Abstract
Noninvasive treatment is recommended as first line for female urinary incontinence (UI). However, surgical procedures are more likely to be implemented to cure UI but are associated with more adverse events. Less invasive operative mesh techniques are relatively effective, but not devoid of complications such as bleeding, bladder perforation, urethral injury, infection, and retention that may require mesh resection. In patients for whom the risks of anesthesia and surgery are too high, a minimally invasive approach is recommended, and further research is needed in terms of more compliant, less invasive, and low-cost methods for the treatment of stress UI and pelvic floor dysfunction.
Contemporary scientific and technological breakthroughs have led to better clinical outcomes with minimally invasive procedures with shorter recovery times and lower implicated costs. In this sense, recent evidence supports laser treatment as an effective and compliant intervention for stress UI. In spite of promising initial results, there is still a need for long-term consistent evidence analyzing laser efficacy and safety in the treatment of female UI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
A wide spectrum of treatments for stress urinary incontinence (SUI) include noninvasive (pelvic floor muscle training, weight reduction, laser interventions), minimally invasive (bulking methods), less invasive (sling and mesh), and invasive surgical procedures. Less invasive operative techniques are relatively effective but are still related to >15% complications (bleeding, erosions, urethral injury, infection, chronic pain, and urine retention) [1], while conventional surgery relates to anesthesia risks and high recurrence rates (25%) [2].
The association between pelvic organ prolapse (POP) and SUI with collagen is well established. The expression levels of Type I and Type III collagen are significantly lower in patients with POP and SUI when compared to the controls (p < 0.01) [3], and pubocervical fasciae of incontinent women have a lower collagen content [4]. Postmenopausal hormonal deficiency with increased degradation of collagen reserve is a possible explanation for the failure of many surgical procedures aimed at correcting prolapse/incontinence with a frequent recurrence of symptoms [5].
Contemporary scientific and technological breakthroughs have led to better clinical outcomes with less invasive procedures with shorter recovery times and lower implicated costs. In this sense, recent evidence supports laser treatment as an alternative and effective intervention for SUI [6].
13.2 Mode of Laser Action and Protocol for Its Use for Stress Urinary Incontinence
Pulsed laser photothermal energy can improve collagen structure and initiate neocollagenesis in the skin [7] and pelvic floor with nearby tissue as well [8]. An increase in temperature breaks up intermolecular cross-links and stabilizes the collagen triple-helix structure, thus resulting in the shortening of collagen fibers with consequent neocollagenesis, elastogenesis, neoangiogenesis, and increased fibroblast pool. In addition, morphometry showed an increase of the volume density of blood capillaries and the thickness of the epithelial layer [9].
The Er:YAG laser SMOOTH® mode beam (Fotona, Slovenia) is strongly absorbed in water which is the major constituent of human soft tissue. Precisely controlled sequences of non-ablative Er:YAG laser pulses are delivered to the intravaginal mucous tissue in order to achieve controlled heating of the collagen in the deeper mucosa layers (lamina propria), without ablation and overheating of the mucosa surface, so avoiding the risk of perforation with accidental lesions of the urethra, bladder, or rectum. Recommended parameters are laser spot size of 7 mm, frequency of 1.6 Hz, and fluence (laser energy emitted per unit area) of 6.0 J/cm2. Mechanical pull of the deeper tissue layers following shrinkage of the upper, photothermally processed tissue layers further contributes to the tightening effect [10]. No general anesthesia is needed. The lower vaginal third and introitus can be covered with a thin layer of anesthetic cream. Treatment regimen consists of three sessions 3–4 weeks apart. When the process of neocollagenesis is well on its way, and assuming the patient Collagen Remodeling Capacity (CRC) was not fully reached during the first session, with the second and third session, some previously not affected collagen fibers are additionally captured. Minor side effects include sensation of warmth, increased vaginal discharge, and rarely transient urge urinary incontinence [11].
CO2 laser system MonaLisa Touch® (DEKA, Florence, Italy) intravaginal therapy is delivered once a month for 3 consecutive months. Recommended settings [12, 13] of the microablative fractional CO2 laser (MFCO2-Laser) are as follows: D-pulse mode, dot power 40 W, dwell time 1000 μs, and dot spacing 1000 μm for the treatment of the vaginal canal and the dot power 24 W, dwell time, 400 μs, and dot spacing 1000 μm for the treatment of the vaginal introitus. The procedure is performed in the outpatient setting and does not require any specific preparation or anesthesia. Patients are recommended to avoid coital sexual activity for at least 3 days after each laser application as mild inflammatory reaction may last up to 48 h.
13.3 Stress Urinary Incontinence (SUI)
The first pilot study regarding Er:YAG laser for the treatment of female SUI started on September 20, 2009 [14]. The degree of incontinence and its impact on quality of life were assessed with the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF) [15] tool, where a maximum score of 21 represents permanent incontinence.
