Abstract
Small cell lung cancer (SCLC) is a disease of the smoking population. The incidence decreased to approximately 13% of all lung cancer because of a decrease in the number of cigarette smokers. The majority of patients are diagnosed with advanced stage due to the aggressive behavior. SCLC is highly responsive to combination chemotherapy however the survival time seems dismal because of the high rate of relapsing disease. Many targeted therapy are still ineffective. The immunotherapy might be effective in SCLC but there are only limited data have been reported.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Small cell lung cancer(SCLC) is classified as a neuroendocrine carcinoma. Patients with SCLC are usually diagnosed with advanced stage at presentation because it has aggressive behavior, rapid growth and early spread to distant sites. This chapter will discuss on epidemiology, pathology, stage of disease, diagnostic work up and treatment modalities.
2 Epidemiology
2.1 Incidence and Prevalence
Nearly all patients with small cell lung cancer (SCLC) were or active smokers. SCLC, the incidence has decreased from 25% of all lung cancers in 1993 to approximately 10–15% in 2017 [1,2,3]. This could be explained by the decrease in prevalence of smokers because smoking remains the predominant risk factor for this disease [1]. The prognosis and therapeutic options in SCLC are still limited and the median survival of patients with advanced SCLC with chemotherapy is between 8 and 10 months [4,5,6,7,8,9,10,11].
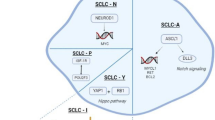
2.2 Genetics in SCLC
There are many studies analyzing SCLC tissue to discover a somatic genetic alteration in SCLC as shown in Table 10.1 [12]. Nearly all SCLCs (75%–90%) show loss of the prominent tumor suppressor protein 53 (TP53), Retinoblastoma 1 (RB1), RASSF1 and FHIT which are poor therapeutic targets.
3 Diagnosis and Staging
The investigations and staging workup for SCLC include, history taking, physical examination, CT, PET or PET/CT, MRI, bone scan, bone marrow aspiration or biopsy which are routinely performed to identify metastasis.
The role of PET or PET/CT scan for initial staging of SCLC has been evaluated in many studies. In summary, it can provide 16% up-stage disease and also 11% of down-stage disease, compared with conventional imaging, which influence the decision making process, approximately 30% change in treatment [13]. Moreover, current study found that patients with limited-stage evaluated by PET achieved an improved disease control and survival comparing with non-PET scan. The OS was 32 months in PET-staged patients and 17 months in non-PET-staged patients (p=0.03). The better intrathoracic disease evaluation may explain these findings [14]. Therefore, in patients with clinically limited-stage SCLC, PET scan is suggested [13].
In the past, SCLC staging was classified into two stages; limited-stage and extensive-stage; however, now a day, it has been inconsistently defined and used. The TNM classification and stage grouping should be applied to SCLC because of presenting significance for prognosis of SCLC and has the advantage of providing a uniform detailed classification of tumor spread [15,16,17]. Limited-stage includes any T, any N, M0, that be safe for definite radiotherapy, except T3-T4 due to multiple lung nodules or lesions and LNs that are too large that do not tolerate the definite radiotherapy. Extensive-stage includes any T, any N, M1a-c or T3-T4 due to multiple lung nodules.
4 Pathology
SCLC has been grouped together with carcinoid tumor and large cell neuroendocrine carcinoma (LCNEC) under pulmonary neuroendocrine tumors (Lung NETs) in 2015 WHO classification [18]. SCLC is a poorly differentiated epithelial tumor of small cells with scant cytoplasm whereas LCNEC is composed of pleomorphic cells with variable amount of granular eosinophilic cytoplasm and round-to-oval vesicular nuclei. LCNEC and SCLC were designated as high-grade full-blown carcinomas with poor prognosis and no significant differences in survival between them (Table 10.2) [19].
4.1 Gross Pathology
SCLCs are usually white-tan, soft, friable perihilar tumors with massive necrosis and often nodal metastasis. They typically spread along bronchi in a submucosal and circumferential fashion with frequently extensive lymphatic invasion.
4.2 Histopathology
The tumors exhibit a wide spectrum of architectures including nest, trabeculae, strands, and rosette formation. Single cell fashion or sheet-like growths without typical neuroendocrine morphology are also common as shown in Fig. 10.1. SCLC cells usually have round, ovoid or spindled nuclei and scant cytoplasm. Characteristic cytologic features include ill-defined cytoplasmic borders, finely granular nuclear chromatin, absent or inconspicuous nucleoli, and prominent nuclear molding. Mitotic rate is high. The diagnosis can be confirmed by using the panel of IHC including chromogranin A, synaptophysin, and CD56. Moreover, using mitotic index (Ki-67) and retinoblastoma protein is applied for prognostic and predictive markers between well differentiated and poorly differentiated lung NETs [20].
