Abstract
Purpose of Review
Occipital neuralgia (ON) and cervicogenic headache (CGH) are secondary headache disorders with occipital pain as a key feature. Due to significant phenotypic overlap, differentiating ON and CGH from primary headache disorders such as migraine or tension-type headache, or other secondary headache disorders, can be clinically challenging. This article reviews the anatomy, clinical features, unique diagnostic considerations, and management approaches relating to ON and CGH.
Recent Findings
Conservative therapeutic approaches are considered first-line. Anesthetic nerve blocks may have a dual role in both supporting diagnosis and providing pain relief. Newer minimally invasive procedures, such as pulsed radiofrequency (PRF) and occipital nerve stimulation (ONS), represent an exciting therapeutic avenue for severe/refractory cases. Surgical interventions should be reserved for select patient populations who have failed all other conservative and minimally invasive options, to be weighed against potential risk.
Summary
ON and CGH represent an ongoing diagnostic challenge. Further studies are required to consolidate efficacy regarding the comprehensive management of ON and CGH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Occipital neuralgia (ON) and cervicogenic headache (CGH) are secondary headache disorders with occipital pain as a key feature. Although there can be some clinical overlap between these two entities, distinct additional features can help clinically with differentiation. In this article, we review the anatomy, clinical features, diagnostic considerations, and management approaches relating to ON and CGH.
Anatomy
Headache in ON and CGH relates mechanistically to convergence between the upper cervical nociceptive afferents and the trigeminal nociceptive afferents in the trigeminocervical complex. This allows for pain arising from the upper cervical nerves to be referred to regions of the head innervated by trigeminal afferents, such as the orbital, frontal, and parietal regions [1,2,3].
Complementary studies have mapped the distribution of referred pain related to the atlantoaxial and zygapophysial (facet) joints of the upper cervical spine. Stimulation at the C1 level experimentally was found to evoke occipital or cervical pain in those without migraine, although was more likely to evoke periorbital and frontal pain in patients with a history of migraine [4••]. C2 and C3 stimulation was found to refer pain to the occipital or cervical region. Although studies have not supported middle or lower cervical lesions (below C4) as being contributory to headache, anastomosis between the spinocervicothalamic tract and the trigeminocervical complex may support this possibility [5•].
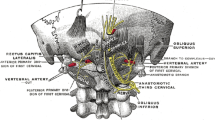
The greater occipital nerve (GON) originates from the medial aspect of the dorsal ramus of C2, where it branches out to become the largest pure sensory nerve in the body. From its origin, the GON travels downward and laterally, bending around the inferior oblique muscle. After traversing the inferior oblique, the GON travels between the inferior oblique and the deep surface of semispinalis capitis, piercing the semispinalis capitis muscle inferior to the inion. The GON then pierces the trapezius aponeurosis, where it exits and travels through multiple superficial branches to supply the integument of the scalp overlying the posterior skull to the vertex. Clinically, the superficial portion of the nerve can be palpated approximately at the medial 1/3rd of a line between the external occipital protuberance (EOP) to the mastoid process (MP) [6, 7]. This is roughly 3 cm below the EOP and 1.5 cm from the midline. There is some inter-individual variability in the anatomy of the GON, particularly along the vertical axis, and this remains a consideration in clinically locating the ON; it may be reasonable to palpate 1–2 cm superiorly and inferiorly to determine the point of tenderness, which can assist in localization.
The lesser occipital nerve (LON) arises from the ventral ramus of C2 and C3 in the cervical plexus. The LON hooks around the spinal accessory nerve and travels upwards along the posterior border of the sternocleidomastoid muscle before exiting through the deep fascia at the occiput. The LON then branches to supply the integument of the scalp, the posterior third of the temporal fossa, and the neck posterior and superior to the auricle. Clinically, the superficial portion of the LON can be palpated at the lateral 1/3rd of the EOP to MP line. This is roughly 4–6 cm below the EOP and 6–7 cm from the midline [8].
The third occipital nerve arises from the dorsal ramus of C3 and innervates the integument of the lower occipital scalp and upper neck.