The inclusion criteria for entering the study were a history of vaginal delivery, SUI, normal cell cytology, negative urine culture, and a vaginal canal, introitus, and vestibule free of injuries and bleeding. The exclusion criteria were severe prolapse and damage of the rectovaginal fascia, urge incontinence, severe neurological conditions associated with incontinence (multiple sclerosis, spinal cord injury, stroke, Parkinson’s disease), neurogenic bladder, insulin-dependent diabetes mellitus, current urinary tract infection, hematuria, age <18 and >70 years, pregnancy, less than 24 weeks after vaginal delivery, body mass index (BMI) >30 kg/m2, intake of photosensitive drugs, injury and/or active infection in the area to be treated, and undiagnosed vaginal bleeding.
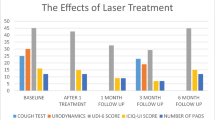
At the first follow-up measurement (1 month after the intervention), the number of those with an ICIQ-UI score = 0 increased from zero to 17/52 (42.3% continent). At the second follow-up measurement, 2–6 months after the intervention, 18/47 (38.3% continent) had an ICIQ-UI score = 0 (Fig. 13.1). From the baseline to the second follow-up, a total of 34/47 (72.3%) of participants experienced improvement, whereas 11/47 (23.4%) experienced no change in the ICIQ-UI score, and two (4.3%) experienced worsening of symptoms. No major adverse events throughout the course of laser treatment and the follow-up period were noticed or reported. The rare mild reported symptoms such as slight edema, vaginal discharge, and transient urgency vanished spontaneously after 8 days.
Kloving’s categories of ICIQ-UI SF score severity at the baseline and follow-up visits. ICIQ-UI International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form, UI urinary incontinence. Reproduced with permission from Fistonic et al. [14]
Ogrinc et al. published a study of 175 women with newly diagnosed SUI (66%) and mixed urinary incontinence (MUI, 34%) [16]. Patients were treated according to the uniform Er:YAG laser protocol. Follow-ups were performed at 2, 6, and 12 months after procedure. Results were based on the Incontinence Severity Index (ISI) and the reduction in ICIQ-UI SF scores. One year after laser treatment, 77% of the SUI patients and only 34% of mixed UI remained continent. No difference in efficacy was noted between pre- and postmenopausal patients.
In another study [17], using the same Er:YAG protocol for SUI, urodynamic studies, pad testing, lower urinary tract symptoms (LUTS), and sexual function were assessed before and after treatment. The authors concluded that the procedure for mild SUI was effective at 6-month follow-up, but was not for the patients with an initial pad weight >10 g. Moreover, it improved LUTS, quality of life (QoL), and sexual function. Urodynamic values did not differ across the timeline. The authors speculate that this paradox originates from tighter and more elastic collagen that acts as a “hammock,” preventing urine leakage and reducing pad weights. Although procedure follow-up was only up to the sixth month, authors summarize that IncontiLase™ should not replace mid-urethral sling (MUS) surgery as the standard therapy for SUI patients who fail to improve following first-line therapy. In addition, authors stress that the injection of bulking agents has been reported to have a cure rate of 53–73.2%, which is better than the cure rate of 39.3% they achieved at a 6-month follow-up with IncontiLase™. In conclusion authors admit that, based on its minimally invasive nature and the lack of significant adverse effects, the IncontiLase™ procedure may be used as an alternative therapy for mild SUI cases.
In a long-term, 24-month follow-up study [18] of 114 postmenopausal women suffering from SUI, the vaginal erbium laser (VEL) treatment induced a significant decrease of baseline ICIQ-SF scores of 12.2 ± 2.5. The ICIQ-UI SF scores remained significantly (p < 0.01) lower than basal values 1 (4.8 ± 1.8), 3 (6.2 ± 1.9), 6 (7.0 ± 2.3), and 12 (8.0 ± 1.8) months after the last VEL application. Scores after 18 (9.3 ± 2.7) and 24 (9.9 ± 2.8) months from the last VEL application were, however, not significantly different from the baseline values. This study shows for the first time that VEL treatment significantly improves the genitourinary syndrome of menopause (GSM) at 12 months after the last laser application, while the effects decrease afterward. The study confirms that VEL is effective in the treatment of GSM, with clinical effects similar to those exerted by established local therapies.
