Abstract
The coral reefs of the Red Sea are host to a diverse fish fauna. Ichthyofauna studies began in the Red Sea during expeditions undertaken by some of the earliest European naturalists. In the more than 200 years that have passed, much has been learned about Red Sea fishes. Nonetheless, many knowledge gaps remain. Although it is a relatively young sea, the geologic history of the Red Sea provides an interesting context for many evolutionary biology studies. The strong environmental gradients within the Red Sea and the broader Arabian region may play a role in structuring some observed biodiversity patterns, perhaps most notably in the context of high numbers of Arabian and Red Sea endemics. As such, Red Sea fishes provide ideal opportunities for connectivity studies, both based on adult movement and larval dispersal patterns. These studies are increasingly important as multiple modern “mega-developments” are planned on Red Sea shores in locations where a lack of scientific information may still hinder conservation efforts and planning for sustainable development. Coupled with increasing pressures from global climate change, each of the Red Sea countries faces unique challenges for the preservation of the rich biological resources for which their reefs are historically known.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Red Sea Ichthyofauna and Movement Ecology
8.1.1 Early Natural Historians and Red Sea Taxonomy
The ichthyofauna of the Red Sea attracted the attention of some of the earliest naturalist historians; several of them spent a great deal of time in the Red Sea, or at least working with material collected from the Red Sea. Peter Forsskäl, a Swedish explorer and naturalist, may have the most unfortunate story, dying near the end of a 7-year journey to what is now called Yemen, but not before sending many preserved specimens back to his mentor, Carl Linnaeus (Hansen 1962). Several fishes bear scientific epithets honoring these naturalists, including Parapeneus forsskali, Thalassoma rueppellii (named for the German Eduard Rüppell, one of the first European naturalists to reach the Gulf of Aqaba), and Lutjanus ehrenbergi (named after Christian Gottfried Ehrenberg, another German naturalist / explorer among the earliest Europeans to study northern Red Sea fauna) (Fig. 8.1). Notably, many of the species were given scientific names derived from local Arabic names for the fishes, such as Acanthurus gahhm, Acanthurus sohal, Hipposcarus harid, Carangoides bajad, Pomacanthus asfur, Lethrinus harak, Lutjanus bohar, and the genus Abudefduf (Fig. 8.2).
A selection of Red Sea fishes bearing scientific names derived from Arabic: (a) the genus Abudefduf, represented here by Abudefduf vaigiensis, (b) Pomacanthus asfur, with the specific epithet named after the Arabic word for “yellow”, (c) Carangoides bajad, taking a specific epithet named after the Arabic word used for most trevallies, (d) Lutjanus bohar, (e) Acanthurus sohal, bearing a specific epithet derived from the Arabic word used for most Acanthurus surgeonfishes, (f) Acanthurus gahhm, (g) Lethrinus harak, and (h) Hipposcarus harid, with a specific epithet named after the Arabic word used for most parrotfishes
As the Red Sea is home to more than 1000 species of fishes (DiBattista et al. 2016b), it is a daunting task to create a field or pictorial guide for the taxonomic diversity of the Red Sea. This chapter is not intended to serve as a field guide, particularly as good examples already exist. Lieske and Myers (2004) offer a very good treatment for most conspicuous reef fishes, including many species from the Gulf of Aden. Users should take care to note that not all species included in this book are found in the Red Sea (i.e., it includes species found in the Gulf of Aden or other parts of Arabia but not found in the Red Sea). Perhaps the most comprehensive and most recent checklist is that provided by Golani and Bogorodsky (2010). Instead of attempting to provide a field guide or a checklist, this chapter instead seeks to review the state of knowledge of Red Sea coral reef fish work, particularly with respect to recent work conducted outside the Gulf of Aqaba (where scientific knowledge has traditionally been more developed than in the main body of the Red Sea (Berumen et al. 2013)).
8.1.2 Fishes and Movement Ecology
Fishes provide many ideal model systems for investigations in the broad domain of movement ecology. For the study of basic biogeographic patterns, the state of knowledge in many other organisms is not yet sufficient even to describe basic distribution patterns. Nonetheless, a review by DiBattista et al. (2016b) assembled information from the few Red Sea groups for which sufficient checklists were available. Recent efforts to understand Red Sea fishes in a broader context were captured in a special issue of the Journal of Biogeography highlighting numerous studies (not exclusively of fishes) from the Red Sea and western Indian Ocean (Berumen et al. 2017). However, the taxonomy of Red Sea fishes is far from perfect. In fact, detailed studies of less-conspicuous groups (e.g., blennies and gobies) are very few; unsurprisingly, the few works to delve into these groups indicate that the Red Sea ichthyofauna may yet hold much more diversity (more on this in Sect. 8.3.3).
On the subject of Red Sea evolutionary biology, fishes again provide one of the most useful study systems. DiBattista et al. (2016a) provides perspective on the potential origins of Red Sea fauna, and particularly the potential reasons that high levels of endemism emerged in the region. The Red Sea’s unique conditions (see Chap. 1) create an important opportunity to investigate adaptation mechanisms to climate change; modern-day conditions in the Red Sea may reflect future scenarios in other oceans, and Red Sea fauna may therefore provide insights (particularly genetic) to the adaptive capacity of reef fauna elsewhere (ReFuGe 2020 Consortium 2015).
In general reef ecology, fishes are also frequent model organisms. When considering the movement ecology of fishes, temporal and spatial scales are important. For many species of reef fishes, the largest distances that individuals will move are realized during the larval phase (Green et al. 2015). Unfortunately, acquiring empirical measurements of the movement patterns of larval fishes poses major practical challenges, primarily due to their small size, the quantities typically produced, and the naturally high mortality rates larvae experience during their pelagic dispersal phase (Thorrold et al. 2007). The movements of many adult fishes can be studied using a variety of techniques and off-the-shelf equipment, although these are typically time-intensive and expensive endeavors. In the Red Sea, there are examples of ecological studies at most scales, although the work may only have taken place with a limited number of species or in a limited number of places. This chapter will touch on various ecological aspects of Red Sea fishes in three broad areas (biodiversity patterns, genetic connectivity, and ecological work) and will conclude with comments on conservation and associated challenges in the region.
8.2 Biodiversity Patterns
8.2.1 Latitudinal/Longitudinal Gradients
Despite the Red Sea’s strong environmental gradients (see Chap. 1) and a long history of research on fishes in the Red Sea, there are few publications examining fish assemblages from a latitudinal perspective. While fish community composition does seem to gradually change along most gradients of the Red Sea, there is likely more difference between reefs across the continental shelf (Khalil et al. 2017) than observed along latitudinal gradients, which is a well-established pattern seen in other reef systems (e.g., Aguilar-Perera and Appeldoorn 2008; Malcolm et al. 2010).
Surveys covering conspicuous fish species on offshore reefs from Al Wajh (26.8°N latitude) to the southern Farasan Banks (18.6°N latitude) (see Fig. 8.3) suggest that overall fish community assemblages do not differ greatly among reefs at the edge of the continental shelf across this span (Roberts et al. 2016). A slight shift in community composition in the central-northern portion of the Red Sea was attributed, in part, to the influence of few taxa with narrow range limits and with relatively low abundances. The butterflyfishes (Chaetodontidae) and angelfishes (Pomacanthidae) are good examples of groups with species following this pattern. Surveys of inshore reef crests from the Gulf of Aqaba (29°N latitude) to the Gulf of Aden (12°N latitude) revealed a shift in these taxa in the central Red Sea (around 20°N latitude) (Roberts et al. 1992). Two species of butterflyfish, Chaetodon paucifasciatus and Chaetodon austriacus, were present only on central and northern reefs while Chaetodon trifasciatus, Chaetodon melannotus, Chaetodon fasciatus, Chaetodon auriga, and Pygoplytes diacanthus all showed marked decreases in abundance towards the south. Other species, including Chaetodon mesoleucos, Chaetodon larvatus, Pomacanthus asfur, and Pomacanthus maculosus showed the opposite trend.
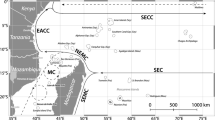
Map of the Red Sea highlighting key features referenced in this chapter. Aquatic features are indicated in blue text; terrestrial features are indicated in black text. The circle drawn around the Farasan Banks indicates the approximate location of the extensive network of more than 100 reefs spread through this area. (Map data sources are ESRI and M. Campbell)
These patterns may have influenced the demarcation of the Red Sea into two Marine Ecoregions of the World by Spalding et al. (2007), splitting the Red Sea roughly in half at ~ 20°N, although subsequent community analyses suggest the appropriate division may be closer to 17°N. A comparison of coastal coral reef communities (including corals, benthic invertebrates, and fishes) found that sites between the Gulf of Aqaba and the central Red Sea were relatively uniform, while Farasan Island communities were distinctly separate (Sawall et al. 2014). These community differences were attributed to a greater abundance of predators and herbivores and lower abundance of small planktivorous fishes in the lower latitudes. The shallow, turbid, and patchy reef structure of the reefs in the Farasan Islands area likely supports a distinctly different assemblage of fishes than the more uniform reefs found in the central and northern Red Sea (Roberts et al. 1992).
A recent study comparing cryptobenthic fishes between the central Red Sea and the Farasan Islands found marked differences in fish abundance and species richness driven by habitat characteristics and productivity (assessed using chlorophyll a values) (Coker et al. 2018). The widening of the continental shelf in the southern part of the Red Sea results in expansive shallow patchy reef systems across the shelf, similar to habitats found on inshore and midshelf reefs of the central Red Sea. Coupled with the influence of Indian Ocean water influx through the Strait of Bab al Mandab, these conditions make the Farasan Islands a distinctly different habitat among Red Sea regions.
A broader analysis by Khalaf & Kochzius (2002), including detailed surveys in the Gulf of Aqaba, supported the suggestion that there are gradual latitudinal shifts in reef fish assemblage from north to south, identifying the clear difference between the Red Sea and the Gulf of Aden / southern Arabian regions. However, Roberts et al. (2016) suggest that the latitudinal shifts become less well-defined with increased distance from shore, possibly indicating that the factors structuring fish assemblage (e.g., habitat variables) have greater change from inshore to offshore sites than they do from north to south (at a given distance from shore). Patterns of prevalent cross-shelf effects have been found in other reef systems (Aguilar-Perera and Appeldoorn 2008; Malcolm et al. 2010). This is also seen in Red Sea reefs, characterized by an increase in herbivore and planktivorous fish diversity in the offshore reefs compared to inshore reefs (Khalil et al. 2017). Coker et al. (2018) compared cryptobenthic fish assemblages across inshore, midshelf, and offshore reefs. The authors found that differences in fish assemblages were driven by proximity to shore, likely due to the change in habitat quality along this gradient. Mechanisms driving the fish assemblage changes are likely associated with distance from shore (Khalil et al. 2017; Coker et al. 2018).
From these few studies, we can say that fish assemblages are not distinctly different from the Gulf of Aqaba to the central Red Sea (Khalaf and Kochzius 1992; Sawall et al. 2014; Roberts et al. 2016). There is indication that the Farasan Islands support an assemblage most different to the rest of the Red Sea, though more investigation is necessary. The well-established pattern of more pronounced differences in assemblages across reefs longitudinally than across latitudes and as seen in reefs such as the Great Barrier Reef, also hold true in the Red Sea thus far.
8.2.2 Understudied Regions of the Red Sea
Many parts of the Red Sea remain poorly studied. For example, the southernmost reaches of the Red Sea contain perhaps the most unique reef habitats (see Chap. 1), but these are among the least-represented among Red Sea reef fish publications. This includes the Farasan Islands in the southern Saudi Arabian Red Sea (and extending into Yemeni waters) and the Dahlak Archipelago in the Eritrean Red Sea. Combined, these two coastal and offshore systems contain more than 200 islands and host a variety of marine biota. Many of the islands are fringed with shallow reefs. Some of the islands, particularly to the far west of the Farasan Islands, have well-developed coral reefs. Multiple groups have conducted surveys of the reef habitats in this region, including the Living Oceans Foundation (Bruckner et al. 2011), and have arrived at the conclusion that the reef communities are unique among Saudi Arabian reef systems (e.g., Sheppard and Sheppard 1991; Sheppard et al. 1992). The reefs are subject to far more sedimentation than most other Saudi Red Sea reefs, the water is consistently more turbid, and remote sensing data indicates very high productivity in this region (Raitsos et al. 2013; Racault et al. 2015). Some of the reefs in this area are largely dominated by macroalgae. In these respects, the Farasan Islands region has greater affinities with the Gulf of Aden region reefs than with the remainder of Red Sea reefs (Sheppard and Sheppard 1991). In some ways, the southern Red Sea islands may functionally be more like inshore, coastal reefs, even though they are relatively distant (>100 km) from the mainland coast.
The southern Red Sea also hosts the largest area of shallow soft-bottom habitats in Saudi Arabia, and is home to some of the only major trawling operations in the country. Although these trawling operations primarily target shrimp, there is some catch of bottom fishes. In recent years, the armed conflict in Yemen has severely hindered any scientific progress in the Yemeni Red Sea. Border tensions exist between Eritrea and most of its neighboring countries, resulting in similarly restricted access (or no access) to its territorial waters. The active geological fault in the southern Red Sea has even given rise to new islands, which could be the subject of fascinating study (to observe primary colonization, etc.), but due to their location in Yemeni waters, work to date has been limited to satellite observations (Xu et al. 2015).
8.3 Genetic Connectivity
8.3.1 Genetic Barriers in the Red Sea
As discussed in Sect. 8.2.1 above, there is mixed evidence for a strong faunal change at the proposed 20°N boundary of Spalding et al. (2007). Few genetic surveys have directly tested for the presence of this barrier, but they provide equally mixed results. Clear signals of a genetic break at 20°N have been shown for an anemonefish (Nanninga et al. 2014; Saenz-Agudelo et al. 2015) and a sponge (Giles et al. 2015). These same patterns seem to exist also for an anemone (Entacmea quadricolor (Emms 2015)) and two damselfishes (Dascyllus marginatus (Robitzch 2017) and Dascyllus trimaculatus (Salas De la Fuente 2017)). However, work in other species has failed to detect this signal, including Chaetodon species and Ctenochaetus striatus (JD DiBattista et al. unpublished), Dascylllus aruanus (Robitzch 2017), two anemone species (Heteractis magnifica and Stichodactyla haddoni (Emms 2015)), and a coral (Pocillopora verrucosa (Robitzch et al. 2015)). Taken together, there does not seem to be a clear connection between the presence of a genetic break at 20°N and biological traits such as pelagic larval duration or spawning mode. At least two of the studied species suggest that environmental characteristics play an important role in shaping gene flow near 20°N (Nanninga et al. 2014; Giles et al. 2015; Saenz-Agudelo et al. 2015), but recent modeling work suggests that oceanographic patterns are tightly linked with genetic similarity among populations (Raitsos et al. 2017).
