Abstract
An increasing amount of waste is produced year over year, without any signs of slowing down. However, by reorienting the perspective, organic residual waste can be seen as a valuable source of nutrients and carbon that should be valorized, instead of a waste product to be disposed of. This chapter covers the main methods of converting waste into value-added products. Two main categories of waste conversion technologies are explored: thermochemical and biochemical. Thermochemical conversion technologies include incineration, gasification, pyrolysis, and torrefaction, while biochemical conversion technologies include anaerobic digestion, fermentation, composting, and landfills with gas capture. Additional technologies for nutrient recovery as marketable end products following thermochemical and biochemical conversion are also discussed, including phosphorus (P) extraction, ammonia stripping and absorption, precipitation/crystallization, and membrane filtration. Carbon dioxide capture and valorization is also briefly explored. This chapter aims at providing general information on these technologies and the products that can be obtained through their use.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Organic wastes
- Nutrient recovery
- Carbon recovery
- Biorefinery
- Thermochemical conversion
- Biochemical conversion
- Landfill gas capture
1 Introduction
In 2012 the global production of municipal solid waste was of 1.3 billion tonnes per year, and it is estimated that, with the increase in population and urbanization, this amount will increase to 2.2 billion tonnes per year by 2025. Of this, approximately 40–50% is organic biodegradable matter, such as food, wood, garden and lawn clippings, and human and animal waste (Hoornweg and Bhada-Tata 2012; David 2013). These materials present a valuable source of nutrients such as phosphorus (P), potassium (K), and nitrogen (N), as well as a variety of minerals, on top of organic carbon (C). For example, food waste, similarly to most organic waste sources, is rich in carbon, usually between 40 and 55% (Adhikari et al. 2008). It can contain around 8% hydrogen (H) and 2–6% nitrogen (Vakalis et al. 2016), and other nutrients are often found below 1% (Zhang et al. 2007). However, this composition varies widely based on location and waste type (Mor et al. 2006; Siddiqui et al. 2011). With the impending nutrient depletion, it is of utmost importance to be able to recover these valuable resources from our waste (Elser and Bennett 2011). A variety of technologies currently exist to transform organic waste into value-added products, such as composting and biomethanation. This chapter will go over a variety of nutrient and carbon recovery and treatment alternatives, looking at both thermochemical and biochemical conversion processes.
In this chapter, nutrients will refer mainly to the substances used by plants to ensure their growth and maintenance. The nutrients which are necessary for proper plant life are boron (B), calcium (Ca), carbon, chlorine (Cl), copper (Cu), hydrogen, iron (Fe), magnesium (Mg), manganese (Mn), molybdenum (Mo), nickel (Ni), nitrogen, oxygen (O), potassium, sodium (Na), silicon (Si), sulfur (S), and zinc (Zn). There are a variety of ways to classify these nutrients. One form of classification is by the amount required, sorting them into either macronutrients (Ca, C, H, K, O, Mg, N, P, S) or micronutrients (B, Cl, Cu, Fe, Mn, Mo, Ni, Na, Si, Zn). They can also be classified by their roles in metabolic processes or by the location and method of their uptake (Engels et al. 2012).
2 Thermochemical Conversion
Thermochemical conversion processes are commonly used to recover energy from waste using high temperatures. The following is a short overview of thermal conversion technologies which include incineration, gasification, pyrolysis, and torrefaction.
A brief reminder of combustion can be useful to better understand the following processes. Combustion is the chemical reaction of a fuel in the presence of an oxidizer, most commonly oxygen, and heat. The fuel and the oxidizer react together to form new chemical substances, most often combinations of both, such as the combustion of hydrogen (H2) and oxygen (O2) to form water (H2O). It can also include combinations of the oxidizer alone, for example, when air is used as an oxidizer, the nitrogen (N2) and oxygen within it can react to form nitrous oxides (NOX). Combustion can either be complete or incomplete. When there is sufficient oxidizer to allow for all the fuel to be fully oxidized, the combustion is said to be complete. Therefore, the complete combustion of hydrocarbons (CnHn + 2) with oxygen will produce water and carbon dioxide (CO2). Incomplete combustion occurs when there is not enough oxidizer to burn all the fuel. In this case, part of the fuel is only partially oxidized, which will lead to the production of hydrogen, carbon, and carbon monoxide (CO). These products can still be oxidized so, depending on the context, an incomplete combustion can either be desirable (to produce fuel) or undesirable (loss of energy).
2.1 Incineration
Incineration consists of oxidizing the waste source, leading to a highly exothermic process which produces flue gas, ash, and heat. The heat is used to produce steam which in turn is sent to a turbine to produce electricity or used directly as process steam. Incineration has seen widespread use throughout Europe and became popular due to restrictions on landfill use, allowing the reduction of more than 90% of the waste’s volume (Chandler et al. 1997). The ash produced from the combustion process can be recovered to recycle nutrients such as phosphorus, metals, and other noncombustible materials (Zacco et al. 2014). Incineration also provides the ability to treat medical and potentially pathogenic waste. However, a wide variety of gases are released during the process, including sulfur dioxide (SO2), NOX, nitrous oxide (N2O), hydrogen chloride (HCl), hydrogen fluoride (HF), CO, CO2, dioxins, furans, polycyclic aromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), and heavy metals (Jay and Stieglitz 1995; Di Maria and Micale 2015). Many of these gases have important greenhouse effects and potential health impacts; however proper flue gas and fly ash treatment should alleviate these issues. For these reasons, there still exists widespread debate over the environmental and social acceptability of these processes.
Following the incineration process , it is possible to recover some nutrients from the ash, most notably phosphorus. Phosphorus contained in organic waste may either be in organic form, which is poorly absorbed by plants, or in inorganic form, which is more easily assimilated by plants (Cieślik and Konieczka 2017). It is necessary to remove heavy metals from the ashes before, during, or after P recovery, which can be achieved by separation methods such as washing and leaching, solidification treatments, and thermal treatments (Zacco et al. 2014).
The phosphorus from the ashes can be extracted through a variety of techniques. The two main methods for phosphorus recovery include thermal conversion, which aims at enriching the phosphorus in the ash, and leaching, which aims at extracting the phosphorus from the ash (Tan and Lagerkvist 2011). Thermal conversion in the context of P recovery is notably performed in a circulating fluidized bed boiler. It can be achieved through three different methods, including biomass combustion with phosphorus in the ash as direct source, biomass combustion followed by a dry process ash for P recovery, and biomass combustion followed by a wet process ash for P recovery. Thermal conversion leads to a high recovery rate from biomass, with up to 98% of the phosphorus retained in the ashes, while other interesting macronutrients such as Ca, K, and Mg may be retained as well. Phosphorus is mainly distributed in submicron ash particles, which may also contain sulfur, silicon, and sodium, with their concentration increasing as the particle size decreases. Temperature and oxygen content also influence the phosphorus content in ashes, with higher combustion temperatures and oxygen content leading to higher P content. Increasing oxygen content also leads to higher P content in particles within the micronic scale. The main obstacle of thermal conversion is the bioavailability of the resulting phosphorus, most of which is recovered in a form that is poorly available to plants. However, bioavailability can be improved with a second thermochemical step (dry process ash), where the phosphorus is transferred into mineral phases available for plants (Adam et al. 2009). Otherwise, the phosphorus obtained from the thermal conversion can be extracted by leaching (wet process ash ) (Tan and Lagerkvist 2011).
Phosphorus recovery by leaching can be done by different means, such as bioleaching, which involves microorganisms; supercritical extraction, which involves supercritical water; and chemical extraction, which involves acids such as hydrochloric acid and sulfuric acid leaching (Tan and Lagerkvist 2011).