Several other observational studies in which Er:YAG was used for mild to moderate SUI also showed improvement of SUI symptoms [19,20,21]. Up to date, there is only one patient-blinded randomized controlled trial for SUI [22] consisting of 114 women patients receiving a single-session of non-ablative thermal-only Er:YAG laser treatment. This study reported improvement of SUI symptoms, QoL (ICIQ-UI SF), and sexual function (PISQ12 and FSFI) in premenopausal parous women. A 21.4% (12/56) of the laser group patients were continent 3 months after treatment according to ICIQ-UI SF (score = 0) in comparison to only 3.6% (2/56) continence in the sham control group. Covariates age, BMI, and parity had no significant effect on the outcome. All pelvic floor muscle variables, derived from perineometry studies (duration and maximum pressure), showed a significant improvement in the laser group but not in the sham control group.
Carbon (CO2) laser has been used for the treament of GSM, particularly focusing on the vulvovaginal atrophy segment [8]. To date, very few studies regarding CO2 laser treatment of SUI have been published.
Isaza et al. [23] used the SmartXide2 V2LR fractional microablative CO2 laser system (MonaLisa Touch™; Deka, Florence, Italy) in a prospective study of 161 postmenopausal women suffering from mild SUI. Patients received four sessions 30–45 days apart. SUI was evaluated using the 1-h pad test and the ICIQ-UI SF at the baseline and at 12, 24, and 36 months of follow-up. Basal ICIQ-UI SF score (14.34 ± 2.65) significantly decreased 12 (7.09 ± 1.1, p < 0.001), 24 (7.49 ± 0.94, p < 0.001), and 36 months (6.76 ± 0.82, p < 0.001) of follow up. The 1-h pad test reduced from 9.89 ± 0.57 g at the baseline to 3.52 ± 1.89 g, 3.55 ± 1.88 g, and 3.72 ± 2.05 g at 12, 24, and 36 months, respectively (all p < 0.001). Histology analyzed pretreatment and 6 weeks after the first treatment showed essentially thicker epithelium with a higher population of intermediate and superficial cell shedding. Nevertheless, such multiple ablative vaginal treatments raise concerns regarding the possibility of vaginal scarring and infection, which can be reduced with the use of a non-ablative treatment [22].
The prospective observational study regarding the efficacy of fractional CO2 laser in postmenopausal women with moderate to severe clinical signs of GSM [24] showed significant improvement of dyspareunia, dryness, burning, itching, dysuria, urgency, and SUI scores assessed by standard questionnaires. Participants received intravaginal therapy, once a month for 3 consecutive months, with a CO2 laser system (MonaLisa Touch®, DEKA, Florence, Italy). As a secondary outcome, authors noted that urinary symptoms improved, as scores of the urinary and QoL questionnaires significantly decreased. ICIQ-UI SF at the baseline was 8.1 ± 5.6 vs. 3.4 ± 4.3 at the 3rd month of follow-up. All participants showed a >5-point improvement in the King’s Health Questionnaire (KHQ) score, which includes psychometric aspects of urinary incontinence. Despite this, authors concluded that factors predictive of ideal CO2 laser therapy candidates were not identified. Considering predictive, preventive, and personalized medicine (PPPM) current goal is not only to predict the risk of an adverse clinical event but also benefits [25]. A recent study [26] identified predictors for the segment of patients achieving optimal short term Er:YAG laser treatment outcomes. The best results after Er:YAG laser treatment of SUI should be expected in younger women (<47.5 years) with a body mass index of <23.3, average newborn birth weight of >3.6 kg, ICIQ-UI at a baseline of <10, and a perineometer squeeze duration at a baseline of >3.51 s.
However, despite the rigorous selection of patients, in a certain group, laser treatment will not succeed. Namely, SUI is not only induced by urethral hypermobility, as a result of weakening or disruption of the pelvic floor musculature and/or pubourethral ligament, but also due to the weakening of the urethral sphincter, resulting in more severe intrinsic sphincter deficiency (ISD) [27]. The urethral sphincter function depends on the muscular component; the rhabdosphincter, extending along 60–70% of the urethra length; and the mucosal or intrinsic component, extending across the urethra and contributing to urethral closure [28]. Gaspar et al. [29, 30] hypothesized that by targeting the mucosal component of the urethral sphincter, urethral coaptation ability would be able to increase. Authors used the novel Er:YAG intraurethral cannula (IntraLase™, Fotona, Slovenia). As assessed by a questionnaire addressing QoL (ICIQ-UI SF) and the 1-h pad test, therapeutic efficacy was measured at 3 and 6 months after the procedure. ICIQ-SF scores improved by an average of 64% at 3 months and by 40% at 6 months. The 1-h pad test showed a reduction of the quantity of leaked urine by 59% at 3 months and by 42% at 6 months.
Patel [31] enrolled women whose urodynamic studies showed a maximal urethral closure pressure (UCP) of less than 40 cm H2O. Subjects received three fractional CO2 laser treatments 4 weeks apart. Three months posttreatment, urodynamic reevaluation showed an increase in maximal UCP from 19 to 33 cm H2O at pretreatment to 45–73 cm H2O posttreatment.