Small cell carcinoma (a) Solid sheets and occasional trabeculae of densely packed malignant cells showing scant cytoplasm, finely granular chromatin (Hematoxylin and eosin 200×). (b) Neoplastic cells show round nuclei with finely granular chromatin, absence of nucleoli and scant cytoplasm. High mitotic rate is typical feature (Hematoxylin and eosin 400×)
5 Clinical Presentation
SCLC is characterized by more aggressive behavior and early development of widespread metastases. The proportion of new cases in limited stage SCLC is approximately 40%. When compared with NSCLC, SCLC is more responsive to chemotherapy and radiation initially but relapse occurs quickly, with a 5-year survival rate < 10% [1]. Brain metastases are common in SCLC, approximately 10–14% of SCLC patients have brain metastases at the time of diagnosis [21].
Paraneoplastic syndromes such as Cushing syndrome, carcinoid syndrome, Lambert-Eaton myasthenia syndrome, dermatomyositis, thrombocytosis or thromboembolism are more commonly presentations in SLCL than those in NSCLC, especially in Cushing syndrome (up to 50% of SCLCs) or SIADH (Syndrome of Inappropriate Antidiuretic Hormone, up to 45%) [22]. Other clinical presentations in NSCLC also can present in SCLC such as chronic cough, hemoptysis, or chest pain. Because SCLC is usually located at the central part of the respiratory airway, superior vena cava syndrome is also more common than in NSCLC.
6 Treatment Approaches
Treatment modalities of SCLC include chemotherapy, radiotherapy, radiosurgery and surgery. Chemotherapy and radiotherapy have a primary role, however, for curative-intent, especially in limited-disease; surgery or radiosurgery should be considered.
6.1 Surgery
Radiotherapy and chemotherapy are primary treatments of SCLC, however, surgery may have a role in early disease. Recent study using propensity matching analysis compared OS between surgical treatment(2,619 patients) and chemotherapy-based non-surgical treatment (27,375 patients) of stage I-III SCLC from National Cancer Database found that surgery was associated with longer survival for Stage I (median OS 38.6 months vs. 22.9 months, HR 0.62 95%CI 0.57-0.69), but survival differences were attenuated for Stage II (median OS 23.4 months vs. 20.7 months, HR 0.84 95%CI 0.70-1.01) and IIIA (median OS 21.7 vs. 16.0 months, HR 0.71 95%CI 0.60-0.83). In analyses by T and N stage, longer OS was observed in resected T3/T4 N0 patients (median OS 33.0 vs. 16.8 months, p=0.008) and node positivity (N1+ 24.4 vs. 18.3 months p=0.03; N2+ 20.1 vs. 14.6 months p=0.007). In the subgroup analysis of stage I/II patients, patients underwent lobectomy with adjuvant chemotherapy was associated with significantly longer survival (median OS 48.6 vs. 28.7 months, p<0.0001) than those with CCRT without surgery. They concluded that surgical resection is associated with significantly longer survival for early SCLC [23]. A large population data- base, US population-based database from 1988 to 2002 with 14,179 SCLC patients and 863 (6.1%) of these who underwent surgery were analyzed. Surgical was more commonly performed in limited-disease and had longer survival than in the non-surgical group. Patients with localized disease underwent lobectomy had a median survival of 65 months and a 5-year OS of 52.6% whereas patients who had regional disease had a median survival of 25 months and a 5-year OS rate of 31.8%. Only N 2 disease patients received a benefit from adjuvant radiotherapy [24]. Another larger database, The National Cancer Institute Surveillance Epidemiology and End Results (SEER) database from 1988 to 2004 with 1,560 stage I SCLC patients was analyzed to evaluate outcomes between surgical and non-surgical groups. They found that the 5 year survival in patients who underwent lobectomy with postoperative radiotherapy was comparable with those without postoperative radiotherapy (50% versus 57%, respectively) [25]. The ACCP guideline 2013 and NCCN guideline 2017 summarized that surgical resection is recommended in patients with clinical stage I (T1-T2, N0, M0 disease) SCLC after being fully evaluated in distant metastasis and invasive mediastinal staging (head MRI/CT and PET or abdominal CT plus bone scan) and these patients should receive platinum-based adjuvant chemotherapy if pathologic nodal negative, and concurrent chemotherapy with mediastinal radiotherapy if nodal positive. Other indications for surgery in SCLC include 1) solitary pulmonary nodule cytologically diagnosed as SCLC (small cytologic samples may be typical or atypical carcinoid tumors; 2) having combined histology tumors (SCLC and NSCLC); 3) persistent local disease after chemoradiotherapy (possible NSCLC component) if operability and resectability; and 4) new metachronous tumor in small-cell survivor (after complete re-staging) that may be a new NSCLC [3, 26].
