Abstract
Vaginal atrophy occurring during menopause is closely related to the dramatic decrease in ovarian estrogens due to the loss of follicular activity. Particularly, significant changes occur in the structure of the vaginal mucosa, with consequent impairment of many physiological functions. In this study, carried out on bioptic vaginal mucosa samples from postmenopausal, nonestrogenized women, we present microscopic and ultrastructural modifications of vaginal mucosa following fractional carbon dioxide (CO2) laser treatment. We observed the restoration of the vaginal thick squamous stratified epithelium with a significant storage of glycogen in the epithelial cells and a high degree of glycogen-rich shedding cells at the epithelial surface. Moreover, in the connective tissue constituting the lamina propria, active fibroblasts synthesized new components of the extracellular matrix including collagen and ground substance (extrafibrillar matrix) molecules. Differently from atrophic mucosa, newly-formed papillae of connective tissue indented in the epithelium and typical blood capillaries penetrating inside the papillae, were also observed. Our morphological findings support the effectiveness of fractional CO2 laser application for the restoration of vaginal mucosa structure and related physiological trophism. These findings clearly coupled with striking clinical relief from symptoms suffered by the patients before treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vaginal mucosa atrophy is one of the most characteristic consequences accompanying menopause. It is due to menstrual cycle arrest and to the depletion of ovarian follicle maturation. The related hormonal decline induces structural modifications to the constitutive tissues of the vaginal mucosa resulting in several symptoms including dryness, itching, burning, dysuria and dyspareunia [1, 2]. These symptoms account for a consequent decline in the quality of life in 30 % of menopausal women with diverse clinical symptoms and dermatological features [1–3]. Recurrent infections are also occurring as the result of a decrease in mucosal lactobacilli and an increase in pathogenic bacteria consequent to a decrease in mucosal glycogen delivery [2]. Nowadays, women reach menopause usually at around the age of 51 and, in Europe, their average life expectancy is about 80 [4]. Owing to these data, a woman spends a significant part of her life in the absence of autologous production of estrogens. Aging-related changes, mainly affecting the skin, have been successfully treated in many human and animal organs by using carbon dioxide (CO2) fractional laser [5, 6]. These studies showed microscopic and ultrastructural evidences of morphologic changes following fractional CO2 laser application on atrophic postmenopausal vaginal mucosa. The aim of our study was to identify fractional CO2-induced morphological modifications related to the expression of possible regenerative mechanisms in the vaginal atrophic mucosa, supporting structural restoration and a renewed physiological condition.
Materials and methods
The present study was performed according to the Declaration of Helsinki and approved by the local Institutional Review Board. All patients signed the informed consent.
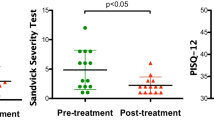
Among 50 postmenopausal women affected by severe symptoms and selected for a pilot clinical study [7], five nonestrogenized patients, aged between 54 and 63 (57 ± 1.7 [mean ± standard error of the mean]), who reached menopause between 44 and 53 years (47.6 ± 1.6 [mean ± standard error of the mean]) and presented severe atrophy symptoms, were recruited for this histological study. The inclusion criteria were as follows: vaginal dryness, burning, itching, disuria and dyspaurenia (painful sexual intercourse). Exclusion criteria were as follows: hormone therapy (local or systemic) in the last 12 months, use of vaginal moisturizers and active genital infections. The pilot clinical study, involving 50 women [7], was performed on the basis of subjective and objective evaluations of the treatment (e.g. satisfaction with the laser procedure due to symptom improvement) owing to the Vaginal Health Index Score parameters (elasticity, fluid volume, pH, epithelial integrity and moisture). The pilot study showed a striking amelioration of vaginal health in a very high percentage of women (84 %) that were also satisfied with the procedure outcome. Vaginal Health Index Score (VHIS) and intensity of vaginal atrophy symptoms data showed a significant improvement (p < 0.001) in all symptoms: dryness (80 %), burning (90 %), hitching (80 %), dysuria (74 %) and dyspareunia (100 % of sexually active women).