Chemical leaching is the most common leaching method used for phosphorus recovery, where P extraction can be achieved with a fairly low consumption of chemicals, using only certain acids (Tan and Lagerkvist 2011). In general, higher temperature and acid concentration lead to higher rates of P recovery. In fact, a pH of 1 may be required to extract over 75% of the phosphorus. Solid-phase extraction of phosphorus by chemical leaching can be combined with precipitation, which is normally done by dissolution of the acidic extract containing the phosphorus, followed by a filtration (Kalmykova and Fedje 2013; Petzet et al. 2012). A series of multiple dilution-filtration cycles may be necessary to allow the extraction of every precipitation preceding the precipitation of phosphorus. An alternative to acid leaching is alkaline leaching, which uses bases such as sodium hydroxide. A two-step leaching process where acid leaching is followed by alkaline leaching may be done, which may lead to superior extraction performance under optimal conditions.
Supercritical extraction aims at enhancing the extraction of phosphorus through supercritical water oxidation (Tan and Lagerkvist 2011). In fact, supercritical water oxidation leads to higher release of phosphorus than conventional chemical leaching with normal acid concentrations. However, supercritical extraction may require pretreatments of the ashes as they may contain heteroatoms such as chlorine, sulfur, and phosphorus which may create a highly corrosive medium during supercritical water oxidation. Cost is also a concern due to the use of supercritical water and the potential need of pretreatments.
Bioleaching is unconventional as it is done prior to combustion and is only indirectly focused on the recovery of phosphorus. It involves bioacidifying microorganisms, notably sulfur-oxidizing microorganisms, for the extraction of heavy metals from soil and sludge mixtures (Shanableh and Omar 2003). Bioleaching is known to be subject to considerable losses of phosphorus and nitrogen during heavy metal removal, as it extracts a significant portion of those nutrients along with the heavy metals during the leaching. For example, 76% of phosphorus and 38% of nitrogen may be lost during bioleaching of heavy metals when using sulfur as a substrate (Shanableh and Ginige 1999). However, this problem can be attenuated by selecting the right substrate for bioleaching. For example, using FeSO4 ·7H2O as a substrate reduces the loss of phosphorus to 45%, while using FeS2 as a substrate reduces the loss of phosphorus and nitrogen to less than 6 and 15% (Wong et al. 2004). It is interesting to note that increasing solid content of biomass leads to better solubilization of heavy metals but also phosphorus (Tan and Lagerkvist 2011). Furthermore, lower sludge pH leads to faster solubilization, while higher pH tends to reduce P losses. In the context of bioleaching, using phosphate-solubilizing bacteria can also be of interest to supply plants with phosphorus from sources that are poorly available.
Another method proposed for phosphorus recovery is the fractionation method , where the ashes are agitated in an acidic solution followed by a series of centrifugations which leads to the extraction of phosphorus from the solids into the supernatant (Kleemann et al. 2017). Phosphorus can also be extracted from ashes by an electrodialytic separation process, which aims at extracting phosphates through ion exchange induced by an electric potential difference, as well as removing heavy metals (Guedes et al. 2014).
2.2 Gasification
Gasification is the process of transforming waste into syngas, a gas which can be used to produce fuels, chemicals, or synthetic natural gas. Whereas a complete combustion is desired during incineration, gasification comprises of an incomplete combustion using an amount of oxidant lower than the amount required for stoichiometric combustion. As a result, the waste becomes gasified into syngas, which contains unoxidized products which can be used as energy sources for later use. The resulting syngas is rich in H2 and CO, with some methane (CH4), but is generally contaminated and necessitates cleaning before being usable (Yan et al. 2010). The largest challenge faced by gasification processes, other than cleaning the syngas, is the need for a heterogeneous waste source (Asadullah 2014).
Furthermore, the solid by-product of gasification is biochar (Hansen et al. 2015). Biochar is rich in nutrients and has been found to increase their retention, thus decreasing leaching and increasing the nutrient availability for plants when applied to soil (Oram et al. 2014; Yuan et al. 2016). Other than its agricultural applications, biochar can also be burned to release energy or as an adsorbent for environmental applications, similarly to activated carbon (Xie et al. 2015).
As is the case for incineration, P recovery from ashes is also possible for gasification (Atienza–Martínez et al. 2014). Chemical leaching for a few hours can recover over 90% of the phosphorus with sulfuric acid, but extending the leaching duration does not significantly affect the recovery rate. A similar load of oxalic acid leads to comparable results as sulfuric acid, but may recover close to 100% of the phosphorus when leaching is done for an extended period of time.
An alternative to combustion for gasification of sewage sludge is supercritical water gasification (Acelas et al. 2014). A key advantage of supercritical water gasification of sewage sludge is that it does not require drying of the sludge, which can avoid the costs of such pretreatments. In addition to the production of biogas, a solid and a liquid residue is also produced, both containing nutrients. Almost all of the N, K, and Cl are contained in the liquid phase, while the solid phase contains almost all of the Ca, P, and Si, leading to an effective separation of nutrients. However, the process is ineffective for separation of S as it is evenly distributed between the two phases. The liquid and solid phases may be separated by filtration, and the solid phase can then undergo a chemical leaching treatment for P extraction. Increasing leaching duration of the solid residue has little impact, generally leading to a minor increase of the percentage of phosphorus recovery. Leaching temperature has a significant impact on leaching performance. A temperature of 400 °C is sufficient to ensure a recovery of at least 80% of the phosphorus, while increasing the temperature to 600 °C leads to a recovery rate close to 90%, if not higher. Once again, oxalic acid leads to a higher recovery rate than sulfuric acid. When compared to leaching of combustion ash, leaching of supercritical water gasification residue leads to higher P yields from recovery. This improvement in recovery performance may be attributed to the formation of different chemical compounds during the thermal treatment. While combustion leads to the formation of organic compounds with P-C bonds, which are inert to acid treatments, supercritical water gasification produces a higher amount of inorganic phosphorus in the form of calcium, aluminum, and iron phosphates, which are more easily dissolved during the leaching treatment.
2.3 Pyrolysis
Pyrolysis is the decomposition of material at high temperatures in the absence of oxygen. This process allows the conversion of waste into syngas (gas), biofuels (liquid), and biochar (solid) (Laird et al. 2009). Unlike the previous two processes, this technology is not a combustion-based process and is endothermic. By increasing the temperature with the absence of an oxidant, the chemical bonds in the organic matter are broken down. Therefore, the longer organic chains are decomposed into simpler compounds, resulting in the production of CO, H2, and hydrocarbons (Jahirul et al. 2012). The proportion of gas, liquid, or solid product is determined by the operating conditions and the type of feedstock. Similarly to gasification, cleaning of the syngas and feedstock quality are some of the most limiting factors to the widespread use of this technology (Carpenter et al. 2014). Pyrolyzed char also benefits from a superior porosity, increasing the interest for adsorption applications (Kleemann et al. 2017).
Recovery of P from pyrolyzed biochar is a possibility, which differs significantly from P recovery from incinerated ashes in terms of performance (Kleemann et al. 2017). First of all, pyrolysis has lower P enrichment capacity relative to sludge than incineration, with P concentrations being enriched by less than three times in pyrolyzed char compared to an enrichment of about seven times in the case of ashes from incineration. Furthermore, P extraction by leaching seems to be less effective on pyrolyzed char, as a lower proportion of P extracted from the former is obtained at optimal acid concentration for incinerated ashes. However, an increase of acid concentration for the leaching process leads to an improvement in P extraction from pyrolyzed char. As a result, optimal extraction from pyrolyzed ashes by leaching requires a higher concentration of acid than for incinerated ashes. Pyrolyzed char contains a significant residual concentration of N, but contains much less heavy metals than incinerated ashes, as a large amount of them are volatilized in the pyrolysis process. The type of acid used for leaching may have an impact on P recovery performance. For example, using oxalic acid leads to higher phosphorus yields than when sulfuric acid is used (Atienza–Martínez et al. 2014).