6.2 Chemo-Radiotherapy
Two meta-analysis confirmed addition of thoracic radiotherapy improves local control and OS compared with combination chemotherapy alone. The first 11 randomized trials demonstrated absolute increase in OS of 5.4% at 2-years survival [27]. The second 13 randomized trials demonstrated absolute increase in OS of 5.4% from 15% to 20.4% at 3-years [28]. Cisplatin-etoposide concurrent with radiotherapy is more effective than sequential chemo-radiotherapy (median survival of 27.2 months VS 19.7 months, 5-year survival of 23.7% VS 18.3%) [29]. One phase III trial reported superior 5-year OS with twice-daily radiotherapy (1.5 Gy twice-daily, 30 fraction) compared with once-daily (1.8 Gy, 25 fractions) of 26% versus 16% [30]. The optimal timing of the concurrent radiotherapy should be initiated as early as possible. Two meta-analyses showed improvement of 2-year survival with early chemo-radiotherapy compared with late chemo-radiotherapy [31, 32]. On the other hand, recent phase III study demonstrated that patients with limited-stage SCLC receiving late thoracic radiotherapy (TRT) (concurrent TRT start at the third cycle) seemed to be non-inferior to early radiotherapy in term of the complete response rate (late versus early; 38% vs. 36%) and less neutropenic fever [33].
TRT in the patients with extensive-stage SCLC after chemotherapy and prophylactic cranial irradiation (PCI) was assessed from retrospective study showed the median PFS 4.2 months and OS 8.3 months [34]. The data from phase III study stated that patients with extensive-stage SCLC receiving chemotherapy and PCI slightly increase 1-year survival from 28% to 33% and significant prolong 2-year OS from 3% to13% in TRT arm without severe toxic effect [35].
6.3 Prophylactic Cranial Irradiation (PCI)
Brain metastases developed in about 30% of patients [36]. Survival after relapse is generally poor, with a median survival of approximately 4 months. Chemotherapy does not reduce the incidence of brain metastases [37]. PCI in patients that achieve complete response (CR) or near CR in Limited-stage SCLC showed a significant decrease in the incidence of brain metastases at 3 years (33.3% VS 58.6%) [38, 39] and improved quality of life and 5-year survival (22–26%) [40]. Total dose of PCI 24–36 Gy, with once-daily or twice-daily fractions equal to 2–3 Gy/day; PCI and concomitant chemotherapy can increase toxicity and should be avoided [21]. In extensive stage, PCI significantly decrease the risk of symptomatic brain metastases (40.4–14.6% at 1 year) and improved the 1-year survival (13.3–27.1%) with median OS 5.42 and 6.74 months in the PCI arm [41]. In 2015, the study from japan also investigated the effect of PCI in Limited-SCLC, if SBRT is available and patients can be followed-up with MRI every 3 months, PCI may be not necessary [42]. However, the brain imaging is not part of standard follow-up examination. The results from recent systematic review and meta-analysis indicated that PCI decrease brain metastasis and improve survival in SCLC patients even if the elderly patients. Therefore, PCI should be taken into consideration for all patients who achieve response to first-line chemotherapy and are in a good general condition [43].
6.4 Chemotherapy
Combination chemotherapy has been the main treatment option in extensive-stage SCLC. A meta-analysis of 19 randomized trials with a total of 4,054 patients demonstrated prolonged OS of patients receiving a cisplatin-containing regimen versus a regimen containing others alkylating agents [44]. Cisplatin-etoposide is the standard regimen for extensive-stage SCLC with high response rate 60–80%, median survival 8–10 months [4,5,6,7,8,9,10,11]. There were 3 RCTs studied in combination of cisplatin-irinotecan compared with cisplatin-etoposide in extensive-stage SCLC, the first study from Japan Clinical Oncology Group (JCOG) demonstrated improvement of response rate (67.5–84.4%), PFS (4.8–6.9 months), median survival (9.4–12.8 months) in cisplatin-irinotecan arm [9]; however, the others were not confirmed to be superior in cisplatin-irinotecan combination in terms of response rate, PFS and OS [6,7,8]. The randomized Phase 3 trial from Japan, limited-stage SCLC who achieved no progression after concurrent chemoradiation with cisplatin-etoposide, cisplatin-irinotecan consolidation failed to demonstrate improvement of median overall survival compared with cisplatin-etoposide consolidation (2.8 years versus 3.2 years) [45]. Cisplatin is associated with more GI adverse effects, neurotoxicity, and renal function impairment, and its administration requires a prolonged hydration, but carboplatin is associated with more myelosuppression [46]. Recently meta-analysis of individual patient data shows that carboplatin-based regimens appear to be equally effective in terms of OS, PFS, and ORR compared with cisplatin-based combinations for the first-line therapy of SCLC [47]. A randomized Phase III trial in Scandinavian countries compared an irinotecan plus carboplatin regimen with an oral etoposide plus carboplatin in extensive-stage SCLC, that demonstrated carboplatin plus irinotecan prolonged median survival (7.1–8.5 months), improved 1 year survival (24–34%) with a slightly better quality of life [48]. The increase in toxicity with an addition of a third agent (ifosfamide or paclitaxel) to cisplatin-etoposide did not improve the overall survival [49,50,51,52]. To date, no molecularly targeted agents have yielded a prolonged survival in patients with SCLCs.
