Abstract
Vasospasm after subarachnoid hemorrhage (SAH) has been studied, but the mechanisms remain to be unveiled. Tenascin-C (TNC), which is a matricellular protein and reported to increase in spastic cerebral artery wall after SAH, is a ligand for both Toll-like receptor 4 (TLR4) and epidermal growth factor receptor (EGFR). Our previous studies suggested the involvement of TNC and these receptors in vasoconstriction or vasospasm after SAH. In this study, we investigated whether upregulation of TNC and TLR4 is observed and if an EGFR inhibitor has suppressive effects against them in a mice endovascular perforation SAH model. At 24 h after SAH, TNC and TLR4 expressions were widely observed in spastic cerebral arteries, and these expressions were suppressed by the administration of an EGFR inhibitor. From these results, EGFR inhibitors possibly suppress the expression of not only EGFR but also TLR4 at least partly through regulating TNC upregulation. More studies are needed to clarify the precise mechanisms linking these receptors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cerebral vasospasm remains an important prognostic factor after aneurysmal subarachnoid hemorrhage (SAH), but the mechanisms are still not well unveiled [1] . Tenascin-C (TNC) is a matricellular protein and one of ligands for epidermal growth factor receptor (EGFR) and Toll-like receptor 4 (TLR4) [2] . Our previous studies showed that TNC was suggested to be involved in vasospasm development in both patients [3] and an experimental animal model [4] . TLR4 activation was also suggested to be involved in vasoconstriction or vasospasm development [1, 5, 6] . Another our previous study showed that administration of recombinant TNC , which consisted of epidermal growth factor (EGF)-like repeats only and did not contain TLR4-binding sites, brought cerebral vasoconstriction in healthy rats [5] . Surprisingly, an anti-TLR4 agent had the most therapeutic effects against this vasoconstriction, and therefore TNC upregulation and subsequent TLR4 activation were suggested [7] . In this study, we investigated whether an EGFR inhibitor has suppressive effects against TNC and TLR4 expression in in vivo SAH models.
Materials and Methods
All procedures were approved by the Animal Ethics Review Committee of Mie University and were carried out in accordance with the institution’s guidelines for animal experiments.
SAH Modeling and Study Protocol
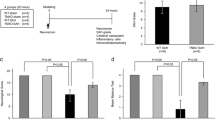
Mice (C57BL/6 N, 25–30 g, male) underwent endovascular perforation SAH or sham modeling as previously described [1] . Briefly, mice were anesthetized, positioned supinely, and skin incision was made at the midline of the neck to expose the left carotid artery. A 4–0 monofilament with a sharpen tip was inserted from the left external carotid artery (ECA) into the left internal carotid artery and push further to perforate the bifurcation of anterior cerebral artery and middle cerebral artery. Then the filament was withdrawn and the stump of ECA was coagulated. The wound was sutured. The sham group underwent the same procedure as described above except for perforating the artery. At 30 min after surgery, vehicle or drug was administrated intraventricularly. After evaluating neurological scores, mice were sacrificed at 24 h after modeling, and then assessment of SAH grade and immunohistochemistry were performed (Fig. 1). Mice were assigned to SAH-vehicle, SAH-drug, and sham groups (n = 3/group).
Intraventricular Injection
Intraventricular injection of vehicle or drug was performed as previously described [1] . Mouse was set on the stereotactic head holder, and using a surgical microscope (Zeiss, Germany), a midline frontoparietal skin incision was performed. A burr hole was perforated at 0.2 mm caudal and 1.0 mm lateral (left) to the bregma. The needle of Hamilton syringe was inserted 2.2 mm below the horizontal plane of the bregma, and 2 μL of vehicle (dimethyl sulfoxide, DMSO) or drug diluent (AG1478; 1 mM diluted in DMSO; Cayman; cat#10010244) was injected intraventricularly, and the wound was sutured.
SAH Grade
SAH grading was performed as previously described [1] . The basal cistern was divided into six segments, and each segment was allotted a grade from 0 to 3 depending on the amount of SAH. A total score ranging from 0 to 18 was determined by summing the scores. Mice with moderate SAH grade (8–12) were used for experiments as the SAH groups.
Neurological Score
Neurological impairments were blindly evaluated as previously described [1] . Neurological scores (3–18) were determined by summing up six test scores (spontaneous activity, spontaneous movement of four limbs, forepaw outstretching, climbing, body proprioception, and response to whisker stimulation).