While there are some species that exhibit this genetic break, it is not typically reflected in a presence / absence change (see Sect. 8.2.1). A more interesting barrier is perhaps the Strait of Bab al Mandab, the narrow opening dividing the Red Sea from the Gulf of Aden (and the wider Indian Ocean), which is the most common range limit for the majority of Arabian endemics (DiBattista et al. 2016a). Approximately half of the species investigated so far have shown signatures of restricted gene flow between the Red Sea and the Gulf of Aden. Recent and unpublished data suggest that this structure is explained to some extent by historical interruption of gene flow, followed by secondary contact. As with the putative barrier at 20°N, evidence for disruption of gene flow between populations at either side of this strait is divided. Several species of fish show genetic structure between populations in Djibouti and populations in the southern or central Red Sea (Saenz-Agudelo et al. 2015; Salas De la Fuente 2016; DiBattista et al. 2017; Robitzch 2017), and some anemone species show similar genetic structure (Emms 2015). However, there are also several fishes for which this pattern is not the case (DiBattista et al. 2017). Although there is limited data available, no consistent pattern has emerged, and it appears that there is not a single biological characteristic that can explain the observed patterns. Indeed, the evolutionary history of Red Sea fauna may be rather complicated and each species may have a unique story (DiBattista et al. 2013, 2016a). Further work with additional species (and application of next-generation sequencing technologies) may reveal common histories for some groups of fishes.
8.3.2 East-West Connectivity
While questions about genetic connectivity along the latitudinal gradient of the Red Sea have received limited attention (Sect. 8.3.1 above), even fewer studies have explicitly tested whether connectivity across the Red Sea (east-west connectivity) is occurring. The geography and oceanography of the Red Sea make this a reasonable possibility; the typical width of the Red Sea is ~200–300 km, and the Red Sea is characterized by periodic basin-width eddies hypothetically capable of facilitating the transport of larvae across these distances (Zhan et al. 2014; Yao et al. 2014). A recent modeling study confirmed the potential for cross-sea connections of larval particles and found correspondence with available genetic data for clownfish (Raitsos et al. 2017). This work demonstrates that the eddies and cross-basin currents should be sufficient to link reefs on opposite sides of the Red Sea on a regular basis. The eddies are somewhat ephemeral (Zhan et al. 2014), and the timing of spawning in most Red Sea reef fishes is not clear (see Sect. 8.4.4 below), but the ‘average’ oceanography appears to be conducive to genetic mixing, even for species with a short pelagic larval duration, such as clownfish (Nanninga et al. 2014; Saenz-Agudelo et al. 2015; Raitsos et al. 2017). Some groupers, which have longer pelagic larval durations than clownfish, also exhibit genetic patterns suggesting east-west connectivity (Priest et al. 2016). The timing of spawning and interactions with the hydrodynamic conditions present during the larval dispersal phase (as opposed to time-averaged conditions) can have substantial influence over specific dispersal potential. Empirical measurements of specific dispersal events are uncommon, but application of genetic parentage analysis has proven to be powerful in this regard (e.g., Harrison et al. 2012; Almany et al. 2017). To our knowledge, parentage analysis has only been conducted in one study in the Red Sea (Nanninga et al. 2015). Based on modeled hydrodynamics of the inferred spawning dates, most of the clownfish larvae would have been advected out of the study area, corresponding with the lack of parent-offspring matches in the study (Nanninga et al. 2015). However, additional modeling work suggested that if the study had focused on a reef located further inshore, a greater portion of the larvae may have been locally retained and self-recruitment may have been more prominent (Nanninga 2013). The potential for connections across the width of the Red Sea, especially if they occur on a regular basis, has important implications for conservation as healthier populations could reseed heavily exploited populations (see Sect. 8.5.2) on opposite sides of the Red Sea.
8.3.3 Genetic Identification of Cryptobenthic Species
Cryptobenthic fishes are generally characterized as fishes that have a proximate association with the benthos and attain body lengths ≤50 mm (Ackerman and Bellwood 2000; Depczynski and Bellwood 2003). These fishes are often cryptic in nature and coloration, hence they are often overlooked or excluded during standard visual reef fish censuses. Despite their small size, this group can be strikingly abundant and diverse across coral reefs. By some estimates this group contributes approximately 50% of the individual fish abundance and 10% of the overall reef fish biomass on coral reefs (Ackerman and Bellwood 2000). Additionally, a large proportion of these fishes exhibit high fecundity, growth, and metabolic rates (Hernaman and Munday 2005, 2007; Depczynski and Bellwood 2006; Depczynski et al. 2007). Due to the rates at which these fishes are preyed upon, they play a disproportionate role in the transfer of energy in reef food webs. In addition to being prey items for larger fishes, cryptobenthic fishes may also play other important functional roles (Goatley and Brandl 2017). However, logistical constraints limit the number of studies that include or focus on cryptobenthic fishes and subsequently impact our understanding of their ecology.
The Red Sea is no exception; few studies have examined cryptobenthic fishes in Arabian waters. The family Gobiidae has been the subject of some study (Herler and Hilgers 2007; Herler 2007), but only recently studies have begun to investigate community-level composition of this assemblage among different habitats (Troyer et al. 2018; Coker et al. 2018). Importantly, the application of molecular tools to identify species (DNA barcoding) has enabled community-level and ecological investigations even though morphology-based taxonomy remains problematic for these fishes (Troyer 2017; Coker et al. 2018; see also Tornabene et al. 2013) There are many undescribed species and very few morphological identification keys are available for Red Sea specimens (Troyer 2017). Fortunately, each new study that combines morphology and genetic analyses steadily contributes to global genetic databases (such as GenBank) and helps to slowly fill some of the many gaps in coverage of Red Sea species (Troyer 2017; Robitzch 2017; Isari et al. 2017a, b; Coker et al. 2018). Barcoding is not a panacea (Rubinoff 2006), but the technique can be a valuable component of an integrated approach (DeSalle 2006).
Standardized field sampling suggests that cryptobenthic fish communities differ along a latitudinal gradient and with distance from shore (Coker et al. 2018). The Red Sea’s environmental gradients (Raitsos et al. 2013; see also Chap. 1) and are predicted to influence species composition and abundance through direct (e.g., temperature, salinity, productivity) and indirect variables (habitat availability, predation pressure). Given the size of individuals within this group, microhabitat is likely to explain finer-scale spatial patterns (see Troyer 2017) while environmental variables are likely driving larger-scale patterns. Given the importance of this group, future work is needed in the Red Sea to better understand biodiversity, spatial patterns, and ecosystem processes. The work so far on these fishes, and particularly the molecular barcoding work, suggests that there are many new fishes (some not yet recorded from the Red Sea and many others probably new to science) to be discovered.
8.3.4 Inter-Species Genetic Variation and Cryptic Speciation
The uniqueness of the Red Sea fauna is only apparent in comparison to the fauna of the seas outside of the Red Sea. The Red Sea is undoubtedly an important biodiversity hotspot among the entire western Indian Ocean (DiBattista et al. 2016b), but there are important unanswered questions as to why this is the case (DiBattista et al. 2016a). Many Red Sea populations may have colonized the Red Sea and then had to adapt to its unique environmental conditions, effectively diverging from the “parent” populations in the Indian Ocean, but there is also evidence that some Indian Ocean species have their origins within the Red Sea, challenging the historical assumption that peripheral seas rarely “export” biodiversity (Bowen et al. 2013). What exactly drives the generation of diversity within the Red Sea is still not well understood, but it could be that novel genes and adaptations emerge to cope with typical Red Sea conditions (see ReFuGe 2020 Consortium 2015), which might otherwise be considered “harsh” in other parts of the Indo-Pacific.
When widespread species have been examined with samples both from within the Red Sea and outside the Red Sea, the patterns of intra-specific genetic variation are unpredictable. In some species, the Red Sea populations appear to show evidence of contemporary genetic exchange with other western Indian Ocean populations (e.g., Abudefduf vaigiensis, DiBattista et al. 2017), while other species show unexpected divergence dating far beyond recent sea level minima (when the Red Sea would have been quite, but not completely, isolated from the Gulf of Aden and the rest of the Indian Ocean (DiBattista et al. 2016a)). Examples of the latter case include Chaetodon melannotus (DiBattista et al. 2017) and Mulloidichthys flavolineatus (Fernandez-Silva et al. 2015, 2016). In some cases, the isolation appears to be so complete that the species should likely be considered separate species yet to be described, such as Pygoplites diacanthus (DiBattista et al. 2013; Coleman et al. 2016) and Cephalopholis hemistiktos (Priest et al. 2016). In the context of Red Sea fishes, there can therefore be some semantic confusion with regards to “cryptic species”. One definition applies to the preceding examples, and is taxonomic in nature, wherein populations have species-level divergence but have evaded detection by taxonomists because the morphology has not diverged (at least obviously enough to have been recognized). Another definition of “cryptic species” is functional or ecological in nature; the Red Sea has many fishes that, due to their size, coloration, or behavior, are difficult to detect in visual surveys, and are often overlooked or understudied (see Sect. 8.3.3).
While the aforementioned studies have examined a small number of species in some detail, the results indicate that there is no single explanation for the evolutionary history of Red Sea ichthyofauna (DiBattista et al. 2016a). We therefore thought it would be useful to broadly assess the genetic “connectedness” of Red Sea fishes using samples from within the Red Sea compared to samples from outside the Red Sea (using Indian Ocean sites when available). For species endemic to the Red Sea, we included samples from sister species (or at least congeners). For many species, prior genetic data was publicly available in the NCBI GenBank repository (http://www.ncbi.nlm.nih.gov/genbank/) (specifically, the mitochondrial gene cytochrome oxidase I (COI) “barcoding” marker), but for many species that were not available, we sequenced new samples. For these species, we used a small (~2mm2) piece of fin tissue and extracted DNA following the “HotSHOT” protocol (Truett et al. 2000; Meeker et al. 2007). The COI barcoding fragment was amplified using the primers FishF2/FishR2 (Ward et al. 2005). PCR products were sequenced in the forward direction with fluorescently labeled dye terminators following the manufacturer’s protocols (BigDye, Applied Biosystems Inc., Foster City, CA, USA) and were analyzed using an ABI 3130XL Genetic Analyzer (Applied Biosystems) in the King Abdullah University of Science and Technology (KAUST) Biosciences Core Laboratory. (Details of the samples used, including accession numbers for existing and newly-generated sequences, are available in Table 8.1 and Appendix 1) Sequences were aligned using Geneious R8 (Biomatters Ltd., Auckland, New Zealand) and divergence was calculated using the Kimura 2-parameter model (K2P) in MEGA 6.0 (Tamura et al. 2013). The results of this comparison show that there do not seem to be any obvious family-specific or genus-specific patterns of genetic relatedness. For species that occur inside and outside of the Red Sea (Appendix 1), there were varying levels of differentiation, and there were a few species exhibiting quite high values (e.g., Bothus pantherinus). Several explanations are possible: among other possibilities, the values may be the result of as-yet undetected cryptic speciation, the samples could have been mis-identified, or intraspecific variation may be quite high in general. Values for sister species comparisons (Table 8.1) were, as expected, generally higher than the intraspecific comparisons. There were several interesting species pairs for which the K2P values were very low (e.g., Pseudochromis fridmani + Pseudochromis sankeyi and Chaetodon austriacus + Chaetodon melapterus). These pairs may be in the early stages of speciation (e.g., Waldrop et al. 2016).
8.4 Ecology
8.4.1 Application of Stable Isotope Techniques to Red Sea Fishes
Stable isotope analyses have been traditionally used to track the movements of fishes through natural isotope gradients, or isoscapes, via analysis of the calcified earbones (“otoliths”) (Campana and Thorrold 2001; Thorrold et al. 2001; Kennedy et al. 2002; Elsdon et al. 2008). While these studies have provided useful insights on the movements of marine organisms, there are some notable challenges to using stable isotope from fish otoliths. Bulk isotope values can be affected by fish metabolism (Kalish 1991; Stephenson et al. 2001), environmental conditions (Mulcahy et al. 1979), and changes in dissolved inorganic carbon δ13C values (Schwarcz et al. 1998). There is also difficulty with associating any changes in otolith δ13C values with either changes in basal resource use or trophic shifts (Post 2002), and this is particularly apparent when working with species that undergo ontogenetic shifts in habitat use, as many coral reef fishes do (Cocheret de la Moriniére et al. 2002; Kimirei et al. 2013). However, the use of compound-specific stable isotope analysis (CSIA) of essential amino acids (EAAs) may help to circumvent these complexities. Essential amino acids are those that most animals, including fishes, have lost the ability to synthesize at sufficient rates for survival (Borman et al. 1946; Reeds 2000), therefore EAAs must be assimilated through the fishes’ diets. Once taken up, EAAs remain virtually unaltered biochemically, so that fractionation factors between food and consumers are essentially zero (Hare et al. 1991; McMahon et al. 2011a). This means δ13C values of a consumer’s EEAs represent the isotopic signatures of the primary producers (e.g. plants, algae, and microbes) at the bottom of the food web. When this information is combined with known isoscapes across marine environments, it allows for the possibility to track movements through habitats, provided the fish is present long enough to incorporate the isotopic signature of its habitat. The use of CSIA-EAA to investigate residency, ontogenetic movement, and food web ecology has been pioneered in studies of fishes from the Red Sea (McMahon et al. 2011a, b, 2012, 2016).