In second-line chemotherapy, Patients with small-cell lung cancer (SCLC) that progress after first-line chemotherapy have a poor prognosis and the evidence of a benefit from second-line (SL) chemotherapy is limited depend on the response and duration of response of previous platinum base chemotherapy. If the interval of disease remission from first-line chemotherapy is less than 3 months (resistant or refractory disease) the response of second-line is very poor. In case of the relapse time of disease is greater than 3 months (sensitive disease) the response of second-line will be expected around 25%. A meta-analysis in 21 studies published between 1984 and 2011 demonstrated the response rate to second-line treatment is 27.7% in sensitive disease and 17.9% in refractory disease. The median survival time was 7.7 months and 5.4 months respectively [53].
Relapse SCLC patients who received oral topotecan experienced an improved median survival time compared with the best supportive care alone (25.9 weeks versus 13.9 weeks) P=0.01 [54]. Cyclophosphamide, doxorubicin, and vincristine (CAV) was as effective as topotecan in second line therapy with median survival 24.7 weeks [55]. Another randomized trial, oral topotecan demonstrated activity and tolerability similar to IV topotecan in chemotherapy-sensitive SCLC patients and offered [56] 33 weeks and 35 weeks respectively [57]. A multicenter phase III trial from Japan (JCOG0605) reported the efficacy between combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed SCLC and found that OS was significantly longer in the combination chemotherapy group (median 18.2 months, 95% CI 15.7-20.6) than in the topotecan group (12.5 months, 10.8–14.9; hazard ratio 0.67, 90% CI 0.51-0.88; p=0.0079). The adverse events such as Grade 3 or 4 febrile neutropenia, or grade 3 or 4 thrombocytopenia were not significantly different [58].
6.5 Immunotherapy
Cancer immunotherapies aim to stimulate immune responses, thereby inhibiting the tumor from escaping immune surveillance. Two well-characterized checkpoint pathways include the cytotoxic T-lymphocyte antigen-4 protein (CTLA-4) and programmed cell death-1 protein receptor (PD-1) and ligand (PD-L1) pathways.
There was the phase I/II trial studied in patients with progressive SCLC who were previously treated with platinum-based chemotherapy and were not tested for PD-L1 expression, the objective response rate of nivolumab monotherapy and combination with ipilimumab were 18% and 17% and the OS were 4.4 months and 8.2 months respectively and the toxicity were manageable [59, 60]. The phase Ib trial studied in patients with SCLC prior receiving platinum-based chemotherapy, the observed objective response rate in patients who had expression of PD-L1 positivity at the 1% cut off threshold evaluated by IHC treated with pembrolizumab monotherapy was 33% and the median survival was 9.7 months [61, 62]. Phase II trial studied in the combination of ipilimumab with paclitaxel and carboplatin in two alternative regimens; 1) phased ipilimumab (placebo+paclitaxel/carboplatin followed by ipilimumab + paclitaxel / carboplatin), 2) concurrent ipilimumab (ipilimumab+paclitaxel/carboplatin followed by placebo + paclitaxel/carboplatin) or, and placebo. The median PFS time and median OS time were 5.2, 3.9 and 5.2 months and median OS of 12.9, 9.1 and 9.9 months, respectively [63].
7 Conclusion
In summary, SCLC is an aggressive cancer. Most of patients are in the extensive stage at first presentation. Combination chemotherapy can achieve high overall response rates, but the duration of response is still short. It is important to seek effective targeted therapies to treat SCLC. Although targeted therapy drugs are widely investigated in NSCLC, currently, there are no approved for SCLC. The early phase clinical studies have demonstrated that immunotherapies targeting immune-checkpoint inhibition may improve survival. Further research on immune-checkpoint inhibition will be necessary to improved outcome in patients with SCLC.
Key Points
-
Small cell lung cancer (SCLC) is a high grade neuroendocrine tumor which related with history of smoking.
-
Surgical approach is not the standard treatment due to the aggressive behavior of tumor and majority of patient were diagnosed with metastatic disease. However, surgical resection followed by platinum-based chemotherapy be considered in patients with stage I SCLC.
-
Small cell lung cancer (SCLC) is the highly sensitive chemotherapy disease. Although combination chemotherapy can achieve high rate of response of tumor but the prognosis still poor due to high rate of the disease relapse.