2.4 Torrefaction
Torrefaction is a form of pyrolysis at lower temperatures. The aim of this technology is to improve the properties of biomass for energy generation, converting the biomass into a coal-like material. Torrefied biomass has a variety of interesting qualities, notably exhibiting a hydrophobic behavior, an inhibited biological decomposition (i.e., it doesn’t rot), an improved grindability, and a higher heating value (Acharya et al. 2012). As is the case for pyrolysis, syngas and biofuels are also produced during torrefaction, but are viewed mostly as by-products, the gas usually being directed back into the process to provide heat. The biochar produced from torrefaction can then be used for combustion or gasification or reused as a soil amendment.
The following Table 1 presents a brief overview of some important technical, economic, and environmental data pertaining to these technologies.
3 Biochemical Conversion
Whereas the previously discussed technologies utilized heat to convert biomass into energy and value-added products, the following technologies use microbial processes to achieve the same goal. It is important to note, however, that these processes are limited to biodegradable waste, reducing their range of application compared to technologies such as incineration.
3.1 Anaerobic Digestion
Anaerobic digestion is the natural process by which microorganisms break down organic material in the absence of oxygen. Examples of organic waste that can be processed by anaerobic digestion include animal manures, food scraps, fats, oils and greases, industrial organic residuals, and wastewater and sewage sludge, among others. Anaerobic digestion produces biogas, which is mainly composed of methane and carbon dioxide and can be used as an energy source or as a precursor for other chemical compounds, and a by-product known as the digestate, which can be valorized as bedding for livestock, fertilizers, and soil amendments, among others. Biogas is generally composed of around 60% methane and 40% carbon dioxide, with possible traces of hydrogen sulfide (H2S), nitrogen, and hydrogen (H2) and traces of oxygen, carbon monoxide, ammonia (NH3), and certain volatile organic compounds (Noyola et al. 2006). Biogas should not be confused with syngas, the latter being composed mainly of H2, CO, and CO2. Anaerobic digestion is done in four key steps (Bajpai 2017). The first step is hydrolysis by microorganisms which break down carbohydrates into forms digestible by other microorganisms. The second step is known as acidogenesis, where acidogenic bacteria convert the hydrolyzed sugars into higher organic acids such as propionic acid and butyric acid. The next step is acetogenesis, where organic acids are converted into acetic acid by acetogenic bacteria, with hydrogen as a main by-product. The final step of anaerobic digestion is methanogenesis, consisting of the conversion of acetic acid into methane and carbon dioxide by methanogenic bacteria. As stated previously, the solid or liquid mixture remaining after the anaerobic digestion process is known as the digestate.
The anaerobic digestion process can be operated in three main temperature ranges, including thermophilic (50–60 °C), mesophilic (25–40 °C), and psychrophilic (15–25 °C) digestion (DeBruyn and Hilborn 2007). The higher operating temperature of the thermophilic range causes the microorganisms to break down organic matter more rapidly, with an average retention time of 3–5 days, and leads to higher production of biogas. However, more energy consumption and greater insulation are required. The mesophilic range operates at a lower temperature, meaning that more time is required for the microorganisms to break down organic material, with a retention time of 15–20 days or more. However, they are known to be more robust to temperature upsets. The psychrophilic system operates at room temperature, meaning that it has the lowest energy requirement of the three temperature ranges, but breaking down of organic matter takes longer, with retention times usually spanning over a month or two (Lettinga et al. 2001).
Prior to anaerobic digestion, substrates and feedstocks are subject to pretreatments in order to enhance performance (Ariunbaatar et al. 2014). Pretreatments include mechanical treatments to increase contact surface between substrate and bacteria; thermal treatments to remove pathogens, enhancing dewatering performance and reducing viscosity of the digestate; as well as chemical treatments for early breakdown of organic compounds and biological treatments for general performance enhancement in certain steps. Anaerobic digestion can be operated in “dry” or “wet” conditions.
3.1.1 Dry Anaerobic Digestion
Dry anaerobic digestion operates at a high total solid content, generally over 20% (Angelonidi and Smith 2015). It is mainly used for feedstocks such as leaves, grass, straw, garden waste, wood waste, as well as chicken slurry and food waste with high solid content (Steffen et al. 1998). Dry anaerobic digestion has a good number of advantages over wet digestion, including lower power and heat requirements, high tolerance to solid contaminants (sand, fibers, large particles, etc.), less critical equipment required (pumps, agitation systems, feeding equipment, etc.), less maintenance, low water consumption, arguably greater flexibility over the type of feedstock accepted, shorter retention times, more flexible management of the end product, and being less complex overall than wet anaerobic digestion (Angelonidi and Smith 2015; ADEME Bourgogne 2013). The main disadvantages of dry anaerobic digestion include the need for special technologies for loading and unloading of the digester, the need to manage the variations of biogas and heat production, uneven mixing of the substrate, lower methane production in comparison to wet anaerobic digestion, and high requirements in structure material.
Industrial dry anaerobic digesters are similar to plug flow digestors as they are generally horizontal and cylindrical, but they may be agitated and are often operated in batch (Cho et al. 2013; Guendouz et al. 2010). Dry anaerobic digesters can also be constructed in the form of a silo or a garage (ADEME Bourgogne 2013). The silo form is known to be simple, robust, and cheaper, but the garage form is easier to operate as opening and closing its doors for loading and unloading are simpler than removing and reinstalling the silo’s cover to do so from above. Dry anaerobic digesters in the form of smaller and easily transportable containers have also been proposed, which would allow transportation as the digestion takes place, but installation costs are currently higher than conventional dry anaerobic digesters.
3.1.2 Wet Anaerobic Digestion
Wet anaerobic digestion operates at a low total solid content, generally less than 20% (Angelonidi and Smith 2015). The main advantages over dry anaerobic digestion include higher rate of biogas produced per tonne of waste treated, lower investment and operating costs, better energy balance, lower parasitic energy consumption, arguably greater flexibility over the type of feedstock accepted, integration of gas buffer, and biological desulfuration (Angelonidi and Smith 2015; ADEME Bourgogne 2013). Improvements in energy efficiency and biogas production rates may be attributed to optimal mixing, which is usually lacking in dry anaerobic digestion processes (Lindmark et al. 2014). The main disadvantages of wet anaerobic digestion include the need to add liquid when dry mixtures are used, the important need for mixing equipment, the significant energy requirement for pumps and agitators, and the risk of solid deposition and scum layer formation. Digestate produced from wet anaerobic digestion has lower dry matter content compared to digestate formed from dry anaerobic digestion. Digestate with low solid content can complicate storage and transportation for land application of digestate and hence require additional investments for further processing of the product, such as solid-liquid separation, drying, and/or nutrient extraction equipment. It is interesting to note, however, that wet anaerobic digestion plants may benefit from a more advantageous energy balance and economic performance overall compared to dry anaerobic digestion, despite these disadvantages (Angelonidi and Smith 2015). Since undigested sewage sludge usually has low solid content (generally ≤1–7%) (Ontario Ministry of the Environment 2008a), wet anaerobic digestion is ideal in this context. However, since higher solid content leads to lower digester sizing and energy requirements, and higher biogas production rates, dewatering the sludge prior to digestion may be desirable (Yi et al. 2014; Ontario Ministry of the Environment 2008b).
