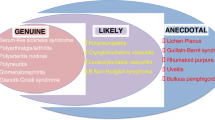
Abstract
The hepatitis C virus (HCV) is a hepatotropic virus that causes liver cirrhosis and hepatocellular cancer, but it is now considered a systemic disease because of the additional HCV-associated extrahepatic manifestations (EHMs) that occur. HCV infection is a global health problem, with 150–170 million people being chronically infected. It is estimated that about 350,000 patients die from HCV-related complications. However, the risks of mortality and morbidity are underestimated because studies do not take into account extrahepatic outcomes of chronically infected HCV patients. Extrahepatic complications of HCV infection have been shown to be more prevalent in large cohort studies, where two thirds of patients chronically infected with HCV infection have experienced EHMs (Cacoub et al., Arthritis Rheum 42:2204–2212, 1999; El-Serag et al., Hepatology 36:1439–1445, 2002). Some of these EHMs are well documented and more common, while others are rare or their association with HCV is unproven. HCV-associated autoimmune or lymphoproliferative disorders, from benign mixed cryoglobulinemia to frank lymphomas, have been reported. More recently, many other extrahepatic HCV-related disorders have been reported, including cardiovascular, renal, metabolic, and central nervous system diseases. Viral eradication of HCV has significantly reduced the rates of hepatic and extrahepatic deaths (Cacoub et al., Arthritis Rheum 42:2204–2212, 1999; Adinolfi et al., World J Gastroenterol 20:3410–3417, 2014; Zignego et al., Dig Liver Dis 39:2–17, 2007; Lee et al., J Infect Dis 206:469–477, 2012; Omland et al., Clin Gastroenterol Hepatol 9:71–78, 2011; Uto et al., Hepatology 50:393–399, 2009; Hsu et al., Hepatology 59:1293–1302, 2014). The aim of this chapter is to give a brief objective approach to the epidemiology, pathogenesis, and treatment of HCV-associated EHMs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
The hepatitis C virus (HCV) infection is a global health problem. It is estimated that 150–170 million people are chronically infected. It is a hepatotropic virus that causes liver cirrhosis and hepatocellular cancer. Beside its hepatic manifestations, it is considered a systemic disease because of the additional HCV-associated extrahepatic manifestations (EHMs). It is estimated that about 350,000 patients die from HCV-related complications. However, the risks of mortality and morbidity are underestimated because studies do not take into account extrahepatic outcomes of chronically infected HCV patients. Extrahepatic complications of HCV infection have been shown to be more prevalent in large cohort studies, where two thirds of patients chronically infected with HCV infection have experienced EHMs [1, 2]. Some of these EHMs are well documented and more common, while others are rare or their association with HCV is unproven. HCV-associated autoimmune or lymphoproliferative disorders, from benign mixed cryoglobulinemia to frank lymphomas, have been reported. More recently, many other extrahepatic HCV-related disorders have been reported, including cardiovascular, renal, metabolic, and central nervous system diseases. Viral eradication of HCV has significantly reduced the rates of hepatic and extrahepatic deaths [1, 3,4,5,6,7,8]. In this chapter, it is aimed to give a brief objective approach to the epidemiology, pathogenesis, and treatment of HCV-associated EHMs.
10.2 Lymphoproliferative Disorders
10.2.1 Essential Mixed Cryoglobulinemia
Essential mixed cryoglobulinemia (EMC) is also called type II cryoglobulinemia. EMC can lead to the deposition of circulating immune complexes in small-to-medium-sized blood vessels. EMC vasculitis involves mainly the skin, the joints, the peripheral nervous system, and the kidneys [9]. Cryoglobulinemia is described by the presence of circulating immunoglobulins, which precipitate at cold temperatures and dissolve with rewarming. More than 90% of patients with EMC are infected with hepatitis C virus (HCV), and half of all patients with HCV have cryoglobulins. All patients with EMC should be tested for HCV infection [10]. The presentation of EMC is variable, ranging from mild disease (purpura, arthralgia) to fulminant disease (glomerulonephritis, extensive vasculitis). The main symptoms are asthenia, myalgia, purpura, arthralgia, peripheral neuropathy, and glomerulonephritis. EMC typically manifests as recurrent palpable purpura and peripheral neuropathy [9, 11].