-
Prophylaxis cranial irradiation (PCI) can reduce incidence of brain metastases in both limited-stage and extensive-stage SCLC who achieve response to the initial systemic chemotherapy.
-
Many targeted therapy have been studied but seem to still be ineffective.
-
The immunotherapy has been proposed and might be the new hope in treatment of SCLC and have to be further explore in the future.
Multiple-Choice Questions
-
1.
Which is the 5-year survival of resectable of small cell lung cancer?
-
A.
10–20%
-
B.
20–30%
-
C.
30–50%
-
D.
50–70%
-
E.
70–80%
-
A.
-
Answer: D
-
Surgery is not the mainstay treatment option for SCLC. In case of localized disease underwent lobectomy had median survival of 65 months and a 5-year OS of 52.6%.
-
2.
Which is the 5-year survival of regional disease of small cell lung cancer?
-
A.
10–20%
-
B.
20–30%
-
C.
30–50%
-
D.
50–70%
-
E.
70–80%
-
A.
-
Answer: C
-
Radiotherapy and chemotherapy are primary treatments of SCLC. Patients with regional disease had a median survival of 25 months and 5-year OS of 31.8%.
-
3.
Which of the following is the most common paraneoplastic syndrome in SCLC?
-
A.
Hypercalcemia
-
B.
Cushing syndrome
-
C.
Carcinoid syndrome
-
D.
Paraneoplastic encephalomyelitis
-
E.
Paraneoplastic cerebellar degeneration
-
A.
-
Answer: B
-
The two most common paraneoplastic syndromes for SCLC are Cushing syndrome and SIADH. Humoral hypercalcemia of malignancy is the common paraneoplastic syndrome of squamous cell carcinoma.
-
4.
A 60-year-old man presented with chronic cough and was diagnosed with limited-stage SCLC.
He has good performance status. The basic laboratory is within normal limit.
What of the following is the most appropriate treatment?
-
A.
Cisplatin and etoposide chemotherapy
-
B.
Cisplatin and etoposide chemotherapy follow by thoracic radiation
-
C.
Cisplatin and etoposide chemotherapy with concurrent thoracic radiation
-
D.
Carboplatin and etoposide chemotherapy with concurrent thoracic radiation
-
E.
Cisplatin and etoposide chemotherapy follow by PCI if response to chemotherapy
-
A.
-
Answer: C
-
The standard therapy for patients with limited stage-SCLC who still have good performance status is chemotherapy with concurrent radiation with cisplatin/etoposide.
-
5.
A 70-year-old man with 40-pack-year smoking history presented with alteration of consciousness. MRI of brain was normal. CXR showed left hilar mass. The additional laboratory test showed Na 116 mEq/L. Otherwise blood chemistries are within normal limits. Bronchoscope with biopsy was done. Which of the following is most likely pathologic result?
-
A.
Lung cancer with positive EGFR mutation
-
B.
Lung cancer with positive KRAS mutation
-
C.
Lung cancer with positive synaptophysin IHC
-
D.
Lung cancer with positive ALK rearrangement
-
E.
Neoplastic cells with positive CD117 IHC
-
A.
-
Answer: C
-
SIADH is the more common paraneoplastic syndrome for SCLC than NSCLC. EGFR mutation, KRAS mutation, ALK rearrangement, and positive CD117 IHC are usually found in NSCLC, whereas, positive synaptophysin IHC is usually found in SCLC.
-
6.
Which of the following is the most common genetic alteration in Small cell lung cancer?
-
A.
MYC overexpression
-
B.
PTEN deletion
-
C.
P53 mutation
-
D.
c-MET amplification
-
E.
PARP1 over expression
-
A.
-
Answer: C
-
Nearly all SCLCs patient have the somatic genetic alteration and loss of the prominent tumor suppressor protein 53 (TP53).
-
7.
Which of the following statement is correct regarding the role of prophylactic cranial irradiation (PCI) in SCLC?
-
A.
PCI reduce the incidence of brain metastases in limited-stage and extensive-stage SCLC
-
B.
PCI improves 2-year survival in patients with extensive-stage SCLC who response to frontline chemotherapy
-
C.
PCI improves 3-year survival in all patients with limited-stage SCLC
-
D.
Patients who receive PCI therapy have no long-term cognitive defects
-
E.
All of the above
-
A.
-
Answer: A
-
PCI reduce the incidence of brain metastases in both limited and extensive stage SCLC and prolong 3-year OS for limited-stage SCLCs who achieve complete response or nearly complete response. PCI also improve 1-year for extensive-stage SCLCs who achieve response from initial combination chemotherapy.
-
8.