The two main types of digester used for wet anaerobic digestion include completely mixed and plug flow systems (DeBruyn and Hilborn 2007). Complete-mixed digesters consist of a large tank where fresh organic material is continuously mixed with partially digested material. This type of configuration is recommended for mixes with lower dry solid content (3–10%) such as pig and cow slurry, animal blood, whey, and fermentation slops (Chen and Neibling 2014; Steffen et al. 1998). Complete-mixed digesters can either operate in the mesophilic or thermophilic range , with a retention time generally ranging from 10 to 25 days. Plug flow digesters are continuously fed and consist of long channels in which feedstock moves through as it gets digested. This system is recommended for the treatment of material with higher dry solid content (11–14%) such as chicken slurry and food wastes, as well as pig and cow slurry with higher solid content (Steffen et al. 1998). They usually operate in the mesophilic range, with the retention time ranging from 15 to 30 days. Other key examples of wet digesters include covered lagoons and fixed-film digesters (Chen and Neibling 2014). As its name suggests, the covered lagoon is a large in-ground lagoon with a gastight cover. Covered lagoons are used for mixes with very low solid content (0.5–2%), making them ideal for raw sludge, as well as whey and fermentation slops with the lowest solid content (Steffen et al. 1998). They generally operate in the psychrophilic temperature range as they are not heated, meaning that they mostly operate in warmer regions where the temperature range can be reliably maintained. Fixed-film digesters are basically columns packed with such media as wood chips and plastic rings. The packing supports a biofilm of bacteria, with methane-forming microorganisms growing on the media. This type of digester is also used for very low solid contents (1–2%) and benefits from a very short retention time (2–6 days). However, the media is vulnerable to plugging by solids. The wet anaerobic digestion process is usually operated in continuous flow as it produces more biogas, and the operating costs are lower in comparison to a batch operation due the avoidance of the complications from restarting the system from cold (World Energy Council 2016).
3.1.3 Nutrient Recovery
Following the digestion process, it is possible to extract end products with higher nutrient concentration through implementation of nutrient recovery technologies (NRTs) . For some liquid waste streams, it is possible to apply these technologies without passing through the anaerobic digestion process first, but process efficiency will be improved following digestion (Vaneeckhaute et al. 2017). The following is a quick overview of the most established NRTs to date. For detailed technical and economic information, reference is made to the literature review on NRTs for digestate treatment presented in Vaneeckhaute et al. (2017).
-
Chemical crystallization: Crystallization/precipitation can be induced through a number of methods such as modification of temperature, solubility, and charges. Chemical crystallization can be used to produce struvite (MgNH4PO4∙6H2O) and calcium phosphates (CaHPO4∙2H2O or Ca5(PO4)3OH) from liquid digestate. Both products are valuable synthetic fertilizer substitutes (Vaneeckhaute et al. 2017). By increasing the pH of the liquid fraction through the addition of magnesium (MgO/MgCl2) or calcium hydroxide (Ca(OH)2) and sodium hydroxide (NaOH), crystallization is achieved (Rahman et al. 2014). Struvite and calcium phosphates (brushite and hydroxyapatite) are of growing interest due to the depletion of rock phosphate and will likely become the chief phosphate fertilizers (Massey et al. 2007). They also contain other valuable minerals such as Mg, N, and Ca. Struvite precipitation allows for low nitrogen loss, around 5% (Kataki et al. 2016). Furthermore, struvite tends to offer some advantages over chemical phosphorus fertilizers, such as allowing for a slow release of nutrients and having a high hardness, allowing for easy transportation and storage (Latifian et al. 2012). It can also be applied to soils at greater quantities and lower frequency than conventional fertilizers (Li and Zhao 2003).
-
Gas stripping and absorption: Ammonia (NH3) stripping and absorption is one of the more established technologies for N recovery. The process involves passing the liquid fraction through a packed bed tower. Some of the ammonia is transferred from the liquid phase to the gas phase, and this gas is sent to an air scrubber. The air scrubber puts the NH3 in contact with a liquid phase, commonly H2SO4, to recover a concentrated solution of ammonium sulfate ((NH4)2SO4). Ammonium sulfate can be used as a fertilizer, being a good source of N and S (Arthington et al. 2002).
-
Membrane separation: Membrane filtration involves passing the digestate through microfiltration, nanofiltration, ultrafiltration, and/or using reverse osmosis. The filtration process allows for the production of process water and a nutrient (N and K)-rich concentrate when using reverse osmosis (Vaneeckhaute et al. 2017; Drosg et al. 2015).
3.2 Fermentation
Fermentation is the use of microorganisms to convert organic matter in absence of oxygen into acids or alcohols, notably ethanol and lactic acid, as well as hydrogen. Microorganisms can also be selected to favor methane production, in which case the process is equivalent to biomethanation. Ethanol and hydrogen are clean fuels of high interest, notably for the transportation sector, while lactic acid is a versatile chemical with a long history of applications in the food, cosmetic, and pharmaceutical industries. The most important application of fermentation is for the production of bioethanol, which is usually done by yeast fermentation either in continuous or batch configuration. Although, batch fermentation is usually the preferred method due to the lower risk of contamination. The two main types of reactor used for fermentation are the continuous stirred tank reactor (CSTR) and the plug flow reactor (PFR) (Brethauer and Wyman 2010). In the CSTR, the composition in the reactor tank is homogenous if the reactor is ideally stirred, meaning that the composition is identical not only within the reactor but also to the outgoing flow. For the PFR, the reactants are pumped through a tube with the fermentation proceeding as the biomass travels through the reactor. In the case of an ideal PFR, a uniform velocity profile across the radius diffusion and negligible diffusion in the axial direction are expected. The fermentation reactor can either be single-stage or multistage, with the latter having the option of either being set up in series or in parallel. It is interesting to note that a large number of CSTRs in series may have similar performance to a single PFR.
Bioethanol production is mainly done from starchy substrates such as corn, wheat, rice, barley, and potatoes, as well as from sucrose-containing feedstocks such as sugar beet, sweet sorghum, and sugarcane (Patni et al. 2013). In fact, the world’s leading sources of ethanol production are corn from the United States and sugarcane from Brazil (Crago et al. 2010). However, there is an increasing interest for the use of organic waste such as lignocellulosic biomass (dry plant matter) for bioethanol production, which would avoid the use of edible crops for bioethanol production. Lignocellulosic biomass is mainly composed of cellulose, hemicellulose, and lignin. Major sources of lignocellulosic biomass include wood, agricultural residues, herbaceous plants, municipal solid wastes, as well as algae. Bioethanol production from cellulosic feedstocks consists of four key steps (Limayem and Ricke 2012). The first step is a pretreatment consisting of enzymatic, thermal, and acid treatments. The treated biomass is then hydrolyzed into free monomer molecules readily available for fermentation conversion to bioethanol. The hydrolysis is either done through acidic or enzymatic reactions. The hydrolyzed sugars then undergo the fermentation in presence of microorganism, where they can be converted into alcohol, as well as into lactic acid or other end products, depending on the microorganisms and fermentation condition used. The final step is focused of the separation and purification of the ethanol produced from water or other by-products through a distillation process.
Nutrients such as nitrogen can be recovered from fermentation waste by electrodialysis (Lee et al. 2003). Otherwise, there is room for valorization of fermentation waste, which can be used as a metal biosorbent (Vijayaraghavan et al. 2008; Gulati et al. 2002) or as a concrete biomodifier, for example (Bolobova and Kondrashchenko 2000).
3.3 Composting
Composting is the aerobic biological decomposition of organic waste such as food or plant material by bacteria, fungi, worms, and other organisms into compost. Compost is rich in carbon and nutrients and therefore presents benefits when applied to soils by increasing the nutrient supply, sequestering carbon, acting as a pesticide, increasing crop yield, decreasing soil erosion, increasing soil workability, and increasing the nutritional quality of crops (Lazcano et al. 2014; Lairon 2011). Composting can also be undertaken in anaerobic conditions (Minale and Worku 2014), in which case it is equivalent to the anaerobic digestion treated earlier. This section will only focus on aerobic composting. Following the anaerobic digestion process described previously, the digestate can be composted to increase its suitability for use on agricultural land (Bustamante et al. 2012; Bustamante et al. 2013).