In the present study, we report the microscopic and ultrastructural findings obtained from the vaginal mucosa biopsies of five women selected for the severity of their symptoms (listed above) before and after laser treatment.
Biopsies
A surgical tool to perform biopsies was used on the lateral vaginal wall for the collection of samples which were immediately immersed in the fixative solution and processed for light and electron microscopy. The dimensions of mucosal samples did not exceed 1 × 1.5 × 1.5 mm3. In order not to re-biopsy the same site, before treatment and 1 month post-treatment, biopsies were taken from the left lateral vaginal wall at a distance of approximately 20 mm. Two months post-treatment, biopsies were taken from the opposite (right) vaginal lateral wall. Specific care was used for a correct orientation of specimens in the embedding and sectioning procedures in order to have sections perfectly perpendicular to the mucosal surface.
Laser
We used a laser source in the far infrared (10,600 nm), capable of delivering energy fluences above 100 mJ per pulse, with a pulse duration of 1,000 μs (SmartXide2 V2LR, Monalisa Touch®, DEKA, Florence, Italy). The laser settings were tuned to a single spot size of 200 μm, and an opto-mechanical scanner XY was used to induce a punctiform sequential targeting in each energy release over a vaginal mucosa area of 1 cm2 at a time, thus covering the entire vaginal surface. The specific fractioning map was important because the tissue, surrounding each single spot area, allows a rapid and complete epithelial repair. Another important peculiarity of the laser system is pulse shaping specifically tuned for treatment of vaginal mucosa (Deka-Pulse [DP]). The shape of this pulse is divided into two distinct areas. The first, set at 60 µs, has a peak power such as to perform an immediate vaporization of the epithelium not very rich in water and poor heat conductor. A second part of the pulse is defined by the operator and ranges from 100 to 2,000 µs with a peak power selectable from a range between 3 and 40 W. This power is tuned to issue a suitable thermal energy transfer to the connective tissue in order to stimulate the reparative action. Pulse energies ranged from 50 to about 150 mJ with residence times of approximately 1 ms. The laser beam was reflected by a 45° mirror at the tip of the handpiece probe in order to direct laser energy perpendicularly to the vaginal mucosal surface and to prevent any irradiation on the uterine cervix. The probe was inserted and rotated along the vaginal canal, in order to treat the whole mucosal surface. Treatments were performed in our outpatient department without anaesthesia since they are completely painless. Biopsies were taken from each patient before treatment and after 30 and 60 days from different sites in the vaginal right and left lateral walls in order to exclude re-biopsies on the same site. Each biopsy was then processed for light and electron microscopy evaluations.
Light microscopy
Mucosal biopsies were immediately immersed in a 4 % paraformaldehyde/phosphate buffer solution for 24 h and then processed (dehydration, paraffin embedding and sectioning) for light microscopy. Some sections were stained with haematoxylin and eosin, and the other sections were treated with the periodic acid-Schiff (PAS) reagent for glycogen staining.
Electron microscopy
Fixation was performed by immersion of bioptic samples in 2.5 % glutaraldehyde (EM grade), 4 % paraformaldehyde in 0.1 M sodium cacodylate buffer solution (pH 7.3) for 6 h at 4 °C. Samples were then postfixed for 2 h in osmium tetroxide 1.33 % in 0.1 M s-collidine buffer, dehydrated in a graded series of ethanol (30, 50, 70, 90 and 100 %) and finally embedded in epoxy resin Epon 812. Semithin (0.2 μm) and ultrathin (40–60 nm) sections were obtained at the ultramicrotome Reichert Ultracut S provided with a diamond knife. Semithin sections were stained with toluidine blue, and ultrathin sections, previously collected on 200-μm mesh grids, were counterstained with lead citrate and uranyl acetate. A Zeiss EM 10 transmission electron microscope (Carl Zeiss, Oberkochen, Germany), operating at 80 kV with an objective aperture of 30/60 μm, was used for direct observation on electron micrographs. Images were recorded on Kodak 4489 Electron Image film (31/4 × 4″) and finally digitized with a Epson Perfection V750 Pro scanner at 1,200 dpi.