The analysis of δ13C values of essential amino acids in Red Sea fishes has expanded our understanding of fish residency and ontogenetic movements. CSIA-EAA has been utilized to study residency patterns of coral reef fish in the Red Sea, providing information applicable to coastal ecosystems across the globe. McMahon et al. (2011b) documented the advantage of CSIA-EAA compared to traditional bulk analysis for determining habitat use in economically important fishes. Although isotopic differences between mangrove and seagrass habitats have been previously documented (Marguillier et al. 1997; Layman 2007), McMahon et al. (2011b) failed to find any clear relationship between habitat residency and bulk isotope δ13C values. Bulk isotope values were only able to distinguish between ocean basins rather than specific habitats, while EAA δ13C values provided sufficient resolution to reliably distinguish between mangrove and seagrass habitats (McMahon et al. 2011b), including across ocean basins.
The application of CSIA to otolith EAAs has also revealed plasticity in the ontogenetic movements between coastal ecosystems of reef fish. Past studies have documented the importance of coastal habitats (e.g., mangrove and seagrass beds) as nurseries for coral reef fishes (Adams et al. 2006; Nagelkerken et al. 2008), though most of these studies have inferred this relationship by analyzing size-frequency distributions and relative densities of juvenile fishes (Nagelkerken et al. 2000; Cocheret de la Moriniére et al. 2002). A Red Sea study was the first to quantify the contribution of different juvenile habitats to adult fish populations via CSIA-EAA of otoliths (McMahon et al. 2012). By assessing EEAs in material from the core of the otoliths (i.e., the material deposited as a juvenile), McMahon et al. (2012) assigned adult fishes into different juvenile habitats. In addition to documenting movements of economically important snappers among coastal habitats in the Red Sea, the study more generally emphasized the importance of seascape configuration as a factor driving ontogenetic movement patterns.
Densities of Ehrenberg’s snapper (Lutjanus ehrenbergii) were found to be highest on shelf reefs near shore, which also happened to have the greatest levels of connectivity between coastal wetland habitats and other shelf reefs. This finding lends empirical support to others that have found higher fish biomass on reefs closer to coastal habitats (Nagelkerken et al. 2000; Mumby et al. 2004). While these snapper are able to migrate from coastal habitats to shelf reefs, there does appear to be a break in connectivity at the shelf edge, where snappers cannot or will not migrate beyond. Red Sea oceanic reefs were dominated by snappers that had settled directly onto these types of reefs, despite the complete absence of juveniles from extensive visual surveys. A small portion (<30%) of snappers on offshore reefs were also found to have migrated from a large island near the shelf edge, crossing deep water and making horizontal movements of at least 30 km. McMahon et al. (2012) demonstrated not only a plasticity in ontogenetic movements of snappers, but also the ability to migrate large distances between coastal wetlands and reef habitats. The role of seascape configuration plays an important role in structuring how snapper, or any fish, may be able to move between coastal habitats. In light of planned coastal developments in the Red Sea (see Sect. 8.5.3), understanding linkages between coastal habitats and nearby reef fish populations will be important to consider.
While isotopic studies from the Red Sea have demonstrated patterns of residency and connectivity in coral reef fishes, the more traditional use of isotopic analyses has been to tease apart information about resource usage. Isotopic approaches have been especially useful in reconstructing the diets of important fishery species (e.g., cod, Hanson and Chouinard 2002). Several isotopic studies have documented reliance on microbially-processed carbon in mangrove ecosystems (Bouillon et al. 2002; Kieckbusch et al. 2004; Kristensen et al. 2017), raising interesting questions about the structure of some marine food webs. In the Red Sea, mangrove-derived carbon contributes little to the diets of coastal snappers compared to other locations (e.g., the Pacific coast of Panama and the Caribbean) (McMahon et al. 2011b). The reduced reliance on mangrove-derived carbon in the Red Sea is potentially due to the relatively diminutive mangrove stands that typically exist on a narrow strip of coastal land, as opposed to the more extensive forests found at some non-Red Sea sites that spend more time submerged and accessible for fishes (McMahon et al. 2011b).
In addition to documenting differences in food webs between broad ocean basins, CSIA is revealing how resource use can change among reefs in the Red Sea. Using a CSIA-EAA analysis of fish muscle samples, McMahon et al. (2016) documented changes in the basal nutrient source that supports Red Sea coral reef fishes. Some functional groups of Red Sea fishes exhibited consistency in their nutritional ecology while other groups appeared to be flexible. Highly specialized functional groups, including obligate corallivorous butterflyfish (Chaetodon trifascialis), algal-farming damselfish (Stegastes nigricans), and detritivorous surgeonfish (Ctenochaetus striatus) show little change in the main nutrient source they rely on across the seascape from shelf to oceanic reefs. Several species were more variable in their resource usage across reefs, though they were generally reliant on mostly a single basal food source. Planktivorous damselfish (Amblyglyphidodon indicus) were found to rely almost equally on carbon sources from macroalgae and phytoplankton on shelf reefs, while these fish on oceanic reefs sourced nearly all their carbon from phytoplankton production. Lutjanus ehrenbergii also showed a similar pattern, being reliant mostly on macroalgae production on shelf reefs and switching to phytoplankton carbon on oceanic reefs (see Figure 4 in McMahon et al. 2016). Giant moray eels (Gymnothorax javanicus) relied mostly on phytoplankton-derived carbon on both shelf and oceanic reefs, though they had a greater phytoplankton reliance on oceanic reefs. The pattern for many species to increase reliance on phytoplankton-derived carbon on oceanic reefs is likely not unique to the Red Sea (e.g., Wyatt et al. 2012; Letourneur et al. 2013). Given the lack of terrestrial/freshwater input into the Red Sea, the patterns documented by McMahon et al. (2016) are likely to be slightly different in other reef systems as runoff and riverine outflow can alter food web nutrient dynamics (e.g., Dromard et al. 2013; Letourneur et al. 2013; Docmac et al. 2017). CSIA-EAA represents a powerful technique for determining broad differences in the nutrient sources supporting coral reefs in oligotrophic systems such as the Red Sea.
While McMahon et al. (2016) have demonstrated the utility of CSIA-EEA for determining broad differences in highly dissimilar functional groups, the approach also has the potential to identify subtler nutritional differences within functional groups than previous techniques. Robust differences have been shown in the δ13C isotope values of EEAs from basal food sources, including various tropical marine algae (Larsen et al. 2009, 2012, 2013). The technique is sensitive enough to discriminate isotope values between similar algae and bacterial species, indicating that CSIA-EEA could be used to determine fine-scale differences in the nutritional ecology of functional groups that may normally be missed in traditional feeding observation or stomach content analysis (Bearhop et al. 2004; Larsen et al. 2012). Indeed, in the Red Sea, preliminary analysis of fishes within the functional group of herbivores have found discreet differences in the nutritional ecology of herbivorous fishes (Tietbohl 2016). Fishes that appear to have nearly identical feeding habits show robust and distinct clustering from other species. The approach even clearly separates scraping and excavating parrotfish species, which implies these fish are actually using different nutritional sources within the turf algae they feed in together. Distinctions among functional (sub)groups of parrotfishes have been previously suggested (Clements et al. 2016); CSIA-EAA of Red Sea parrotfishes may be able to definitively show these differences and further attribute the differences to the use of isotopically distinct food sources. Broader application in other geographic regions will provide important comparisons and determine the generality of Red Sea trends for reef systems in other parts of the world.
8.4.2 Megafauna Movements
Reports of whale sharks (Rhincodon typus) impaled on the bows of steams ships, including four incidents from the Red Sea and Gulf of Aden, make up some of the earliest published records of these sharks in the Arabian region (Gudger 1940). These instances resulted in Gudger concluding, “whale sharks must surely abound in this region” (Gudger 1938). Following these reports, sporadic sightings of whale sharks were recorded throughout the region, but research was limited. Whale shark research within the region began to increase with the discovery of a juvenile male dominated whale shark aggregation in the Gulf of Tadjura, Djibouti (Rowat et al. 2007). Several years later, a juvenile whale shark aggregation with sexual parity was described within the Red Sea along the central Saudi Arabian coast approximately 200 km south of Jeddah (Berumen et al. 2014; Cochran et al. 2016). Historically, work on sharks in the Red Sea has been sparse and generally concentrated in the Gulf of Aqaba (Spaet et al. 2012), but efforts over the last decade have begun to fill in vital knowledge gaps for select elasmobranch species.
The only known Saudi Arabian whale shark aggregation takes place at a nearshore reef, locally known as Shib Habil, which lies approximately 4 km from the coast of the small town of Al Lith. Whale sharks are commonly encountered here from March through May (Berumen et al. 2014). In addition, reef mantas (Mobula alfredi, following the taxonomic synonymization of the genus Manta (White et al. 2017)) are occasionally encountered alongside whale sharks and commonly at the surrounding nearshore reefs (Braun et al. 2014; Berumen et al. 2014). Despite their similar habitat use near Al Lith during the spring, the two species show distinct differences in movement patterns the rest of the year. Mobula alfredi movements were restricted to coastal areas and reefs primarily within the Al Lith region, which was confirmed by acoustic monitoring (Braun et al. 2014; Braun et al. 2015). Similar restricted coastal movements of M. alfredi have been documented using satellite tags at a large manta aggregation in Dunganab Bay along the Sudanese coast (Kessel et al. 2017). One manta at this location was the first (and currently the only) documented M. alfredi x Manta (now Mobula) birostris hybrid (Walter et al. 2014; Kessel et al. 2017).
In contrast to the mantas, whale sharks leave the Al Lith region outside of the aggregation season. Most satellite-tagged sharks (39 of 47) made basin-scale movements throughout the southern Red Sea. Seasonal variation was present, with sharks preferring the central Red Sea in the spring and shifting to the south-central and far southern Red Sea during the summer, fall, and into the winter months (Berumen et al. 2014). These high-use areas include waters of multiple countries including Saudi Arabia, Sudan, Yemen, and Eritrea, highlighting the need for international cooperation to protect such highly mobile species. Only three of the whale sharks moved into the northern Red Sea, but tagged sharks ventured as far north as Sharm el-Sheikh on the Sinai Peninsula (see Fig. 8.3). Five sharks left the Red Sea and passed through the Gulf of Aden into the northwestern Indian Ocean (Berumen et al. 2014). On-going photo identification efforts and monitoring of the aggregation site have not identified these sharks as returning to the Al Lith region after exiting the Red Sea. Limited satellite tagging data is available from the Djibouti aggregation, with only one track showing short term movements of a single individual around the Gulf of Tadjoura (Rowat et al. 2007). On the other side of the Arabian Peninsula, a presumed pregnant female shark was tagged in Qatari waters and was tracked moving toward the Gulf of Aden. The shark traveled at least 2640 km over 37 days, with the tag detaching between the Somali coast and the main island of Socotra (Robinson et al. 2017).
Photo-identification of whale sharks from 2010 through 2017 at the Shib Habil aggregation has resulted in the identification of 147 unique individuals in the Al Lith region. Cochran et al. (2016) described the population structure at Shib Habil using the 136 individuals identified from 305 encounters between 2010 and 2015. The population exhibited sexual parity and all individuals were immature based on size estimate and male clasper morphology. Daily abundances at the aggregation site were estimated as 15 to 34 individuals with individual residence times of 4–44 days (Cochran et al. 2016).
An international database, Wildbook for Whale Sharks (whaleshark.org), invites researchers and citizen scientists to submit photos of whale sharks from anywhere in the world. Suitable images are used for photo-identification and are then cross-referenced against the entire database. At the end of 2017, Wildbook had a total of 585 Red Sea whale shark encounters submitted from dive companies, tourists, and researchers. There are sightings from all Red Sea nations except for Eritrea. Shib Habil has the most encounter records with 318, an expected result considering the area has been regularly monitored by researchers since 2010. However, there are only six reported encounters for the rest of Saudi Arabia, which is likely due to the lack of local knowledge about the database (and not necessarily reflective of an absence of whale sharks).
The second highest number of encounters, 208, comes from Egypt. The remaining countries all have very low numbers of encounters recorded. The satellite tagging results of Berumen et al. (2014) suggest that the lack of records in Wildbook arises from a similar unawareness of the database and far fewer tourists in other areas. Egypt is a well-known Red Sea diving destination and many dive companies report sightings directly to Wildbook. The Red Sea Sharks Monitoring Programme (redseasharks.org), primarily operating at dive sites throughout the Egyptian Red Sea, maintains photo-identification databases for three species of shark, including oceanic whitetips (Carcharhinus longimanus), grey reef sharks (Carcharhinus amblyrhynchos), and silky sharks (Carcharhinus falciformis). The website also directs those interested in submitting whale shark and manta photos to Wildbook and Manta Matcher (mantamatcher.org), respectively. In addition to identifying >1000 individual sharks, the Red Sea Sharks Monitoring Programme has identified sightings of other species, such as scalloped hammerheads (Sphyrna lewini), pelagic threshers (Alopias pelagicus), and whitetip reef sharks (Triaenodon obesus).
A global genetic analysis also suggests regular connections of whale sharks between the Red Sea and the Indian Ocean. Very little genetic structure was detected within the Indo-Pacific, including samples from the Saudi Arabian aggregation (Vignaud et al. 2014). A follow-up study added additional locations by using DNA sequences obtained from copepod ectoparasites of whale sharks (Pandarus rhincodonicus), but found a similar genetic pattern (Meekan et al. 2017). Both studies show slight genetic structure between the Indo-Pacific and the Atlantic Ocean whale shark populations, and relative homogeneity within the Indo-Pacific.
Limited data is currently available on the identity of potential food sources that whale sharks target throughout the Red Sea, including at the Shib Habil aggregation site. Preliminary plankton tows collected next to feeding sharks have resulted in a near-monoculture of the sergestid shrimp (Lucifer hanseni) and in one case, copepods (Acartia spp.). These limited results suggest that, as described in Rohner et al. (2015), whale sharks most likely do not target one specific food source but rather target dense patches of prey without specific preferences. In 2016, 83% of the 53 encounters involved sharks feeding either at or just below the surface. This suggests that Shib Habil hosts a feeding aggregation, especially considering that the sharks are immature (based on size and clasper morphology in males) and breeding is therefore unlikely (Cochran et al. 2016). It is not clear what may drive the presumably high densities of prey that whale sharks feed upon in such a concentrated area (Hozumi 2015), although regional productivity may play a role (Racault et al. 2015). It also remains unclear if the mantas are targeting the same food source, or why the whale sharks venture so much farther from the site compared to the mantas. Understanding these drivers may become increasingly important if Saudi Arabia intends to develop marine ecotourism (see Sect. 8.5.3) in the near future; whale shark aggregations lend themselves to such initiatives, and can be sustainable if appropriate guidelines are adopted (e.g., Rowat and Engelhardt 2007; Catlin and Jones 2010).