Generally, the composting process is divided into four stages: mesophilic, thermophilic, cooling, and maturation (Chen et al. 2011; Tortosa et al. 2017). Composting is started by mesophilic organisms operating between 25 and 45 °C. The decomposition of the biomass leads to a rapid increase in the temperature of the compost, reaching 50–60 °C within the first 72 h. At these higher temperatures, thermophilic organisms take the lead in decomposing the materials. This second stage lasts significantly longer than the first one (several days or weeks). The high temperatures during this stage lead to the added benefit of destroying pathogens, weed seeds, and phytotoxic compounds. However, proper temperature control is crucial. If the compost pile goes above 70 °C, there is a high risk of killing the thermophilic organisms, as well as increasing the risk of fires. Partway through this second stage, the decomposition of the compost will render less nutrients and energy available to sustain the microorganisms. The temperature will start to decrease progressively, leading into the third stage of composting: the cooling stage. During this stage, the temperature continually decreases, leading mesophilic microbial organisms to take back the reins. Once the temperature stabilizes, the final stage, known as maturation, begins. By the end of this process, which can last several months and even more than a year, the original biomass will have been decomposed into compost which can be spread over agricultural land or for home use. The first two phases are often referred to as the active phase, whereas the final two are known as the curing phase. Overall, the composting process can last between 3 months and 2 years (United States Environmental Protection Agency 2018).
Throughout the whole of this process, it is paramount that the compost receives oxygen. If proper aeration of the compost pile is not provided, the microorganisms will quickly use up all the oxygen, causing the pile to transition into an anaerobic environment. Therefore, the composting process would be drawn out and take much longer due to non-optimal operating conditions. To ensure proper aeration, there are a variety of techniques that can be used: aerated windrow composting, aerated static pile composting, and in-vessel composting (Neher et al. 2013).
Aerated windrow composting consists in making long rows, known as windrows, of organic waste and aerating the piles by turning them frequently (Kuchenrither et al. 1985). On the other hand, aerated static pile composting uses one large pile of organic waste, supplemented with bulking material such as wood chips, paper, and cardboard, to create spacing within the pile and allow air to circulate through it. Pipes can be used to draw air in or out of the pile (Ekinci et al. 2017).
For in-vessel composting, the organic waste is placed into an enclosed vessel that allows control of the air flow and temperature. The organic waste is mixed by mechanical means. This method allows for a reduction of space compared to the previous two methods, a strong control of process parameters and a control of odors, and offers the added benefit of potential integration with anaerobic digestion. Indeed, some processes have been developed wherein, following anaerobic digestion, the same reactor is used to allow aeration of the organic waste (Walker et al. 2009).
In all these cases, composting remains the process of decomposing organic waste, meaning that a significant amount of greenhouse gases (GHGs) are produced. It is therefore necessary to treat the air adequately to control these gases, most notably nitrous oxides and methane (Nigussie et al. 2016). Furthermore, a large amount of the initial nitrogen is lost during the process, being converted into NH3, NOx, N2O, and N2, which become more volatile at higher temperatures (thermophilic stage). Composting tends to have high levels of N loss, ranging from 16 to 88% depending on the waste source, therefore reducing the quality of the compost and producing undesired gases (Vu et al. 2015). It is estimated that 35–70% of this loss occurs during the thermophilic active phase (Chowdhury et al. 2014).
There exists another form of composting which allows circumventing some of these issues. Vermicomposting focuses on the use of worms for decomposition at temperatures between 12 and 25 °C with the help of microorganisms (Ndegwa and Thompson 2001). This type of decomposition takes place in a psychrophilic temperature range, avoiding many of the problems previously identified. The worms are inserted into the organic matter, which has been supplemented with bulking agents to make it more attractive to the earthworms (Lim et al. 2016), and are left to eat and digest it. The product of vermicomposting is also somewhat different from normal compost. It is known as vermicompost (or casting) and tends to be richer in nutrients, which are also more readily accessible by the plants, than compost (Lazcano et al. 2009). Vermicompost also has added benefit for soil and plant life due to some by-products excreted by the worms, such as mucus and urine which are rich in carbon and ammonium and urea, respectively (Quaik and Ibrahim 2013). By operating at lower temperatures than the thermophilic stages of composting, vermicomposting also allows for a reduction of nitrogen loss and methane emissions (Nigussie et al. 2016). However, these low temperatures also hinder the ability to destroy pathogens observed during composting (Ndegwa and Thompson 2001).
Composting can lead to the production of leachate, which arises when water passes through and leaches out some of the contents from the waste (Chatterjee et al. 2013). This liquid tends to be high in nitrogen and organic matter. Depending on regulatory constraints, this leachate can potentially be used as a fertilizer (Romero et al. 2013). It could therefore be possible to treat the leachate to produce struvite through precipitation and/or to use ammonia stripping and absorption to produce ammonium sulfate. The production of these solid fertilizers could cut down on the issues related with transportation and storage of a liquid.
Overall, composting is a relatively easy to implement method that allows for both carbon and nutrient recovery but also produces an important amount of GHGs which must be considered. The long curing time can be viewed as a drawback to this process; however, its general acceptability and ease of use when compared to anaerobic digestion make it an alluring treatment and valorization method.
3.4 Landfills with Gas Capture
A landfill is a site for the disposal of waste materials by burial. Landfills are a significant source of greenhouse gas emissions, with the organic materials decomposing in them producing a gas comprised of around 50% methane and 50% carbon dioxide with trace amounts of non-methane organic compounds (Themelis and Ulloa 2007). This gas mixture is known as landfill gas (LFG) . Gas capture technologies can be integrated within the landfills to capture the greenhouse gases from LFG. Landfills with gas capture are largely focused on capturing the methane, but the carbon dioxide could technically be captured as well.
To capture the LFGs, wells are installed throughout the waste in either vertical or horizontal configurations. These wells extract the gases by producing a pressure gradient by using blowers or air compressors. The difference in pressure decreases as the distance from the well increases, meaning that proper well spacing is necessary to achieve efficient gas capture. The landfill can be covered or left open to the air, but open landfills are less efficient at capturing the gas (approximately 17% less efficient). However, methane content is reduced by around 6% in closed landfills (Powell et al. 2016).
Nutrient recovery from landfills is also a possibility (Li and Zhao 2003; Kulikowska and Klimiuk 2008). Landfill leachate, similarly to composting leachate, can be a good source of nutrients, such as nitrogen (ammonia) and phosphorus. Therefore, struvite precipitation and ammonia stripping can be excellent techniques to valorize this by-product.
4 Chemical Conversion
Biomass can be converted into biofuels using purely chemical reactions. Key reactions involved in the conversion of biomass includes hydrolysis, dehydration, isomerization, aldol condensation, reforming, hydrogenation or hydrogenolysis, and oxidation (Jiang et al. 2016). The most common reaction used in bioenergy contexts is transesterification, which converts triglycerides (oils, fats, and greases) into biodiesel. With the help of an acid or base catalyst, fatty acids from oils, fats, and greases are bonded to alcohol, which reduces the viscosity of the fatty acids and makes them combustible, thus producing biodiesel. The transesterification reaction produces glycerol as a by-product, which leaves room for valorization such as biofuel production by glycerol fermentation as well as hydrogen production by glycerol steam reforming.
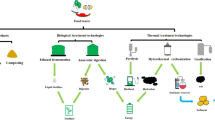
Based on the above discussion, Fig. 1 provides a summary overview of the principal thermochemical and biochemical conversion technologies for nutrient and carbon recovery from organic waste, as well as the main recovered end products.