13.4 Overactive Bladder
Perino et al. [32] analyzed the effect of CO2 laser treatment in postmenopausal women with overactive bladder (OAB) symptoms (≥8 times micturition/24 h) and ≥1 symptoms of GSM (itching, burning, reduced lubrication, superficial and/or severe dyspareunia) in the previous 3 months. OAB symptoms were assessed using the validated Overactive Bladder Questionnaire Short Form (OAB-Q SF). Results at 1-month follow-up after the third laser session indicated a significant reduction of the number of micturitions and number of urge episodes (p < 0.0001). Since atrophy of muscles and reduction of collagen content may be important factors in the increased prevalence of urinary incontinence, authors stress that fractional CO2 laser system can irradiate deeper layers of the vaginal wall, ultimately enhancing tissue trophysm and reactivating the extracellular matrix and collagen synthesis, with beneficial effects in the three layers of the vaginal wall, in contrast to estrogens or other local therapies that only treat the epithelium.
Besides improvement of SUI episodes using the Er:YAG protocol, Tien and coauthors [17] also found a positive effect over OAB, as evidenced by the improvements in the Urgency Severity Scale Questionnaire (USS), the Overactive Bladder Symptoms Score Questionnaire (OABSS), nicturia episodes, and daytime frequency episodes. Since the majority of women with stress predominant MUI experience significant improvement in OAB symptoms following incontinence surgery [33], authors speculate that their findings may be at least partly related to SUI improvements following laser treatment.
In patients with SUI, urine leakage into the proximal urethra may stimulate urethral afferents and facilitate the voiding reflex [34]. Lin et al. [35] hypothesized that laser therapy may slightly increase the entire urethral pressure, including proximal urethral pressure, and in turn alleviate OAB symptoms due to a reduction of the bladder reflex response observed in SUI patients. Their treatment consisted of two sessions, 4 weeks apart using the Er:YAG laser (XS Dynamis, Fotona, Slovenia). OABSS scores were significantly improved at 3-month follow-up (p < 0.027), especially in terms of urinary frequency (p < 0.001). However, symptom scores were not sustained at the 12-month follow-up. By most patients’ report, the optimal therapeutic effect was maintained for the duration of 3–6 months, similar to results observed by Fistonic et al. [14] (2–6 months). Neocollagenesis induced by Er:YAG SMOOTH® mode can change the composition of the pelvic floor structures and thus increase pressure over the entire length of the urethra. In SUI patients, the increased proximal urethral pressure may alleviate OAB symptoms by reducing the bladder reflex response.
13.5 Vaginal Microbiota
An effect of laser on vaginal microbiota has been reported by Athanasiou et al. They assessed the effect of microablative fractional CO2 laser (MFCO2-laser) therapy on the vaginal microenvironment of postmenopausal women [13]. Findings suggest that in their sample of 53 postmenopausal women with moderate to severe GSM symptoms, the completion of three laser therapies (at monthly intervals) significantly increased Lactobacillus (p < 0.001) and normal flora (p < 0.001), which decreased vaginal pH from a mean of 5.5 ± 0.8 (baseline) to 4.7 ± 0.5 (third month, p < 0.001). The prevalence of Lactobacillus changed from a baseline value of 30–79% at 3 months. Clinical signs and symptoms of bacterial vaginosis, aerobic vaginitis, or candidiasis did not appear in any participant. Although significant decreases were observed only for E. coli and Mobiluncus, there was a trend of lower growth of all Lactobacillus antagonists.
Authors suggest that the observed increase of the normal vaginal epithelial cells confirms the results of the histological study of Zerbinati et al. [36] where it was demonstrated that one of the effects of the MFCO2-Laser therapy on the vaginal mucosa was the high degree of epithelial exfoliation, with superficial cells filled with glycogen shedding at the epithelial surface. In conclusion, authors believe that MFCO2-Laser therapy is a promising treatment for the improvement of postmenopausal vaginal health, aiding the repopulation of the vagina with normally existing Lactobacillus species and reconstituting the normal flora as that observed in the premenopausal status.
13.6 Histology
Histological changes in the epithelium and lamina propria, following fractional CO2 laser treatments (CO2 RE Intima, Syneron Candela, Wayland, MA), correlated with clinical findings of vaginal hydration and pH in SUI patients [37]. At the 3-month follow-up, biopsies showed increased collagen and elastin staining, as well as a thicker epithelium with an increased number of cell layers and a better degree of surface maturation (increase in the ratio of parabasal, intermediate, and superficial cells showing an estrogenic effect). At the 6-month follow-up, histology showed increased submucosal vascularity with increased collagen and elastin deposits (Fig. 13.2).
