Abstract
Occipital neuralgia, also known as Arnold’s neuralgia, is a pathology that occurs in 0.1–4.7% of patients with cephalalgia [1]. It is defined by the International Headache Society (IHS) [2] as paroxysmal, shooting or stabbing pain lasting from a few seconds to minutes and starting in the occipital region before radiating within (and often beyond) the distribution of the greater occipital nerve (i.e., Arnold’s nerve, emanating from the C2 and C3 roots), lesser occipital nerve (emanating from the C2 root), or third occipital nerve (from the C3 root). These attacks are sometimes triggered by cold or by cervical movements. Tenderness to palpation of the emergence of the occipital nerve is frequently observed. The neurological examination was normal apart from occasional subjective anomalies (dysesthesia or hypoesthesia affecting a part of the scalp). Between attacks, there is occasionally a persistence of dull headache with variable characteristics (mimicking migraine, tension-type headache, cervical headache, or headache from overuse of analgesics, which are the main differential diagnoses). The syndrome is usually improved by infiltration of the nerve with local anesthetic. Cervicogenic headache (term introduced by Bovim et al. [3]) is precisely defined by the IHS as pain referred from the neck and perceived in one or more regions of the head and/or the face compatible with a cervical spine origin and can be abolished by a block of the cervical spine. But many chronic cervicogenic headaches became occipital neuralgias without efficient treatment and occipital neuralgias are frequently accompanied by tenderness of the neck muscles.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Occipital neuralgia, also known as Arnold’s neuralgia, is a pathology that occurs in 0.1–4.7% of patients with cephalalgia [1]. It is defined by the International Headache Society (IHS) [2] as paroxysmal, shooting or stabbing pain lasting from a few seconds to minutes and starting in the occipital region before radiating within (and often beyond) the distribution of the greater occipital nerve (i.e., Arnold’s nerve, emanating from the C2 and C3 roots), lesser occipital nerve (emanating from the C2 root), or third occipital nerve (from the C3 root). These attacks are sometimes triggered by cold or by cervical movements. Tenderness to palpation of the emergence of the occipital nerve is frequently observed. The neurological examination was normal apart from occasional subjective anomalies (dysesthesia or hypoesthesia affecting a part of the scalp). Between attacks, there is occasionally a persistence of dull headache with variable characteristics (mimicking migraine, tension-type headache, cervical headache, or headache from overuse of analgesics, which are the main differential diagnoses). The syndrome is usually improved by infiltration of the nerve with local anesthetic. Cervicogenic headache (term introduced by Bovim et al. [3]) is precisely defined by the IHS as pain referred from the neck and perceived in one or more regions of the head and/or the face compatible with a cervical spine origin and can be abolished by a block of the cervical spine. But many chronic cervicogenic headaches became occipital neuralgias without efficient treatment and occipital neuralgias are frequently accompanied by tenderness of the neck muscles.
Apart from these cases where the link between a recognized cervical lesion and the headache appears to be certain or probable, can occipital or occipitofrontales headaches be imputed to infraradiologiques lesions or functional joint disturbances in high cervical level? Many authors say so without proof. Bogduk and Marsland [4] suffer from C2 to C3 joints in seven out of ten patients with a fairly banal, occipital and sub-occipital, probably unilateral, irradiating to the forehead, associated with one or more suggestive facts of a cervical origin, such as a traumatic antecedent, aggravation of the pain by the movement of the neck, or a local sensitivity to pressure. The advanced evidence is the complete and reproducible effect of an anesthetic block of the C2–C3 occipital nerve.