8.4.3 Lessepsian Migrants
In addition to natural connectivity and movement patterns in the Red Sea, there is an important anthropogenically-induced connection in the far north of the Red Sea. The Suez Canal provides connectivity between the fauna of the Indo-West Pacific and Mediterranean biogeographical provinces (Por 1978). Since the opening of the canal in 1869, approximately 450 species of marine organisms (Bernardi et al. 2016), including 106 species of fishes (Rothman et al. 2016; Golani et al. 2017), have invaded the Mediterranean Sea from the Red Sea. The phenomenon, termed “Lessepsian migration” (named after the engineer Ferdinand de Lesseps, who supervised the construction of the canal), has been well-documented (Por 1978), particularly for fish taxa (Golani 1998; Golani and Appelbaum-Golani 2010; Azzurro et al. 2016). The canal has no locks or dams, providing little barrier to dispersal along the corridor. Two hypersaline lakes, known as the “Bitter Lakes”, may have initially acted as an ecological barrier to dispersal. However, the salinity of these lakes has gradually equalized with the Red Sea over time (Edwards 1987); the large number of species successfully colonizing the Mediterranean is evidence of the ineffectiveness of the barrier. Despite the migratory pathway permitting bi-directional movement of marine fauna, only a few species have been confirmed as “reverse Lessepsian migrants” that immigrate from the Mediterranean and colonize the Red Sea (Ben-Tuvia 1971; Spanier and Galil 1991; Golani 1998, 1999). The largely unidirectional nature of Lessepsian migration may be attributed to the existence of unsaturated ecological niches in the Mediterranean and the competitive superiority and pre-adaptation of species originating in the highly diverse tropical Red Sea compared to those of a temperate origin (Golani 1999). Consequently, Lessepsian migrants are of significant ecological and economic concern, in some instances resulting in the displacement and local extirpation of native fish species in the Mediterranean (Galil et al. 2015). For example, the goldband goatfish, Upeneus moluccensis, a widespread Indo-Pacific species that invaded the Mediterranean via the Suez Canal, has largely replaced the native red mullet, Mullus barbatus, in Levantine fisheries (Goren and Galil 2005). Dramatic declines in biogenic habitat complexity, biodiversity, and biomass in the Levantine basin have also been attributed to Lessepsian invaders from the Red Sea. Research suggests the herbivorous invaders Siganus luridus and Siganus rivulatus are responsible for the rapid shift from well-developed native algal assemblages to “barrens” in the Mediterranean rocky infralittoral ecosystem (Sala et al. 2011). Some invasion events are relatively well-documented, and provide ideal opportunities to study the genetics associated with a rapid colonization of a new area. The bluespotted cornetfish (Fistularia commersonii) took 130 years to enter the Mediterranean, but only 4 years to expand as far as any other Lessepsian invaders had been recorded (Tenggardjaja et al. 2013). A new expansion of the Suez Canal was completed in late 2016, raising concerns of even further invasions to come (Galil et al. 2015).
8.4.4 Larval Ecology and Recruitment of Reef Fishes
The diversity of a larval fish pool, combined with species-specific distribution patterns, may provide useful information on spawning seasons and recruitment patterns of fish. Knowledge of such patterns facilitates efforts for ecosystem conservation and fisheries management, yet the research on the ecology of early life-history stages of fish in the Red Sea is still in its infancy. High species diversity and a paucity of diagnostic morphological characteristics for larval life stages of reef fishes have been among the major bottlenecks in larval ecological research in tropical waters (Leis 2014). These biological obstacles are further exacerbated by a lack of marine research opportunities and infrastructure in several Red Sea countries (see Sect. 8.2.2 above).
The primary sources of ichthyoplankton knowledge in the Red Sea are a few academic theses on larval fish taxonomy and ecology in the northern Red Sea, specifically from the Jordanian Gulf of Aqaba and Egyptian coastal waters (Abu El-Regal 1999, 2008; Froukh 2001). These studies identified larval stages at broad taxonomic levels (i.e., family level) and made predictions of potential fish spawning seasons.
The advancement of species identification through molecular techniques has boosted multi-species Red Sea ichthyoplankton studies (Isari et al. 2017a, b; Robitzch 2017; Kimmerling et al. 2018). Combining morphological characterization with DNA barcoding, Isari et al. (2017a) determined the larval fish diversity and assemblage variation throughout an annual cycle in coral reef waters of the central Saudi Arabian Red Sea using bongo net tows. Genetic analyses revealed high species richness in the area, and high water temperatures during the year appeared to be the main driver associated with the numerical increase of larvae in many families. Examination of coral reef fish recruitment patterns using light traps on coral reefs in the same area by Robitzch (2017) revealed a seasonal peak in the fall and early winter (i.e., October, November, and December) for most of the dominant families (e.g., Labridae and Gobiidae). Interestingly, other species appear to have spawning peaks during the cooler months of the year (e.g., Amphiprion bicinctus (Nanninga et al. 2015) and Scarus niger (Isari et al. 2017a)), which could likely reflect differentiation in reproductive thermal optima among species. Unfortunately, for many species, there is not even sufficient information to make an educated guess about the timing (or locations) of their spawning events.
Interestingly, larval fish collections by nets and light traps are now revealing previously unknown aspects of Red Sea fish biodiversity. Based on morphological criteria, new Schindleria records have been reported in the northern Red Sea (Abu El-Regal and Kon 2008; Fricke and Abu El-Regal 2017a, b). Genetic markers support a striking species richness of gobies in the central part of the basin (Isari et al. 2017a, 2017b), while high-throughput metabarcoding in ichthyoplankton collections from Gulf of Aqaba has been suggested as a promising tool in assessing the diversity of larval fish community at a species-level (Kimmerling et al. 2018).
Besides larval stages per se, studies on juveniles may also be informative regarding important ecological processes taking place during the larval phase. For instance, the duration of the pelagic larval phase and factors that may influence species recruitment across the Red Sea have been assessed on postlarval stages of pomacentrid species (Ben-Tzvi et al. 2007, 2008; Robitzch et al. 2016). These works showed a decrease in pelagic larval duration towards the southern Red Sea, mostly associated with the increase in food availability and water temperature (Racault et al. 2015; Robitzch et al. 2016), while increased downwelling current flow in the Gulf of Aqaba was associated with an enhancement of recruitment events (Ben-Tzvi et al. 2007). Otolith micro-chemistry analyses of newly-settled damselfishes at the Gulf of Aqaba have provided information on larval dispersal trajectories, showing heterogeneity in the dispersal routes that supply local populations (Ben-Tzvi et al. 2008; Ben-Tzvi et al. 2012). Coupling genetic analyses with biophysical dispersal models has verified a large scale of spatial dispersal of larval anemonefish in the central Red Sea (Nanninga et al. 2015; Raitsos et al. 2017; see also Sect. 8.3.2 above).
Despite the recent and growing interest in larval fish ecology in the Red Sea, thorough baseline data are missing. Much of the basic fish biology, larval biology and ecology, and other dynamics related to reproduction and recruitment processes remain unstudied or poorly known. Increased knowledge of early and late larval stages will improve our understanding of spawning, recruitment, and connectivity patterns, which are crucial components of effective management plans (McCook et al. 2009). Molecular techniques may be highly helpful in future studies to reveal not only the hidden diversity in Red Sea ichthyofauna (Kimmerling et al. 2018), but will also improve our knowledge of larval dispersal trajectories and their influence in population dynamics.
8.4.5 Particularly Understudied Areas
In terms of geography and depth of coverage in many topics, our knowledge of Red Sea fishes is in early stages. Nonetheless, there are some areas that are even less well understood ecologically, and some of these are noteworthy. This is not intended to be an exhaustive list, but we have highlighted some areas of potential interest that warrant future study.
8.4.5.1 Mesophotic Coral Ecosystems
In terms of reef habitats, depths greater than ~30 m are rarely the subject of thorough study, and only a portion of the limited studies address fishes inhabiting these depths (Hinderstein et al. 2010; Kahng et al. 2010). Such systems, termed “mesophotic coral ecosystems” (MCEs) are of increasing interest for several reasons, including the potential for reefs at these depths to serve as refugia from climate change and increasing temperatures in shallower reef systems. However, the technical challenge of accessing these depths (beyond the depth at which standard scuba diving can be conducted for any reasonable amount of time) remains a limiting factor. Often when deep-diving resources are available, such as remotely operated vehicles or manned submersibles, the target depths are deeper than the lower limit of MCEs. Only a handful of mesophotic reef studies have been conducted in the Red Sea. The Gulf of Aqaba was explored in seminal studies (Fricke and Schuhmacher 1983; Fricke and Hottinger 1983; Fricke and Knauer 1986), primarily with respect to the distributions of stony corals. More recent work has employed technical diving techniques and has focused on fishes in the Gulf of Aqaba (e.g., Brokovich et al. 2007, 2008, 2010). In terms of fishes, almost no other MCEs have been described in any detail in the Red Sea.
8.4.5.2 Al Wajh Lagoon Reefs
The Red Sea is often referred to as an extreme environment because it has summer sea surface temperatures regularly exceeding 30 °C and salinity often above 40 ppt (Ngugi et al. 2012; see also Chap. 1). Within the Red Sea basin, there are several coastal lagoon systems; these are often quite shallow and have limited water exchange with the broader Red Sea. These lagoons potentially experience even greater temperature and salinity ranges (due to reduced water exchange and increased evaporation) that could significantly influence benthic and fish communities. Most lagoons are small and support marginal reefs, however, there is one notable exception. The Al Wajh (sometimes transliterated from Arabic as “Wadj” or “Wahdj”) lagoon system in the north-central region of the Red Sea (Fig. 8.3) is a distinct habitat that differs greatly from the adjacent deep, clear waters of the Red Sea basin. It is approximately 1500 km2, consists of approximately 50 islands, and is contained within a barrier reef system with three very small channels providing limited hydrodynamic links to the broader Red Sea. Although tidal fluctuations are generally quite small (rarely more than 10s of cm, and often completely masked by wind-driven basin-wide shifts in sea level (Edwards 1987)), these narrow channels experience strong currents due to the volume of water in the lagoon. The lagoon is relatively shallow (mostly <30 m in depth) with a sandy substrate and shallow, patchy coral reefs.
While no temporal in situ environmental measurements have been reported from within the lagoon, SST satellite data (MODIS) reveals that temperature fluctuations are greater than the adjacent Red Sea basin with maximum summer temperatures up to 1 °C warmer and winter temperatures up to 3 °C cooler (Calder Atta, unpublished data). In January-February of 2016, several of the authors (MLB, THST, RSH, MDT, AK, and DJC) participated in an exploratory survey in the Al Wajh lagoon and experienced unexpectedly cold water temperatures, typically as low as 17–18 °C during dives at 10–15 m depth. It is conceivable that the lagoon may likewise reach peak temperatures well above 33 °C in summer. These extreme temperature ranges likely have an influence on fish communities, both directly and indirectly. Increased temperature ranges have been shown to directly influence metabolic rates, movement, and growth rates of fishes (Munday et al. 2008). In this regard, the Al Wajh lagoon may be more like the Arabian Gulf (see Sale et al. 2011), and only a subset of Red Sea fauna may be able to tolerate such large fluctuations in environmental conditions. Furthermore, the difference in the benthic reef communities (which have not yet been fully documented) may further influence the fish fauna, as indirect effects through changes in habitat are also expected to modify fish abundance and community structure (Wilson et al. 2006; Pratchett et al. 2008). The possibility of yet-undiscovered endemic species cannot be ruled out, as even the Gulf of Aqaba has endemic species (DiBattista et al. 2016b). While the Gulf of Aqaba is twice as large (~3100 km2), it has a much wider connection with the Red Sea (>5 km wide, compared to <1 km for Al Wajh). The Living Oceans Foundation included the Wajh lagoon in their habitat-mapping and groundtruthing of select areas of the Red Sea (Bruckner et al. 2011), but little data about the fish fauna from this unique habitat is available. This unique environment warrants future investigation to better understand how species present in this region adapt and cope in an extreme environment with implications to climate change within the region and globally.
8.5 Conservation Status and Future Challenges
The lack of historical data available on reef health (coral and fish communities) in the Red Sea presents challenges when assessing the current status of reefs, and, like many other regions, the Red Sea suffers from shifting baselines (Price et al. 2014). Nonetheless, consistent fish harvesting and recent disturbances suggest that this region is not immune to large-scale degradation and that it faces the same global threats (e.g., climate change, overfishing, coastal development, etc.) as reefs around the world. One notable exception is that terrestrial impacts (through fresh water input and nutrient runoff) are limited or inconsequential across many regions of the Red Sea due to limited rainfall and an absence of any permanent rivers entering the Red Sea. Nonetheless, inputs related to coastal development, fishing pressure, and increasing sea temperatures appear to be the main modern threats to reef-associated fishes of the Red Sea (Kotb et al. 2008; Wilkinson 2008; Furby et al. 2013; Spaet and Berumen 2015).
8.5.1 Bleaching and Thermal Stress
Historical information on coral bleaching in the Red Sea is limited, with some of the earliest reports of widespread bleaching documented during 1998 (in Egypt, Eritrea, Saudi Arabia, Sudan, and Yemen). This coincides with the global bleaching event at the time (see Hoegh-Guldberg 1999) and implies that while the Red Sea reefs experience higher water temperatures than other reef systems, they are not immune to the influences of global climate change (see also Cantin et al. 2010). Further bleaching has been reported in 2007 (Egypt), 2010 (Saudi Arabia), 2012 (Egypt), and more recently, large-scale coral loss was observed in 2015 throughout the southern reefs of Saudi Arabia (Osman et al. 2018; see also Chap. 3). Limited in situ data about flow-on effects restrict our understanding of how fish communities are influenced following disturbances, however, declines in coral cover and benthic structure are well-known to negatively affect many fish (Wilson et al. 2006; Pratchett et al. 2008).
