Abstract
Acute mesenteric ischemia results from inadequate blood supply to the small intestine. Resultant ischemic and inflammatory changes lead to bowel injury. If not treated promptly, bowel necrosis can occur. Prognosis is typically poor with a high mortality if not treated. Contrast-enhanced computed tomography angiography (CTA) is the recommended imaging modality for assessment of acute mesenteric ischemia. Aside from identifying vascular etiologies such as thrombus and calcifications, CT is helpful in assessing the bowel wall. Prompt identification of the etiology of acute mesenteric ischemia is essential in determining a treatment algorithm. A multidisciplinary approach to include vascular surgery, general surgery, critical care, and interventional radiology is important. The goal of this section is to provide a better understanding of the radiologist’s role for the imaging and treatment of acute mesenteric ischemia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute mesenteric ischemia
- Endovascular
- Computed tomography Angiography
- Thrombolysis
- Suction embolotherapy
Introduction
Mesenteric ischemia is a medical condition that is precipitated by inadequate blood supply to the small intestine. The inadequacy of blood supply can lead to both reversible and irreversible injury to an affected small intestine segment. The ischemic injury progresses from cellular damage to intestinal necrosis and can ultimately cause death if left untreated with mortality rates between 30% and 90% [1, 2]. Mesenteric ischemia has both acute and chronic forms.
The acute form is associated with severe abdominal pain and can often result in death. The chronic form of mesenteric ischemia has a more gradual course and usually presents with gradually increasing postprandial, abdominal pain and unintentional weight loss. Inadequacy of blood perfusion to the small intestine can be due to a disruption of either venous or arterial blood supply. The disruption most commonly develops secondary to embolism and is followed by thrombosis, nonocclusive ischemia, and less frequently venous thrombosis. For patients with mesenteric venous thrombosis and nonocclusive mesenteric ischemia, treatment is most commonly a conservative measure unless the stage of ischemia is sufficiently advanced. In recent years, interventional radiology procedures have provided a potential lifesaving remedy to acute mesenteric ischemia (AMI) [1,2,3,4].
Epidemiology
Advanced age is a risk factor for mesenteric ischemia. Partially occlusive disease of the visceral arteries is a common finding in elderly patients and is due to atherosclerotic disease. Up to 10% of autopsy studies have shown atherosclerotic disease in the mesenteric vessels [5]. Females are affected more than males with a ratio of approximately 3:1 [6]. Other risk factors include smoking, hyperlipidemia, diabetes, and sedentary lifestyle [7]. The total number of deaths associated with AMI has declined from 12.9 to 5.3 per million from 2000 and 2012 [8]. Non-acute mesenteric ischemia can remain asymptomatic until two or more mesenteric vessels become involved secondary to the development of collateral vessels. The acute form typically involves acute thrombosis of the superior mesenteric artery (SMA).
Relevant Anatomy
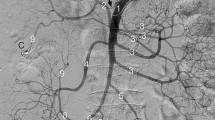
Imaging of the mesenteric viscera is commonly done using computed tomography or via angiography (Fig. 29.1). Table 29.1 is from the 2018 American College of Radiology Appropriateness Criteria in the imaging of acute mesenteric ischemia. Computed tomography angiography (CTA) is the preferred imaging modality for the assessment of acute mesenteric ischemia. At our institution, we prefer CTA over MRA given the faster acquisition time, less effect of respiratory artifact, and better assessment of atherosclerotic calcifications. MRA is useful in patients who are unable to receive iodinated contrast. A meta-analysis to determine the diagnostic accuracy of contrast agent-enhanced multi-detector computed tomography between 1996 and 2009 showed a pooled sensitivity of 93% and a pooled specificity of 96% [9]. The sensitivity and specificity of 3D contrast MRA are approximately 95% [10]. Limitations of MRI include limited number of MRI-compatible pacemakers, claustrophobia, lengthy examination time, and the inability to assess patients with mesenteric stents secondary to artifact.
The relevant anatomy for AMI cases can be divided based upon the gut region supplied by specific arterial flow as well as the collateral flow.
-
First, the foregut: Distal esophagus to the ampulla of Vater between the second and third portions of the duodenum supplied by the celiac artery with collateral connections from the pancreaticoduodenal arteries and far more rarely the arc of Buhler distally.