Results
Premenopausal vaginal mucosa is formed by a squamous stratified epithelium and a lamina propria of connective tissue which is rich in vessels and often projecting into the underside of the epithelium with papillae rich in blood capillaries. During the menopause, follicular function arrest in the ovary is responsible for important modifications in the genital tract, particularly vaginal atrophy [2]. Vaginal mucosa atrophy is characterized by a significant thinning of vaginal epithelium with reduction in and/or absence of papillae and related vessels. In the connective tissue, fibroblasts are characterized by a small cytoplasm frequently elongated as very thin projections and a poor content of organelles, particularly those involved in the synthesis of the molecular components of the extracellular matrix.
Light microscopy
Pre-treatment
Before treatment in all biopsies from the atrophic vaginal mucosa, the stratified squamous epithelium appeared very thin and formed by few cell layers (5–10) with a flat and even basal surface lacking in connective tissue indentations in the papillae (Fig. 1a). In the poorly vascularized connective tissue, fibres were organized in very thin bundles, often intermingled and presenting various orientations. In the sections treated with the PAS method, the epithelial cells appeared very poor in glycogen and limited to few intermediate and superficial cells (Fig. 2a).
Haematoxylin and eosin staining of a patient’s vaginal mucosa before treatment (a, c) and 2 months after treatment (b, c). a In the atrophic mucosa, the epithelium is very thin since it is formed by few layers of cells, and the epithelial-connective junction presents an even feature; b the epithelium appears much thicker since it is constituted by many layers with visible large intermediate and shedding superficial cells. The underlying connective tissue is provided with papillae indenting the epithelial-connective junction. c Comparative microphotographs (before and 2 months after treatment) of atrophic vaginal mucosa biopsies demonstrating striking structural recovery features 2 months after treatment in all the patients
Histochemical PAS reaction for glycogen identification (red) in the vaginal epithelium before treatment (a) and 2 months after treatment (b). a Atrophic mucosa: glycogen is present in a few superficial cells of the thin epithelium; b remark on high content of glycogen in the epithelial intermediate and superficial layers. Deep papillae of connective tissue indenting the epithelium are also clearly observable
One- and 2-month follow-up
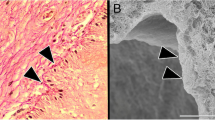
Striking morphological modifications of mucosa structural recovery were already observable at 1-month follow-up and also maintained after 2 months post-treatment without any distinguishable and/or quantifiable differences. The typical features are reported in Fig. 1b. The squamous stratified epithelium appeared very thick, formed by many cell layers (20–40) giving cells to upper layers for differentiation (in the intermediate layers) and superficial shedding. Basal layer cells appeared closely packed as in any continuously renewing stratified epithelium. The intermediate layer cells appeared enlarged, with the nucleus surrounded by a wide cytoplasm. In the most superficial layer, many wide cells, shedding from the epithelial surface into the vaginal lumen, were observable. The basal surface of the epithelium appeared characteristically indented for the presence of numerous papillae of connective tissue, projecting onto the underside of the epithelium giving an uneven appearance to the epithelial-connective tissue junction. In the sections treated with the PAS method, a large amount of glycogen was present in the cytoplasm of the intermediate layer cells and the numerous exfoliating cells of the superficial layer (Figs. 2b and 3). Inside the papillae, elongated blood capillaries were also easily identifiable (Fig. 3).