5 Understanding the Carbon Balance and Adding Value through Carbon Capture Technologies
Many of the technologies that have been presented in this section aim at converting biomass into energy by either burning it directly (incineration) or by using it to produce various gases which are then destined for combustion (gasification, pyrolysis, biomethanation, LFG capture). Many of these alternatives are considered to be eco-friendly or “green” when compared to simply burying waste in a landfill. However, some may wonder why this is the case; at the end of the day, the gases are still being combusted and released into the atmosphere. The answer to this can be found when examining the carbon balance of the alternatives.
The carbon cycle refers to the biogeochemical cycle of carbon on Earth. It is separated into three subcategories, which includes two types of biological carbon cycle (terrestrial and oceanic) and geological carbon cycle. The carbon balance refers to the exchange between these categories. While the carbon cycle as a whole is a self-sustainable cycle that converts or captures as much CO2 as it produces or emits, greenhouse gas emissions from human activities create a surplus of carbon dioxide to the atmosphere (Falkowski et al. 2000). This disturbs the natural carbon balance, thereby causing a continuous increase of atmospheric CO2 concentrations, contributing to global warming and climate change. Major sources of anthropogenic greenhouse gas emissions include energy supply, transport, industries, forestry and agriculture, as well as commercial and residential sectors (Victor et al. 2014). Although biomass actively absorbs CO2 from the atmosphere through photosynthesis as it grows, the CO2 is released back to the atmosphere as biodegradation of biomass takes place, combined with methane and other gases. While decomposition is largely carbon-neutral when considering biomass growth, a reduction of these emissions into the atmosphere could be valuable in the current fight against global warming. By burning syngas, biogas, or LFG, the methane which would be released to the atmosphere is converted into CO2, which has a global warming potential much lower than methane.
It is possible to move the carbon balance to a point of “negative emissions” by integrating bioenergy with carbon capture and storage (BECCS) technologies (Biorecro 2010). Carbon capture and storage, or CCS, is mainly used for natural gas or coal power plants, consisting of capturing CO2 emissions and storing them underground (such as in oil wells, coal seams and saline aquifers) to prevent them from reaching the atmosphere, thus causing a net reduction of greenhouse gas emissions from those energy sources (Ketzer et al. 2012). Other key applications for carbon capture and storage include cement, metal, and chemical industries. Carbon capture is mainly done by gas-liquid absorption, but other key methods proposed include adsorption, membrane separation, chemical looping combustion, cryogenic distillation, hydrate-based separation, and enzymatic capture (Leung et al. 2014). BECCS consist in implementing such technologies for the capture and storage of CO2 emissions from combustion, fermentation, putrefaction, biodegradation, and other biological processes (Biorecro 2010). BECCS could also be implemented for the combustion of biogas produced from biomethanation. The captured CO2 can then be valorized, which consists of using or transforming the CO2 for other purposes, or stored under bedrock, leading to permanent storage of carbon dioxide that natural carbon sinks and valorization may not provide. Geological storage of CO2 emissions can be combined with mineralization, which converts CO2 into solid minerals, thus preventing any potential risk of CO2 leakage from the storage sites (Matter et al. 2016).
CO2 can be valorized in a number of ways. It can be used directly to supplement plant growth in greenhouses (Jaffrin et al. 2003), to aid in enhanced oil recovery by using it to displace oil (Whittaker and Perkins 2013), to act as a coolant or refrigerant (Lorentzen 1994), to act as a flame retardant for fire extinguishers (United States Department of Labor 2002), and for carbonation in food and beverage (Energy Research Centre of the Netherlands 2002), just to name a few. Otherwise, CO2 can be converted into cements (Biello 2008), plastics, fuels, and a variety of useful chemicals, such as carboxylates, lactones, carbamates, urea, isocyanates, carbamate, alcohols, ethers, and hydrocarbons, or serve as a catalyst or a co-reactant for the conversion of organic compounds (Olajire 2013). Carbon dioxide can even be used to generate energy or electricity, thus turning unwanted greenhouse gas emissions into a renewable energy source. Energy can either be generated through electrochemical conversion of CO2 that generates electricity (Al Sadat and Archer 2016) or in the form of supercritical CO2 as a substitute to water for steam turbines, such as those used for natural gas, coal, nuclear, geothermal, and solar power plants (Ahn et al. 2015).
References
Acelas NY, López DP, Brilman DW, Kersten SR, Kootstra AMJ (2014) Supercritical water gasification of sewage sludge: gas production and phosphorus recovery. Bioresour Technol 174:167–175
Acharya B, Sule I, Dutta A (2012) A review on advances of torrefaction technologies for biomass processing. Biomass Convers Biorefin 2:349–369
Adam C, Peplinski B, Michaelis M, Kley G, Simon F-G (2009) Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag 29:1122–1128
ADEME Bourgogne (2013) La méthanisation agricole en voie sèche discontinue. Agence de l’Environnement et de la Maîtrise de l’Energie
Adhikari BK, Barrington S, Martinez J, King S (2008) Characterization of food waste and bulking agents for composting. Waste Manag 28:795–804
Ahn Y, Bae SJ, Kim M, Cho SK, Baik S, Lee JI, Cha JE (2015) Review of supercritical CO2 power cycle technology and current status of research and development. Nucl Eng Technol 47:647–661
Al Sadat WI, Archer LA (2016) The O2-assisted Al/CO2 electrochemical cell: A system for CO2 capture/conversion and electric power generation. Sci Adv 2:e1600968
Angelonidi E, Smith SR (2015) A comparison of wet and dry anaerobic digestion processes for the treatment of municipal solid waste and food waste. Water Environ J 29:549–557
Arena U (2012) Process and technological aspects of municipal solid waste gasification. A review. Waste Manag 32:625–639
Ariunbaatar J, Panico A, Esposito G, Pirozzi F, Lens PN (2014) Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl Energy 123:143–156
Arthington J, Rechcigl J, Yost G, McDowell L, Fanning M (2002) Effect of ammonium sulfate fertilization on bahiagrass quality and copper metabolism in grazing beef cattle 1, 2. J Anim Sci 80:2507–2512
Asadullah M (2014) Barriers of commercial power generation using biomass gasification gas: a review. Renew Sustain Energy Rev 29:201–215
Atienza–Martínez M, Gea G, Arauzo J, Kersten SR, Kootstra AMJ (2014) Phosphorus recovery from sewage sludge char ash. Biomass Bioenergy 65:42–50
Bajpai P (2017) Basics of anaerobic digestion process. In: Anaerobic technology in pulp and paper industry. Springer, Berlin
Biello D (2008) Cement from CO2: a concrete cure for global warming? Sci Am 7:61
Biorecro AB (2010) Global status of BECCS projects 2010. Global CCS Institute, Melbourne
Bolobova A, Kondrashchenko V (2000) Use of yeast fermentation waste as a biomodifier of concrete. Appl Biochem Microbiol 36:205–214
Brethauer S, Wyman CE (2010) Continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour Technol 101:4862–4874