2 Anatomy and Physiopathology
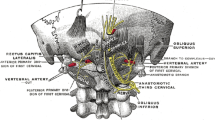
Anatomy of the occipital nerves includes three nerves: the greater, the lesser occipital nerve and the great auricular nerve. Sometimes, a branch from C3 root reaches the midline occipital region. The course of the major occipital nerve is formed by the ramus dorsalis of the C2 nerve root. The first part runs between the origin of the nerve and the musculus obliquus capitis inferior underneath with the nerve makes its first bend in a medial direction. The second part of the nerve runs cranially between the musculus semispinalis capitis on the one side and the musculus obliquus capitis inferior, musculus rectus capitis posterior, and the musculus rectus capitis anterior on the other side. When perforating the musculus semispinalis capitis toward the surface, the nerve makes its second bend in a lateral direction. The third part of the nerve runs further laterally where the aponeurosis of the musculus trapezius is perforated and the nerve begins its subcutaneous course. The nerve usually divides into branches after perforating the aponeurosis (cf. Fig. 15.1).
There are various potential causes of irritations: vascular, neurogenic, muscular, and osteogenic:
-
1.
Vascular: irritation of the nerve by aberrant vertebral artery, dural arteriovenous fistula …
-
2.
Neurogenic: Schwanoma, C2 myelitis, multiple sclerosis
-
3.
Muscular and osteogenic: C1/C2 athrosis, atlodendal sclerosis, cervical chondroma, exuberant callus formation.
The nature of this neuralgia is not well understood. The etiology is variable with idiopathic or secondary cases: post-traumatic, compressive (C1–C2 osteoarthritis, inflammation within a context of rheumatoid polyarthritis or spondyloarthropathy, ligamento-muscular, vascular, or tumoral), postsurgical (treatment of Chiari malformation, surgery of the cervical spine with a posterior approach, surgery of the posterior fossa).
3 Treatments
There are a variety of treatments for occipital neuralgia, ranging from medical treatment to invasive surgery. Medical treatments include analgesics, antidepressants, anti-epileptics, topical agents, physiotherapy, osteopathy, and acupuncture. Local infiltrations of anesthetics or corticosteroids are proposed in cases refractory to medical treatment. If this fails, more invasive treatments are used, such as pulsed radiofrequency, occipital nerve neurolysis, selective rhizotomy at C1–C3 and C2 ganglionectomy [3,4,5,6,7].
A variety of surgical treatments do exist, but they remain imperfect in terms of pain control and may also induce side effects, such as deafferentation pain. Neuromodulation techniques offer a new approach for the treatment of occipital neuralgia. These techniques have already proved effective for migraine and cluster headache [8,9,10,11,12,13,14,15].
In 2011, Slavin [16] traced the history of peripheral nerve stimulation. Its use for the treatment of chronic pain was proposed for the first time by En 1967 by Wall and Sweet with the first implantations made in 1962 by Shelden. The author had shown that the stimulation of peripheral nerves caused a suppression of the perception of pain. Semi-experimental use of this technique continued for 15–20 years. At the end of the year 1980, peripheral nerve stimulation became a recognized surgical technique. At the end of the year 1990, Weiner and Reed proposed the use of such a percutaneous technique of inserting an electrode near the occipital nerves to treat neuralgia occipital. Weiner has shown that this percutaneous stimulation technique is simple and effective. This exploratory work marked the beginning of the modern era of peripheral nerve stimulation. Occipital nerves stimulation is proved to be efficient in occipital neuralgias and cervicogenic headache.
The physiopathologic mechanisms underlying the efficacy of occipital stimulation are still not well known. The gate control theory of Wall and Melzack could play a part in the analgesic action, but is unlikely to be the sole explanation. An involvement of the trigemino-cervical complex most likely contributes to the analgesic effect and partly explains the analgesic effect of occipital stimulation in refractory chronic headaches [17,18,19].
