used therapeutic option to prevent nephrotoxicity.
To our knowledge, this is the first study which investigates the protective effect of NAC on colistin-induced nephrotoxicity. Colistin-induced renal toxicity may be attributable to oxidative damage, and administration of NAC seems to have a beneficial role in restoring kidney injury. Further research is warranted to enlighten us about the possible effect, appropriate dose, and duration of the NAC therapy on colistin-induced nephrotoxicity.
References
Michalopoulos AS, Falagas ME (2008) Colistin and polymyxin B in critical care. Crit Care Clin 24:377–391
Michalopoulos AS, Tsiodras S, Rellos K, Menzelopoulos S, Falagas ME (2005) Colistin treatment in patients with ICU-acquired infections caused by multidrug resistant gram negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect 11:115–121
Zavacki AP, Goldani LZ, Li J, Nation RL (2007) Polymyxin B for the treatment of multidrug resistant pathogens: a critical review. J Antimicrob Chemother 60:1206–1215
Falagas ME, Kasiakou SK (2005) Colistin: the revival of polymyxins for the management of multidrug resistant gram negative bacterial infections. Clin Infect Dis 40:1333–1341
Aitio ML (2005) N-acetylcysteine—passé-partout or much ado about nothing? Br J Clin Pharmacol 61:5–15
Petronilho F, Constantino L, de Souza B, Reinke A, Martins MR, Fraga CM, Ritter C, Dal-Pizzol F (2009) Efficacy of the combination of N-acetylcysteine and desferrioxamine in the prevention and treatment of gentamicin-induced acute renal failure in male Wistar rats. Nephrol Dial Transplant 24:2077–2082
Bagshaw SM, McAlister FA, Manns BJ, Ghali WA (2006) Acetylcysteine in the prevention of contrast induced nephropathy: a case study of the pitfalls in the evaluation of evidence. Arch Intern Med 166:161–166
McCarty KS Jr, Miller RS (1985) Estrogen receptor analysis. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109:716–721
Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, Gregorakos L (2003) Intravenous colistin in the treatment of sepsis from multidrug resistant gram negative bacilli in critically ill patients. Crit Care 7:R78–R83
Falagas ME, Kasiakou SK (2006) Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10:R27
Reed MD, Stern RC, O’Riordan MA, Blumer JL (2001) The pharmacokinetics of colistin in patients with cystic fibrosis. J Clin Pharmacol 41:645–654
Falagas ME, Rizos M, Bliziotis IA, Rellos K, Kasiakou SK, Michalopoulos A (2005) Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect Dis 5:1
Dekhuijzen PNR (2004) Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J 23:629–636
Senoglu N, Yuzbasioglu MF, Aral M, Ezberci M, Kurutas EB, Bulbuloglu E, Ezberci F, Oksuz H, Ciragil P (2008) Protective effects of N-acetylcysteine and beta-glucan pretreatment on oxidative stress in cecal ligation and puncture model of sepsis. J Invest Surg 21:237–243
Erturk E, Cekic B, Geze S, Kosucu M, Coskun I, Eroglu A, Ulusoy H, Mentese A, Karahan C, Kerimoglu S (2009) Comparison of the effect of propofol and N-acetyl cysteine in preventing ischaemia-reperfusion injury. Eur J Anaesthesiol 26:279–284
Ali BH, Al Salam S, Al-Husseini I, Nemmar A (2009) Comparative protective effect of N-acetyl cysteine and tetramethylpyrazine in rats with gentamicin nephrotoxicity. J Appl Toxicol 29:302–308
Luo J, Tsuji T, Yasuda H, Sun Y, Fujigaki Y, Hishida A (2008) The molecular mechanisms of the attenuation of cisplatin-induced acute renal failure by N-acetylcysteine in rats. Nephrol Dial Transplant 23:2198–2205
Ghaznavi R, Kadkhodaee M (2007) Comparative effects of selective and non-selective nitric oxide synthase inhibition in gentamicin-induced rat nephrotoxicity. Arch Toxicol 81:453–457
Acknowledgments
The authors do not have any personal relationships to disclose which may affect the present work. The study was supported by a grant from the Internal Medicine Postgraduate Education Society (IMSED).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ozyilmaz, E., Ebinc, F.A., Derici, U. et al. Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine?. Intensive Care Med 37, 141–146 (2011). https://doi.org/10.1007/s00134-010-2038-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2038-7