A 60-year-old man diagnosed with extensive-stage SCLC presented with a bulky hilar lung mass and mediastinal LN metastases. After frontline chemotherapy, the CT scan showed nearly complete response and MRI brain showed no brain metastasis. He still has a good performance status. Which of the following is the appropriate standard of care?
-
A.
Clinical observe
-
B.
MRI brain every 3 months
-
C.
Prophylactic cranial radiation
-
D.
Thoracic radiation to the residual tumor
-
E.
Maintenance chemotherapy with oral etoposide
-
A.
-
Answer: C
-
Prophylactic cranial irradiation for extensive-stage SCLC patients who achieved nearly complete response after systemic chemotherapy should be considered in patients who still have good performance status. MRI after complete treatment is not the routine surveillance imaging in SCLCs.
-
9.
Which is the response rate of second-line chemotherapy in sensitive-disease small cell lung cancer?
-
A.
10%
-
B.
15–25%
-
C.
35–45%
-
D.
50–60%
-
E.
70–80%
-
A.
-
Answer: B
-
The response rate of combination platinum doublet chemotherapy in first line treatment are 60–80%, however for the second line treatment, the response rate is not very impressive even in the sensitive-disease, the response is rate only 25%.
-
10.
A 57-year-old man without comorbid disease and good functional status presented with incidental solitary lung nodule at right upper lobe. Complete diagnostic work-up were done and found small cell lung cancer, clinical stage T1aN0M0. Which is the appropriate treatment in this patient?
-
A.
Chemotherapy alone
-
B.
Chemotherapy and followed by radiotherapy
-
C.
Lobectomy with systematic lymph node dissection
-
D.
Lobectomy with systematic lymph node dissection followed by chemotherapy
-
E.
Lobectomy with systematic lymph node dissection followed by chemoimmunotherapy and radiotherapy
-
A.
-
Answer: D
-
According to current recommendation from ASCO, ACCP, and NCCN guideline, surgical resection followed by platinum-based adjuvant chemotherapy is recommended in patients with stage I SCLC. No previous studies have found significant advantage in the use of postoperative radiotherapy for stage I disease after R0 resection.
Clinical Case
A 65-year-old man presented with chronic cough and weight loss. The CT scan was done showed the 4 cm left upper lobe mass with multiple lung nodule bilaterally. The MRI brain showed a single 2 cm brain metastasis without neurological symptom. The bronchoscopy and biopsy was done and diagnose to be small cell carcinoma. The patient still do the daily life activity by himself and the blood tests for complete bold count and chemistry profiles are within normal limited.
Question and Comments
-
1.
Dose this patient need to start brain radiation first?
-
2.
Which modality should be consider to treat the brain metastasis?
-
3.
Which regimen should be first-line chemotherapy?
Comments
-
1.
According to NCCN guideline version 2.2018, the extensive-stage small cell lung cancer with asymptomatic brain metastasis should be start treatment with platinum-doublet systemic chemotherapy first with whole-brain radiation after completion of systemic therapy.
-
2.
The brain metastasis in small cell lung cancer should be treated with whole-brain radiation rather than surgery or stereotactic radiotherapy/radiosurgery alone, because the patient tend to develop multiple brain metastases. However, stereotactic radiotherapy/radiosurgery could be the option in patients who underwent prophylactic cranial irradiation or whole-brain radiation.
-
3.
The cisplatin and etoposide is the most commonly used combination. However, for the patient who are cisplatin ineligible or elderly patients, carboplatin could be used. There are meta-analysis show that carboplatin-based regimens appear to be equally effective in terms of OS, PFS, and ORR with different toxicity profiles.