Bustamante M, Alburquerque J, Restrepo A, De la Fuente C, Paredes C, Moral R, Bernal M (2012) Co-composting of the solid fraction of anaerobic digestates, to obtain added-value materials for use in agriculture. Biomass Bioenergy 43:26–35
Bustamante M, Restrepo A, Alburquerque J, Pérez-Murcia M, Paredes C, Moral R, Bernal M (2013) Recycling of anaerobic digestates by composting: effect of the bulking agent used. J Clean Prod 47:61–69
Carpenter D, Westover TL, Czernik S, Jablonski W (2014) Biomass feedstocks for renewable fuel production: a review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem 16:384–406
Chandler AJ, Eighmy TT, Hjelmar O, Kosson D, Sawell S, Vehlow J, Van der Sloot H, Hartlén J (1997) Municipal solid waste incinerator residues. Elsevier, Amsterdam
Chatterjee N, Flury M, Hinman C, Cogger CG (2013) Chemical and physical characteristics of compost leachates. A review report prepared for the Washington State Department of Transportation. Washington State University
Chen L, Neibling H (2014) Anaerobic digestion basics. University of Idaho Extension, Moscow, p 6
Chen L, De Haro M, Moore A, Falen C (2011) The composting process: dairy compost production and use in Idaho CIS 1179. University of Idaho, Moscow
Cho S-K, Im W-T, Kim D-H, Kim M-H, Shin H-S, Oh S-E (2013) Dry anaerobic digestion of food waste under mesophilic conditions: performance and methanogenic community analysis. Bioresour Technol 131:210–217
Chowdhury MA, de Neergaard A, Jensen LS (2014) Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 97:16–25
Cieślik B, Konieczka P (2017) A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. J Clean Prod 142:1728–1740
Crago CL, Khanna M, Barton J, Giuliani E, Amaral W (2010) Competitiveness of Brazilian sugarcane ethanol compared to US corn ethanol. Energy Policy 38:7404–7415
David A (2013) Technical document on municipal solid waste organics processing. Environment Canada
DeBruyn J, Hilborn D (2007) Anaerobic digestion basics. Ontario Ministry of Agriculture, Food and Rural Affairs, Guelph, ON, p 10
Di Maria F, Micale C (2015) Life cycle analysis of incineration compared to anaerobic digestion followed by composting for managing organic waste: the influence of system components for an Italian district. Int J Life Cycle Assess 20:377–388
Drosg B, Fuchs W, Al Seadi T, Madsen M, Linke B (2015) Nutrient recovery by biogas digestate processing. IEA Bioenergy, Dublin, pp 7–11
Ekinci K, Tosun I, Seyit Ahmet I, Memici M, Kumbul BS (2017) Design and construction of a pilot scale aerated static pile composting systems. Sci Papers Ser E Land Reclam Earth Observ Survey Environ Eng 6:7–12
Elser J, Bennett E (2011) Phosphorus cycle: a broken biogeochemical cycle. Nature 478:29
Energy Research Centre of the Netherlands (2002) Workshop on carbon capture and storage. Intergovernmental Panel on Climate Change, Geneva
Engels C, Kirkby E, White P (2012) Mineral nutrition, yield and source–sink relationships. Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier, Amsterdam
Falkowski P, Scholes R, Boyle E, Canadell J, Canfield D, Elser J, Gruber N, Hibbard K, Högberg P, Linder S (2000) The global carbon cycle: a test of our knowledge of earth as a system. Science 290:291–296
Guedes P, Couto N, Ottosen LM, Ribeiro AB (2014) Phosphorus recovery from sewage sludge ash through an electrodialytic process. Waste Manag 34:886–892
Guendouz J, Buffière P, Cacho J, Carrère M, Delgenes J-P (2010) Dry anaerobic digestion in batch mode: design and operation of a laboratory-scale, completely mixed reactor. Waste Manag 30:1768–1771
Gulati R, Saxena R, Gupta R (2002) Fermentation waste of Aspergillus terreus: a potential copper biosorbent. World J Microbiol Biotechnol 18:397–401
Hansen V, Müller-Stöver D, Ahrenfeldt J, Holm JK, Henriksen UB, Hauggaard-Nielsen H (2015) Gasification biochar as a valuable by-product for carbon sequestration and soil amendment. Biomass Bioenergy 72:300–308
Hoornweg D, Bhada-Tata P (2012) What a waste: a global review of solid waste management. World Bank, Washington, DC
Jaffrin A, Bentounes N, Joan AM, Makhlouf S (2003) Landfill biogas for heating greenhouses and providing carbon dioxide supplement for plant growth. Biosyst Eng 86:113–123
Jahirul MI, Rasul MG, Chowdhury AA, Ashwath N (2012) Biofuels production through biomass pyrolysis—a technological review. Energies 5:4952–5001
Jay K, Stieglitz L (1995) Identification and quantification of volatile organic components in emissions of waste incineration plants. Chemosphere 30:1249–1260
Jiang Y, Wang X, Cao Q, Dong L, Guan J, Mu X (2016) Chemical conversion of biomass to green chemicals. Sustainable production of bulk chemicals. Springer, Berlin
Kalmykova Y, Fedje KK (2013) Phosphorus recovery from municipal solid waste incineration fly ash. Waste Manag 33:1403–1410
Kataki S, West H, Clarke M, Baruah DC (2016) Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour Conserv Recycl 107:142–156
Ketzer JM, Iglesias RS, Einloft S (2012) Reducing greenhouse gas emissions with CO2 capture and geological storage. In: Handbook of climate change mitigation. Springer, Berlin
Kleemann R, Chenoweth J, Clift R, Morse S, Pearce P, Saroj D (2017) Comparison of phosphorus recovery from incinerated sewage sludge ash (ISSA) and pyrolysed sewage sludge char (PSSC). Waste Manag 60:201–210
Kuchenrither R, Martin W, Smith D, Williams D (1985) Design and operation of an aerated windrow composting facility. J Water Pollut Control Feder 57:213–219
Kulikowska D, Klimiuk E (2008) The effect of landfill age on municipal leachate composition. Bioresour Technol 99:5981–5985
Laird DA, Brown RC, Amonette JE, Lehmann J (2009) Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod Biorefin 3:547–562
Lairon D (2011) Nutritional quality and safety of organic food. Sustainable agriculture, vol 2. Springer, Berlin
Latifian M, Liu J, Mattiasson B (2012) Struvite-based fertilizer and its physical and chemical properties. Environ Technol 33:2691–2697
Lazcano C, Arnold J, Zaller J, Martín JD, Salgado AT (2009) Compost and vermicompost as nursery pot components: effects on tomato plant growth and morphology. Span J Agric Res 944:951
Lazcano C, Martínez-Blanco J, Christensen TH, Muñoz P, Rieradevall J, Møller J, Antón A, Boldrin A, Nuñez M (2014) Environmental benefits of compost use on land through LCA–a review of the current gaps. Proceedings of the 9th International Conference on Life Cycle Assessment in the Agri-Food Sector (LCA Food 2014), San Francisco, California, USA, 8–10 October, 2014. American Center for Life Cycle Assessment
Lee H-J, Oh S-J, Moon S-H (2003) Recovery of ammonium sulfate from fermentation waste by electrodialysis. Water Res 37:1091–1099
Lettinga G, Rebac S, Zeeman G (2001) Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol 19:363–370
Leung DY, Caramanna G, Maroto-Valer MM (2014) An overview of current status of carbon dioxide capture and storage technologies. Renew Sustain Energy Rev 39:426–443
Li X, Zhao Q (2003) Recovery of ammonium-nitrogen from landfill leachate as a multi-nutrient fertilizer. Ecol Eng 20:171–181
Lim SL, Lee LH, Wu TY (2016) Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. J Clean Prod 111:262–278
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Progr Energ Combust Sci 38:449–467
Lindmark J, Eriksson P, Thorin E (2014) The effects of different mixing intensities during anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag 34:1391–1397
Lorentzen G (1994) Revival of carbon dioxide as a refrigerant. Int J Refriger 17:292–301