4 Indications for Occipital Stimulation
4.1 Evaluation of the Disease
Patients who suffer from refractory occipital neuralgias according to the IHS criteria will be candidate to occipital stimulation. It is a major criterion to have homogenous studies, but it is not sufficient. Occipital neuralgias must be refractory to medical treatment (association of neuropathic medication like antiepileptic, and/or antidepressant, and/or antalgic treatment like paracetamol, tramadol, or morphine) and pain management in a pain Unit, including multidisciplinary approach, physiotherapy, block test, radiofrequency rhizolysis, and/or corticosteroid infiltration of C2. An essential prerequisite to ensure an acceptable benefit/adverse effect balance is that the patient must have been informed, before any decision to operate, about the possible beneficial effects and possible complications of surgery, and must have realistic – and not unrealistic – expectations in relation to this type of treatment. ON must be chronic (duration of the disease up to 6 months). Pain must be neuropathic pain associated or not with cervicogenic headache. Pain could be unilateral or bilateral. A cranial and cervical spine MRI must be done to eliminate etiology of pain which could be relevant to a surgical treatment (tumors, aneurysms, and spine instability)
4.2 Preoperative Clinical and Psycho-Social Evaluation
This evaluation is essential before considering any invasive procedure for the treatment of pain, in order to select the best candidates for these techniques, to inform the patient about the potential benefits, and to limit the patient’s expectations of a miracle cure. This assessment needs to confirm the refractory nature of the neuropathic pain, to identify the presence of painful physical comorbidities (particularly an association with fibromyalgia) and/or psychological comorbidities, to record the efficacy of previous drug and non-drug treatments, and to determine the absence of general contraindication to neurostimulation procedures (coagulopathy, severe cardiac disease, chronic infections, and other comorbidities). Finally, functional surgery must not be considered if the patient has a life expectancy of less than 6 months.
A thorough psychological and/or psychiatric assessment is very important prior to any interventional technique. Ideally, the opinion of the psychologist or psychiatrist should be integrated into the multidisciplinary consultation meeting. In particular, it is important to evaluate patient’s expectations, fears, and beliefs in relation to the stimulation technique, as well as the presence of any severe or decompensate psychiatric co-morbidity (particularly severe depression or a psychotic illness); any addictive behaviors contraindicating a surgical procedure; a poor compliance and/or insufficient understanding of the treatment and a lack of social and/or family support; and any litigation, which should be settled, whenever possible, before any operative procedure and/or patients awaiting financial compensation.
5 Surgical Techniques
5.1 General Considerations
These stimulation techniques appear to be useful in various refractory neuropathic pain indications, as long as there is some preservation of sensation in the painful area. However, large-scale randomized controlled studies are necessary to confirm the maintenance of the efficacy and to validate emerging indications.
Occipital nerve stimulation (ONS, performed for the first time by Weiner and Reed [20]) is a simple technique that can be performed under local or general anesthesia. It consists of subcutaneous placement of a flat or cylindrical stimulation electrode in contact with the occipital nerves via a retromastoid or midline incision. The electrode is then connected to a stimulator placed under the skin in the infraclavicular or abdominal region.
The patient is placed in a prone position and the first stage of the procedure is performed under mild sedation or local anesthesia to monitor the patient’s feedback and ensure the most optimal coverage of the painful areas by stimulation. After disinfection and sterile draping, a curved needle is inserted at the emergence of Arnold’s nerve through midline or lateral incision. Fluoroscopic and/or echography could be used to be sure of the position of the lead with one or more contacts crossing the nerve. The electrode is sutured in place with nonabsorbable sutures using two plastic anchors. If the trial is successful, defined as more than 50% of pain reduction, the second stage (implantation of the pulse generator) is done under general anesthesia. A subcutaneous pocket is created below the clavicula, on the flank, on the buttock according to the center habits. Extension cables, if needed, are tunneled and connecting electrode and pulse generator. Otherwise, leads are directly connected to the pulse generators.
Jennifer Sweet [21] proceeds to a systematic review of occipital nerve stimulation in refractory occipital neuralgias. Nine series were analyzed: five retrospective studies, three prospective, and one unknown.
We can add our personal retrospective study performed on 60 patients with intractable occipital neuralgias treated with peripheral nerve stimulation (PNS) was performed during the period from October 2008 to October 2014. Pain evaluation, location, duration and cause, and previous treatment were analyzed. Evaluations included a visual analogue scale (VAS) before and 6 months after PNS implantation, the Medication Quantification Scale (MQS) before and 6, 12 months after implantation and at the last follow-up, failure of medical treatment and a multidisciplinary approach to pain. External trials with transcutaneous electrical nerve stimulation (TENS) were performed to evaluate if the trial is successful (patient reported at least 30% decrease of pain on VAS during 1 month). Sixty patients were implanted.