Epidemiology and Demographics
Clinical Features
ON is classified in the International Classification of Headache Disorders, 3rd Edition (ICHD–3) [12] as a headache characterized by paroxysmal shooting or stabbing pain over the posterior scalp, in the distribution of the occipital nerve. This may be in the distribution of the greater occipital nerve, the lesser occipital nerve, or both. Pain from ON typically radiates from the suboccipital region towards the vertex and is unilateral in 85% of patients [13]. The pain may be severe, and paroxysmal attacks may last seconds to minutes. In between paroxysms, there can be a persistent dull ache over the occipital nerve territory, and there may be corresponding dysesthesia or allodynia. Continuous occipital pain in the absence of any associated dysesthesia or allodynia should raise suspicion for possible referral of pain from the cervical structures. There is typically tenderness over the affected nerve branches and there may be trigger point tenderness at the emergence of the greater occipital nerve or in the C2 distribution. There also may be tingling evoked by light pressure or percussion over the nerve, known as Tinel’s sign. Pain with hyperextension or rotation of the neck when patients are in bed may also be a feature, known as the pillow sign [8].
Due to interneural connections in the trigeminal spinal nuclei through the trigeminocervical complex, pain from ON may be referred to the ipsilateral temporal, frontal, or orbital areas. ON may be a consequence of a known underlying irritant to the occipital nerve; in the absence of this, it may be considered idiopathic.
ON must be carefully differentiated from pain in the occiput referred from the atlantoaxial or upper zygapophyseal joints, which may point to CGH as a more appropriate diagnosis. CGH is caused by a disorder or lesion within the cervical spine or soft tissues within the neck. Headache must demonstrate a temporal relationship with the cervical disorder to support causality. Cervical range of motion may be reduced, and headache can be made worse with provocation maneuvers of the neck. Demonstration of a cervical disorder on imaging may be supportive of a diagnosis of CGH, but does not establish firm evidence of causation. Causation should be considered in context of the clinical presentation and suspected underlying disorder. Tumors, fractures, infections, cervical spondylosis, osteochondritis, and rheumatoid arthritis of the spine have not been formally validated as a cause for headache, but may support causation for CGH in certain individual cases. Due to the convergence of cervical and trigeminal nociception, upper cervical myelopathy (C1, C2, C3) may be causal for headache [10, 14••]. In those with cervical myelopathy or radiculopathy, up to 88% may report headache [15].
Both CGH and ON may respond to local anesthetic blockade of the occipital nerves (greater, lesser, or both). While this intervention is sensitive, it is not specific. Many other primary headache disorders including migraine, tension-type headache, and cluster headache may also demonstrate a similar response. Clinically, there may be significant overlap in phenotype between ON, CGH, migraine, and tension-type headache with pericranial tenderness. Neck pain can be a predominant feature in up to 68% of patients with migraine and tension-type headache [14••]. In the case of migraine, neck pain may occur as a prodromal symptom, intra-attack, or as a postdromal symptom [15]. Due to connections with cranial nerve VIII (vestibulocochlear), IX (glossopharyngeal), and X (vagus), symptoms such as tinnitus, dizziness, and nausea may be features of both ON and CGH [16]. However, these features are less prominent in ON and CGH than in migraine. While migraine tends to be unilateral shifting, ON and CGH tend to be side-locked, and CGH is often triggered by head movement. Pain radiation in ON and CGH tends to be posterior-anterior. Whereas in migraine, the pain tends to be more anterior, and if there is radiation it tends to be anterior-posterior. ON and CGH should also be differentiated from other secondary disorders such as headache attributed to cervical dystonia, headache attributed to Chiari, headache attributed to cervical artery or vertebral dissection, headache attributed to whiplash injury, congenital malformations, space-occupying or destructive lesions, or infection [17].
Diagnostic Considerations
The diagnostic criteria for ON are outlined in the ICHD-3 [12] and summarized in Table 1. Diagnostic criteria for CGH were previously put forward by the Cervicogenic Headache International Study Group (CHISG) in 1998 with a broader definition, allowing for the presence of photophobia, phonophobia, and nausea [18]. This broader definition was felt to lead to over classification of CGH, or conversely, the missed diagnosis of migraine. The ICHD-3 has promulgated a more limited definition for CGH, requiring the anatomic dependency on a cervical pathology, although neck pain is not required for diagnosis [12]; see Table 2.
Investigations
A detailed history and examination should be the starting point for the clinician. Additional maneuvers on physical examination should include movement tests of the cervical spine, such as passive flexion, extension, and rotation; segmental palpation of the cervical facet joints; and assessment for palpation tenderness over the GON, LON, and upper cervical muscle groups [19].