In addition to coral loss, direct effects of climate change are predicted to have significant ramifications for fishes through increased water temperature and changes in ocean acidification (Munday et al. 2008). Fishes in the Red Sea are already existing in relatively high water temperatures, and several fishes may already be living beyond or at their thermal maxima for some periods of the year. It is unclear if fishes are already thermally stressed, or if fishes within the Red Sea have adapted to cope with greater temperature anomalies. Increased water temperatures can influence latitudinal distributions, depth structure, activity, growth, and metabolic processes (Booth et al. 2011; Johansen and Jones 2011; Nowicki et al. 2012). Latitudinal gradients in temperature, along with extreme regions like the Al Wajh lagoon (see Sect. 8.4.5.2), provide natural environments in the Red Sea to investigate the effects and adaption to future climate change scenarios.
8.5.2 Fisheries
The extraction of fishes by artisanal fisheries has historically been an integral component of food security in the Red Sea. Methods such as larger trawlers have recently been introduced in regions amendable to this method (e.g., southern Red Sea), however most fishing efforts employ more traditional methods, such as hook and line, gill nets, and traps (Tesfamichael and Pauly 2016). Accurate catch data in the Red Sea is difficult to source, particularly at a local scale (e.g., at the level of detail of individual fishing ports or landing sites) (Jin et al. 2012). As coastal populations increase, so will the demand for fish-based protein and associated catch rates, particularly in regions with large populations. Fishing pressure varies among countries (and among regions within countries) based on population, resources, and culture (Tesfamichael and Pauly 2016). Current estimates suggest that most targeted fishes are overfished in the Red Sea, with some groups, such as sharks, significantly reduced from historical numbers (Tesfamichael 2012; Spaet and Berumen 2015). Most fishers employ multi-gear, multi-species operations with no regional fisheries management organization oversight, and even bans on catching protected species are not enforced (Spaet et al. 2016). Some regions, such as Sudan, appear to experience lower levels of fishing pressure. A recent study comparing fish communities among comparable offshore reefs in south-central Saudi Arabia to reefs in Sudan revealed significantly lower abundance and biomass levels on Saudi Arabian reefs (Kattan et al. 2017). The cumulative evidence suggests that Saudi Arabian reefs generally experience heavy fishing pressure (e.g., Jin et al. 2012), however, this could be even higher in more populated regions (e.g., near Jeddah) and on reefs closer to shore. Data is lacking for catch rates, and is also deficient for more nuanced details such as the number of days at sea, discards, distance traveled, gear use, and targeted events (e.g., spawning aggregations). For example, in the southern islands of Saudi Arabia, longnose parrotfish (Hipposcarus harid, see Fig. 8.2h) are targeted in shallow waters during spawning aggregations (Gladstone 2006; Spaet 2013). These gaps in data need to be addressed if plans for sustainable fisheries are to be developed for future generations, in addition to simply maintaining the current level of associated goods and services that reef fisheries supply for Red Sea countries. The narrowness of the Red Sea (Morcos 1970) presents the potential for cross-basin connectivity through larval dispersal, specifically facilitated by periodic oceanographic features (Raitsos et al. 2017; see Sect. 8.3.2). This potential connectivity implies that regions of low fishing pressure (e.g., Sudanese reefs) could serve as a replenishment source for regions with depleted fish stocks (e.g., Saudi reefs) or for regions impacted by severe disturbances (e.g., recent mortality in the Farasan Banks due to bleaching, see Chap. 3).
While larval dispersal may provide some reason for optimism for reef fisheries, some highly mobile species (e.g., tunas or whale sharks, see Sect. 8.4.2) would benefit from formal management at the level of the entire Arabian Peninsula (e.g., Spaet et al. 2015). Fortunately, there does not currently appear to be a targeted fishery for mantas or whale sharks, two species of potential ecotourism value. Mobula alfredi is listed as vulnerable with on the IUCN Red List and Rhincodon typus is listed as endangered, with species population trends considered to be declining (Marshall et al. 2011; Pierce and Norman 2016). Surveys at the main Jeddah fish market revealed no manta or whale sharks (bi-monthly surveys between 2011–2013), however, two species of mobulid ray were found (6 Mobula thurstoni and 1 Mobula kuhlii) (Spaet and Berumen 2015). The fishing fleet within the Al Lith area (near the whale shark aggregation site), like most of Saudi Arabia, is dominated by artisanal fishers using hand lines (e.g., Jin et al. 2012); mantas and whale sharks are not targeted. In 2011, one whale shark (previously tagged at the aggregation site) was accidentally captured in a gill net and died as a result (Cochran et al. 2016). Although it appears that bycatch in this form is rare, it is unclear if such instances would normally be reported. The nearshore location of Shib Habil and its proximity to the local port puts the mantas and sharks at risk from outboard motor strikes (Braun et al. 2015). Approximately half of all sharks encountered at the aggregation site have scars, with 15% of the scars apparently resulting from propeller trauma (Cochran et al. 2016). A manta aggregation in Dunganab Bay, Sudan, falls within a marine protected area that was declared a UNESCO World Heritage Site in 2016 (Kessel et al. 2017), affording the individuals at that location protection. Unfortunately, other elasmobranchs do not enjoy such reprieve and appear to be heavily impacted by fishing activies (Spaet and Berumen 2015; Spaet et al. 2016; see also Sect. 8.5.5).
8.5.3 Coastal Development, Ecotourism, and Saudi Arabia’s Vision 2030
One Red Sea nation is poised to launch some of the most ambitious development projects ever undertaken. The Kingdom of Saudi Arabia has released and identified the nation’s “Vison 2030”, which outlines major economic goals for the country (details are available at http://vision2030.gov.sa). Among the many plans outlined, there are several coastal developments in northwestern Saudi Arabia that each have the potential to influence reefs in this area (ranging from the Al Wajh lagoon to Egyptian side of the Gulf of Aqaba). These coastal projects are described as “Giga-Projects” by the Saudi government’s Public Investment Fund (www.pif.gov.sa/pifprograms/vrp_en). The NEOM project envisions a world-leading “smart city” occupying 438 km of coastline, sprawling into Egypt and Jordan. Among other lofty goals, the NEOM project has a plan to achieve a productive city with the highest per capita GDP in the world. A second coastal giga-project, known as “The Red Sea Project”, is based in the Al Wajh lagoon area. This project focuses much more specifically on diversifying tourism activities in Saudi Arabia (projections include reaching 90,000 visitors to the Al Wajh lagoon’s islands annually by 2022 and 1 million visitors annually by 2035). Eco-tourism and water sports are explicitly named among the attractions. The proposed scale and pace of development would set numerous records, especially considering the near-complete lack of infrastructure present in this region. These giga-projects will provide interesting case studies for years to come – hopefully they provide examples of ‘successes’ to serve as models for other regional developments.
Among the major goals of the new vision is an increased tourism sector, including the general introduction of tourism visas (reported to begin in April 2018). At this time, there is no mention of directly exploiting mantas or whale sharks. However, these species both readily lend themselves to ecotourism endeavors and are attractive targets in various locations worldwide. Access to the Al Lith whale shark aggregation site is relatively limited, despite the reef’s proximity to shore, because there is currently only one dive operation in the Al Lith area with a limited number of vessels. Light ecotourism focused on the whale sharks has been ongoing since 2012. At present, there is no formal code of conduct for interactions with either species, which could lead to conflicts should tourism begin to increase. Whale sharks and mantas have the potential to play a role in sustainable development of the regional economy, but precautions must be taken to ensure the long-term viability and minimal risk to the animals. Some valuable lessons could be learned from Sudan. Sudanese reefs were brought to the world’s attention following Jacques Cousteau’s 1964 documentary, “World Without Sun”, which documents the Cousteau team’s adventures living underwater in the Conshelf 2 station. Today, the majority of international marine ecotourism in Sudan is centered around liveaboard dive boats and has grown rapidly (Chekchak 2013). In 2000, there were 8 liveaboard boats operating out of Port Sudan, but by 2017, there were 15 (with 7 boats from outside of Sudan). Between 2500–4000 divers visit annually (mostly from Europe), generating an estimated US$15–17 million per year in gross income, including tourism fees (Chekchak 2013). Tourism is the largest source of income for the Sanganeb Atoll Marine National Park (see Sect. 8.5.5). Nonetheless, marine tourism in Sudan can still be considered under-developed, and fortunately there seem to be minimal impacts on the conditions of the reefs or their resident fish communities. Many of the diving tourists to Sudan are attracted by the still-healthy populations of reef sharks (Hussey et al. 2013; Spaet et al. 2016).
8.5.4 Aquaculture
Al Lith is near Saudi Arabia’s largest prawn farm, part of the National Aquaculture Group (NAQUA). The prawn farm pumps water into the initial stages of the farm and then uses a gravity-driven system to distribute the water. The effluent drains immediately adjacent to the sole marina available for visitor access to the region, and is directly inshore from the whale shark aggregation site. The prawn farm in Al Lith was established before focused study began on either the mantas or whale sharks, hindering a full understanding of the potential impacts (see also Hozumi 2015). NAQUA has recently introduced several sets of open-ocean fish cages growing barramundi (Lates calcarifer, a non-native species) approximately 15 km north of Shib Habil.
One aspect of Vision 2030 is continued and rapid development of aquaculture along the Saudi Arabian coast. There are at least two factors that will drive a major increase in demand for marine protein in the proposed plan for Saudi Arabia: a need to establish greater levels of food security (i.e., less reliance on imported foods) and an increase in international tourism and luxury seaside resorts, both of which can be expected to create demand for local seafood. A 2016 study identifying suitable potential sites for finfish cages along the Saudi Arabian coast suggested that the two southern-most sites in the study have the most potential (Salama et al. 2016). These locations were chosen due to their distance from industrial and residential areas (Salama et al. 2016), but they also align with nearshore reefs shown to be frequently used by M. alfredi (Braun et al. 2014). The tagging studies (see Sect. 8.4.2) can be used to inform development along the Saudi coastline, much like Kessel et al. (2017) focused on habitat use of the reef mantas in Sudan where development is being considered within the protected area.
8.5.5 Existing Protected Areas
Of all the countries bordering the Red Sea, Egypt and Sudan appear to have relative success in implementing and enforcing some forms of marine resource protection. Between 1983 and 2006, the Egyptian Environmental Affairs Agency (EEAA) declared the following areas as national parks or protected areas: Ras Mohamed, Nabq, and Abu Galum in the Sinai Peninsula, as well as Elba, Wadi El Gemal, and the Red Sea North Islands in the Red Sea Governorate (www.eeaa.gov.eg). These protected areas include both terrestrial and marine components and enjoy varying degrees of protection. While some tourism activities are permitted in each of these parks, entry usually requires special permits, and extractive activities (e.g., fishing) are prohibited. Outside the borders of protectorates, fishing regulations also prohibit the fishing of sharks and endangered species. Before the Arab Spring political uprising of 2011, the enforcement of protective regulations was carried out partially by rangers appointed by a branch of the EEAA and partially by the coast guard and the military. The current status of enforcement is unclear, although the same entities remain responsible. Anecdotal evidence from within the diving community in Sinai and Hurghada suggests possible higher levels of non-compliance by fishermen post-2011 as well as some potential positive impacts on coral reefs due to the reduction in tourism in recent years.
Sudan is home to some of the healthiest reefs in the Red Sea with relatively intact populations of sharks (Hussey et al. 2013; Spaet et al. 2016) and other top predators (Kattan et al. 2017). Currently, two marine protected areas exist in attempt to recognize and preserve the biodiversity and unique natural resources found along the coast of the Red Sea State: (1) Sanganeb Atoll Marine National Park was established in 1990, encompassing 22 km2 around a prominent deepwater atoll, and (2) Dunganab Bay and Mukkawar Island National Park, a 2800 km2 reserve established in 2004 that includes a mosaic of undisturbed coral reef, mangrove, seagrass, and intertidal mudflat habitats. These habitats collectively support regionally significant populations of endangered dugongs, sharks, manta rays, dolphins, nesting sea turtles, and birds (sudanmarineparks.info). Together these two sites were declared a World Heritage Site in July 2016. A management structure for these parks is in place, but faces three major challenges: (1) there is no broad community involvement, (2) it is missing a general facility for monitoring and enforcement, and (3) it lacks the capacity to absorb future growth in the region (Chekchak and Klaus 2013). Fortunately, hitherto underdeveloped levels of tourism (Chekchak and Klaus 2013) and fisheries (Tesfamichael and Pitcher 2006) have resulted in relatively minimal impacts to Sudanese marine resources. Some degree of self-policing by the local liveaboard dive boats creates a kind of de facto protection force, as the quality of the reefs is a driving factor in the success of the local ecotourism industry (see Sect. 8.5.3). With increasing interest in coastal development, fisheries, and tourism to the region, however, much effort is required to plan and coordinate for the long-term health of these fragile marine ecosystems (Chekchak 2013; Chekchak and Klaus 2013).
The Kingdom of Saudi Arabia, on the other hand, which controls most of the eastern coast of the Red Sea, has declared only two marine protected areas (MPAs), both of which currently appear to be little more than paper parks: the Farasan Islands and the island of Um Al-Qamari. The Farasan Islands (3310 km2, see Fig. 8.3) were officially declared as protected in 1996 (Wood 2007). The islands are known to host a unique seasonal aggregation of the parrotfish Hipposcarus harid (Gladstone 1996; Spaet 2013). This MPA briefly enjoyed some success due to strong initial community involvement. However, its success was short-lived, as lack of long-term training and awareness programs for local rangers, combined with growing commercial fisheries in the area, led to a decline in the effectiveness of this MPA (Gladstone 2000). The island of Um Al-Qamari (located near Al-Qunfidhah in the Farasan Banks) was declared a protectorate in 1977, much earlier than the Farasan Islands, with an area of 2 km2 (Wood 2007). It was designated to protect a resident population of seabirds, and it is not clear whether any enforcement of protection currently takes place on the island or the surrounding waters. In addition to these declared MPAs, Saudi Arabia issued a royal decree in 2008 putting a total ban on the fishing of sharks (Spaet et al. 2016). However, little to no enforcement of this ban takes place; shark fishing occurs on a daily basis, and hundreds of sharks are landed in Saudi fish markets every month (Spaet and Berumen 2015).