5.2 Paddle Lead vs. Percutaneous Lead
Paddle or percutaneous leads can be inserted via a retromastoid or a midline approach.
Retromastoid approach could be used for unilateral or bilateral approach. Several authors described a retromastoid approach to lead placement: Weiner and Reed [20], Melvin Jr et al. [22], Slavin et al. [23], Oh et al. [24], Kapural et al. [25] and Johnstone and Sundaraj [26] described implantation of paddle lead via a midline approach. Slavin used the two approaches: lateral if unilateral lead and midline if bilateral occipital neuralgias with percutaneous lead.
In our series, we use percutaneous lead and paddle lead with the two approaches like Slavin et al. [23].
In my opinion, paddle lead could be fixed and avoid some side effects like migration, but the inconvenient of paddle lead is to require dissection of surrounding tissues in contrast to percutaneous lead which is inserted via a needle. The needle is bent to conform to the occipital region as described by Weiner and Reed, but this process is imperfect, as the curve of the occipital region is not uniform. This is the major challenge of the future lead to be curve, flexible, and adapted to the occipital region (Fig. 15.2).
5.3 Local Versus General Anesthesia
This criterion is left to the appreciation of each according to local habits. If the percutaneous electrodes can be inserted under local anesthesia, it is more complicated for the surgical electrodes.
Weiner and Reed [20] described electrode placement under local anesthesia. The advantage of the local anesthesia is to be able to test the correct positioning of the paresthesia induced by the electrode covered the painful territory by the paresthesia. However, experienced physicians now perform the procedure under general anesthesia [23, 27, 28]. For a review, see the article of Paemeleire and Bartsch [19].
5.4 Fluoroscopy Alone or Fluoroscopy Plus Ultrasonography
A study was published on this subject [29]. This is a retrospective study of 21 patients with 53 electrodes. Patients had refractory occipital neuralgia for which an indication of occipital stimulation had been retained. The aim of the study was to compare two groups: patients with ONS placed with fluoroscopy and patients with ONS placed with ultrasonography and fluoroscopy. There were no statistical differences between the two groups. However, it could be useful to use ultrasonography to assess the depth of the lead. Indeed, subcutaneous stimulation is effective only if it is well positioned between the dermis and the hypodermis in order to stimulate the receptors of specific fibers linked to the tact; otherwise, there is a risk of stimulation of the muscles with contractures and unpleasant feelings. The stimulation should not be too superficial because there is a risk of skin erosion by the electrode (Fig. 15.3).
-
1.
Sense of hair
-
2.
Sense of touch
-
3.
Epicritic sense
-
4.
Pressure sense
-
5.
Thermic sense
-
6.
Coarse sense
-
7.
Pain
-
8.
Protopathic sense
6 Results and Complications
6.1 Results
ONS is an effective treatment to refractory occipital neuralgias. All the articles and review provide class III-level evidence. Only three articles concern prospective study with small numbers of patients (24 patients). There is heterogeneity in evaluating the results with the use of different scales (Short-Form Mac gill questionnaire, the Visual Analog scale, the Present Pain Index, The Pain Disability Index, the percentage of pain decreased). Randomized prospective studies are needed to assess long-term results. ONS provides good results in patents with occipital neuralgia and cervicogenic headache (approximately 80% of patients experience improvement) (Weiner and Reed [20], Melvin Jr et al. [22], Slavin et al. [23], Oh et al. [24], Johnstone and Sundaraj [26], Abhinav et al. [30], Palmisani et al. [31]), and postoperative or posttraumatic occipital neuralgia (Kapural et al. [25]).
Our series is the larger series in the world. Mean Vas decreased dramatically after ONS implantation from 8.35 preoperatively to 2.32 postoperatively. The case series of Abhinav is particularly impressive with patient totally pain free. The results seem to be sustained in the long term. The study of Weiner and Reed had the more important follow-up with a mean of 2 years, ranging from 1.5 to 5.5 years.