Imaging is required for the evaluation of patients with suspected ON or CGH. Ultrasound may be a useful technique to evaluate the course of the occipital nerve from its origin at the C2 nerve root until it becomes subcutaneous at the trapezius aponeurosis. This may identify a site of entrapment, corresponding with an enlarged, swollen nerve appearance. However, evaluation with ultrasound relies on expertise in performing the test and interpreting results, which may render it a less valuable diagnostic modality for most clinicians. X-rays of the cervical spine may reveal changes suggestive of arthritis or cranio-cervical instability. Computed tomography (CT) should be considered particularly when there is a high index of suspicion for an osseous pathology.
Magnetic resonance imaging (MRI) remains the imaging modality of choice, as it allows for high-quality visualization of both the cervical spine as well as the surrounding occipital and cervical soft tissues [20•].
It should be noted that osteoarthritic changes are common with advancing age, and the presence of these findings alone on imaging is not diagnostic for CGH. In our experience, over-investigating a patient with a clinical history consistent with a primary headache disorder such as migraine carries the risk of discovering these incidental changes. This has the potential for harm in creating patient doubt around the initial primary headache diagnosis. For this reason, there should be a reasonable clinical suspicion of CGH prior to initiating these investigations.
Management
Management of ON and CGH should start with structured education and support, and creating a therapeutic partnership with the patient to empower the patient to take an active role in the plan of care. Functional outcomes are improved when patients, particularly those with chronic pain (> 3 months) related to their headache disorder, are empowered with the information, skills, and confidence to manage the biopsychosocial impact of their condition. Any underlying or contributory pathology should be appropriately addressed and managed [21].
The various approaches for the management of ON and CGH are discussed below.
Non-pharmacologic
Non-pharmacologic management strategies for both ON and CGH include massage, cool compresses, cranio-cervical exercises, and physiotherapy to improve posture [22, 23]. A randomized-control trial looking at spinal manipulation therapy (SMT) in a cohort of highly selected patients with CGH, in the absence of contraindications to SMT, suggested a linear dose-response relationship between SMT therapeutic sessions and days with CGH, with a reduction in headache days sustained to 52 weeks after the start of therapy [24]. However, potential benefits should be considered against the potential for adverse events and the additional cost and time associated with this intervention. Rare but potentially serious adverse events reported with cervical spine manipulation include traumatic myelopathy and stroke [25, 26]. For this reason, SMT involving the cervical spine is not routinely recommended.
Transcutaneous electrical nerve stimulation therapy (TENS) has been used in the conservative management of CGH, with reported benefit [27•, 28]. Case reports also suggest possible benefit in ON [29, 30]. However, given the inherent difficulty of realistic placebo and blinding in TENS studies, results should be interpreted cautiously [31].
Although the evidence for non-pharmacologic strategies is limited, it is nevertheless comparable or greater than evidence in the literature for the other more invasive therapeutic options, discussed below [32].
Pharmacologic
Pharmacologic therapies for both ON and CGH are largely based on case reports and have not been systematically evaluated. Medications used include NSAIDs, tricyclic antidepressants such as amitriptyline, muscle relaxants such as baclofen, and anticonvulsants such as gabapentin or carbamazepine [13, 24, 33, 34]. Opioids do not have a role in the management of ON or CGH due to lack of evidence for benefit and risk of side effects and dependence. All patients using analgesic therapy for ON and CGH require comprehensive education around safe limitations for use and prevention of medication overuse headache.
Proinflammatory mediators such as cytokines and TNF-α have been hypothesized to be involved in the pathophysiology of CGH. A small open-label pilot study in six patients with CGH found that infliximab treatment, which targets TNF-α, was associated with rapid and sustained effects on headache pain scores and self-administered analgesic consumption. However, further studies are certainly needed to cautiously explore these results [35].
Botulinum toxin A has been used in the treatment of several primary headache disorders, the most evidence-based of which is in the treatment of chronic migraine. The mechanism of benefit is not well understood. One hypothesis surrounds the possible inhibitory effects on sensory nerve mediators implicated in pain such as substance P, calcitonin gene-related peptide (CGRP), and glutamate. In the case of ON and CGH, there is postulation that injection into the nerve entrapment or irritation site may result in direct local inhibition of neurogenic inflammation, and decreased activity in a dynamic range of neurons for indirect inhibition of central sensitization [36]. However, there are a paucity of studies to support the use of botulinum toxin A in the management of CGH or ON. In one randomized controlled trial, botulinum toxin A for CGH was not shown to have a clinically relevant or statistically significant role in the treatment of CGH. A subsequent Cochrane review further supported the lack of evidence for benefit of botulinum toxin A use in CGH [37, 38].