(a) Pretreatment histology of a 59-year-old woman. (b) At 8 months post-baseline, histological findings showed increased submucosal vascularity, as well as increased collagen deposits and elastic fibers. Reproduced with permission from Samuels and Garcia [37]
Histological study of vaginal wall biopsies showed signs of neocollagenesis, elastogenesis, neoangiogenesis, reduction of epithelial degeneration and atrophy, and an increase of the fibroblast population after non-ablative Er:YAG laser SUI treatment (IncontiLase™, Fotona, Slovenia) [38].
13.7 Non-ablative Photothermal Er:YAG Laser and Microablative Fractional CO2 Laser in SUI Treatment: Differences
Lasers used in SUI treatment emit thermal energy at the different wave lengths (Er:YAG 2940 nm; CO2 10,600 nm), but they both induce similar changes related to increased tissue trophysm such as retraction of collagen, neocollagenesis, elastogenesis, enhanced density of connective particles, and neovascularization. CO2 laser thermal action spreads to the depth of 50–125 μm in the vaginal tissue, causing superficial vaporization. Under the same conditions, Er:YAG laser reaches only 5–20 μm in depth with no ablation at all [8].
Er:YAG laser has 10–15 times the affinity for water absorption compared with carbon dioxide laser. Mucous membranes have a very high percentage of water, which is a good target for the Er:YAG laser beam. Because of the extremely high absorption in water, the incident photon energy is almost totally attenuated in the first few micrometers of the tissue, producing at appropriate parameters a very controlled column of ablation with an extremely narrow band of secondary coagulation. This process has been known as residual thermal damage (RTD) [39]. This translates into shorter down time with quicker healing and has been the cornerstone for the use of the Er:YAG in full-face ablative laser resurfacing when compared to the CO2 laser, which has a much larger RTD zone [40]. That was a rationale for Lee to use Er:YAG in the treatment of the vaginal relaxation syndrome [41]. The authors emphasize that the depth control associated with the Er:YAG wavelength offers major benefits as ablative damage depth is minimized. Multiple micro-pulses create a shallow, few μm-thick epidermal windows in the vaginal epithelium with minimal RTD, and subsequent micro-pulses create a pulse-stacking effect without further ablation but with thermal buildup down into the lamina propria, increasing the RTD zone. Only Er:YAG laser is characterized by the critical temperature above ablation temperature, making this laser the safest medical laser for dual-tissue regeneration mechanism (DRM) non-ablative resurfacing (Fig. 13.3) [42].
Critical temperature depends on penetration depth. Only Er:YAG laser is characterized by the critical temperature above ablation temperature. Reproduced with permission from Lukac et al. [42]
Athanasiou et al. [13] stress out, based on Hutchinson-Colas et al. [43] and Helbig et al. [44], that erbium YAG laser only has a thermal effect, while the MFCO2-Laser has both ablative and thermal effects, thus stimulating heat shock proteins and other factors (e.g., TGF-β), promoting neocollagenesis and neoangiogenesis, and consequently resulting in tissue rejuvenation.
13.8 Review Papers
Most review articles discuss different methodological aspects of the published studies regarding the use of laser for the treatment of the GSM, vaginal relaxation syndrome, and urinary incontinence [11, 45,46,47,48,49,50]. Conte et al. [51] reviewed the use of laser for the treatment of female SUI in seven prospective, single-centered, and non-comparative studies without control groups. All studies used Er:YAG or a CO2 laser. Primary outcomes were ICIQ-UI SF scores in six studies and the pad test in one. Follow-up ranged from 5 to 36 months. Improvement rates ranged from 62 to 78%. No major adverse events were noted. Minor side effects included sensation of warmth, increased vaginal discharge, and transient urge urinary incontinence. Authors stand for more rigorous and adequately powered trials to assess the benefits and adverse event profile of laser treatment of SUI, as compared with other minimally invasive procedures.
An updated technical bulletin on laser for GSM and SUI was prepared by the urogynecology committee and approved by the Board of the Society of Obstetricians and Gynaecologists of Canada [52]. Analyzing contemporary reviews on the SUI topic, conclusion is that short-term observational studies of small patient number with the use of intravaginal laser have demonstrated improvements in symptoms of SUI. Evidence is insufficient to offer intravaginal laser therapy as an effective modality for the treatment of SUI compared to alternate managements such as pelvic floor physiotherapy, incontinence pessaries, or surgery.