Higher magnification of the superficial part of vaginal mucosa 2 months after treatment. Histochemical PAS reaction for glycogen identification (red). The high content of glycogen in the epithelial cells of the intermediate and superficial layers and the numerous large cells shedding at the epithelial surface are clearly visible. The epithelial-connective junction is well identified by the PAS positivity of the basal membrane (red line). In the connective tissue of the papilla, small vessels penetrating the papilla are clearly identifiable
Electron microscopy
Under the electron microscope, at 1- and 2-month follow-up, fibroblasts appeared very rich in organelles. The extension of the rough endoplasmic reticulum was particularly remarkable with wide flattened membranous cisternae and many electron-dense attached ribosomes (Fig. 4a, b). A peculiar ultrastructural feature was the presence of fine filamentous material inside cisternae and dilated vesicles with ribosomes attached to the membranes (Fig. 4a). The lumen of these structures was filled with the same fine filamentous material realistically representing the molecular precursors of the fibrillar components of the extracellular matrix. This material, synthesized by polyribosomes at the surface of the rough endoplasmic reticulum membrane, passed along the extension of the cisternae and was temporary stored into the vesicles, “waiting” to be transmitted through intermediate vesicles to the Golgi apparatus for the delivery in the surrounding extracellular matrix. Such material was most recently synthesized, ready to be transferred to the Golgi apparatus for concentration, glycosilation and extracellular release in most of secretory cells and also in fibroblasts owing to a well-known mechanism [8]. Typical Golgi complexes, formed by stacks of flattened cisternae and vesicles, were also recognizable (Fig. 4b). These ultrastructural features observed in the vaginal mucosa connective tissue after fractional CO2 laser treatment strongly support the idea of a fibroblast stimulation that produces new molecular components of the extracellular matrix, i.e. the fibrillar (collagen) and the amorphous ground substance components including glycosaminoglycanes, proteoglycans and multiadhesive glycoproteins.
Electron microscopy of vaginal mucosa connective tissue 2 months after treatment. a In fibroblasts, a widely extended rough endoplasmic reticulum, constituted by membranes with attached ribosomes forming cisternae and dilated vesicles (V), is visible. The content of these structures is constituted by the molecular precursors of the fibrillar components of the extracellular matrix. Some mitochondria are also detectable; b fibroblasts are rich in organelles: profiles of the rough endoplasmic reticulum with a medium electron density content, and some elements of the Golgi apparatus with associated dilated vesicles are observable. N: nucleus, RER: rough endoplasmic reticulum, M: mitochondria, G: Golgi apparatus
Discussion
The comparative morphology among samples of atrophic vaginal mucosa, pre- and post-treatment with fractional CO2 laser, gives the first microscopic and ultrastructural evidence of fractional CO2 laser effectiveness in restoring atrophic vaginal mucosa structure to a non-atrophic one which couples with a highly significant relief from clinical symptoms observed before treatment [7]. Our microscopic and ultrastructural findings demonstrate the recovery of the whole structures supporting a full functionality in the epithelial and connective tissue compartments since the restored structure represents a restored physiological condition. We found peculiar microscopic and ultrastructural findings after treatment as follows:
-
1.
A thicker (no more atrophic) epithelium with a basal layer of closely packed (proliferating) cells.
-
2.
A significant storage of glycogen in the large epithelial cells forming the intermediate and superficial layers as expression of a restored differentiative mechanism specifically oriented to the synthesis of glycogen
-
3.
A high degree of epithelial exfoliation with superficial cells filled with glycogen shedding at the epithelial surface
-
4.
In fibroblasts, extended rough endoplasmic reticulum and vesicles for the synthesis and storage of procollagen molecules and a well-developed Golgi apparatus with associated vesicles for the glycosilation of proteins and the synthesis of molecular components of the ground substance, such as glycoproteins, proteoglycans and multiadhesive glycoproteins [8, 9], are highly represented. These features support the idea of an active structural machine for the synthesis of molecular components of the extracellular matrix
-
5.
A rich content of blood vessels in the connective tissue stimulating and supporting the activity of fibroblasts and capillaries penetrating the newly-formed papillae into the underside of the very thick epithelium for a better metabolic support.