Occipital nerve block injections with botulinum toxin A were evaluated in two small case series, with some suggested improvement in the sharp lancinating pain of ON and less for the dull aching pain [39, 40].
Interventional
Anesthetic block of the greater and/or lesser occipital nerves are often used both diagnostically and therapeutically [12, 41]. However, evidence is limited as most studies are non-controlled [42].
Occipital nerve blocks with or without corticosteroids yield transient benefit in most, with 15–36% sustaining extended relief for several months [8, 43]. Sustained benefit may relate to inhibitory interactions between the afferent inputs into the cervical dorsal horn and trigeminal nucleus caudalis from the occipital nerve [44].
Repeated injections may contribute to a more sustained benefit of headache relief [45]. There are no studies to suggest that the addition of steroids increases the efficacy or duration of response in ON or CGH, and the use of corticosteroids confers additional risk of adverse reactions, alopecia, cutaneous atrophy, and Cushing syndrome if systemically absorbed.
While most clinicians perform GON and LON blocks without image guidance, the use of bedside sonography allows for the anesthetic block to be placed at a more proximal site (between C1/C2) or targeted directly at the site of visualized entrapment. However, studies are needed to determine if there is any benefit to a more proximal or targeted block, as compared to the traditional “blind” block at the nuchal line [20•, 46].
Facet block or anesthetic block of the upper cervical nerves with corticosteroid has also been used as a therapeutic approach in CGH. Intra-articular corticosteroid injections may be beneficial in reducing short-term pain, but may have lesser benefit long-term [47, 48].
Surgical
Minimally Invasive
For patients failing the above interventions, minimally invasive surgical options include neuromodulation with subcutaneous occipital nerve stimulation (ONS), or pulsed radiofrequency (PRF) therapy.
PRF therapy exposes the nerve to high-voltage radiofrequency pulses, which is hypothesized to induce an inhibitory electrical field around the nociceptive afferents, disrupting pain transmission and potentiation [48, 49]. PRF has been described as a promising alternative to the traditional lesioning procedures, outlined below, which carry the risk of post-procedure deafferentation pain [50].
Three observational cohort studies have evaluated the use of PRF in ON, suggesting short-term benefit [51]. In a retrospective analysis, 51% of ON patients treated with PRF reported a > 50% reduction in pain relief at a 3-month follow-up. In this cohort, traumatic etiology, response to occipital nerve block, and multiple rounds of PRF treatment seemed to predict better treatment response [52]. Another prospective study found similar results, with just over half of patients reporting a decrease in pain and resultant medication use [53]. Although these studies support short-term benefit with reduction in pain, the lack of randomized control studies and variation in technique limit a wide application of this treatment modality.
With respect to CGH, a recent systematic review analyzed the data for PRF in CGH and found very limited benefit for PRF. Although several case reports suggest benefit, no high-quality randomized controlled trials or strong non-randomized trials have been conducted to support the use of this intervention [54•, 55, 56•].
Radiofrequency ablation (RF ablation) is a minimally invasive lesioning procedure, selectively destroying A-delta and C pain fibers via thermocoagulation. Initial studies assessing RF ablation demonstrated a lack of benefit for cervicogenic headaches; however, these were targeting the mid-to-lower cervical spine (C3/4 through C6/7) [57]. RF ablation has also been used for ON specifically involving the third occipital nerve [58]. A more recent study including patients with ON and CGH who had failed all other conservation therapies suggested that RF ablation of the C2 dorsal root ganglion and/or third occipital nerve may provide greater than 50% pain relief in the vast majority of recipients, lasting up to 5–6 months. However, the rate of adverse events was not insignificant, ranging from 12 to 13% [59, 60].
ONS involves the subcutaneous insertion of electrodes in the C1/C2 region of the posterior cervical spine and is attractive as a nondestructive, reversible therapeutic approach for refractory ON. The therapeutic mechanism is aligned with the gate theory of pain. Studies have generally been concordant in showing a 62.5–100% improvement in symptomatology post-procedure [61,62,63,64,65]. Complications related to ONS include lead migration (4%), post-surgical infection (12%), and less commonly, lead fracture or disconnection (2%) [8]. The Congress of Neurological Surgeons 2015 Guideline supports the use of ONS for treatment of intractable occipital neuralgia (level III) [66••].