Skin manifestations present in EMC include; palpable purpura, chronic cutaneous ulcers, Raynaud’s phenomenon, and acrocyanosis, which may evolve into digital ulcerations. Purpura often involves the lower legs and can leave brown spots on the skin after it resolves. Skin biopsy samples generally show cutaneous vasculitis with destruction of blood vessels, with a neutrophilic infiltration in and around the vessel wall. HCV-associated proteins have been observed in vasculitic skin biopsy samples. Vasculitis can lead to ischemic necrosis, skin ulceration, and necrosis of digits. Arthralgia is reported in 40–80% of HCV-infected patients with EMC and is bilateral, symmetrical, and nondeforming. Joint pains involve mainly the knees and hands, and less commonly the elbows and ankles. Rheumatoid factor (RF) is established in 70–80% of patients with EMC. Anti–cyclic citrullinated peptide (anti-CCP) antibodies are usually absent in patients with HCV. Neurological manifestations vary from sensory axonopathy to mononeuritis multiplex. Vasculitic lesions affecting the vasa nervorum manifest as peripheral neuropathy, which frequently affects lower-extremity peripheral nerves. The most frequent form is a distal sensory or sensory–motor polyneuropathy, presenting with painful, asymmetrical paresthesia. Multiple mononeuropathy may be seen rarely. The most common renal involvement is acute or chronic type I membranoproliferative glomerulonephritis with subendothelial deposits. The most common presentation is proteinuria with microscopic hematuria, a variable degree of renal insufficiency, and hypertension [12,13,14].
EMC is diagnosed on the basis of the history, clinical manifestations, and laboratory tests. Hypocomplementemia, especially low C4 and cryoglobulin levels, are detected in the laboratory. Elevations in the erythrocyte sedimentation rate and C-reactive protein levels can be present, as can normocytic anemia. A purpuric skin lesion biopsy shows leukocytoclastic vasculitis [15, 16].
Treatment of underlying HCV can suppress the manifestations of vasculitis. Achievement of a sustained virological response (SVR) with pegylated interferon plus ribavirin has been shown to improve HCV-associated EMC manifestations [17], but it has also been reported that sofosbuvir-based direct-acting antiviral regimens are more effective than pegylated interferon plus ribavirin [18, 19]. The main indication for immunosuppressive therapy such as rituximab is progressive systemic disease affecting the kidneys, nervous system, gastrointestinal tract, skin, or digits. A plasma exchange may be required in some patients. The prognosis is variable.
10.2.2 Lymphoma
A causative association between HCV and non-Hodgkin lymphoma (NHL) has been shown in recent studies. HCV monoinfection doubles the risk of developing NHL [20, 21]. The most common HCV-associated lymphoproliferative disorders include diffuse large B cell lymphoma, marginal-zone lymphoma, lymphoplasmacytic lymphoma, splenic lymphoma with villous lymphocytes, and extranodal marginal-zone B cell lymphoma of mucosa-associated lymphoid tissue, as well as primary hepatic lymphoma [20, 22,23,24].
Marginal-zone lymphoma appears to be the most frequently encountered low-grade B cell lymphoma in HCV patients. The risk of lymphoma may be related to cryoglobulinemia. Between 8% and 10% of patients with type II EMC develop B cell NHL after a long-term infection [25]. Unexplained anemia or development of lymphadenomegaly in patients with HCV and clinically active cryoglobulinemia should increase suspicion in terms of underlying lymphoproliferative disease. There is some evidence that HCV therapy decreases the risk of lymphoma, as shown in a small number of studies in which antiviral therapy resulted in regression of lymphoma [26, 27]. In a case report, follicular lymphoma remission was achieved in a patient whose HCV was successfully treated with direct-acting antiviral therapy [24].