Our microscopic and ultrastructural findings strongly support a metabolic reactivation of the connective components of the vaginal mucosa due to a direct photobiomodulation effect with remodelling of collagen following application of fractional laser nonablative photothermolysis, as previously reported in the skin [10]. In the application of fractional CO2 laser to the human skin [11], the local increase in different cytokines has been histochemically demonstrated, particularly transforming growth factor-β (TGF-β; stimulating matrix proteins, such as collagen), basic fibroblast growth factor (bFGF; stimulating angiogenetic activity with endothelial cell migration and proliferation), epidermal growth factor (EGF; stimulating the re-epithelization), platelet-derived growth factor (PDGF; stimulating fibroblasts to produce extracellular matrix components) and vascular endothelial growth factor (VEGF; regulating vasculogenesis and angiogenesis). The increase in these factors, as observed in the human skin [11], well couples with the activation of epithelial cell differentiation mechanisms in the vaginal atrophic mucosa. The expression of these mechanisms is starting in the first suprabasal layers of cells in the epithelium (glycogen synthesis) and in the connective tissue, with the formation of new vessels and the stimulation of fibroblast activity. Other studies on human skin strongly support the increase in growth factors following CO2 laser application [12, 13]. Further significant data on human skin following CO2 laser treatment were obtained using reverse transcriptase real-time polymerase chain reaction technology and immunohistochemistry [14] through the assessment of the levels of messenger RNAs (mRNAs) for procollagen, metalloproteinases (MMPs) involved in the renewal of collagen and primary interleukin-1β, tumour necrosis factor-α, and profibrotic cytokine TGF-β1. The highly significant reported increase in the levels of the different mRNAs following the CO2 laser application to the skin also suggests a stimulation of tissue renewal mechanisms in the vaginal mucosa. A parallel synergic mechanism for the stimulation of connective tissue matrix remodelling by fibroblasts following CO2 laser application is the activation of heat shock proteins (HSPs). Immunohistochemical studies for HSP (specifically HSP47) revealed a persistent collagen response lasting on human skin at least 3 months following CO2 treatment [15]. HSP47 is a chaperone of collagen [16, 17] localized in the rough endoplasmic reticulum, with an important role in the early stages of collagen biosynthesis [18]. Epithelial cell differentiation in the vaginal epithelium leads to the formation of glycogen and acid mucins which are released at the mucosal surface, favouring a physiological balance due to lactobacilli repopulation and competition with pathogenic bacteria [2, 19]. Moreover, in order to better understand the possible activation of regenerative mechanisms responsible for the restoration of the atrophic vaginal mucosa after fractional CO2 laser treatment, we must consider the absorption of energy by the mucosal tissues and the related controlled increase in mucosal temperature. The main “chromophore” for the specific wavelength of the CO2 laser is water. It is crucial to consider that the healthy premenopausal vaginal mucosa (epithelial cells and the ground substance of the connective tissue) is highly hydrated, and the mucosal dryness is one of the main causes of the menopause discomfort and symptomatology [20]. The specific fractional feature of the instrument and the range of the most suitable power levels have been determined during our preliminary ex vivo experiments on human vaginal mucosa (unpublished data). Instrument parameters are crucial to maximize the treatment efficacy, limiting the side effects in order to induce a full restoration of epithelial and connective tissue structure and trophism and to prevent an energetic surcharge due to an excessively high increase in tissue temperature. This could constitute, at the same time, an environmental condition dangerous for cell survival. Following treatment, our morphological findings support an increase in fibroblast