Abbreviations
- CT:
-
Computed Tomography
- GI:
-
Gastrointestinal
- IASLC:
-
International Association Study of Lung Cancer
- JCOG:
-
Japan Clinical Oncology Group
- LCNEC:
-
Large Cell Neuroendocrine Carcinoma
- NET:
-
Neuroendocrine Tumor
- OS:
-
Overall Survival
- PCI:
-
Prophylactic Cranial Irradiation
- PET:
-
Positron Emission Tomography
- RCT:
-
Randomized Controlled Trial
- SCLC:
-
Small Cell Lung Cancer
- SIADH:
-
Syndrome of Inappropriate Antidiuretic Hormone
- TRT:
-
Thoracic Radiotherapy
References
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A et al (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24(28):4539–4544
Riaz SP, Luchtenborg M, Coupland VH, Spicer J, Peake MD, Moller H (2012) Trends in incidence of small cell lung cancer and all lung cancer. Lung Cancer 75(3):280–284
Low M, Ben-Or S (2018) Thoracic surgery in early-stage small cell lung cancer. Thorac Surg Clin 28(1):9–14
Evans WK, Osoba D, Feld R, Shepherd FA, Bazos MJ, DeBoer G (1985) Etoposide (VP-16) and cisplatin: an effective treatment for relapse in small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 3(1):65–71
Fukuoka M, Furuse K, Saijo N, Nishiwaki Y, Ikegami H, Tamura T et al (1991) Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 83(12):855–861
Hanna N, Jr PAB, Langer C, Einhorn L, Jr TG, Beck T et al (2006) Randomized phase iii trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 24(13):2038–2043
Jr PNL, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE et al (2009) Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 27(15):2530–2535
Schmittel A, Sebastian M, Fischer von Weikersthal L, Martus P, Gauler TC, Kaufmann C et al (2011) A German multicenter, randomized phase III trial comparing irinotecan-carboplatin with etoposide-carboplatin as first-line therapy for extensive-disease small-cell lung cancer. Ann Oncol 22(8):1798–1804
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A et al (2002) Irinotecan plus Cisplatin compared with Etoposide plus Cisplatin for extensive small-cell lung cancer. N Engl J Med 346(2):85–91
Roth BJ, Johnson DH, Einhorn LH, Schacter LP, Cherng NC, Cohen HJ et al (1992) Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol Off J Am Soc Clin Oncol 10(2):282–291
Sundstrom S, Bremnes RM, Kaasa S, Aasebo U, Hatlevoll R, Dahle R et al (2002) Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol Off J Am Soc Clin Oncol 20(24):4665–4672
Kahnert K, Kauffmann-Guerrero D, Huber RM (2016) SCLC-State of the art and what does the future have in store? Clin Lung Cancer 17(5):325–333
Jett JR, Schild SE, Kesler KA, Kalemkerian GP (2013) Treatment of small cell lung cancer. Chest 143(5):e400S–ee19S
Xanthopoulos EP, Corradetti MN, Mitra N, Fernandes AT, Kim M, Grover S et al (2013) Impact of PET staging in limited-stage small-cell lung cancer. J Thorac Oncol 8(7):899–905
Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A et al (2016) The International Association for the study of lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 11(3):300–311
Rami-Porta R (2016) Staging manual in thoracic oncology, 2nd edn. Editorial Rx Press, North Fort Myers, Aurora, CO
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R et al (2007) The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2(8):706–714
Pelosi G, Sonzogni A, Harari S, Albini A, Bresaola E, Marchio C et al (2017) Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res 6(5):513–529
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB et al (2015) The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10(9):1243–1260
Pavel ME, Singh S, Strosberg JR, Bubuteishvili-Pacaud L, Degtyarev E, Neary MP et al (2017) Health-related quality of life for everolimus versus placebo in patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT-4): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18(10):1411–1422
Pottgen C, Eberhardt W, Stuschke M (2004) Prophylactic cranial irradiation in lung cancer. Curr Treat Options in Oncol 5(1):43–50
Ost DE, Jim Yeung S-C, Tanoue LT, Gould MK (2013) Clinical and organizational factors in the initial evaluation of patients with lung cancer. Chest 143(5):e121S–ee41S
Wakeam E, Acuna SA, Leighl NB, Giuliani ME, Finlayson SRG, Varghese TK et al (2017) Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: a propensity-matched analysis of survival. Lung Cancer 109:78–88
Schreiber D, Rineer J, Weedon J, Vongtama D, Wortham A, Kim A et al (2010) Survival outcomes with the use of surgery in limited-stage small cell lung cancer. Cancer 116(5):1350–1357
Yu JB, Decker RH, Detterbeck FC, Wilson LD (2010) Surveillance epidemiology and end results evaluation of the role of surgery for stage i small cell lung cancer. J Thorac Oncol 5(2):215–219
de Hoyos A, DeCamp MM (2014) Surgery for small cell lung cancer. Thorac Surg Clin 24(4):399–409
Warde P, Payne D (1992) Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 10(6):890–895
Pignon J-P, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL et al (1992) A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327(23):1618–1624
Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S et al (2002) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol Off J Am Soc Clin Oncol 20(14):3054–3060
Turrisi AT, Kim K, Blum R, Sause WT, Livingston RB, Komaki R et al (1999) Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340(4):265–271
Fried DB, Morris DE, Poole C, Rosenman JG, Halle JS, Detterbeck FC et al (2004) Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol 22(23):4837–4845
Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, Kester A, Rutten I, Lambin P (2007) Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev 33(5):461–473