-
Next, the midgut: Ampulla of Vater to the splenic flexure of the colon supplied by the SMA with collateral supply from pancreaticoduodenal arteries and occasionally the arc of Buhler proximally, the marginal artery of Drummond, and arc of Riolan distally (Figs. 29.2 and 29.3).
-
The hindgut: Splenic flexure of the colon to the distal sigmoid colon supplied by the inferior mesenteric artery (IMA) with collateral supply from the marginal artery of Drummond proximally and the superior hemorrhoidal to middle hemorrhoidal arteries distally.
-
Finally, the cloacal derivatives: Distal sigmoid colon to the anus supplied by branches of the internal iliac arteries with collateral supply from the middle and superior hemorrhoidal arteries proximally.
Of note, the arc of Riolan is different from other collateral flows as it may provide flow in either direction between the proximal SMA and IMA. The flow to these regions accounts for 10–35% of resting cardiac output and can increase by as much as 200% postprandially [3].
The SMA is the most commonly affected artery in cases of AMI and its branches are often the ischemic culprit. The SMA originates from the anterior aspect of the abdominal aorta inferior to the celiac trunk. In adults it typically arises at the L1 vertebral level. The SMA then traverses in an anteriorly inferior manner and then passes posteriorly to the neck of the pancreas and splenic vein. Typically the superior mesenteric vein can also be found running to the right of the SMA. Once the SMA passes from under the neck of the pancreas, it then begins diverging into smaller branches. The first branch is the inferior pancreaticoduodenal artery, which supplies the head of the pancreas and the inferior portion of the duodenum. Next are various intestinal arteries that supply parts of the ileum and jejunum. The SMA also has three colic branches; however, some patients may not possess all three due to anatomic variance. The first colic branch is the ileocolic artery which supplies the distal ileum, cecum, and appendix. Next is the right colic artery which typically supplies the ascending colon. Finally, the middle colic artery arises from the SMA and supplies the transverse colon. It is important to note that many patients may lack an artery and receive collateral supply from one or more alternative arteries [3].
Acute Mesenteric Ischemia
The advent of minimally invasive techniques and improved diagnostic capabilities has allowed for additional treatment options for patients with AMI. Although there is significant mortality and morbidity associated with AMI, it has become one of the leading diagnoses that is successfully treated with early endovascular technique. The majority of AMI cases are caused by arterial emboli from cardiac arrhythmias, such as atrial fibrillation, with one study quoting approximately 40–50% attributable to cardiac emboli [11]. Arterial thrombosis is the next most common cause for AMI where preexisting atherosclerotic lesions create a nidus for acute occlusion. This thrombosis accounts for roughly 25% of cases. The role of local thrombolysis is aimed at early intervention to prevent the formation of irreversible necrosis via prolonged ischemia due to embolic or thrombotic events. For this reason, in stable patients with AMI, it is paramount to acquire CT angiography to confirm diagnosis and follow with prompt intervention [3, 4, 11, 13]. The notable other forms of mesenteric ischemia are nonocclusive mesenteric ischemia (accounting for approximately 20% of cases) and venous mesenteric thrombosis (accounting for approximately 10% of cases) [12].
Clinical Presentation
The diagnosis of acute mesenteric ischemia is relatively uncommon, and subsequently recording data for its prevalence has been challenging. Many patients present with suspected AMI and are later found to have an alternative diagnosis. One study found that as little as 19% of suspected cases of AMI were actually confirmed to have AMI. Patients greater than the age of 65 are seemingly at the greatest risk of developing AMI with 20% of suspected cases, although patients between the ages of 35 and 65 accounted for 18%. One study indicates that alternative broad diagnoses such as gastrointestinal, hepatopancreaticobiliary, cardiopulmonary, genitourinary, and vascular conditions are far more common in patients less than the age of 35. Recent research indicates that there are no significant differences in diagnosis of AMI between men and women. Interestingly, one study concluded that inpatients were more likely to develop AMI than patients presenting to the emergency department [13].