activity which is involved in the production of new connective tissue matrix molecular components, restoration of vascular supply, and an increase in epithelial cell activity with an enhanced synthesis, storage and release of glycogen. The ultrastructutral features of fibroblasts after fractional CO2 laser treatment of atrophic vaginal mucosa also suggest a direct involvement in the restoration/renewal of the ground substance. In spite of its poor morphology (it appears as an amorphous substance in the histological preparations), it is often forgotten in many discussions. A correct chemical composition of the ground substance guarantees the correctness of the tropocollagen molecule packing (fibrillogenesis) which the specific mechanical properties of collagen are depending on. Moreover, renewal of the ground substance components means a new synthesis and release of glycosaminoglycans, glycoproteins and multiadhesive glycoproteins. The main structural characteristic of these macromolecules consists in an extreme richness of superficial polar groups capable of linking high amounts of water which leads to a significant increase in tissue hydration (mucosal swelling) and a related increase in nutrient permeability that can move from blood vessels to tissues. The epithelium, whose trophism is enhanced, is also particularly sensitive to this better nutritional condition. This happens not only for the amelioration of the supply of water, ions and nutrients but also for a better diffusion of hormones produced by the reduced/residual activity of the ovaries and other organs or nongonadal tissues such as adipose tissue [21]. In the childbearing age, the vaginal epithelium undergoes cyclical changes corresponding to the ovarian cycle while, after the menopause, oogenesis and the endocrine functions stop [2, 20]. After treatment, a large amount of glycogen is stored in the epithelial cells of the intermediate and superficial layers with shedding at the epithelial surface. As epithelial cells exfoliate and die, their glycogen content is released on the inner vaginal surface. Glycogen is hydrolized to glucose, feeding Lactobacilli vaginalis with the production of lactic acid. Lactic acid acidifies the vaginal transudate fluid moistening the mucosal surface and inhibiting, at the same time, the colonization of yeasts and potentially pathogenic bacteria [2]. In summary, the most significant microscopic and ultrastructural features, observed following vaginal mucosa fractional CO2 laser treatment, are a restoration of vaginal mucosa structure to a premenopausal condition without any hormone therapy.
Conclusions
In conclusion, this study highlights the specific effects of fractional CO2 laser application on vaginal mucosa, consisting of microscopic and ultrastructural modifications closely related to wavelength, profile pulse and pulse width used. Firstly, the wavelength used, i.e. 10,600 nm, strongly absorbed by the chromophore water, allows a selective targeting of mucosal tissues object of a minor nonspecific thermal damage associated with a particular profile pulse [5]. This profile (DP) has been specifically designed for treatment of vaginal mucosa and is characterized by an initial peak power of 60 μs followed by a longer low-power tail of about 1 ms. In this way, the application of laser pulse can result primarily in an athermal ablation of epithelial layer surface (low water content) and a release of sub-ablative thermal energy in the underlying connective tissue richer in water, without any uncontrolled thermal damage. These effects are guaranteed by the specific distribution map of laser beam spots of the opto-mechanical scanner used. Our results strongly support the hypothesis that a new production of collagen and ground substance components within the connective tissue and glycogen and acidic mucins within the epithelium can rebalance and restore vaginal mucosa from atrophy induced by the absence of ovarian estrogens, resulting in a highly significant improvement in clinical symptoms [7]. Our hypothesis opens new perspectives on the use of electromagnetic radiation to re-establish structural and physiological conditions in the atrophic vaginal mucosa.