Although the studies evaluating ONS for CGH are scant, the case reports seem to suggest potential benefit [67, 68•, 69, 70•]. Further studies are warranted to clarify clinical translation.
Invasive
More invasive surgical approaches such as neurolysis, posterior partial rhizotomy, and dorsal root entry zone lesioning, have mixed results which should be weighed against the possibility for poor longevity and frequent, often significant, side effects [71,72,73].
Neurolysis of the GON root in ON may provide effective short-term pain relief in selected patients. Several factors have been suggested as correlating with positive outcome including tenderness over the GON, positive response to anesthetic blockade of the GON, history of direct occipital trauma, and pre-operative care under a neurologist or pain specialist. However, the long-term benefit of neurolysis has been questioned, with one study suggesting up to 92% of patients experienced recurrence of symptoms by 1 year [71].
Conclusion
Occipital or neck pain and headache are common complaints that can be multifactorial and challenging to accurately diagnose. The convergence of the upper cervical segment nociceptive afferents in the trigeminocervical complex underlies the anatomical basis for both headache and neck pain to frequently co-exist. Due to significant phenotypic overlap, differentiating ON and CGH from primary headache disorders such as migraine or tension-type headache, or other secondary headache disorders, can be clinically challenging. A detailed history, physical examination with special maneuvers, and appropriate investigations can be informative. Prompt and accurate diagnosis is critical, as pain can be refractory, lead to disability, and reduce quality of life. In the management of ON and CGH, conservative therapeutic approaches are considered first-line. Anesthetic nerve blocks may have a dual role in both supporting diagnosis and providing pain relief. Importantly, response to anesthetic nerve blocks should not be considered pathognomonic or differentiating for ON or CGH, as the specificity is poor, and other primary and secondary headache disorders may also respond. Minimally invasive procedures such as PRF and ONS are an exciting therapeutic avenue for severe/refractory cases, as they are generally well-tolerated; however, more studies are needed to consolidate efficacy. Surgical interventions should be reserved for select patient populations who have failed all other conservative and minimally invasive options, to be weighed against potential risk.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bogduk N. The neck and headaches. Neurol Clin N Am. 2004;22(1):151–71.
Bogduk N. Cervicogenic headache: anatomic basis and pathophysiologic mechanisms. Curr Pain Headache Rep. 2001;5(4):382–6.
Goadsby PJ, Ratsch T. On the functional neuroanatomy of neck pain. Cephalalgia. 2008;28(suppl 1):1–7.
•• Johnston MM, Jordan SE, Charles AC. Pain referral patterns of the C1 to C3 nerves: implications for headache disorders. Ann Neurol. 2013;74(1):145–8 Insights on the patterns of pain referral from the upper cervical nerves.
• Shimohata K, Hasegawa K, Onodera O, Nishizawa M, Shimohata T. The clinical features, risk factors, and surgical treatment of cervicogenic headache in patients with cervical spine disorders. Headache. 2017;57(7):1109–17 Cross-sectional study describing the clinical features of cervicogenic headache and the prevalence in patients with cervical myelopathy/radiculopathy.
Levin M. Nerve blocks in the treatment of headaches. Neurotherapeutics. 2010;7(2):197–2013.
Shin KJ, Kim HS, Kwon HJ, Yang HM. Anatomical consideration of the occipital cutaneous nerves and artery for the safe treatment of occipital neuralgia. Clin Anat. 2018;31(7):1058–64.
Choi II, Jeon SR. Neuralgias of the head: occipital neuralgia. J Korean Med Sci. 2016;31(4):479–88.
Koopman JS, Dieleman JP, Huygen FJ, de Mos M, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain. 2009;147(1–3):122–7.
Sjaastad O, Bakketeig LS. Prevalence of cervicogenic headache: Vaga study of headache epidemiology. Acuta Neurol Scand. 2008;117(3):173–80.
Knackstedt H, Bansevicius D, Aaseth K, Grande RB, Lundqvist C, Russel MB. Cervicogenic headache in the general population: the Akershus study of chronic headache. Cephalalgia. 2010;30(12):1468–76.
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018 ;38(1):1–211.
Bogduk N, Govind J. Cervicogenic headache: an assessment of the evidence on clinical diagnosis, invasive tests, and treatments. Lancet Neurol. 2009;8(10):959–68.
•• Ashina S, Bendtsen L, Lyngberg AC, Lipton RB, Hajiyeva N, Jensen R. Prevalence of neck pain in migraine and tension-type headache: A population study. Cephalalgia. 2015;35(3):211–9 Evaluation of the prevalence of coexisting neck pain in primary headache disorders.