HCV infection may increase the risk of hepatotoxicity associated with treatment for lymphoma. The presence of HCV infection was associated with a high incidence of severe hepatotoxicity (with a hazard ratio of 15) in patients with diffuse large B cell lymphoma who were treated with rituximab-containing chemotherapy regimens. Thus, hepatic function should be carefully monitored in HCV-positive patients receiving immunochemotherapy [28].
10.3 Dermatological Diseases
10.3.1 Porphyria Cutanea Tarda
Porphyrias are inherited or acquired metabolic disorders caused by reduced activity of enzymes in heme and porphyrin synthesis. Porphyria cutanea tarda (PCT), which is also called symptomatic porphyria, comprises a group of diseases resulting from an inherited (autosomal-dominant) or acquired deficiency of hepatic uroporphyrinogen decarboxylase enzyme (UROD). UROD is the fifth enzyme in the heme synthetic pathway and catalyzes the decarboxylation of uroporphyrinogen to coproporphyrinogen. Reduced activity of UROD causes a subsequent build-up of uroporphyrinogen in the blood and urine [29, 30].
There are two types of PCT. Type 1 is also called the acquired type and accounts for nearly 80% of all PCT cases. It occurs in predisposed individuals with deficient activity of the enzyme in the liver, which is triggered by exposure to liver toxins (hepatotoxic aromatic hydrocarbons, alcohol), drugs (estrogens), cigarette smoking, dialysis, and hepatopathic viruses, with HCV at the forefront [31]. Type 2 is an inherited form, and UROD mutation is inherited in an autosomal-dominant form. Type 2 accounts for nearly 20% of all PCT cases, and there is decreased enzymatic activity in all tissues [32, 33].
Clinical symptoms of PCT are observed when hepatic UROD activity falls below 20% of normal. Accumulation of porphyrinogens causes formation of uroporphyrin and hepatocarboxyl porphyrins, and the conversion continues with the help of different enzymes and modifications by intestinal bacteria. Ultimately, porphyrins are transported from the liver to the skin and lead to phototoxicity. These phototoxic porphyrins damage membranes, lipids, and proteins [34].
A strong association between the acquired form of PCT and HCV infection has been shown in several studies. Although there was marked geographic variability, a mean HCV prevalence of 50% was reported of patients with PCT. All patients with PCT should be screened for HCV infection and should have a comprehensive assessment of liver function, as well as assessment for other potential disease associations, including HIV infection, iron overload, and hemochromatosis (with homeostatic iron regulator (HFE) mutation testing). On the other hand, in patients with active HCV-related hepatic disease, routine testing for porphyrin metabolism is not recommended, as there is only a 5% reported prevalence of preclinical or overt PCT in the HCV-infected patient population. The possible mechanisms associating HCV with PCT have not been clearly identified; one possible mechanism may include HCV-induced production of reactive oxygen species, downregulating hepcidin and causing hepatic iron overload, rather than a direct effect on the enzymatic pathway. Iron overload (hepatic siderosis) is a critical pathogenetic event, disrupting the enzymatic activity of UROD by inducing the formation of an intracellular inhibitor, probably derived from hydroxymethylbilane and/or uroporphyrinogen [35,36,37,38].
The skin and liver are the two main sites affected by acquired PCT. Skin disease is characterized by photosensitivity and skin fragility, in which exposure to the sun and/or minor trauma can cause skin erythema and the development of vesicles and bullae that may become hemorrhagic. Hyperpigmentation, hypopigmentation, hirsutism, sclerodermatous changes, hypertrichosis, alopecia, and onycholysis may develop with the passage of time. Sun-exposed areas, such as the backs of the hands, forearms, face, neck, and feet, are more prone to photodamage. Skin lesions may be painful, and scarring of the lesions may progress to contractions and calcifications that resemble systemic scleroderma. Chronic liver disease is common in type 2 PCT. Liver biopsy shows a wide range of changes, including steatosis, mild to severe inflammation, hepatic fibrosis, and cirrhosis. It has also been reported that patients with PCT have an increased risk of developing hepatocellular carcinoma [39,40,41,42,43].