The Canadian group [53] summarized Er:YAG laser therapy as a minimally invasive, alternative treatment option for female SUI and that laser therapy has yielded reasonable initial outcomes with an acceptable low cost and safety profile. At the same time, authors emphasized that it was not clear which group of patients will respond better to this therapy, as the mechanism of action was still somewhat vague. In this sense, a recently published predictive model [26] detects patients’ baseline characteristics that may aid at achieving the best results after Er:YAG laser treatment for SUI. A clinically relevant decrease in the ICIQ-UI (minimum important difference, MID) of >30% can be predicted based on age, body mass index, average newborn birth weight, perineometer squeeze duration, and pre-intervention ICIQ-UI score. In addition, the first single-blinded sham study regarding Er:YAG laser treatment for SUI [22] moderately reduced frequent criticism on the lack of randomized controlled trials in this field. Results showed a significant improvement in 114 women at the 3rd month follow-up, with a single-session treatment with non-ablative thermal-only Er:YAG laser.
A systematic critical review published in July 2018 analyzed 17 eligible studies including 652 (12 studies) and more than 240 patients (5 studies) treated with Er:YAG-laser and CO2 laser, respectively. Authors concluded that the use of laser for women with urinary incontinence seems a promising minimally invasive alternative to the current standard therapies. On the other hand, there is still lack of evidence showing long-term safety and effectiveness. Additionally, all reviewed studies included only patients with primary urinary incontinence, so there is no information about these treatments on patients previously treated with surgery [54].
Upon the review of the literature derived from the PubMed database up to July 2018, using the key words “laser” and “urinary incontinence”, 326 articles were found. After exclusion for incontinence in male, laser use in surgery, and non-laser techniques, 34 articles met criteria regarding laser use in women with urinary incontinence. Twelve of them were review articles and 22 were original papers. Laser effect in SUI patients was analyzed in 19 and OAB patients in three articles. Authors used Er:YAG laser in 16 studies, while CO2 laser was used in six studies. A total of 1310 patients were enrolled at the 1–36 months of follow up (average 7.9 months).
Although laser is an attractive [45] novel, non-hormonal but expensive [47] new technology for the treatment of the GSM, additional studies are needed to explore the long-term safety and efficacy of various laser therapies for genitourinary symptoms as most of published evidence rely on short-term follow-up (1–6 months). Three studies extended to 12–24 months (Er:YAG) [16, 18, 35] and 1–36 months (CO2) [23]. To date, only one patient-blinded randomized controlled laser sham trial for SUI, using Er:YAG laser, has been published [22]. Also, future studies need to be designed taking mentioned considerations into account, including the histological assessment performed immediately after treatment, which will help compare morphology at baseline with changes in the vaginal architecture following laser procedure [41]. Next studies should focus on the individual patient level in order to predict personal risk or benefit based on the decision of undergoing a given proposed procedure [26, 55]. Simultaneously, predictive systems may impact public health policies in terms of prevention [56].
Abbreviations
- BMI:
-
Body mass index
- CRC:
-
Collagen Remodeling Capacity
- Er:YAG:
-
Erbium:yttrium-aluminum-garnet
- FSFI:
-
Female sexual function index
- GSM:
-
Genitourinary syndrome of menopause
- ICIQ-UI SF:
-
International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form
- ISD:
-
Intrinsic sphincter deficiency
- ISI:
-
Incontinence Severity Index
- LUTS:
-
Lower urinary tract symptoms
- MUS:
-
Mid-urethral sling
- OAB:
-
Overactive bladder
- OABSS:
-
Overactive Bladder Symptoms Score Questionnaire
- PISQ12:
-
Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire
- POP:
-
Pelvic organ prolapse
- QoL:
-
Quality of life
- RTD:
-
Residual thermal damage
- SUI:
-
Stress urinary incontinence
- UCP:
-
Urethral closure pressure
- UI:
-
Urinary incontinence
- VEL:
-
Vaginal erbium laser
References
Blaivas JG, Purohit RS, Benedon MS, Mekel G, Stern M, Billah M, et al. Safety considerations for synthetic sling surgery. Nat Rev Urol. 2015;12:481–509.
Lazarou G, Minis E, Grigorescu B. Outcomes of stress urinary incontinence in women undergoing TOT versus Burch colposuspension with abdominal sacrocolpopexy. Int Urogynecol J. 2019;30:245–50.
Han L, Wang L, Wang Q, Li H, Zang H. Association between pelvic organ prolapse and stress urinary incontinence with collagen. Exp Ther Med. 2014;7:1337–41.
Rechberger T, Postawski K, Jakowicki JA, et al. Role of fascial collagen in stress urinary incontinence. Am J Obstet Gynecol. 1998;179:1511–4.
Tinelli A, Malvasi A, Rahimi S, Negro R, Vergara D, Martignago R, Pellegrino M, Cavallotti C. Age-related pelvic floor modifications and prolapse risk factors in postmenopausal women. Menopause. 2010;17:204–12.
Bergsland J. Major innovations and trends in the medical device sector. Acta Inform Med. 2012;20:44–6.
El-Domyati M, Abd-El-Raheem T, Medhat W, et al. Multiple fractional erbium: yttrium-aluminum-garnet laser sessions for upper facial rejuvenation: clinical and histological implications and expectations. J Cosmet Dermatol. 2014;13:30–7.
Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137–59.
Lapii GA, Yakovleva AY, Neimark AI. Structural reorganization of the vaginal mucosa in stress urinary incontinence under conditions of Er:YAG laser treatment. Bull Exp Biol Med. 2017;162:510–4.
Fistonić N, Fistonić I, Guštek ŠF, Turina ISB, Marton I, Vižintin Z, Kažič M, Hreljac I, Perhavec T, Lukač M. Minimally invasive, non-ablative Er:YAG laser treatment of stress urinary incontinence in women-a pilot study. Lasers Med Sci. 2016;31:635–43.
Gambacciani M, Palacios S. Laser therapy for the restoration of vaginal function. Maturitas. 2017;99:10–5.
Salvatore S, Nappi RE, Zerbinati N, Calligaro A, Ferrero S, Origoni M, Candiani M, Leone Roberti Maggiore U. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363–9.
Athanasiou S, Pitsouni E, Antonopoulou A, Zacharakis D, Salvatore S, Falaggas ME, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19:512–8.
Fistonic N, Fistonic I, Lukanovic A, Findri Gustek S, Sorta Bilajac T, Franic D. First assessment of short-term efficacy of Er:YAG laser treatment on stress urinary incontinence in women: prospective cohort study. Climacteric. 2015;18(Suppl 1):37–42.
Klovning A, Avery K, Sandvik H, Hunskaar S. Comparison of two questionnaires for assessing the severity of urinary incontinence: the ICIQ-UI SF versus the incontinence severity index. Neurourol Urodyn. 2009;28:411–5.
Ogrinc BU, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689–97.
Tien YW, Hsiao SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28:469–76.
Gambacciani M, Levancini M, Russo E, Vacca L, Simoncini T, Cervigni M. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148–52.
Pardo J, Sola V, Morales A. Treatment of female stress urinary incontinence with erbium YAG laser in non-ablative mode. Eur J Obstet Gynecol Reprod Biol. 2016;204:1–4.
Leshunov EV, Martov AG. Application of laser technologies for treatment of urinary stress incontinence in women of reproductive age. Urologiia. 2015;1:36–40.
Neimark AI, Yakovleva AY, Lapii GA. Outcomes of ER:YAG laser treatment of stress urinary incontinence in women. Urologiia. 2018;2:20–5.
Blaganje M, Šćepanović D, Žgur L, Verdenik I, Pajk F, Lukanović A. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153–8.
Isaza PG, Jaguszewska K, Cardona JL, Lukaszuk M. Long-term effect of thermoablative fractional CO2 laser treatment as a novel approach to urinary incontinence management in women with genitourinary syndrome of menopause. Int Urogynecol J. 2018;29:211–5.
Pitsouni E, Grigoriadis T, Tsiveleka A, et al. Microablative fractional CO2-laser therapy and the genitourinary syndrome of menopause: an observational study. Maturitas. 2016;94:131–6.
Golubnitschaja O, Kinkorova J, Costigliola V. Predictive, preventive and personalised medicine as the hardcore of “horizon 2020”: EPMA position paper. EPMA J. 2014;5:6.
Fistonić I, Fistonić N. Baseline ICIQ-UI score, body mass index, age, average birth weight, and perineometry duration as promising predictors of the short-term efficacy of Er:YAG laser treatment in stress urinary incontinent women: a prospective cohort study. Lasers Surg Med. 2018;50:636–43.
Lane TM, Shah PJR. Valsalva leak point pressure in the evaluation of stress urinary incontinence. Braz J Urol. 2000;26:420–5.
McGuire EJ, Fitzpatrick CC, Wan J, et al. Clinical assessment of urethral sphincter function. J Urol. 1993;150:1452–4.
Gaspar A, Brandi H. Non-ablative erbium YAG laser for the treatment of type III stress urinary incontinence (intrinsic sphincter deficiency). Lasers Med Sci. 2017;32:685–91.
Gaspar A, Maestri S, Silva J, Brandi H, Luque D, Koron N, Vižintin Z. Intraurethral erbium:YAG laser for the management of urinary symptoms of genitourinary syndrome of menopause: a pilot study. Lasers Surg Med. 2018;50:802–7.
Patel F. The effects of RF excited fractional CO2 laser on the vaginal canal in treating stress urinary incontinence [2G]. Obstet Gynecol. 2017;129:S71–2.
Perino A, Cucinella G, Gugliotta G, Saitta S, Polito S, Adile B, Marci R, Calagna G. Is vaginal fractional CO2 laser treatment effective in improving overactive bladder symptoms in post-menopausal patients? Preliminary results. Eur Rev Med Pharmacol Sci. 2016;20:2491–7.