Treatment Overview
Originally the treatment of mesenteric ischemia was limited to open surgical repair; however, in the last 15 years, development of endovascular procedures has provided an additional option for this disease. Endovascular treatment began with percutaneous dilation of the SMA in 1980 and sent placement in 1992 [14]. Catheter-directed thrombolysis has been a commonly used technique in SMA thrombolysis. However, given the long treatment interval needed to achieve lysis, suction embolectomy is becoming more popular [13].
Although new techniques have emerged that may benefit the patient, it is important to identify the cases that will provide maximal benefit with little risk. Patients with advanced stage disease, frank peritonitis, or hemodynamic instability may not benefit from an endovascular technique and are better suited for laparotomy.
With enhanced imaging techniques, the role of endovascular techniques for AMI has been instrumental for reducing morbidity and mortality for affected patients. Intestinal surgery for AMI is aimed at resecting necrosis. If the diagnosis is made before this process occurs, then endovascular techniques aimed at re-vascularizing the acute thrombosis can provide significant benefit. One study found that active endovascular-first strategy resulted in a 42% overall mortality rate – which is in stark contrast to a similar study in Finland that produced a mortality rate of 82% without this intervention [15]. However, many studies have concluded that when AMI is significantly severe or present in fragile patients, supportive care is often the best therapy due to the futility of any surgical intervention [4, 13, 15].
Endovascular Treatments
The technical treatment of AMI has multiple endovascular modalities available. The most common are as follows: catheter-directed thrombolysis, suction embolotherapy, balloon angioplasty with or without stenting, and mechanical vacuum-assisted removal (AngioVac). To perform these procedures, the patient is positioned on the table, and sedation is typically required. Sterile technique is used to access the desired entry point, and a small incision is made at the access site, usually at the common femoral artery using the Seldinger technique [2, 16,17,18,19].
Catheter-directed thrombolysis is a technique that aims to improve blood flow by essentially dissolving obstructive blood clots. This technique requires a catheter to be advanced endovascularly from the accessed site into the SMA. The procedure is completed with cone beam CT-guided imagery to ensure proper placement. The catheter is then advanced to the area of poor circulation. Contrast is then injected through the catheter, and images are taken to precisely pinpoint the exact area of occlusion. Tissue plasminogen activator (tPA) is most commonly injected through an infusion catheter over the course of several hours to dissolve the embolus. Continued monitoring and imaging are carried out throughout the procedure to ensure revascularization is achieved. Once complete, the catheter is removed, and hemostasis is achieved at the access site via manual compression or a mechanical closure device [16, 19].
Suction embolotherapy has emerged as an additional option with the benefit of rapid restoration of blood flow to the bowel. This technique involves advancing a suction catheter through the incision site and traversing the circulatory system to the ischemic area of interest. Arterial access is again achieved using the Seldinger technique, usually through a common femoral or brachial artery approach. A sheath may or may not be used; however, many angiographers use them due to the necessity of multiple catheter changes. Imaging is vitally important for locating the area of interest, and digital subtraction angiography (DSA) has provided fast and accurate diagnoses in acute situations. End-hole catheters are utilized with an inner diameter and taper large enough to prevent occlusion by the embolic clot. Suction is then applied to the catheter and the clot is then removed [2, 18].
Balloon angioplasty is a third option. A guide-wire is navigated to the area of occlusion, and a balloon catheter is then placed over the wire to reach the site. The stenotic area is then expanded via the balloon catheter. The process of inflating and deflating the balloon may be repeated multiple times to achieve the desired expansion of the vessel. As the balloon is inflated, the occlusive material is compressed against the arterial wall. Once this has been achieved, the patient may need a stent to maintain the scaffold structure of the vessel. Balloon-mounted stents are most commonly used. Once the balloon is inflated, the stent is then expanded on the arterial wall and subsequently left in place after the balloon is deflated [2, 19].
AngioVac or mechanically assisted vacuum-assisted cannulation is a relatively new technique that utilizes an external centrifugal pump to remove the embolic material. This procedure requires two insertion sites, one for the AngioVac cannula and one for the reinfusion cannula. A 22 French cannula with an expandable funnel tip is navigated to the ischemic site. Once imaging has confirmed that the tip is proximal to the embolus, the pump is engaged creating a vacuum. The embolus is removed externally, and the blood is reinfused after external filtration of the occlusive materials [2, 20].