Sun JM, Ahn YC, Choi EK, Ahn MJ, Ahn JS, Lee SH et al (2013) Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer†. Ann Oncol 24(8):2088–2092
Yee D, Butts C, Reiman A, Joy A, Smylie M, Fenton D et al (2012) Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother Oncol 102(2):234–238
Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M et al (2015) Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet (London, England) 385(9962):36–42
Schiller JH, Adak S, Cella D, RF DV 3rd, Johnson DH (2001) Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593–a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol Off J Am Soc Clin Oncol 19(8):2114–2122
Thatcher N, Faivre-Finn C, Lorigan P (2005) Lung cancer: Management of small-cell lung cancer. Ann Oncol 16(suppl_2):ii235–iiii9
Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ et al (1999) Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 341(7):476–484
Meert AP, Paesmans M, Berghmans T, Martin B, Mascaux C, Vallot F et al (2001) Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 1:5
Lee JJ, Bekele BN, Zhou X, Cantor SB, Komaki R, Lee JS (2006) Decision analysis for prophylactic cranial irradiation for patients with small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 24(22):3597–3603
Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M et al (2007) Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 357(7):664–672
Ozawa Y, Omae M, Fujii M, Matsui T, Kato M, Sagisaka S et al (2015) Management of brain metastasis with magnetic resonance imaging and stereotactic irradiation attenuated benefits of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. BMC Cancer 15:589
Zhang W, Jiang W, Luan L, Wang L, Zheng X, Wang G (2014) Prophylactic cranial irradiation for patients with small-cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 14:793
Pujol JL (2000) Carestia, Daurès JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 83:8
Kubota K, Hida T, Ishikura S, Mizusawa J, Nishio M, Kawahara M et al (2014) Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): a randomised phase 3 study. Lancet Oncol 15(1):106–113
Go RS, Adjei AA (1999) Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol Off J Am Soc Clin Oncol 17(1):409–422
Rossi A, Maio MD, Chiodini P, Rudd RM, Okamoto H, Skarlos DV et al (2012) Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 30(14):1692–1698
Hermes A, Bergman B, Bremnes R, Ek L, Fluge S, Sederholm C et al (2008) Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol 26(26):4261–4267
Loehrer PJ Sr, Ansari R, Gonin R, Monaco F, Fisher W, Sandler A et al (1995) Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol Off J Am Soc Clin Oncol 13(10):2594–2599
Mavroudis D, Papadakis E, Veslemes M, Tsiafaki X, Stavrakakis J, Kouroussis C et al (2001) A multicenter randomized clinical trial comparing paclitaxel-cisplatin-etoposide versus cisplatin-etoposide as first-line treatment in patients with small-cell lung cancer. Ann Oncol 12(4):463–470
Miyamoto H, Nakabayashi T, Isobe H, Akita H, Kawakami Y, Arimoto T et al (1992) A phase III comparison of etoposide/cisplatin with or without added ifosfamide in small-cell lung cancer. Oncology 49(6):431–435
Niell HB, II JEH, Miller AA, Watson DM, Sandler AB, Kelly K et al (2005) Randomized phase III Intergroup Trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: cancer and leukemia Group B trial 9732. J Clin Oncol 23(16):3752–3759
Owonikoko TK, Behera M, Chen Z, Bhimani C, Curran WJ, Khuri FR et al (2012) A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 7(5):866–872
O’Brien MER, Ciuleanu T-E, Tsekov H, Shparyk Y, Čučeviá B, Juhasz G et al (2006) Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 24(34):5441–5447
von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG et al (1999) Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 17(2):658–667
Taylor M, Antonia S, Bendell J, Calvo E, Jäger D, de Braud F et al (2015) Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Immunother Cancer 3(Suppl 2):P376
Eckardt JR, von Pawel J, Pujol JL, Papai Z, Quoix E, Ardizzoni A et al (2007) Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol 25(15):2086–2092
Goto K, Ohe Y, Shibata T, Seto T, Takahashi T, Nakagawa K et al (2016) Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 17(8):1147–1157
Antonia SJ, Bendell JC, Taylor MH, Calvo E, Jaeger D, Braud FGD et al (2015) Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Clin Oncol 33(15_suppl):7503
Chae YK, Pan A, Davis AA, Mohindra N, Matsangou M, Villaflor V et al (2017) Recent advances and future strategies for immune-checkpoint inhibition in small-cell lung cancer. Clin Lung Cancer 18(2):132–140
Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S et al (2017) Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 Study. J Clin Oncol Off J Am Soc Clin Oncol 35:3823–3829. https://doi.org/10.1200/JCO.2017.72.5069
Ott P, Felip E, Hiret S, Kim D-W, Morosky A, Saraf S et al (2017) OA05.01 pembrolizumab in patients with extensive-stage small cell lung cancer; Updated survival results from KEYNOTE-028. J Thorac Oncol 12(1):S259
Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R et al (2013) Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol: Official Journal of the European Society for Medical Oncology 24(1):75–83
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Suksombooncharoen, T., Tantraworasin, A., Kongkarnka, S., Lertprasertsuke, N., Wannasopha, Y. (2019). Small Cell Lung Cancer. In: De Mello, R., Mountzios, G., Tavares, Á. (eds) International Manual of Oncology Practice. Springer, Cham. https://doi.org/10.1007/978-3-030-16245-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-16245-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16244-3
Online ISBN: 978-3-030-16245-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)