Histology
Mice’s brains were used for making paraffin-embedded coronal sections at bregma +1 mm as previously described [1] . Briefly, at 24 h after modeling, mice were deeply anesthetized with Avertin® (2,2,2-tribromoethanol) solution and perfused with cold phosphate-buffered saline followed by 4% paraformaldehyde for brain fixation. The brains were removed, embedded in paraffin, and cut into 4 μm sections. Sections were first deparaffinized followed by rehydration and heat-induced antigen retrieval in 10 mM citrate butter (pH 6.0). Sections were incubated with rabbit anti-TNC primary antibody (1:50; Santacruz Biotechnology; cat #20932) or rabbit anti-TLR4 primary antibody (1:1000; Abcam; cat#13556) at 4 °C for overnight. Then, sections were incubated with anti-rabbit secondary antibody (Vector; cat#BA-1000) at room temperature for 30 min, incubated with avidin-biotin complex solution (Vector; cat#PK-6100) at room temperature for 30 min, visualized by diaminobenzidine (brown color), and counterstained with hematoxylin. Sections were dehydrated, cleared in xylene, and mounted for observation under light microscope.
Results
Increased Expressions of TNC and TLR4 Were Observed in Spastic Cerebral Arteries After SAH
No mice died before sacrifice in the sham group. Mice in the sham group showed full scores at neurological assessment, while mice in the SAH-vehicle group showed neurological deterioration (data not shown). TNC and TLR4 were almost undetected in cerebral arteries in the sham group (Fig. 2a). In contrast, expressions of these molecules were widely observed in the vascular endothelial cells and smooth muscle cells of spastic cerebral arteries after SAH (Fig. 2a).
Effects of an epidermal growth factor receptor (EGFR) inhibitor (AG1478) on expressions of tenascin-C (TNC) and Toll-like receptor 4 (TLR4) after subarachnoid hemorrhage (SAH). (a) Representative pictures of coronal sections of internal carotid artery. Single arrow, immunoreactive endothelial cells; double arrow, immunoreactive vascular smooth muscle cells. DMSO, dimethyl sulfoxide; bar, 50 μm. (b) Possible links among molecules, which engage in vasospasm development after SAH. MAPK mitogen-activated protein kinase
Administration of EGFR Inhibitor Suppressed Expression of TNC and TLR4 in Cerebral Arteries
Neurological behavior of the SAH-drug group showed improvement compared with that of the SAH-vehicle group (data not shown). In the SAH-drug group, immunoreactivities for TNC and TLR4 on cerebral arteries with improved vasospasm were suppressed mainly in the vascular smooth muscle cells (Fig. 2a).
Discussion
This study showed that after administration of EGFR inhibitor, expressions of TNC and TLR4 were suppressed in cerebral arteries in an experimental SAH model (Fig. 2a).
TNC is a matricellular protein, which is rarely detected in the normal adult tissues [2] . Under pathological conditions, TNC appears and is suggested to be involved in the pathogenesis of various diseases such as lung fibrosis [8] , rheumatoid arthritis [2], and cerebral vasospasm [3, 4] . TNC is also known as a ligand for EGFR and TLR4 [2] .
Our previous studies suggested that TNC was upregulated [4] and that TLR4 was one of the receptors activated in major cerebral arteries after SAH [1, 6] . Positive feedback of TNC was observed in spastic cerebral arterial wall after SAH [4] . Jones et al. suggested that exogenous TNC caused EGFR activation in vascular smooth muscle cells [9] . TNC is thought to be involved in its own protein synthesis at least partly through mitogen-activated protein kinase pathway [3] . Furthermore, another of our previous study showed that EGFR stimulation using recombinant TNC, which had only EGF-like repeats, caused cerebral vasoconstriction in healthy rats [5] and that unexpectedly a TLR4 antagonist had therapeutic effects against this vasoconstriction [7] . Taking these findings into consideration, after activating EGFR, TNC positive feedback and subsequent TLR4 activation were suggested. In this study, diminished expressions of TNC and TLR4 on cerebral artery were observed under the presence of an EGFR inhibitor (Fig. 2a), suggesting the similar reactions occurred in in vivo SAH models (Fig. 2b).