References
Brincat M, Moniz CJ, Studd JW, Darby A, Magos A, Emburey G, Versi E (1985) Long-term effects of the menopause and sex hormones on skin thickness. Br J Obstet Gynaecol 92(3):256–259
Mac Bride MB, Rhodes DJ, Shuster LT (2010) Vulvovaginal atrophy. Mayo Clin Proc 85(1):87–94. doi:10.4065/mcp.2009.0413
Leiblum S, Bachmann G, Kemmann E, Colburn D, Swartzman L (1983) Vaginal atrophy in the postmenopausal woman. The importance of sexual activity and hormones. JAMA 249(16):2195–2198
WHO (2013) European Health Report 2012. Charting the way to well-being
Berlin AL, Hussain M, Phelps R, Goldberg DJ (2009) A prospective study of fractional scanned nonsequential carbon dioxide laser resurfacing: a clinical and histopathologic evaluation. Dermatol Surg 35(2):222–228. doi:10.1111/j.1524-4725.2008.34413.x
Lee CJ, Park JH, Ciesielski TE, Thomson JG, Persing JA (2008) Retinoids, 585-nm laser, and carbon dioxide laser: a numeric comparison of neocollagen formation in photoaged hairless mouse skin. Aesth Plast Surg 32(6):894–901. doi:10.1007/s00266-008-9121-2
Salvatore S, Nappi RE, Zerbinati N, Calligaro A, Ferrero S, Candiani M, Maggiore ULR (2014) A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 17(4):363–369. doi:10.3109/13697137.2014.899347
Alberts B, Johnson A, Lewis Jea (2002) Transport from the ER through the Golgi apparatus. In: Molecular biology of the bell, 4th edition. Garland Science, New York
Stephens DJ (2012) Cell biology: collagen secretion explained. Nature 482(7386):474–475. doi:10.1038/482474a
Tierney EP, Kouba DJ, Hanke CW (2009) Review of fractional photothermolysis: treatment indications and efficacy. Dermatol Surg 35(10):1445–1461. doi:10.1111/j.1524-4725.2009.01258.x
Prignano F, Campolmi P, Bonan P, Ricceri F, Cannarozzo G, Troiano M, Lotti T (2009) Fractional CO2 laser: a novel therapeutic device upon photobiomodulation of tissue remodeling and cytokine pathway of tissue repair. Dermatol Ther 22(Suppl 1):S8–15. doi:10.1111/j.1529-8019.2009.01265.x
Nowak KC, McCormack M, Koch RJ (2000) The effect of superpulsed carbon dioxide laser energy on keloid and normal dermal fibroblast secretion of growth factors: a serum-free study. Plast Reconstr Surg 105(6):2039–2048
Manolis EN, Kaklamanos IG, Spanakis N et al (2007) Tissue concentration of transforming growth factor b1 and basic fibroblast growth factor in skin wounds created with a CO2 laser and scalpel: a comparative experimental study, using an animal model of skin resurfacing. Wound Repair Regen 15:252–257. doi:10.1111/j.1524-475X.2007.00212.x
Orringer JS, Kang S, Johnson TJ, Karimipour DJ, Hamilton T, Hammerberg C, Voorhees JJ, Fisher GJ (2004) Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch Dermatol 140(11):1326–1332. doi:10.1001/archderm.140.11.1326
Hantash BM, Bedi VP, Kapadia B, Rahman Z, Jiang K, Tanner H, Chan KF, Zachary CB (2007) In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med 39:96–107. doi:10.1002/lsm.20468
Ishida Y, Nagata K (2011) Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol 499:167–182. doi:10.1016/B978-0-12-386471-0.00009-2
Tasab M, Batten MR, Bulleid NJ (2000) Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J 19(10):2204–2211. doi:10.1093/emboj/19.10.2204
Dafforn TR, Della M, Miller AD (2001) The molecular interactions of heat shock protein 47 (Hsp47) and their implications for collagen biosynthesis. J Biol Chem 276(52):49310–49319. doi:10.1074/jbc.M108896200
North American Menopause Society (2007) The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause 14:355–369. doi:10.1097/gme.0b013e31805170eb
Farage MA, Maibach HI (2011) Morphology and physiological changes of genital skin and mucosa. Curr Probl Dermatol 40:9–19. doi:10.1159/000321042
Guerre-Millo M (2002) Adipose tissue hormones. J Endocrinol Invest 25(10):855–861
Acknowledgments
NZ, MS, MO, MC, TI, SS, FM and AC contributed equally to this work. Many thanks to Aurora Farina for her skillful technical contribution. This article was not supported by grants.
Conflict of interest
NZ, MS, MO, MC, TI, SS, FM and AC certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Statement of authorship
NZ, MS, MO, MC, TI, SS, FM and AC hereby certify that all work contained in this article is original. The authors claim full responsibility for the content of this article.
Informed consent
Written informed consent was obtained from the patients participating in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zerbinati, N., Serati, M., Origoni, M. et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci 30, 429–436 (2015). https://doi.org/10.1007/s10103-014-1677-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1677-2