It is difficult to conclude whether the surgical electrodes provide a greater benefit in terms of pain reduction, but there seems to be a slight superiority of these. In our series, we use less current with paddle lead and we have no migration with paddle lead.
6.2 Failed Trials
Some patients were not implanted because of failed trial: Melvin 3/14, Slavin 4/14, Johnstone 1/8. We don’t have this problem because we use TENS to preselect the patients [32].
We shaved the patient or use Arnold kit for the TENS. All patients who have more than 30% reduction of pain with TENS were implanted with ONS in one stage (lead and pulse generator at the same time). Patients with negative TENS had a trial with a percutaneous lead to test if there is pain reduction about 50% or more. If the trial is negative, patients do not receive the implanted pulse generator.
6.3 Parameters Settings
In the literature, Oh and Whiting [33] described high-intensity parameters: 8 V, 330 μs, 85 Hz at the beginning, which decreased at 2.5 V, 300 μs, 85 Hz with a cycling mode.
For Slavin et al. [23], pulse width ranged from 90 to 360 μs, the frequency ranged 30 to 90 Hz, and the amplitude ranged from 1 to 4 V.
In the study of Rodrigo-Royo et al. [34], in four cases of cervicogenic headache, pulse width ranged from 210 to 450 μs, the frequency ranged 40 to 60 Hz, and the amplitude ranged from 0.3 to 2.7 V.
The implanted material in our series was obtained from three manufacturers (Medtronic, Boston Scientific, St Jude Medical) and was distributed as follows: three Boston Scientific, five Medtronic, 51 St Jude Medical. Seventeen patients received a rechargeable stimulator.
The stimulation parameters varied between patients:
-
For St Jude Medical: 4.25 mA, 50 Hz, 208 μs, the frequency varied from 30 to 80 Hz, PW ranged from 156 to 256 μs, and current intensity varied from 1.1 to 13.3 mA.
-
For Boston: intensity was 4.7 mA for each side, 50 Hz, 450 μs (1.2 to 17 mA).
-
For Medtronic: mean intensity was 3.4 V, 50 Hz, 210 μs (with intensity varying from 1.2 to 7 V and a current pulse of between 150 and 450 μs).
The highest intensities were observed with four- or eight-lead percutaneous electrodes.
On average, for St Jude Medical, the parameters were 2.25 mA, 208 μs, 58 Hz if a single electrode was implanted (Table 15.1).
6.4 Complications
Complications included lead migration, infection, allergic reaction, pain on the neck or on the pulse generator site. The rate of complications varied from 0 (Abhinav et al. [30]) to 33% (Palmisani et al. [31]).
Lead migration is due to movements of the head. In the all ten studies, migration of the lead occurred in 4.6% (6/131). In all cases, it was percutaneous electrodes. Lead dysfunction (breaking or disconnected electrode was present in the series of Melvin Jr et al. [22] (1/136).
An infection is found in 5.3% of cases. Depending on the series, the infection rate is between 0% and 29% (cf. Table 15.2). It was the most common complication for this procedure. The treatment can be taken locally or, most often, ablation of the stimulator with antibiotic therapy. No consensus is formally established at present to avoid infections. The study of Bendel et al. [36] of 2737 SCS implant procedures identified all procedures complicated by infection (2.45%). Localized incisional pain and wound erythema were the most common presenting signs. Laboratory studies were performed in the majority of patients, but an imaging study was performed in less than half of these patients. The most common causative organism was Staphylococcus aureus and the IPG pocket was the most common site of an SCS-related infection. Explantation was ultimately performed in 52 of the 67 patients (77.6%). Non-explantation salvage therapy was attempted in 24 patients and was successful in resolving the infection in 15 patients without removal of SCS hardware components.
It can be concluded from this study that utilizing an occlusive dressing over the incision in the postoperative period decreases the rate of infection and should become the standard of care. This study also demonstrated the positive impact of postoperative antibiotics in decreasing the rate of infection. There was no difference in the rate of infection between implants performed by physicians of different base specialties, cylinder leads vs. paddle leads, or between different prophylactic antibiotics. Implants performed at academic centers had a higher rate of infection when compared to implants performed in non-academic settings [37].