Inflammation is suggested to cause upregulation of matricellular proteins such as TNC to manage tissue injury [2] . Besides our previous study [4] , TNC positive feedback was reported in experimental animal models of arthritis [2] and epilepsy [10] . Overexpression of TNC could progress diseases in each condition [6] . Therefore, TNC has been suggested to be a therapeutic target to prevent cerebral vasospasm [3, 4] . Our results in this study added a new potential feature of TNC that TNC could exert signaling pathways to upregulate a specific receptor: thus, several working points of inhibitors can be used to block the signaling.
As mentioned in our previous reports [3, 7] , there are many studies suggesting crosstalk signaling between receptors. Interaction between EGFR and TLR4 was also reported [11] . In this study, we could not investigate such crosstalk signalings, but they are possibly involved in vasospasm development after SAH. More studies are needed to unveil the mechanisms.
In conclusion, we showed the possibility that an EGFR inhibitor may have suppressive effects against TLR4 upregulation at least partly via reducing TNC upregulation after SAH.
References
Kawakita F, Fujimoto M, Liu L, Nakano F, Nakatsuka Y, Suzuki H. Effects of Toll-like receptor 4 antagonists against cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol. 2017;54:6624–33.
Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–80.
Suzuki H, Kanamaru K, Shiba M, Fujimoto M, Imanaka-Yoshida K, Yoshida T, Taki W. Cerebrospinal fluid tenascin-C in cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2011;23:310–7.
Shiba M, Suzuki H, Fujimoto M, Shimojo N, Imanaka-Yoshida K, Yoshida T, Kanamaru K, Matsushima S, Taki W. Imatinib mesylate prevents cerebral vasospasm after subarachnoid hemorrhage via inhibiting tenascin-C expression in rats. Neurobiol Dis. 2012;46:172–9.
Fujimoto M, Shiba M, Kawakita F, Liu L, Nakasaki A, Shimojo N, Imanaka-Yoshida K, Yoshida T, Suzuki H. Epidermal growth factor-like repeats of tenascin-C-induced constriction of cerebral arteries via activation of epidermal growth factor receptors in rats. Brain Res. 2016;1642:436–44.
Okada T, Suzuki H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen Res. 2017;12:193–6.
Nakano F, Fujimoto M, Kawakita F, Nakazaki A, Liu L, Nakatsuka Y, Imanaka-Yoshida K, Yoshida T, Suzuki H. Receptors that mediate tenascin-C-induced constriction of cerebral arteries in rats. In: Sasaki T, Ohkuma H, Kanamaru K, Suzuki M, editors. Neurovascular events after subarachnoid hemorrhage. Tokyo: Narunia; 2017. p. 151–6.
Estany S, Vicens-Zygmunt V, Llatjós R, Montes A, Penín R, Escobar I, Xaubet A, Santos S, Manresa F, Dorca J, Molina-Molina M. Lung fibrotic tenascin-C upregulation is associated with other extracellular matrix proteins and induced by TGFβ1. BMC Pulm Med. 2014;14:120.
Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997;139:279–93.
Mercado-Gómez O, Landgrave-Gómez J, Arriaga-Avila V, Nebreda-Corona A, Guevara-Guzmán R. Role of TGFβ signaling pathway on Tenascin C protein upregulation in a pilocarpine seizure model. Epilepsy Res. 2014;108:1694–704.
De S, Zhou H, DeSantis D, Croniger CM, Li X, Stark GR. Erlotinib protects against LPS-induced endotoxicity because TLR4 needs EGFR to signal. Proc Natl Acad Sci U S A. 2015;112:9680–5.
Fujimoto M, Suzuki H, Shiba M, Shimojo N, Imanaka-Yoshida K, Yoshida T, Kanamaru K, Matsushima S, Taki W. Tenascin-C induces prolonged constriction of cerebral arteries in rats. Neurobiol Dis. 2013;55:104–9.
Acknowledgments
This work was supported in part by a grant-in-aid for Scientific Research from Japan Society for the Promotion of Science to Drs. Shiba and Suzuki. We thank Chiduru Yamamoto (Department of Neurosurgery, Mie University Graduate School of Medicine) for her assistance.
Conflict of Interest: The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nakano, F. et al. (2020). Link Between Receptors That Engage in Developing Vasospasm After Subarachnoid Hemorrhage in Mice. In: Martin, R., Boling, W., Chen, G., Zhang, J. (eds) Subarachnoid Hemorrhage. Acta Neurochirurgica Supplement, vol 127. Springer, Cham. https://doi.org/10.1007/978-3-030-04615-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-04615-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04614-9
Online ISBN: 978-3-030-04615-6
eBook Packages: MedicineMedicine (R0)