Vasospasm has been cited as a cause of nonocclusive mesenteric ischemia. Continuous intra-arterial infusion of vasodilator drugs into the SMA has become a therapeutic option, although there is no strong evidence that this intervention prevents vasospasm [1]. Intravenous rather than intra-arterial prostaglandin E1 (PGE1) has demonstrated efficacy in treatment of this vasospasm [21].
Outcomes
The complications from endovascular treatment of AMI range from relatively mild to death. A notably common complication of endovascular treatment of AMI is worsening of bowel necrosis. This is partly due to the inability of the interventional radiologist to visually inspect the bowel. Perhaps the greatest argument for treatment of AMI with an open technique is to allow for visual assessment of potentially affected bowel and signs of necrosis.
Despite early thrombolysis with an endovascular technique, the patient may still suffer bowel necrosis due to the obscure signs and distracting presentation of these critical patients. The rates of bowel resection remain quite high despite the higher rates of treatment of AMI with an endovascular technique [3]. As such the clinicians must still be wary of the need for surgical intervention to assess bowel viability.
Medical assessment of bowel necrosis has historically been monitoring lactate levels. However, several studies have found this lab value to be inaccurate at predicting the need for bowel resection (13). Yet, the amount of patients receiving bowel resection due to mesenteric ischemia has steadily declined since the late 1980s. Multiple studies have found that in the late 1980s, bowel resection rates were approximately 70%, and most recent data suggests 20% to 50% are receiving bowel resections (15). This is most likely attributed to the fact that diagnostic modalities are better at detecting AMI and the endovascular techniques available have provided prompt reperfusion that effectively prevents necrosis. The presentation of AMI is not standardized among patient populations, and there is a multifaceted approach to managing each case with personalized medical needs. However, it is clear that as both radiologic diagnostics and interventional techniques have evolved, patients are experiencing better outcomes [3, 13, 15].
Despite the advantages of endovascular techniques, there are complications to include access site injury (hematoma or pseudoaneurysm) and potential contrast agent-related complications (contrast agent-induced nephropathy and/or allergic reactions). In patients undergoing papaverine or vasodilator therapy, systemic hypotension may develop when the infusion catheter inadvertently disengages from the SMA. Thrombolysis-associated complications include access site bleeding, embolization, and stroke [22].
If angioplasty or stent placement is performed, vessel injury or distal embolization can occur. Factors associated with higher rates of distal embolization are mesenteric occlusion, severe calcification, and lesion length >30 mm [23].
Conclusion
Advanced radiologic modalities aid in the diagnosis of AMI and endovascular techniques can both treat AMI and reduce the need for surgery while improving overall morbidity and mortality. The decision to involve endovascular techniques requires a multidisciplinary approach with a vascular surgeon, a general surgeon, an interventional radiologist, and a critical care physician. As early intervention is best, it is recommended that appropriate consults be made when the suspicion arises and a CTA be obtained quickly to confirm the diagnosis. Patients who are in extremis are more likely to benefit from surgery or supportive care. Interventional and endovascular techniques seem to be best suited in patients who respond to resuscitation or who are hemodynamically normal; however, these patients still require prompt endovascular intervention and/or surgery to determine the viability of the bowel.
References
Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZJ, Civil I, Coccolini F, Leppaniemi A, Peitzman A, Ansaloni L, Sugrue M, Sartelli M, Di Saverio S, Fraga GP, Catena F. Acute mesenteric ischemia: guidelines of the world Society of Emergency Surgery. World J Emerg Surg. 2017;12:38.
Lerardi AM, Tsetis D, Sbaraini S, Angileri SA, Galanakis N, Petrillo M, Patella F, Panella S, Balestra F, Lucchina N, Carrafiello G. The role of endovascular therapy in acute mesenteric ischemia. Ann Gastroenterol. 2017;30(5):526–33.
Sise MJ. Acute mesenteric ischemia. Surg Clin North Am. 2014;94(1):165–81.
Henes FO, Pickhardt PJ, Herzyk A, Lee SJ, Motosugi U, Derlin T, Lubner MG, Adam G, Schon G, Bannas P. CT angiography in the setting of suspected acute mesenteric ischemia: prevalence of ischemic and alternative diagnoses. Abdom Radiol (NY). 2017;24(4):1152–61.