The diagnosis of PCT is typically suspected on clinical grounds and confirmed by the demonstration of markedly elevated urine uroporphyrine and hepatocarboxyl porphyrin levels in symptomatic patients. Another useful tool for diagnosis is the plasma porphyrin fluorescent assay, which has a characteristic peak at 620 nm [44].
The standard of care for PCT includes low-dose antimalarial hydroxychloroquine and phlebotomy—the latter is done to decrease hepatic iron stores. It is also important to avoiding precipitating factors such as sun exposure, alcohol consumption, estrogen use, iron supplementation, smoking, and exposure to polyhalogenated hydrocarbons [45]. In patients with HCV, antiviral therapy seems to heal cutaneous PCT lesions. However, PCT has been independently associated with an insufficient viral response to interferon-alpha treatment [46]. It has been shown that combination of interferon-alpha treatment with iron reduction is more beneficial in patients with HCV infection. New-onset PCT has been observed during treatment with interferon-alpha plus ribavirin [47]. Indeed, ribavirin is known to induce hemolytic anemia, which further aggravates liver iron excess and progression to clinically manifest PCT in predisposed individuals [48]. Finally, despite a lack of data, treatment of PCT with direct-acting antiviral therapy seems to be more effective than interferon-based regimens [49].
10.3.2 Lichen Planus
Lichen planus (LP) is an uncommon disease of the stratified squamous epithelium and is characterized by flat-topped, violaceous, pruritic papules with a generalized distribution. LP may affect the skin, oral cavity, genitalia, scalp, nails, and even the esophagus. It is seen in fewer than 1% of the general population [50]. LP can be seen in patients with particularly advanced liver disease. Although the range of anti-HCV antibodies in patients with LP is 10–40%, a relationship between HCV and LP has not been clearly shown [51]. However, a meta-analysis of 70 studies revealed that the presence of HCV may be used as a predictive marker of LP, because there was an increased risk of LP development in patients with HCV [52].
The frequency of LP in patients with HCV varies geographically. The prevalence of HCV in patients with LP has been reported to be 4% in Europe and 24% in the Middle East. Seroprevalence differences between geographic regions may depend on human leukocyte antigen (HLA) types and what the most common genotypes are in those regions [53]. Recommendations for HCV testing in patients with LP depend on the geographical area of the patient. LP can occur or be exacerbated during interferon-alpha treatment for chronic HCV infection [43]. Data on the effectiveness of interferon-free direct-acting antiviral treatment in patients with HCV and LP are not available.
10.3.3 Necrolytic Acral Erythema
Necrolytic acral erythema is a rare, pruritic, psoriasis-like skin disorder characterized by well-defined, erythematous to hyperpigmented pruritic plaques with variable scale and erosion on the acral surfaces. Necrolytic acral erythema is strongly associated with HCV, often being seen as an early cutaneous marker of this infection [54]. All patients with necrolytic acral erythema should be tested for HCV infection. The pathogenesis of necrolytic acral erythema is unknown, but it is thought to be related to zinc dysregulation, which can occur as a result of hepatitis C–induced metabolic alterations. Improvement in necrolytic acral erythema has been observed in patients treated with interferon-alpha and oral zinc sulfate [55, 56]. Topical and systemic corticosteroids have variable benefits.