8.5.6 Marine Invasive Species
The primary invasion threat Red Sea fish populations presently face appears to be limited to potential escapees from aquaculture operations. There are very few cases of invasive fishes colonizing the Red Sea (e.g., Por 1978). Planned rapid expansion of aquaculture efforts, particularly in Saudi Arabia, includes dramatic increases in open-sea cage farming of fishes (see Sect. 8.5.4). These operations have already commenced near Al Lith (see Fig. 8.3) and near Duba (north of Al Wajh). Adult barramundi (Lates calcifer) are routinely spotted at the Al Lith marina, apparently having escaped from the cages ~15 km to the north. Surveys of coastal reefs in the area, however, have yet to detect any barramundi between the marina and the fish farm (Alex Kattan, unpublished data). Barramundi may require estuarine or riverine areas for successful completion of some parts of the early life cycle (Copland and Grey 1987). The lack of these habitats in the Red Sea may preclude the establishment of a wild barramundi population, but large numbers of escaped barramundi (which are voracious predators) could still exert an impact on native reef fish populations.
As described in Sect. 8.4.3, the Red Sea appears to ‘export’ far more invasive species (into the Mediterranean) than it ‘imports’ (i.e., reverse Lessepsians are rare). It is possible that the relatively high temperatures and salinity levels may present physiological challenges for non-native species. If this is the mechanism reducing Mediterranean immigrants to the Red Sea, it may also be inhibiting potential invasive species that would otherwise arrive via traditional mechanisms (e.g., ship ballast water). These hypotheses remain to be formally tested.
Red Sea fishes have evolved in and adapted to some of the most challenging conditions in which modern coral reef ecosystems appear to be thriving. The opposite sides of the central Red Sea currently offer an interesting contrast that may reflect the impacts of anthropogenic pressures in recent decades. On one side, reef fish communities may be greatly altered by heavy fishing pressure and coastal development. On the other side, a lack of infrastructure and locally-initiated de facto protection may be preserving healthy reef communities, and may even be supplying important larval input to overfished populations across the basin. The anticipated additional future stressors (ranging from local to widespread) may create even more challenging conditions for Red Sea reefs, particularly planned ‘giga-projects’ with the potential to impact large portions of coastline. Responsible and sustainable management of Red Sea reef fish populations will require a more thorough understanding of the status of fisheries, the nuances of local ecology, and various aspects of connectivity. More than 250 years have passed since the first European natural historians began investigations into Red Sea fishes, yet we still have much to learn.
References
Abu El-Regal MA (1999) Some biological and ecological studies on the larvae of coral reef fishes in Sharm El-Sheikh (Gulf of Aqaba-Red Sea). MSc thesis. Suez Canal University, Egypt
Abu El-Regal MA (2008) Ecological studies on the ichthyoplankton of coral reef fishes in Hurghada, Red Sea, Egypt. PhD thesis. Suez Canal University, Egpyt
Abu El-Regal M, Kon T (2008) First record of the paedomorphic fish Schindleria (Gobioidei, Schindleriidae) from the Red Sea. J Fish Biol 72:1539–1543
Ackerman JL, Bellwood DR (2000) Reef fish assemblages: a re-evaluation using enclosed rotenone stations. Mar Ecol Prog Ser 206:227–237
Adams AJ, Dahlgren CP, Kellison GT, Kendall MS, Layman CA, Ley JA, Nagelkerken I, Serafy JE (2006) Nursery function of tropical back-reef systems. Mar Ecol Prog Ser 318:287–301
Aguilar-Perera A, Appeldoorn RS (2008) Spatial distribution of marine fishes along a cross-shelf gradient containing a continuum of mangrove–seagrass–coral reefs off southwestern Puerto Rico. Estuar Coast Shelf Sci 76:378–394
Almany GR, Planes S, Thorrold SR, Berumen ML, Bode M, Saenz-Agudelo P, Bonin MC, Frisch AJ, Harrison HB, Messmer V, Nanninga GB, Priest MA, Srinivasan M, Sinclair-Taylor T, Williamson DH, Jones GP (2017) Larval fish dispersal in a coral reef seascape. Nat Ecol Evol 1:148
Azzurro E, Maynou F, Belmaker J, Golani D, Crooks JA (2016) Lag times in Lessepsian fish invasion. Biol Invasions 18:2761–2772
Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012
Ben-Tuvia AA (1971) On the occurrence of the Mediterranean serranid fish Dicentrarchus punctatus (Bloch) in the Gulf of Suez. Am Soc Ichthyol Herpetol 1971:741–743
Ben-Tzvi O, Kiflawi M, Gildor H, Abelson A (2007) Possible effects of downwelling on the recruitment of coral reef fishes to the Eilat (Red Sea) coral reefs. Limnol Oceanogr 52:2618–2628
Ben-Tzvi O, Kiflawi M, Gaines SD, Al-Zibdah M, Sheehy MS, Paradis GL, Abelson A (2008) Tracking recruitment pathways of Chromis viridis in the Gulf of Aqaba using otolith chemistry. Mar Ecol Prog Ser 359:229–238
Ben-Tzvi O, Abelson A, Gaines SD, Bernardi G, Beldade R, Sheehy MS, Paradis GL, Kiflawi M (2012) Evidence for cohesive dispersal in the sea. PLoS One 7:e42672
Bernardi G, Azzurro E, Golani D, Miller MR (2016) Genomic signatures of rapid adaptive evolution in the bluespotted cornetfish, a Mediterranean Lessepsian invader. Mol Ecol 25:3384–3396
Berumen ML, Hoey AS, Bass WH, Bouwmeester J, Catania D, Cochran JE, Khalil MT, Miyake S, Mughal MR, Spät JL, Saenz-Agudelo P (2013) The status of coral reef ecology research in the Red Sea. Coral Reefs 32:737–748
Berumen ML, Braun CD, Cochran JE, Skomal GB, Thorrold SR (2014) Movement patterns of juvenile whale sharks tagged at an aggregation site in the Red Sea. PLoS One 9:e103536
Berumen ML, DiBattista JD, Rocha LA (2017) Introduction to virtual issue on Red Sea and Western Indian Ocean biogeography. J Biogeogr 44:1923–1926
Booth DJ, Bond N, Macreadie P (2011) Detecting range shifts among Australian fishes in response to climate change. Mar Freshw Res 62:1027–1042
Borman A, Wood TR, Black HC, Anderson EG, Oesterling MJ, Womack M, Rose WC (1946) The role of arginine in growth with some observations on the effects of argininic acid. J Biol Chem 166:585–594
Bouillon S, Raman AV, Dauby P, Dehairs F (2002) Carbon and nitrogen stable isotope ratios of subtidal benthic invertebrates in an estuarine mangrove ecosystem (Andhra Pradesh, India). Estuar Coast Shelf Sci 54:901–913
Bowen BW, Rocha LA, Toonen RJ, Karl SA, ToBo Laboratory (2013) The origins of tropical marine biodiversity. Trends Ecol Evol 28:359–366
Braun CD, Skomal GB, Thorrold SR, Berumen ML (2014) Diving behavior of the reef manta ray links coral reefs with adjacent deep pelagic habitats. PLoS One 9:e88170
Braun CD, Skomal GB, Thorrold SR, Berumen ML (2015) Movements of the reef manta ray (Manta alfredi) in the Red Sea using satellite and acoustic telemetry. Mar Biol 162:2351–2362
Brokovich E, Einbinder S, Kark S, Shashar N, Kiflawi M (2007) A deep nursery for juveniles of the zebra angelfish Genicanthus caudovittatus. Environ Biol Fish 80:1–6
Brokovich E, Einbinder S, Shashar N, Kiflawi M, Kark S (2008) Descending to the twilight-zone: changes in coral reef fish assemblages along a depth gradient down to 65 m. Mar Ecol Prog Ser 371:253–262
Brokovich E, Ayalon I, Einbinder S, Segev N, Shaked Y, Genin A, Kark S, Kiflawi M (2010) Grazing pressure on coral reefs decreases across a wide depth gradient in the Gulf of Aqaba, Red Sea. Mar Ecol Prog Ser 399:69–80
Bruckner A, Rowlands G, Riegl B, Purkis S, Williams A, Renaud P (2011) Khaled bin Sultan Living Oceans Foundation Atlas of Saudi Arabian Red Sea Marine Habitats. Panoramic Press, Phoenix
Campana SE, Thorrold SR (2001) Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can J Fish Aquat Sci 58:30–38
Cantin NE, Cohen AL, Karnauskas KB, Tarrant AM, McCorkle DC (2010) Ocean warming slows coral growth in the central Red Sea. Science 329:322–325
Catlin J, Jones R (2010) Whale shark tourism at Ningaloo Marine Park: a longitudinal study of wildlife tourism. Tour Manag 31:386–394
Chekchak T (2013) Toward a sustainable future for the Red Sea coast of Sudan. Part 2: socio-economic and governance survey. Cousteau Society, New York
Chekchak T, Klaus R (2013) Toward a sustainable future for the Red Sea coast of Sudan. Part 1: coastal and marine habitats survey. Cousteau Society, New York
Clements KD, German DP, Piché J, Tribollet A, Choat JH (2016) Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linnean Soc 120:729–751
Cocheret de la Moriniére E, Ollux BJA, Nagelkerken I, van der Velde G (2002) Postsettlement life cycle migration patterns and habitat preference of coral reef fish that use seagrass and mangrove habitats as nurseries. Estuar Coast Shelf Sci 55:309–321
Cochran JEM, Hardenstine RS, Braun CD, Skomal GB, Thorrold SR, Xu K, Genton MG, Berumen ML (2016) Population structure of a whale shark Rhincodon typus aggregation in the Red Sea. J Fish Biol 89:1570–1582
Coker DJ, DiBattista JD, Sinclair-Taylor TH, Berumen ML (2018) Spatial patterns of cryptobenthic coral-reef fishes in the Red Sea. Coral Reefs 37:193–199
Coleman RR, Eble JA, DiBattista JD, Rocha LA, Randall JE, Berumen ML, Bowen BW (2016) Regal phylogeography: range-wide survey of the marine angelfish Pygoplites diacanthus reveals evolutionary partitions between the Red Sea, Indian Ocean, and Pacific Ocean. Mol Phylogenet Evol 100:243–253
Copland JW, Grey DL (1987) Management of wild and cultured sea bass / barramundi (Lates calcarifer): proceedings of an international workshop held at Darwin, N.T., Australia, 24–30 September 1986. ACIAR Proceedings No. 20, Australian Centre for International Agricultural Research, Canberra
Depczynski M, Bellwood DR (2003) The role of cryptobenthic reef fishes in coral reef trophodynamics. Mar Ecol Prog Ser 256:183–191
Depczynski M, Bellwood DR (2006) Extremes, plasticity, and invariance in vertebrate life history traits: insights from coral reef fishes. Ecology 87:3119–3127
Depczynski M, Fulton CJ, Marnane MJ, Bellwood DR (2007) Life history patterns shape energy allocation among fishes on coral reefs. Oecologia 153:111–120
Desalle R (2006) Species discovery versus species identification in DNA barcoding efforts: response to Rubinoff. Cons Biol 20:1545–1547
DiBattista JD, Berumen ML, Gaither MR, Rocha LA, Eble JA, Choat JH, Craig MT, Skillings DJ, Bowen BW (2013) After continents divide: comparative phylogeography of reef fishes from the Red Sea and Indian Ocean. J Biogeogr 40:1170–1181
DiBattista JD, Choat JH, Gaither MR, Hobbs JPA, Lozano-Cortés DF, Myers RF, Paulay G, Rocha LA, Toonen RJ, Westneat MW, Berumen ML (2016a) On the origin of endemic species in the Red Sea. J Biogeogr 43:13–30
DiBattista JD, Roberts MB, Bouwmeester J, Bowen BW, Coker DJ, Lozano-Cortés DF, Choat JH, Gaither MR, Hobbs JPA, Khalil MT, Kochzius M, Myers RF, Paulay G, Robitzch VSN, Saenz-Agudelo P, Salas E, Sinclair-Taylor T, Toonen RJ, Westneat MW, Williams ST, Berumen ML (2016b) A review of contemporary patterns of endemism in the Red Sea. J Biogeogr 43:423–439
DiBattista JD, Gaither MR, Hobbs J-PA, Saenz-Agudelo P, Piatek MJ, Bowen BW, Rocha LA, Choat JH, McIlwain JH, Priest MA, Sinclair-Taylor T, Berumen ML (2017) Comparative phylogeography of reef fishes from the Gulf of Aden to the Arabian Sea reveals two cryptic lineages. Coral Reefs 36:625–638
Docmac F, Araya M, Hinojosa IA, Dorador C, Harrod C (2017) Habitat coupling writ large: pelagic-derived materials fuel benthivorous macroalgal reef fishes in an upwelling zone. Ecology 98:2267–2272
Dromard CR, Bouchon-Navaro Y, Cordonnier S, Fontaine MF, Verlaque M, Harmelin-Vivien M, Bouchon C (2013) Resource use of two damselfishes, Stegastes planifrons and Stegastes adustus, on Guadeloupean reefs (Lesser Antilles): inference from stomach content and stable isotope analysis. J Exp Mar Biol Ecol 440:116–125
Edwards FJ (1987) Climate and oceanography. In: Edwards AJ, Head SM (eds) Red Sea – (Key environments). Pergamon Books Ltd, Exeter, pp 45–69
Elsdon TS, Wells BK, Campana SE, Gillanders BM, Jones CM, Limburg KE, Secor DH, Thorrold SR, Walther BD (2008) Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanogr Mar Biol Annu Rev 46:297–330
Emms MA (2015) Broad-scale population genetics of the host sea anemone, Heteractis magnifica. MSc thesis. King Abdullah University of Science and Technology, Saudi Arabia
Fernandez-Silva I, Randall JE, Coleman RR, DiBattista JD, Rocha LA, Reimer JD, Meyer CG, Bowen BW (2015) Yellow tails in the Red Sea: phylogeography of the Indo-Pacific goatfish Mulloidichthys flavolineatus reveals isolation in peripheral provinces and cryptic evolutionary lineages. J Biogeogr 42:2402–2413