Foley MI, Moneta GI, About-Zamzam AM Jr, et al. Revascularization of the superior mesenteric artery alone for treatment of intestinal ischemia. J Vasc Surg. 2000;32(1):37–47.
Acosta S, et al. Modern treatment of acute mesenteric ischaemia. Br J Surg. 2014;101(1):e100–8.
Paterno F, et al. The etiology and pathogenesis of vascular disorders of the intestine. Radiol Clin N Am. 2008;46(5):877–85.
Zettervall SL, Lo RC, Soden PA, et al. Trends in treatment and mortality for mesenteric ischemia in the United States from 2000–2012. Ann Vasc Surg. 2016;42:111–9.
Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology. 2010;256(1):93–101.
Schaefer PJ, Pfarr J, Trentmann J, et al. Comparison of noninvasive imaging modalities for stenosis grading in mesenteric arteries. Rofo. 2013;185:628–34.
Jia Z, Jiang G, Tian F, Zhao J, Li S, Wang K, Wang Y, Jiang L, Wang W. Early endovascular treatment of superior mesenteric occlusion secondary to thromboemboli. Eur J Vasc Endovasc Surg. 2014;47(2):196–203.
Bobadilla JL. Mesenteris ischemia. Surg Clin North Am. 2013;93(4):925–40, ix.
Karkkainen JM, Acosta S. Acute mesenteric ischemia (Part I) – incidence, etiologies, and how to improve early diagnosis. Best Pract Res Clin Gastroenterol. 2017;31(1):15–25.
Finch IJ. Use of the Palmaz stent in ostial celiac artery stenosis. J Vasc Interv Radiol. 1992;3(4):633–5. discussion 636–7
Karkkainen JM, Acosta S. Acute mesenteric ischemia (Part II) – vascular and endovascular surgical approaches. Best Pract Res Clin Gastroenterol. 2017;31(1):27–38.
Bjornsson S, Bjorck M, Block T, Resch T, Acosta S. Thrombolysis for acute occlusion of the superior mesenteric artery. J Vasc Surg. 2011;54(6):1734–42.
Sugimoto K, Hofmann L, Razavi M, Kee S, Sze D, Drake M, Semba C. The safety, efficacy, and pharmacoeconomics of low-dose alteplase compared with urokinase for catheter-directed thrombolysis of arterial and venous occlusions. J Vasc Surg. 2003;37(3):512–7.
Suzuki M, Mizunari T, Iwamoto N, Morita A. Embolectomy through aneurysm wall for iatrogenic occlusion of M1 portion during coil embolization: technical note for transaneurysmal embolectomy. World Neurosurg. 2018 Jun;114:113–6.
Colleran R, Kastrati A. Percutaneous coronary intervention: balloons, stents and scaffolds. Clin Res Cadiol. 2018;107(suppl2):55–63.
Pasha AK, Elder MD, Khurram D, Snyder BA, Movahed MR. Successful management of acute massive pulmonary embolism using Angiovac suction catheter technique in a hemodynamically unstable patient. Cardiovasc Revasc Med. 2014 Jun;15(4):240–3.
Mitsuyoshi A, Obama K, Shinkura N, et al. Survival in non-occlusive mesenteric ischemia: early diagnosis by multidetector row computed tomography and early treatment with continuous intravenous high-dose prostaglandin E(1). Ann Surg. 2007;246(2):229–35.
Stone JR, Wilkins LR. Acute mesenteric ischemia. Tech Vasc Interv Radiol. 2015;18(1):24–30.
Oderich GS, Macedo TA, Malgor RD, Bower TC, Vritiska T, Duncan AA, et al. Natural history and predictors of mesenteric artery in-stent restenosis in patients with mesenteric ischemia. J Vasc Surg 2009;49(Suppl 1):A1–22. S1–58, e1–2
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Stevens, B.J., Ching, B.H. (2019). Radiology for Acute Mesenteric Ischemia. In: Lim, R. (eds) Multidisciplinary Approaches to Common Surgical Problems. Springer, Cham. https://doi.org/10.1007/978-3-030-12823-4_29
Download citation
DOI: https://doi.org/10.1007/978-3-030-12823-4_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-12822-7
Online ISBN: 978-3-030-12823-4
eBook Packages: MedicineMedicine (R0)