10.3.4 Psoriasis
Psoriasis is a common, chronic, immune-mediated inflammatory disease, which affects the skin and/or the joints. Psoriasis manifests with well-demarcated erythematous plaques with silver scale. Like other immune-mediated disorders, psoriasis results from a complex interplay between genetic factors and environmental triggers. A few hospital-based clinical studies and observational studies have revealed an association between HCV infection and psoriasis. An increased risk of HCV infection among patients with moderate-to-severe psoriasis and psoriatic arthritis has been reported [57,58,59]. However, according to a population-based database study, psoriasis does not seem to be associated with an increased risk of HCV. A large case–control study has further supported the association between psoriasis and HCV infection. In the same study, there was a significant interaction with smoking—a risk factor common to both psoriasis and progression of HCV-related liver disease [60]. More prospective studies are needed to support the role of HCV in the pathogenesis of psoriasis and to establish whether psoriasis is a true extrahepatic manifestation (EHM) of HCV infection. However, in patients with psoriasis, HCV testing should be done before commencement of systemic immunosuppressive treatment that can cause HCV reactivation [61].
10.4 Autoimmune Disorders
10.4.1 Production of Autoantibodies
The prevalence of circulating autoantibodies is high in patients with chronic HCV infection, and 53% of HCV-infected patients have at least one immunological abnormality. The most common immunological abnormalities include mixed cryoglobulins (50–60%); RF activity (40%); and antinuclear (20–35%), anticardiolipin (10–15%), antithyroid (10%), and anti–smooth muscle antibodies (7%) [1, 62, 63]. The presence of autoantibodies has not been related to findings of a connective tissue disease, except for EMC. However, the presence of antinuclear antibodies is associated with more advanced liver fibrosis and lower serum HCV RNA levels in chronic HCV infection [64]. Possible reasons underlying the mechanism of autoantibody production include HCV-induced overactivation and proliferation of B lymphocytes.
10.4.2 Sjögren Syndrome/Sicca Symptoms
Sjögren syndrome is a systemic chronic inflammatory disorder characterized by lymphocytic infiltrates in exocrine organs. Most patients with Sjögren syndrome present with sicca symptoms such as xerophthalmia (dry eyes) and xerostomia (dry mouth). Sicca symptoms have been reported in 10–30% of HCV-infected patients. However, the prevalence of HCV-infected patients is lower than 5% [62]. In a recent review, the reported prevalence of Sjögren syndrome was 11.9% in patients with HCV (risk ratio 2.29), versus less than 1% in HCV-negative control subjects [65]. Testing for HCV infection is advised in patients with Sjögren syndrome.
10.4.3 Thyroid Disease
Thyroid disorders are found more commonly in patients with chronic HCV infection—particularly in women—than in the general population. About 13% of HCV-infected patents have hypothyroidism, and up to 25% have thyroid antibodies [66]. Interferon-alpha treatment may induce thyroid disease or unmask pre-existing silent thyroidopathies such as Graves disease or Hashimoto thyroiditis [67]. Thyroid function should be tested when a patient is first diagnosed with HCV and it should be monitored during interferon-based treatment.
10.4.4 Arthralgia/Myalgia
Arthralgia has been reported in 6–20% of HCV-infected patients. Arthralgia generally affects the fingers, knees, and back, and is bilateral and symmetrical [1, 68]. Synovitis is generally absent, and there is no evidence of joint destruction. Arthralgia is often seen in patients with EMC, and its presentation may mimic that of rheumatoid arthritis. High rates of RF positivity in HCV-infected patients may lead to misdiagnosis; however, anti-CCP antibody tests are negative in HCV-infected patients—a feature that is useful to differentiate the two diseases. Arthritis unrelated to EMC is rare, affecting small joints associated with carpal tunnel syndrome and palmar tenosynovitis. Myalgia is less frequent, affecting approximately 2–5% of HCV-infected patients [1, 68]. Interferon-alpha therapy, which is no longer used in HCV treatment, can cause arthralgia and myalgia.