Fernandez-Silva I, Randall JE, Golani D, Bogorodsky SV (2016) Mulloidichthys flavolineatus flavicaudus Fernandez-Silva & Randall (Perciformes, Mullidae), a new subspecies of goatfish from the Red Sea and Arabian Sea. ZooKeys 605:131–157
Fricke R, Abu El-Regal M (2017a) Schindleria elongata, a new species of paedomorphic gobioid from the Red Sea (Teleostei: Schindleriidae). J Fish Biol 90:1883–1890
Fricke R, Abu El-Regal MA (2017b) Schindleria nigropunctata, a new species of paedomorphic gobioid fish from the Red Sea (Teleostei: Schindleriidae). Mar Biodivers:1–5. https://doi.org/10.1007/s12526-017-0831-z
Fricke HW, Hottinger L (1983) Coral bioherms below the euphotic zone in the Red Sea. Mar Ecol Prog Ser 11:113–117
Fricke HW, Knauer B (1986) Diversity and spatial pattern of coral communities in the Red Sea upper twilight zone. Oecologia 71:29–37
Fricke HW, Schuhmacher H (1983) The depth limits of Red Sea stony corals: an ecophysiological problem (a deep diving survey by submersible). Mar Ecol 4:163–194
Froukh TJ (2001) Studies on taxonomy and ecology of some fish larvae from the Gulf of Aqaba. MSc thesis. University of Jordan, Jordan
Furby KA, Bouwmeester J, Berumen ML (2013) Susceptibility of central Red Sea corals during a major bleaching event. Coral Reefs 32:505–513
Galil BS, Boero F, Campbell ML, Carlton JT, Cook E, Fraschetti S, Gollasch S, Hewitt CL, Jelmert A, Macpherson E, Marchini A, McKenzie C, Minchin D, Occhipinti-Ambrogi A, Ojaveer H, Olenin S, Piraino S, Ruiz GM (2015) “Double trouble”: the expansion of the Suez Canal and marine bioinvasions in the Mediterranean Sea. Biol Invasions 17:973–976
Giles EC, Saenz-Agudelo P, Hussey NE, Ravasi T, Berumen ML (2015) Exploring seascape genetics and kinship in the reef sponge Stylissa carteri in the Red Sea. Ecol Evol 5:2487–2502
Gladstone W (1996) Unique annual aggregation of longnose parrotfish (Hipposcarus harid) at Farasan Island (Saudi Arabia, Red Sea). Copeia 1996:483–485
Gladstone W (2000) The ecological and social basis for management of a Red Sea marine-protected area. Ocean Coast Manag 43:1015–1032
Goatley CH, Brandl SJ (2017) Cryptobenthic reef fishes. Curr Biol 27:R452–R454
Golani D (1998) Impact of Red Sea fish migrants through the Suez Canal on the aquatic environment of the eastern Mediterranean. Yale F&ES Bull 103:375–387
Golani D (1999) The Gulf of Suez ichthyofauna-assemblage pool for Lessepsian migration into the Mediterranean. Isr J Zool 45:79–90
Golani D, Appelbaum-Golani B (2010) Fish invasions of the Mediterranean Sea: change and renewal. Coronet Books Incorporated, Philadelphia
Golani D, Bogorodsky S (2010) The fishes of the Red Sea – reappraisal and updated checklist. Zootaxa 2463:1–135
Golani D, Massutí E, Quignard J-P, Dulcic J, Azzurro E (2017) CIESM atlas of exotic fishes in the Mediterranean. http://www.ciesm.org/atlas/appendix 1.html
Goren M, Galil BS (2005) A review of changes in the fish assemblages of Levantine inland and marine ecosystems following the introduction of non-native fishes. J Appl Ichthyol 21:364–370
Green AL, Maypa AP, Almany GR, Rhodes KL, Weeks R, Abesamis RA, Gleason MG, Mumby PH, White AT (2015) Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol Rev 90:1215–1247
Gudger EW (1938) Four whale sharks rammed by steamers in the Red Sea region. Copeia 1938(4):170–173
Gudger EW (1940) Whale sharks rammed by ocean vessels: how these sluggish leviathans aid in their own destruction. New Engl Nat 7:1–10
Hansen T (1962) Det Lykkelige Arabien. Gyldendal, Copenhagen. Based on a 1964 translation published as: Arabia Felix: the Danish expedition of 1761–1767. Wm. Collins Sons & Co. Ltd, Glasgow
Hanson JM, Chouinard GA (2002) Diet of Atlantic cod in the southern gulf of St Lawrence as an index of ecosystem change, 1959–2000. J Fish Biol 60:902–922
Hare PE, Fogel ML, Stafford TW, Mitchell AD, Hoering TC (1991) The isotopic composition of carbon and nitrogen in individual amino-acids isolated from modern and fossil proteins. J Archaeol Sci 18:277–292
Harrison HB, Williamson DH, Evans RD, Almany GR, Thorrold SR, Russ GR, Feldheim KA, Van Herwerden L, Planes S, Srinivasan M, Berumen ML, Jones GP (2012) Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr Biol 22:1023–1028
Herler J (2007) Microhabitats and ecomorphology of coral-and coral rock-associated gobiid fish (Teleostei: Gobiidae) in the northern Red Sea. Mar Ecol 28:82–94
Herler J, Hilgers H (2007) A synopsis of coral and coral-rock associated gobies (Pisces: Gobiidae) from the Gulf of Aqaba, northern Red Sea. Aqua J Ichthyol Aquat Biol 10:103–132
Hernaman V, Munday P (2005) Life-history characteristics of coral reef gobies. I. Growth and life-span. Mar Ecol Prog Ser 290:207–221
Hernaman V, Munday P (2007) Evolution of mating systems in coral reef gobies and constraints on mating system plasticity. Coral Reefs 26:585–595
Hinderstein LM, Marr JCA, Martinez FA, Dowgiallo MJ, Puglise KA, Pyle RL, Zawada DG, Appeldoorn R (2010) Theme section on “Mesophotic coral ecosystems: characterization, ecology, and management”. Coral Reefs 29:247–251
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Hozumi A (2015) Environmental factors affecting the whale shark aggregation site in the south central Red Sea. PhD thesis. King Abdullah University of Science and Technology, Saudi Arabia
Hussey NE, Stroh N, Klaus R, Chekchak T, Kessel ST (2013) SCUBA diver observations and placard tags to monitor grey reef sharks, Carcharhinus amblyrhynchos, at Sha’ab Rumi, the Sudan: assessment and future directions. J Mar Biol Assoc UK 93:299–308
Isari S, Pearman JK, Casas L, Michell CT, Curdia J, Berumen ML, Irigoien X (2017a) Exploring the larval fish community of the central Red Sea with an integrated morphological and molecular approach. PLoS One 12:e0182503
Isari S, Pearman JK, Casas L, Michell CT, Curdia J, Berumen ML, Irigoien X (2017b) Integrating morphology and genetics to study the larval community of gobies in the central Arabian Red Sea. Abstracts from the 10th Indo-Pacific Fish Conference, Tahiti, French Polynesia. p83
Jin D, Kite-Powell H, Hoagland P, Solow A (2012) A bioeconomic analysis of traditional fisheries in the Red Sea. Mar Resour Econ 27:137–148
Johansen J, Jones G (2011) Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob Chang Biol 17:2971–2979
Kahng SE, Garcia-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ (2010) Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29:255–275
Kalish JM (1991) δ13C and δ18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects. Mar Ecol Prog Ser 75:191–203
Kattan A, Coker DJ, Berumen ML (2017) Reef fish communities in the central Red Sea show evidence of asymmetrical fishing pressure. Mar Biodivers 47:1227–1238
Kennedy BP, Klaue A, Blum JD, Folt CL, Nislow KH (2002) Reconstructing the lives of fish using Sr isotopes in otoliths. Can J Fish Aquat Sci 59:925–929
Kessel ST, Elamin NA, Yurkowski DJ, Chekchak T, Walter RP, Klaus R, Hill G, Hussey NE (2017) Conservation of reef manta rays (Manta alfredi) in a UNESCO world heritage site: large-scale island development or sustainable tourism? PLoS One 12:e0185419
Khalaf MA, Kochzius M (1992) Community structure and biogeography of shore fishes in the Gulf of Aqaba, Red Sea. Helgol Mar Res 55:252–284
Khalil MT, Bouwmeester J, Berumen ML (2017) Spatial variation in coral reef fish and benthic communities in the central Saudi Arabian Red Sea. PeerJ 5:e3410
Kieckbusch DK, Koch MS, Serafy JE, Anderson WT (2004) Trophic linkages among primary producers and consumers in fringing mangroves of subtropical lagoons. Bull Mar Sci 74:271–285
Kimirei IA, Nagelkerken I, Trommelen M, Blankers P, Van Hoytema N, Hoeijmakers D, Huijbers CM, Mgaya YD, Rypel AL (2013) What drives ontogenetic niche shifts of fishes in coral reef ecosystems? Ecosystems 16:783–796
Kimmerling N, Zuqert O, Amitai G, Gurevich T, Armoza-Zvuloni R, Kolesnikov I, Berenshtein I, Melamed S, Gilad S, Benjamin S, Rivlin A, Moti O, Paris CB, Holzman R, Kiflawi M, Sorek R (2018) Quantitative species-level ecology of reef fish larvae via metabarcoding. Nat Ecol Evol 2:306–316
Kotb MM, Hanafy MH, Rirache H, Matsumura S, Al-Sofyani A, Ahmed A, Bawazir G, Al Horani F (2008) Status of coral reefs in the Red Sea and Gulf of Aden region. In: Wilkinson C (ed) Status of Coral Reefs of the World: 2008. Australian Institute of Marine Science, Townsville, pp 67–78
Kristensen E, Lee SY, Mangion P, Quintana CO, Valdemarsen T (2017) Trophic discrimination of stable isotopes and potential food source partitioning by leaf-eating crabs in mangrove environments. Limnol Oceanogr 62:2097–2112
Larsen T, Taylor DL, Leigh MB, O’Brien DM (2009) Stable isotope fingerprinting: a novel method for identifying plant, fungal, or bacterial origins of amino acids. Ecology 90:3526–3535
Larsen T, Wooller MJ, Fogel ML, O’Brien DM (2012) Can amino acid carbon isotope ratios distinguish primary producers in a mangrove ecosystem? Rapid Commun Mass Spectrom 26:1541–1548
Larsen T, Ventura M, Andersen N, O’Brien DM, Piatkowski U, McCarthy MD (2013) Tracing carbon sources through aquatic and terrestrial food webs using amino acid stable isotope fingerprinting. PLoS One 8:e73441
Layman CA (2007) What can stable isotope ratios reveal about mangroves as fish habitat? Bull Mar Sci 80:513–527
Letourneur Y, De Loma TL, Richard P, Harmelin-Vivien ML, Cresson P, Banaru D, Fontaine MF, Gref T, Planes S (2013) Identifying carbon sources and trophic position of coral reef fishes using diet and stable isotope (δ15N and δ13C) analyses in two contrasted bays in Moorea, French Polynesia. Coral Reefs 32:1091–1102
Lieske E, Myers RT (2004) Coral Reef Guide: Red Sea. HarperCollins Publishers Ltd, London
Malcolm H, Jordan A, Smith SA (2010) Biogeographical and cross-shelf patterns of reef fish assemblages in a transition zone. Mar Biodivers 40:181–193
Marguillier S, van der Velde G, Dehairs F, Hemminga MA, Rajagopal S (1997) Trophic relationships in an interlinked mangroveseagrass ecosystem as traced by δ13C and δ15N. Mar Ecol Prog Ser 151:115–121
Marshall A, Kashiwagi T, Bennett MB, Deakos M, Stevens G, McGregor F, Clark T, Ishihara H, Sato K (2011) Manta alfredi. The IUCN red list of threatened species 2011: e.T195459A8969079
McCook LJ, Almany GR, Berumen ML, Day JC, Green AL, Jones GP, Leis JM, Planes S, Russ GR, Sale PF, Thorrold SR (2009) Management under uncertainty: guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs 28:353–366
McMahon KW, Fogel ML, Johnson BJ, Houghton LA, Thorrold SR (2011a) A new method to reconstruct fish diet and movement patterns from δ13C values in otolith amino acids. Can J Fish Aquat Sci 68:1330–1340
McMahon KW, Berumen ML, Mateo I, Elsdon TS, Thorrold SR (2011b) Carbon isotopes in otolith amino acids identify residency of juvenile snapper (Family: Lutjanidae) in coastal nurseries. Coral Reefs 30:1135–1145
McMahon KW, Berumen ML, Thorrold SR (2012) Linking habitat mosaics and connectivity in a coral reef seascape. Proc Natl Acad Sci USA 109:15372–15376
McMahon KW, Thorrold SR, Houghton LA, Berumen ML (2016) Tracing carbon flow through coral reef food webs using a compound-specific stable isotope approach. Oecologia 180:809–821
Meekan M, Austin CM, Tan MH, Wei NWV, Miller A, Pierce SJ, Rowat D, Stevens G, Davies TK, Ponzo A, Gan HM (2017) iDNA at sea: recovery of whale shark (Rhincodon typus) mitochondrial DNA sequences from the whale shark copepod (Pandarus rhincodonicus) confirms global population structure. Front Mar Sci 4:420
Meeker ND, Hutchinson SA, Ho L, Trede NS (2007) Method for isolation of PCR-ready genomic DNA from zebrafish tissues. BioTechniques 43(5):610–614
Morcos SA (1970) Physical and chemical oceanography of the Red Sea. Oceanogr Mar Biol Annu Rev 8:73–202
Mulcahy SA, Killingley JS, Phleger CF, Berger WH (1979) Isotopic composition of otoliths from a benthopelagic fish, Coryphaenoides acrolepis, Macrouridae: Gadiformes. Oceanol Acta 2:423–427
Mumby PJ, Edwards AJ, Arias-González JE, Lindeman KC, Blackwell PG, Gall A, Gorczynska MI, Harborne AR, Pescod CL, Renken H, Wabnitz CC (2004) Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427:533–536
Munday PL, Jones GP, Pratchett MS, Williams AJ (2008) Climate change and the future for coral reef fishes. Fish Fish 9:261–285
Nagelkerken I, Dorenbosch M, Verberk WCEP, Cocheret de la Moriniére E, van der Velde G (2000) Importance of shallow-water biotopes of a Caribbean bay for juvenile coral reef fishes: patterns in biotope association, community structure and spatial distribution. Mar Ecol Prog Ser 202:175–192
Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM, Sasekumar A, Somerfield PJ (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185