Lampl C, Rudolph M, Deligianni CI, Mitsikostas DD. Neck pain in episodic migraine: premonitory symptom or part of the attack? J Headache Pain. 2015;16:566.
Kuhn WF, Kuhn SC, Gilberstadt H. Occipital neuralgias: clinical recognition of a complicated headache. A case series and literature review. J Orofac Pain. 1997;11(2):158–65.
Blumenfeld A, Siavoshi S. The challenges of cervicogenic headache. Curr Pain Headache Rep. 2008;22(7):47.
Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic headache: diagnostic criteria. Headache. 1998;38(6):442–5.
Van Suijlekom H, Van Zundert J, Narouze S, Van Kleef M, Mekhail N. Cervicogenic headache. Pain Pract. 2010;10(2):124–30.
• Narouze S. Occipital neuralgia diagnosis and treatment: the role of ultrasound. Headache. 2016;56(4):801–7 Review of occipital neuralgia and the use of ultrasound-guided interventional therapies.
Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet. 2004;364(9444):1523–37.
Côté P, Yu H, Shearer HM, Randhawa K, Wong JJ, Mior S, Ameis A, Carroll LJ, Nordin M, Varatharajan S, Sutton D, Southerst D, Jacobs C, Stupar M, Taylor-Vaisey A, Gross DP, Brison RJ, Paulden M, Ammendolia C, Cassidy JD, Loisel P, Marshall S, Bohay RN, Stapleton J, Lacerte M Non-pharmacological Management of Persistent Headaches Associated with neck pain: a clinical practice guideline from the Ontario protocol for traffic injury management (OPTIMa) collaboration. Eur J Pain 2019. doi: https://doi.org/10.1002/ejp.1374.
López-Soto PJ, Bretones-García JM, Arroyo-García V, García-Ruiz M, Sánchez-Ossorio E, Rodríguez-Borrego MA. Occipital Neuralgia: a noninvasive therapeutic approach. Rev Lat Am Enfermagem. 2018;26:e3067.
Haas M, Bronfort G, Evans R, Schulz C, Vavrek D, Takaki L, et al. Dose-response and efficacy of spinal manipulation for care of cervicogenic headache: a dual-center randomized controlled trial. Spine J. 2018;18(10):1741–54.
Van Zagten MS, Troost J, Heeres JG. Cervical myelopathy as complication of manual therapy in a patient with a narrow cervical canal. Ned Tijdschr Geneeskd. 1993;137(32):1617–8.
Biller J, Sacco RL, Albuquerque FC, Demaerschalk BM, Fayad P, Long PH, et al. Stroke. Cervical Arterial Dissections and Assoc Cervical Manip Ther: a statement for healthcare professionals from the American Heart Association/American Stroke Association. 2014;45(10):3155–74.
• Gross A, Langevin P, Burnie SJ, Bédard-Brochu MS, Empey B, Dugas E, et al. Manipulation and mobilisation for neck pain contrasted against an inactive control or another active treatment. Cochrane Database Syst Rev. 2015;23(9):CD004249 Systematic review of mobilisation therapy for neck pain in patients with and without cervicogenic headache.
Chen L, Zhang XL, Ding H, Tao YQ, Zhan HS. Comparative study on effects of manipulation treatment and transcutaneous electrical nerve stimulation on patients with cervicogenic headache. J Chin Integr Med/Zhong Xi Yi. 2007;5(4):403–6.
Ghaly RF, Plesca A, Candido KD, Knezevic NN. Transcutaneous electrical nerve stimulation in treatment of occipital neuralgia: a case report. A A Pract. 2018;11(1):4–7.
Nguyen JP, Nizard J, Kuhn E, Carduner F, Penverne F, Verleysen-Robin MC, et al. A good preoperative response to transcutaneous electrical nerve stimulation predicts a better therapeutic effect of implanted occipital nerve stimulation in pharmacologically intractable headaches. Neurophysiol Clin. 2016;46(1):69–75.
Deyo RA, Walsh NE, Schoenfeld LS, Ramamurthy S. Can trials of physical treatments be blinded? The example of transcutaneous electrical nerve stimulation for chronic pain. Am J Phys Med Rehabil. 1990;69(1):6–10.
Haldeman S, Dagenais S. Choosing a treatment for cervicogenic headache: when? What? How much? Spine J. 2010;10(2):169–71.