10.5 Diabetes Mellitus
10.5.1 Insulin Resistance and Type II Diabetes Mellitus
Several studies have evaluated the associations between chronic HCV infection, insulin resistance (IR), and diabetes mellitus (DM), which have been linked. A meta-analysis of retrospective and prospective studies confirmed a higher risk of development of type II DM in patients with chronic HCV infection (odds ratio 1.68, 95% confidence interval 1.15–2.20) [69]. Some studies have identified risk factors for the development of DM in HCV-infected patients, such as older age, obesity, severe liver fibrosis, and a family history of DM [70]. HCV also increases the risk of developing DM after liver transplantation [71]. HCV has been linked to IR without overt DM [72, 73]. IR may contribute to hepatic fibrosis progression, particularly with HCV genotypes 1 and 4 and with high serum RNA levels [72, 73]. The pathomechanism of HCV-induced IR is yet not fully understood. Successful treatment of HCV may decrease the risk of DM. Achievement of a sustained virological response (SVR) with interferon-based therapy has been associated with a reduced incidence of DM [74, 75]. IR has been shown to decrease in patients who achieved an SVR but not in patients who failed to respond to treatment or relapsed [76].
10.6 Other Manifestations
10.6.1 Renal Disease
Glomerular disease may occur in patients with chronic HCV infection. The pathogenesis appears to be related to the deposition of immune complexes containing anti-HCV and HCV RNA in the glomeruli. Type I membranoproliferative glomerulonephritis associated with EMC is the most common form of renal disease related to HCV infection [77, 78]. The most common presentation is proteinuria with microscopic hematuria and variable degrees of renal insufficiency. The Kidney Disease Improving Global Outcomes (KDIGO) Group suggests that all patients with chronic renal disease should be tested for HCV [79].
10.6.2 Fatigue, Depression, Cognitive Impairment, and Impaired Quality of Life
Hepatitis C virus is associated with various neuropsychiatric disorders. The neurological manifestations of HCV include cognitive impairment, which can lead to brain fog and fatigue, markedly impair the quality of life, and increase the risks of cerebrovascular events and stroke. The exact pathophysiology of neuropsychiatric defects in HCV is not fully explained. The detection of HCV genetic sequences in postmortem brain tissue raises the possibility that the presence of HCV infection in the central nervous system may be related to the reported neuropsychiatric symptoms and cognitive impairment [80]. HCV may directly affect the central nervous system through alterations in serotonergic and dopaminergic transmission, with resultant depressive symptoms [81]. This mechanism may explain other central nervous system symptoms seen in HCV infection, such as fatigue and cognitive impairment [82].
Approximately 60% of HCV-infected patients suffer from sleep disorders, fatigue, and mood disorders. Cognitive impairment has also been described. It is a common symptom in persons with end-stage liver disease. HCV eradication leads to improved cognitive function and improved cerebral metabolism [82, 83]. Patients with SVR demonstrate significant improvements in verbal learning, memory, and visuospatial memory.
Fatigue is one of the most frequent and disabling complaints in patients with HCV (occurring in 50–70%) and an independent predictor of poor health-related quality of life (HRQoL) [84]. Fatigue has been independently associated with female sex, age over 50 years, cirrhosis, and depression. Chronic fatigue is associated with poor sleep quality and increased nocturnal activity in patients with HCV [85].
Before commencement of antiviral treatment, patients with HCV have a lower HRQoL than control subjects [86]. HRQoL worsens with more advanced liver disease and therapy, leading to a reduction in adherence [87]. Viral eradication correlates positively with improvements in HRQoL. Achievement of SVR after 12 weeks of follow-up treatment with sofosbuvir and ribavirin has been associated with improvements in HRQoL [88].