Zyczynski HM, Albo ME, Goldman HB, et al. Change in overactive bladder symptoms after surgery for stress urinary incontinence in women. Obstet Gynecol. 2015;126:423–30.
Geirsson G, Fall M. Reflex interaction between the proximal urethra and the bladder. A clinical experimental study. Scand J Urol Nephrol. 1999;33:24–6.
Lin YH, Hsieh WC, Huang L, Liang CC. Effect of non-ablative laser treatment on overactive bladder symptoms, urinary incontinence and sexual function in women with urodynamic stress incontinence. Taiwan J Obstet Gynecol. 2017;56:815–20.
Zerbinati N, Serati M, Origoni M, Candiani M, Iannitti T, Salvatore S. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429–36.
Samuels JB, Garcia MA. Treatment to external labia and vaginal canal with CO2 laser for symptoms of vulvovaginal atrophy in postmenopausal women. Aesthet Surg J. 2019;39:83–93.
Lapii GA, Yakovleva AY, Neimark AI, Lushnikova EL. Study of proliferative activity of vaginal epithelium in women with stress urinary incontinence treated by Er:YAG laser. Bull Exp Biol Med. 2017;163:280–3.
Price CR, Carniol PJ, Glaser DA. Skin resurfacing with the erbium:YAG laser. Facial Plast Surg Clin North Am. 2001;9:291–302.
Newman JB, Lord JL, Ash K, McDaniel DH. Variable pulse erbium:YAG laser skin resurfacing of perioral rhytides and side-by-side comparison with carbon dioxide laser. Lasers Surg Med. 2000;26:208–14.
Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90° and 360° scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129–38.
Lukac M, Gaspar A, Bajd F. Dual tissue remodeling: non-ablative resurfacing of soft tissues with FotonaSmooth® mode Er:YAG laser. J Laser Health Acad. 2018;1:1–16.
Hutchinson-Colas J, Segal S. Genitourinary syndrome of menopause and the use of laser therapy. Maturitas. 2015;82:342–5.
Helbig D, Bondendorf MO, Grunewald S, et al. Immunohistochemical investigation of wound healing in response to fractional photothermolysis. J Biomed Opt. 2009;14:064044.
Gandhi J, Chen A, Dagur G, Suh Y, Smith N, Cali B, Khan SA. Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol. 2016;215:704–11.
Song S, Budden A, Short A, Nesbitt-Hawes E, Deans R, Abbott J. The evidence for laser treatments to the vulvo-vagina: making sure we do not repeat past mistakes. Aust N Z J Obstet Gynaecol. 2018;58:148–62.
Arunkalaivanan A, Kaur H, Onuma O. Laser therapy as a treatment modality for genitourinary syndrome of menopause: a critical appraisal of evidence. Int Urogynecol J. 2017;28:681–5.
Digesu G, Swift S. Laser treatment in urogynaecology and the myth of the scientific evidence. Int Urogynecol J. 2017;28:1443–4.
Pitsouni E, Grigoriadis T, Falagas ME, Salvatore S, Athanasiou S. Laser therapy for the genitourinary syndrome of menopause. A systematic review and meta-analysis. Maturitas. 2017;103:78–88.
Faubion SS, Sood R, Kapoor E. Genitourinary syndrome of menopause: management strategies for the clinician. Mayo Clin Proc. 2017;92:1842–9.
Conté C, Jauffret T, Vieillefosse S, Hermieu JF, Deffieux X. Laser procedure for female urinary stress incontinence: a review of the literature. Prog Urol. 2017;27:1076–83.
Walter JE, Larochelle A. No. 358-intravaginal laser for genitourinary syndrome of menopause and stress urinary incontinence. J Obstet Gynaecol Can. 2018;40:503–11.
Shamout S, Campeau L. Stress urinary incontinence in women: current and emerging therapeutic options. Can Urol Assoc J. 2017;11(6 Suppl 2):S155–8.
Henriques J, Brandão P, Almeida A, et al. Female urinary incontinence: is laser treatment effective? A systematic review. Obstet Gynecol Int J. 2018;9:227–32.
Sinderman AD, D’Agostino RB Sr, Pencina MJ. The role of physicians in the era of predictive analytics. JAMA. 2015;314:25–6.
Vogenberg FR. Predictive and prognostic models: implications for healthcare decision-making in a modern recession. Am Health Drug Benefits. 2009;2:218–22.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fistonić, I., Fistonić, N. (2019). Laser Treatments in Female Urinary Incontinence. In: Pérez-López, F. (eds) Postmenopausal Diseases and Disorders. Springer, Cham. https://doi.org/10.1007/978-3-030-13936-0_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-13936-0_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13935-3
Online ISBN: 978-3-030-13936-0
eBook Packages: MedicineMedicine (R0)