Vanelderen P, Lataster A, Levy R, Mekhail N, Van Kleef M, Van Zundert J. Occipital neuralgia. Pain Pract. 2010;10(2):137–44.
Dougherty C. Occipital Neuralgia. Curr Pain Headache Rep. 2014;18(5):411.
Martelletti P. Inflammatory mechanisms in cervicogenic headache: an integrative view. Curr Pain Headache Rep. 2002;6(4):315–9.
Volcy M, Tepper SJ, Rapoport AM, Sheftell FD, Bigal ME. Botulinum toxin a for the treatment of greater occipital neuralgia and trigeminal neuralgia: a case report with pathophysiological considerations. Cephalalgia. 2006;26:336–40.
Linde M, Hagen K, Salvesen Ø, Gravdahl GB, Helde G, Stovner LJ. Onabotulinum toxin a treatment of cervicogenic headache: a randomised, double-blind, placebo-controlled crossover study. Cephalalgia. 2001;31(7):797–807.
Langevin P, Peloso PM, Lowcock J, Nolan M, Weber J, Gross A, et al. Botulinum toxin for subacute/chronic neck pain. Cochrane Database Syst Rev 2011;(7):CD008626.
Kapural L, Stillman M, Kapural M, McIntyre P, Guirgius M, Mekhail N. Botulinum toxin occipital nerve block for the treatment of severe occipital neuralgia: a case series. Pain Pract. 2007;7(4):337–40.
Taylor M, Silva S, Cottrell C. Botulinum toxin type-a (BOTOX) in the treatment of occipital neuralgia: a pilot study. Headache. 2008;48(10):1476–81.
Blumenfeld A, Ashkenazi A, Napchan U, Bender SD, Klein BC, Berliner R, et al. Expert consensus recommendations for the performance of peripheral nerve blocks for headaches–a narrative review. Headache. 2013;53(3):437–46.
Ashkenazi A, Blumenfeld A, Napchan U, Narouze S, Grosberg B, Nett R, et al. Peripheral nerve blocks and trigger point injections in headache management: a systematic review and suggestions for future research. Headache. 2010;50(6):943–52.
Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tawfik OM. Occipital nerve blockade for cervicogenic headache: a double-blind randomized controlled clinical trial. Pain Pract. 2006;6(2):89–95.
Goadsby PJ, Hoskin KL, Knight YE. Stimulation of the greater occipital nerve increases metabolic activity in the trigeminal nucleus caudalis and cervical dorsal horn of the cat. Pain. 1997;73(1):23–8.
Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tawfik OM. Repetitive occipital nerve blockade for cervicogenic headache: expanded case report of 47 adults. Pain Pract. 2006;6(4):278–84.
O'Neill F, Nurmikko T, Sommer C. Other facial neuralgias. Cephalalgia. 2017;37(7):658–69.
Zhou L, Hud-Shakoor Z, Hennessey C, Ashkenazi A. Upper cervical facet joint and spinal rami blocks for the treatment of cervicogenic headache. Headache. 2010;50(4):657–63.
Narouze SN, Casanova J, Mekhail N. The longitudinal effectiveness of lateral atlantoaxial intra-articular steroid injection in the treatment of cervicogenic headache. Pain Med. 2007;8(2):184–8.
Byrd D, MacKey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12(1):37–41.
Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications- a review. Acta Neurochir. 2011;153(4):763–71.
Choi HJ, Oh IH, Choi SK, Lim YJ. Clinical outcomes of pulsed radiofrequency neuromodulation for the treatment of occipital neuralgia. J Korean Neurosurg Soc. 2012;51(5):281–5.
Huang JHY, Galvagno SM, Hameed M, et al. Occipital nerve pulsed radiofrequency treatment: a multi-center study evaluating predictors of outcome. Pain Med. 2012;13(4):489–97.
Vanelderen P, Rouwette T, De Vooght P, et al. Pulsed radiofrequency for the treatment of occipital neuralgia: a prospective study with six months of follow-up. Reg Anesth Pain Med. 2010;35(2):148–51.
• Grandhi RK, Kaye AD, Abd-Elsayed A. Systematic review of radiofrequency ablation and pulsed radiofrequency for Management of Cervicogenic Headaches. Curr Pain Headache Rep. 2018;22(3):18 Review of radiofrequency ablation and pulsed radiofrequency in the management of cervicogenic headache.