Nanninga GB (2013) Merging approaches to explore connectivity in the anemonefish, Amphiprion bicinctus, along the Saudi Arabian coast of the Red Sea. Ph.D. Thesis. King Abdullah University of Science and Technology, Saudi Arabia
Nanninga GB, Saenz-Agudelo P, Manica A, Berumen ML (2014) Environmental gradients predict the genetic population structure of a coral reef fish in the Red Sea. Mol Ecol 23:591–602
Nanninga GB, Saenz-Agudelo P, Zhan P, Hoteit I, Berumen ML (2015) Not finding Nemo: limited reef-scale retention in a coral reef fish. Coral Reefs 34:383–392
Ngugi DK, Antunes A, Brune A, Stingl U (2012) Biogeography of pelagic bacterioplankton across an antagonistic temperature-salinity gradient in the Red Sea. Mol Ecol 21:388–405
Nowicki JP, Miller GM, Munday PL (2012) Interactive effects of elevated temperature and CO2 on foraging behavior of juvenile coral reef fish. J Exp Mar Biol Ecol 412:46–51
Osman EO, Smith DJ, Ziegler M, Kürten B, Conrad C, El-Haddad KM, Voolstra CR, Suggett DJ (2018) Thermal refugia against coral bleaching throughout the northern Red Sea. Glob Chang Biol 24:e474–e484
Pierce SJ, Norman B (2016) Rhincodon typus. The IUCN red list of threatened species 2016: e.T19488A2365291
Por FD (1978) Lessepsian migration. The influx of Red Sea biota into the Mediterranean by way of the Suez Canal. Springer Verlag, Berlin
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Pratchett MS, Munday P, Wilson SK (2008) Effects of climate-induced coral bleaching on coral-reef fishes - ecological and economical consequences. Oceanogr Mar Biol Annu Rev 46:251–296
Price A, Ghazi S, Tkaczynski P, Venkatachalam A, Santillan A, Pancho T, Metcalfe R, Saunders J (2014) Shifting environmental baselines in the Red Sea. Mar Pollut Bull 78:96–101
Priest MA, DiBattista JD, McIlwain JL, Taylor BM, Hussey NE, Berumen ML (2016) A bridge too far: dispersal barriers and cryptic speciation in an Arabian Peninsula grouper (Cephalopholis hemistiktos). J Biogeogr 43:820–832
Racault MF, Raitsos DE, Berumen ML, Brewin RJW, Platt T, Sathyendranath S, Hoteit I (2015) Phytoplankton phenology indices in coral reef ecosystems: application to ocean-colour observations in the Red Sea. Remote Sens Environ 160:222–234
Raitsos DE, Pradhan Y, Brewin RJ, Stenchikov G, Hoteit I (2013) Remote sensing the phytoplankton seasonal succession of the Red Sea. PLoS One 8:e64909
Raitsos DE, Brewin RJW, Zhan P, Dreano D, Pradhan Y, Nanninga GB, Hoteit I (2017) Sensing coral reef connectivity pathways from space. Sci Rep 7:9338
Reeds PJ (2000) Dispensable and indispensable amino acids for humans. J Nutr 130:1835S–1840S
ReFuGe 2020 Consortium (2015) The ReFuGe 2020 consortium—using “omics” approaches to explore the adaptability and resilience of coral holobionts to environmental change. Front Mar Sci 2:68
Roberts CM, Shepherd ARD, Ormond RFG (1992) Large-scale variation in assemblage structure of Red Sea butterflyfishes and angelfishes. J Biogeogr 19:239–250
Roberts MB, Jones GP, McCormick MI, Munday PL, Neale S, Thorrold S, Robitzch VSN, Berumen ML (2016) Homogeneity of coral reef communities across 8 degrees of latitude in the Saudi Arabian Red Sea. Mar Pollut Bull 105:558–565
Robinson DP, Jaidah MY, Bach SS, Rohner CA, Jabado RW, Ormond R, Pierce SJ (2017) Some like it hot: repeat migration and residency of whale sharks within an extreme natural environment. PLoS One 12:e0185360
Robitzch VSN (2017) The assessment of current biogeographic patterns of coral reef fishes in the Red Sea by incorporating their evolutionary and ecological background. Ph.D. Thesis. King Abdullah University of Science and Technology, Saudi Arabia
Robitzch V, Banguera-Hinestroza E, Sawall Y, Al-Sofyani A, Voolstra CR (2015) Absence of genetic differentiation in the coral Pocillopora verrucosa along environmental gradients of the Saudi Arabian Red Sea. Front Mar Sci 2:5
Robitzch VSN, Lozano-Cortés D, Kandler NM, Salas E, Berumen ML (2016) Productivity and sea surface temperature are correlated with the pelagic larval duration of damselfishes in the Red Sea. Mar Pollut Bull 105:566–574
Rohner CA, Armstrong AJ, Pierce SJ, Prebble CE, Cagua EF, Cochran JE, Berumen ML, Richardson AJ (2015) Whale sharks target dense prey patches of sergestid shrimp off Tanzania. J Plankton Res 37:352–362
Rothman SBS, Stern N, Goren M (2016) First record of the Indo-Pacific areolate grouper Epinephelus areolatus (Forsskål, 1775) (Perciformes: Epinephelidae) in the Mediterranean Sea. Zootaxa 4067:479–483
Rowat D, Engelhardt U (2007) Seychelles: a case study of community involvement in the development of whale shark ecotourism and its socioeconomic impact. Fish Res 84:109–113
Rowat D, Meekan MG, Engelhardt U, Pardigon B, Vely M (2007) Aggregations of juvenile whale sharks (Rhincodon typus) in the Gulf of Tadjoura, Djibouti. Environ Biol Fish 80:465–472
Rubinoff D (2006) Utility of mitochondrial DNA barcodes in species conservation. Consver Biol 20:1026–1033
Saenz-Agudelo P, DiBattista JD, Piatek MJ, Gaither MR, Harrison HB, Nanninga GB, Berumen ML (2015) Seascape genetics along environmental gradients in the Arabian Peninsula: insights from ddRAD sequencing of anemonefishes. Mol Ecol 24:6241–6255
Sala E, Kizilkaya Z, Yildirim D, Ballesteros E (2011) Alien marine fishes deplete algal biomass in the Eastern Mediterranean. PLoS One 6:e17356
Salama AJ, Satheesh S, Balqadi AA, Kitto MR (2016) Identifying suitable fin fish cage farming sites in the eastern Red Sea Coast, Saudi Arabia. Thalassas Int J Mar Sci 32:1–9
Salas De la Fuente EM (2016) Reef fish population genomics and hybridization using RADSeq: a case study with Dascyllus trimaculatus. PhD thesis. University of Santa Cruz
Sale PF, Feary DA, Burt JA, Bauman AG, Cavalcante GH, Drouillard KG, Kjerfve B, Marquis E, Trick CG, Usseglio P, Van Lavieren H (2011) The growing need for sustainable ecological management of marine communities of the Persian Gulf. Ambio 40:4–17
Sawall Y, Kürten B, Hoang BX, Sommer U, Wahl M, Al-Sofyani A, Al-Aidaroos AM, Marimuthu N, Khomayis HS, Gharbawi WY (2014) Coral communities, in contrast to fish communities, maintain a high assembly similarity along the large latitudinal gradient along the Saudi Red Sea coast. J Ecosyst Ecography S4:003
Schwarcz HP, Gao Y, Campana S, Browne D, Knyf M, Brand U (1998) Stable carbon isotope variations in otoliths of Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 55:1798–1806
Sheppard C, Sheppard AS (1991) Corals and coral communities of Arabia. Fauna Arab 12:3–170
Sheppard C, Price A, Roberts C (1992) Marine ecology of the Arabian region - patterns and processes in extreme tropical Environments. Academic, London
Spaet J (2013) Predictable annual aggregation of longnose parrotfish (Hipposcarus harid) in the Red Sea. Mar Biodivers 43:179–180
Spaet JLY, Berumen ML (2015) Fish market surveys indicate unsustainable elasmobranch fisheries in the Saudi Arabian Red Sea. Fish Res 161:356–364
Spaet JLY, Thorrold SR, Berumen ML (2012) A review of elasmobranch research in the Red Sea. J Fish Biol 80:952–965
Spaet JLY, Jabado RW, Henderson AC, Moore ABM, Berumen ML (2015) Population genetics of four heavily exploited shark species around the Arabian Peninsula. Ecol Evol 5:2317–2332
Spaet JLY, Nanninga GB, Berumen ML (2016) Ongoing decline of shark populations in the Eastern Red Sea. Biol Conserv 201:20–28
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana AL, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573–583
Spanier E, Galil BS (1991) Lessepsian migration: a continuous biogeographical process. Endeavour 15:102–106
Stephenson PC, Edmonds JS, Moran MJ, Caputi N (2001) Analysis of stable isotope ratios to investigate stock structure of red emperor and Rankin cod in northern Western Australia. J Fish Biol 58:126–144
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tenggardjaja K, Jackson A, Leon F, Azzurro E, Golani D, Bernardi G (2013) Genetics of a Lessepsian sprinter: the bluespotted cornetfish, Fistularia commersonii. Isr J Ecol Evol 59:181–185
Tesfamichael D (2012) Assessment of the Red Sea ecosystem with emphasis on fisheries. PhD thesis. University of British Columbia
Tesfamichael D, Pauly D (2016) The Red Sea ecosystem and fisheries. Springer Netherlands, Dordrecht
Tesfamichael D, Pitcher TJ (2006) Multidisciplinary evaluation of the sustainability of Red Sea fisheries using Rapfish. Fish Res 78:227–235
Thorrold SR, Latkoczy C, Swart PK, Jones CM (2001) Natal homing in a marine fish metapopulation. Science 291:297–299
Thorrold SR, Zacherl DC, Levin LA (2007) Population connectivity and larval dispersal using geochemical signatures in calcified structures. Oceanography 20:80–89
Tietbohl MD (2016) Assessing the functional diversity of herbivorous reef fishes using a compound-specific stable isotope approach. MSc thesis. King Abdullah University of Science and Technology, Saudi Arabia
Tornabene L, Ahmadia GN, Berumen ML, Smith DJ, Jompa J, Pezold F (2013) Evolution of microhabitat association and morphology in a diverse group of cryptobenthic coral reef fishes (Teleostei: Gobiidae: Eviota). Mol Phylogenet Evol 66:391–400
Troyer E (2017) Microhabit association of cryptobenthic fishes (Family Gobiidae) in the central Red Sea. MSc thesis. King Abdullah University of Science and Technology, Saudi Arabia
Troyer EM, Coker DJ, Berumen ML (2018) Comparison of cryptobenthic reef fish communities among microhabitats in the Red Sea. PeerJ 6:e5014
Truett G, Heeger P, Mynatt R, Truett A, Walker J, Warman M (2000) Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). BioTechniques 29:52–54
Vignaud TM, Maynard JA, Leblois R, Meekan MG, Vázquez-Juárez R, Ramírez-Macías D, Pierce SJ, Rowat D, Berumen ML, Beeravolu C, Baksay S (2014) Genetic structure of populations of whale sharks among ocean basins and evidence for their historic rise and recent decline. Mol Ecol 23:2590–2601
Waldrop E, Hobbs JPA, Randall JE, DiBattista JD, Rocha LA, Kosaki RK, Berumen ML, Bowen BW (2016) Phylogeography, population structure and evolution of coral-eating butterflyfishes (Family Chaetodontidae, genus Chaetodon, subgenus Corallochaetodon). J Biogeogr 43:1116–1129
Walter RP, Kessel ST, Alhasan N, Fisk AT, Heath DD, Chekchak T, Klaus R, Younis M, Hill G, Jones B, Braun CD (2014) First record of living Manta alfredi × Manta birostris hybrid. Mar Biodivers 44:2016–2001
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD (2005) DNA barcoding Australia’s fish species. Philos Trans Roy Soc B Biol Sci 360:1847–1857
White WT, Corrigan S, Yang L, Henderson AC, Bazinet AL, Swofford DL, Naylor GJP (2017) Phylogeny of the manta and devilrays (Chondrichthyes: mobulidae), with an updated taxonomic arrangement for the family. Zool J Linnean Soc 182:50–75
Wilkinson C (2008) Status of Coral Reefs of the World: 2008. Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre, Townsville
Wilson SK, Graham NAJ, Pratchett MS, Jones GP, Polunin NVC (2006) Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob Chang Biol 12:2220–2234
Wood LJ (2007) MPA global: a database of the world’s marine protected areas. Sea Around Us Project, UNEP-WCMC & WWF. http://www.mpaglobal.org
Wyatt ASJ, Waite AM, Humphries S (2012) Stable isotope analysis reveals community-level variation in fish trophodynamics across a fringing coral reef. Coral Reefs 31:1029–1044
Xu W, Ruch J, Jónsson S (2015) Birth of two volcanic islands in the southern Red Sea. Nat Commun 6:7104
Yao F, Hoteit I, Pratt LJ, Bower AS, Zhai P, Köhl A, Gopalakrishnan G (2014) Seasonal overturning circulation in the Red Sea: 1. Model validation and summer circulation. J Geophys Res 119:2238–2262
Zhan P, Subramanian AC, Yao F, Hoteit I (2014) Eddies in the Red Sea: a statistical and dynamical study. J Geophys Res – Oceans 119:3909–3925
Acknowledgements
Data acknowledgement: This research has made use of data and software tools provided by Wildbook for Whale Sharks, an online mark-recapture database operated by the non-profit scientific organization Wild Me with support from public donations and the Qatar Whale Shark Research Project.
We thank Malek Amr Gusti, Manal Bamashmos, and Prof. Khaled Salama for their assistance with Arabic translations.
We thank the staff of the KAUST Biosciences Core Laboratory for their assistance in the genetic analyses described in Sect. 8.3.3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
8.1 Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Berumen, M.L. et al. (2019). Fishes and Connectivity of Red Sea Coral Reefs. In: Voolstra, C., Berumen, M. (eds) Coral Reefs of the Red Sea. Coral Reefs of the World, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-05802-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-05802-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05800-5
Online ISBN: 978-3-030-05802-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)