Massey MS, Davis JG, Sheffield RE, Ippolito JA (2007) Struvite production from dairy wastewater and its potential as a fertilizer for organic production in calcareous soils. International Symposium on Air Quality and Waste Management for Agriculture, 16–19 September 2007, Broomfield, Colorado. American Society of Agricultural and Biological Engineers
Matter JM, Stute M, Snæbjörnsdottir SÓ, Oelkers EH, Gislason SR, Aradottir ES, Sigfusson B, Gunnarsson I, Sigurdardottir H, Gunnlaugsson E (2016) Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 352:1312–1314
Minale M, Worku T (2014) Anaerobic co-digestion of sanitary wastewater and kitchen solid waste for biogas and fertilizer production under ambient temperature: waste generated from condominium house. Int J Environ Sci Technol 11:509–516
Mor S, Ravindra K, De Visscher A, Dahiya R, Chandra A (2006) Municipal solid waste characterization and its assessment for potential methane generation: a case study. Sci Total Environ 371:1–10
Ndegwa P, Thompson S (2001) Integrating composting and vermicomposting in the treatment and bioconversion of biosolids. Bioresour Technol 76:107–112
Neher DA, Weicht TR, Bates ST, Leff JW, Fierer N (2013) Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS One 8:e79512
Nigussie A, Kuyper TW, Bruun S, de Neergaard A (2016) Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. J Clean Prod 139:429–439
Noyola A, Morgan-Sagastume JM, Lopez-Hernandez JE (2006) Treatment of biogas produced in anaerobic reactors for domestic wastewater: odor control and energy/resource recovery. Rev Environ Sci Biotechnol 5:93–114
Olajire AA (2013) Valorization of greenhouse carbon dioxide emissions into value-added products by catalytic processes. J CO2 Utilization 3:74–92
Ontario Ministry of the Environment (2008a) Design guidelines for sewage works: sludge stabilization
Ontario Ministry of the Environment (2008b) Design guidelines for sewage works: sludge thickening and dewatering
Oram NJ, van de Voorde TF, Ouwehand G-J, Bezemer TM, Mommer L, Jeffery S, Van Groenigen JW (2014) Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agr Ecosyst Environ 191:92–98
Patni N, Pillai SG, Dwivedi AH (2013) Wheat as a promising substitute of corn for bioethanol production. Proc Eng 51:355–362
Petzet S, Peplinski B, Cornel P (2012) On wet chemical phosphorus recovery from sewage sludge ash by acidic or alkaline leaching and an optimized combination of both. Water Res 46:3769–3780
Powell JT, Townsend TG, Zimmerman JB (2016) Estimates of solid waste disposal rates and reduction targets for landfill gas emissions. Nat Clim Change 6:162–165
Quaik S, Ibrahim MH (2013) A review on potential of vermicomposting derived liquids in agricultural use. Int J Sci Res Publ 3:1–6
Rahman MM, Salleh MAM, Rashid U, Ahsan A, Hossain MM, Ra CS (2014) Production of slow release crystal fertilizer from wastewaters through struvite crystallization—a review. Arab J Chem 7:139–155
Romero C, Ramos P, Costa C, Márquez MC (2013) Raw and digested municipal waste compost leachate as potential fertilizer: comparison with a commercial fertilizer. J Clean Prod 59:73–78
Samolada M, Zabaniotou A (2014) Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag 34:411–420
Shanableh A, Ginige P (1999) Impact of metals bioleaching on the nutrient value of biological nutrient removal biosolids. Water Sci Technol 39:175–181
Shanableh A, Omar M (2003) Bio-acidification and leaching of metals, nitrogen, and phosphorus from soil and sludge mixtures. Soil Sediment Contam 12:565–589
Siddiqui Z, Horan N, Anaman K (2011) Optimisation of C: N ratio for co-digested processed industrial food waste and sewage sludge using the BMP test. Int J Chem React Eng 9:1–9
Steffen R, Szolar O, Braun R (1998) Feedstocks for anaerobic digestion. Institute of Agrobiotechnology Tulin, University of Agricultural Sciences, Vienna
Tan Z, Lagerkvist A (2011) Phosphorus recovery from the biomass ash: a review. Renew Sustain Energy Rev 15:3588–3602
Themelis NJ, Ulloa PA (2007) Methane generation in landfills. Renew Energy 32:1243–1257
Tortosa G, Castellano-Hinojosa A, Correa-Galeote D, Bedmar EJ (2017) Evolution of bacterial diversity during two-phase olive mill waste (“alperujo”) composting by 16S rRNA gene pyrosequencing. Bioresour Technol 224:101–111
United States Department of Labor (2002) Portable fire extinguishers—1910.157. Occupational Safety and Health Standards
United States Environmental Protection Agency (2018) Types of Composting and Understanding the Process [Online]. Accessed May 2018
Vakalis S, Sotiropoulos A, Moustakas K, Malamis D, Vekkos K, Baratieri M (2016) Characterization of hotel bio-waste by means of simultaneous thermal analysis. Waste Biomass Valorization 7:649–657
Vaneeckhaute C, Lebuf V, Michels E, Belia E, Vanrolleghem PA, Tack FM, Meers E (2017) Nutrient recovery from digestate: systematic technology review and product classification. Waste Biomass Valorization 8:21–40
Victor D, Zhou D, Ahmed E, Dadhich P, Olivier J, Rogner H-H, Sheikho K, Yamaguchi M (2014) Climate change 2014: mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change: Introductory chapter. Cambridge University Press, Cambridge
Vijayaraghavan K, Lee MW, Yun Y-S (2008) Evaluation of fermentation waste (Corynebacterium glutamicum) as a biosorbent for the treatment of nickel (II)-bearing solutions. Biochem Eng J 41:228–233
Vu QD, de Neergaard A, Tran TD, Hoang HTT, Vu VTK, Jensen LS (2015) Greenhouse gas emissions from passive composting of manure and digestate with crop residues and biochar on small-scale livestock farms in Vietnam. Environ Technol 36:2924–2935
Walker L, Charles W, Cord-Ruwisch R (2009) Comparison of static, in-vessel composting of MSW with thermophilic anaerobic digestion and combinations of the two processes. Bioresour Technol 100:3799–3807
Whittaker S, Perkins E (2013) Technical aspects of CO2 enhanced oil recovery and associated carbon storage. Global CCS institute, Melbourne
Wong J, Xiang L, Gu X, Zhou L (2004) Bioleaching of heavy metals from anaerobically digested sewage sludge using FeS2 as an energy source. Chemosphere 55:101–107
World Energy Council (2016) World energy resources, waste to energy, 2016
Xie T, Reddy KR, Wang C, Yargicoglu E, Spokas K (2015) Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Environ Sci Technol 45:939–969
Yan F, Zhang L, Hu Z, Cheng G, Jiang C, Zhang Y, Xu T, He P, Luo S, Xiao B (2010) Hydrogen-rich gas production by steam gasification of char derived from cyanobacterial blooms (CDCB) in a fixed-bed reactor: influence of particle size and residence time on gas yield and syngas composition. Int J Hydrogen Energy 35:10,212–10,217
Yi J, Dong B, Jin J, Dai X (2014) Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: performance and microbial characteristics analysis. PLoS One 9:e102548
Yuan H, Lu T, Wang Y, Chen Y, Lei T (2016) Sewage sludge biochar: nutrient composition and its effect on the leaching of soil nutrients. Geoderma 267:17–23
Zacco A, Borgese L, Gianoncelli A, Struis RP, Depero LE, Bontempi E (2014) Review of fly ash inertisation treatments and recycling. Environ Chem Lett 12:153–175
Zhang R, El-Mashad HM, Hartman K, Wang F, Liu G, Choate C, Gamble P (2007) Characterization of food waste as feedstock for anaerobic digestion. Bioresour Technol 98:929–935
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Walling, E., Babin, A., Vaneeckhaute, C. (2019). Nutrient and Carbon Recovery from Organic Wastes. In: Bastidas-Oyanedel, JR., Schmidt, J. (eds) Biorefinery. Springer, Cham. https://doi.org/10.1007/978-3-030-10961-5_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-10961-5_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10960-8
Online ISBN: 978-3-030-10961-5
eBook Packages: EnergyEnergy (R0)