Gabrhelik T, Michalek P, Adamus M. Pulsed radiofrequency therapy versus greater occipital nerve block in the management of refractory cervicogenic headache—a pilot study. Prague Med Rep. 2011;112(4):279–87.
Halim W, Chua NH, Vissers KC. Long-term pain relief in patients with cervicogenic headaches after pulsed radiofrequency application into the lateral atlantoaxial (C1-2) joint using an anterolateral approach. Pain Pract. 2010;10(4):267–71.
Lord SM, Barnsley L, Wallis B, McDonald G, Bogduk N. Percutaneous radiofrequency neurotomy for chronic cervical zygapophyseal joint pain. N Engl J Med. 1996;335(23):1721–6.
Govind J, King W, Bailey B, Bogduk N. Radiofrequency neurotomy for the treatment of third occipital headache. J Neurol Neurosurg Psychiatry. 2003;74(1):88–93.
Hamer JF, Purath TA. Response of cervicogenic headaches and occipital neuralgia to radiofrequency ablation of the C2 dorsal root ganglion and/or third occipital nerve. Headache. 2014;54(3):500–10.
Park SW, Park YS, Nam TK, Cho TG. The effect of radiofrequency neurotomy of lower cervical medial branches on cervicogenic headache. J Korean Neurosurg Soc. 2011;50(6):507–11.
Keifer OP Jr, Diaz A, Campbell M, Bezchlibnyk YB, Boulis NM. Occipital nerve stimulation for the treatment of refractory occipital neuralgia: a case series. World Neurosurg. 2017;105:599–604.
Palmisani S, Al-Kaisy A, Arcioni R, Smith T, Negro A, Lambru G, et al. A six year retrospective review of occipital nerve stimulation practice–controversies and challenges of an emerging technique for treating refractory headache syndromes. J Headache Pain. 2013;14:67.
Johnstone CS, Sundaraj R. Occipital nerve stimulation for the treatment of occipital neuralgia-eight case studies. Neuromodulation. 2006;9(1):41–7.
Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58(1):112–9.
Melvin EA Jr, Jordan FR, Weiner RL, Primm D. Using peripheral stimulation to reduce the pain of c2-mediated occipital headaches: a preliminary report. Pain Physician. 2007;10(3):453–60.
•• Sweet JA, Mitchell LS, Narouze S, et al. Occipital nerve stimulation for the treatment of patients with medically refractory occipital neuralgia: congress of neurological surgeons systematic review and evidence-based guideline. Neurosurgery. 2015;77(3):332–41 Systematic review and guideline recommendations for the use of occipital nerve stimulation in the management of occipital neuralgia.
Sjaastad O, Fredriksen T, Jorgensen JV. Electrical stimulation in headache treatment. For separate headache(s) or for headache generally? Funct Neurol. 2009;24(1):53–9.
• Eghtesadi M, Leroux E, Fournier-Gosselin MP, Lespérance P, Marchand L, Pim H, et al. Neurostimulation for refractory Cervicogenic headache: a three-year retrospective study. Neuromodulation. 2018;21(3):302–9 Retrospective review of occipital nerve stimulation in the management of cervicogenic headache.
Rodrigo-Royo MD, Azcona JM, Quero J, Lorente MC, Acin P, Azcona J. Peripheral neurostimulation in the management of cervicogenic headache: four case reports. Neuromodulation. 2005;8(4):241–8.
Jasper JF, Hayek SM. Implanted occipital nerve stimulators. Pain Physician. 2008;11(2):187–200.
Bovim G, Fredriksen TA, Stolt-Nielsen A, Sjaastad O. Neurolysis of the greater occipital nerve in cervicogenic headache. A follow up study. Headache. 1992;32(4):175–9.
Wilhour D, Nahas S. The neuralgias. Curr Neurology Neurosci Rep. 2018;18(10):69.
Schrot RJ, Mathew JS, Li Y, Beckett L, Bae HW, Kim KD. Headache relief after anterior cervical discectomy: post hoc analysis of randomized investigational device exemption trial: clinical article. J Neurosurg Spine. 2014;21(2):217–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Headache
Rights and permissions
About this article
Cite this article
Barmherzig, R., Kingston, W. Occipital Neuralgia and Cervicogenic Headache: Diagnosis and Management. Curr Neurol Neurosci Rep 19, 20 (2019). https://doi.org/10.1007/s11910-019-0937-8
Published:
DOI: https://doi.org/10.1007/s11910-019-0937-8