7 Conclusion
The efficacy of occipital stimulation in occipital and cervicogenic neuralgias seems undeniable in view of the results of the literature. It’s a simple, reversible, minimally invasive technique with few complications. The majority of complications are related to electrode migration and infection. It can be avoided with new design of cylindrical electrodes. Prospective studies are needed to assess these results.
References
Kuhn WF, Kuhn SC, Gilbertstadt H. Occipital neuralgias: clinical recognition of a complicated headache. J Orofac Pain. 1997;11:158–65.
Headache Classification Committee of the International Classification Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2013;33:629–808.
Bovim G, Fredriksen TA, Stolt-Nielsen A, Sjaastad O. Neurolysis of the greater occipital nerve in cervicogenic headache. A follow up study. Headache. 1992;32:175–9.
Bogduk N, Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine. 1988;13(6):610–7.
Dubuisson D. Treatment of occipital neuralgia by partial posterior rhizotomy at C1-3. J Neurosurg. 1995;82:581–6.
Gille O, Lavignolle B, Vital JM. Surgical treatment of greater occipital neuralgia by neurolysis of the greater occipital nerve and sectioning of the inferior oblique muscle. Spine. 2004;29:828–32.
Lozano AM, Vanderlinden G, Bachoo R, Rothbart P. Microsurgical C-2 ganglionectomy for chronic intractable occipital pain. J Neurosurg. 1998;89:359–65.
Stechison MT. Outcome of surgical decompression of the second cervical root for cervicogenic headache. Neurosurgery. 1997;40:1105–6.
Wilbrink LA, Teernstra OP, Haan J, van Zwet EW, Evers SM, Spincemaille GH, et al. Occipital nerve stimulation in medically intractable, chronic cluster headache. The ICON study: rationale and protocol of a randomised trial. Cephalalgia. 2013;33:1238–47.
Goadsby PJ. Analysis of occipital nerve stimulation in studies of chronic migraine and broader implications of social media in clinical trials. Cephalalgia. 2013;33:214–5.
Burns B, Watkins L, Goadsby PJ. Treatment of medically intractable cluster headache by occipital nerve stimulation: long-term follow-up of eight patients. Lancet. 2007;369:1099–106.
Schwedt TJ, Dodick DW, Hentz J, Trentman TL, Zimmerman RS. Occipital nerve stimulation for chronic headache—long-term safety and efficacy. Cephalalgia. 2007;27:153–7.
Fontaine D, Vandersteen C, Magis D, Lanteri-Minet M. Neuromodulation in cluster headache. Adv Tech Stand Neurosurg. 2015;42:3–21.
Magis D, Allena M, Bolla M, De Pasqua V, Remacle JM, Schoenen J. Occipital nerve stimulation for drug-resistant chronic cluster headache: a prospective pilot study. Lancet Neurol. 2007;6:314–21.
Dodick DW, Silberstein SD, Reed KL, Deer TR, Slavin KV, Huh B, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2015;35:344–58.
Slavin KV. History of peripheral nerve stimulation. Prog Neurol Surg. 2011;24:1–15.
Goadsby PJ, Knight YE, Hoskin KL. Stimulation of the greater occipital nerve increases metabolic activity in the trigeminal nucleus caudalis and cervical dorsal horn of the cat. Pain. 1997;73:23–8.
Piovesan EJ, Kowacs PA, Tatsui CE, Lange MC, Ribas LC, Werneck LC. Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia. 2001;21:107–9.
Paemeleire K, Bartsch T. Occipital nerve stimulation for headache disorders. Neurotherapeutics. 2010;7:213–9.
Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217–21.
Sweet JA, Mitchell LS, Narouze S, Sharan AD, Falowski SM, Schwalb JM, et al. Occipital nerve stimulation for the treatment of patients with medically refractory occipital neuralgia: Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline. Neurosurgery. 2015;77(3):332–41.
Melvin EA Jr, Jordan FR, Weiner RL, Primm D. Using peripheral stimulation to reduce the pain of C2-mediated occipital headaches: a preliminary report. Pain Physician. 2007;10(3):453–60.
Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58(1):112–9.
Oh MY, Ortega J, Bellotte JB, Whiting DM, Aló K. Peripheral nerve stimulation for the treatment of occipital neuralgia and transformed migraine using a c1-2-3 subcutaneous paddle style electrode: a technical report. Neuromodulation. 2004;7(2):103–12.
Kapural L, Mekhail N, Hayek SM, Stanton-Hicks M, Malak O. Occipital nerve electrical stimulation via the midline approach and subcutaneous surgical leads for treatment of severe occipital neuralgia: a pilot study. Anesth Analg. 2005;101(1):171–4.
Johnstone CS, Sundaraj R. Occipital nerve stimulation for the treatment of occipital neuralgia-eight case studies. Neuromodulation. 2006;9(1):41–7.
Paemeleire K, Van Buyten JP, Van Buynder M, Alicino D, Van Maele G, Smet I, et al. Phenotype of patients responsive to occipital nerve stimulation for refractory head pain. Cephalalgia. 2010;30(6):662–73.
Trentman TL, Zimmerman RS, Dodick DW, Dormer CL, Vargas BB. Occipital nerve stimulator placement under general anesthesia: initial experience with 5 cases and review of the literature. J Neurosurg Anesthesiol. 2010;22(2):158–62.
Jones JH, Brown A, Moyse D, Qi W, Roy L. Survival analysis of occipital nerve stimulator leads placed under fluoroscopic guidance with and without ultrasonography. Pain Physician. 2017;20(7):E1115–21.
Abhinav K, Park ND, Prakash SK, Love-Jones S, Patel NK. Novel use of narrow paddle electrodes for occipital nerve stimulation--technical note. Neuromodulation. 2013;16(6):607–9.
Palmisani S, Al-Kaisy A, Arcioni R, Smith T, Negro A, Lambru G, et al. A six year retrospective review of occipital nerve stimulation practice--controversies and challenges of an emerging technique for treating refractory headache syndromes. J Headache Pain. 2013;14:67.
Nguyen JP, Nizard J, Kuhn E, Carduner F, Penverne F, Verleysen-Robin MC, et al. A good preoperative response to transcutaneous electrical nerve stimulation predicts a better therapeutic effect of implanted occipital nerve stimulation in pharmacologically intractable headaches. Neurophysiol Clin. 2016;46(1):69–75.
Oh M, Whiting D. Minimally invasive peripheral nerve stimulation for the treatment of occipital neuralgia. Pain Section Newsletter, October 1999, CNS Meeting.
Rodrigo-Royo MD, Azcona JM, Quero J, Lorente MC, Acín P, Azcona J. Peripheral neurostimulation in the management of cervicogenic headache: four case reports. Neuromodulation. 2005;8(4):241–8.
Magown P, Garcia R, Beauprie I, Mendez IM. Occipital nerve stimulation for intractable occipital neuralgia: an open surgical technique. Clin Neurosurg. 2009;56:119–24.
Bendel MA, O’Brien T, Hoelzer BC, Deer TR, Pittelkow TP, Costandi S, et al. Spinal cord stimulator related infections: findings from a multicenter retrospective analysis of 2737 implants. Neuromodulation. 2017;20(6):553–7. https://doi.org/10.1111/ner.12636.
Hoelzer BC, Bendel MA, Deer TR, Eldrige JS, Walega DR, Wang Z, et al. Spinal cord stimulator implant infection rates and risk factors: a multicenter retrospective study. Neuromodulation. 2017;6:558–62. https://doi.org/10.1111/ner.12609.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Raoul, S., Slavin, K.V. (2020). Neuromodulation in Cervicogenic Headache and Occipital Neuralgia. In: Lambru, G., Lanteri-Minet, M. (eds) Neuromodulation in Headache and Facial Pain Management. Headache. Springer, Cham. https://doi.org/10.1007/978-3-030-14121-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-14121-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14120-2
Online ISBN: 978-3-030-14121-9
eBook Packages: MedicineMedicine (R0)