10.6.3 Cardiovascular Disease
Chronic HCV infection can trigger cardiovascular disease and has been associated with increased accelerated atherosclerosis [89]. The prevalence rates of carotid artery plaques and carotid intima–media thickening have been shown to be four times higher in HCV-infected patients than in control subjects [90]. Increased cardiovascular mortality (1.5–25 times higher), as well as higher incidence rates of cerebrovascular disease and acute coronary syndromes, have been noted in HCV-seropositive patients [91]. In addition, an increased rate of peripheral arterial disease in patients with chronic HCV infection has been revealed. The pathogenesis associating HCV and acceleration of atherosclerosis has not been fully elucidated. However, HCV may induce production of proatherogenic cytokines. Rates of cardiovascular events such as acute coronary syndrome and ischemic stroke have been shown to be significantly lower in patients treated with pegylated interferon plus ribavirin than in untreated patients [3]. Although an association between HCV and increased cardiovascular risk has been found in some studies, a correlation between them has not been clearly established [92, 93]. Atherosclerosis in patients with HCV is probably a result of the aforementioned insulin resistance, metabolic disturbance, and proinflammatory cytokine action.
Abbreviations
- Anti-CCP:
-
Anti–cyclic citrullinated peptide
- DM:
-
Diabetes mellitus
- EHM:
-
Extrahepatic manifestation
- EMC:
-
Essential mixed cryoglobulinemia
- HCV:
-
Hepatitis C virus
- HFE:
-
Homeostatic iron regulator
- HLA:
-
Human leukocyte antigen
- HRQoL:
-
Health-related quality of life
- IR:
-
Insulin resistance
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- LP:
-
Lichen planus
- NHL:
-
Non-Hodgkin lymphoma
- PCT:
-
Porphyria cutanea tarda
- RF:
-
Rheumatoid factor
- SVR:
-
Sustained virological response
- UROD:
-
Uroporphyrinogen decarboxylase
References
Cacoub P, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment virus C. Arthritis Rheum. 1999;42(10):2204–12.
El-Serag HB, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36(6):1439–45.
Adinolfi LE, et al. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20(13):3410–7.
Zignego AL, et al. Extrahepatic manifestations of hepatitis C virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39(1):2–17.
Lee MH, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206(4):469–77.
Omland LH, et al. Increased mortality among persons infected with hepatitis C virus. Clin Gastroenterol Hepatol. 2011;9(1):71–8.
Uto H, et al. Increased rate of death related to presence of viremia among hepatitis C virus antibody–positive subjects in a community-based cohort study. Hepatology. 2009;50(2):393–9.
Hsu YC, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59(4):1293–302.
Cacoub P, et al. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis. 2016;3(1):3–14.
Lunel F, et al. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterology. 1994;106(5):1291–300.
Terrier B, Cacoub P. Renal involvement in HCV-related vasculitis. Clin Res Hepatol Gastroenterol. 2013;37(4):334–9.
Cacoub P, et al. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. 2014;46(Suppl 5):S165–73.
Ferri C, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33(6):355–74.
Cacoub P, et al. Rheumatologic manifestations of hepatitis C virus infection. Clin Liver Dis. 2017;21(3):455–64.
Sene D, et al. Longterm course of mixed cryoglobulinemia in patients infected with hepatitis C virus. J Rheumatol. 2004;31(11):2199–206.
Pietrogrande M, et al. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus–infected patients. Autoimmun Rev. 2011;10(8):444–54.
Saadoun D, et al. Antiviral therapy for hepatitis C virus–associated mixed cryoglobulinemia vasculitis: a long-term followup study. Arthritis Rheum. 2006;54(11):3696–706.
Sise ME, et al. Treatment of hepatitis C virus–associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology. 2016;63(2):408–17.
Emery JS, et al. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am J Gastroenterol. 2017;112(8):1298–308.
Giordano TP, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297(18):2010–7.
Duberg AS, et al. Non-Hodgkin’s lymphoma and other nonhepatic malignancies in Swedish patients with hepatitis C virus infection. Hepatology. 2005;41(3):652–9.
Tursi A, et al. Detection of HCV RNA in gastric mucosa–associated lymphoid tissue by in situ hybridization: evidence of a new extrahepatic localization of HCV with increased risk of gastric malt lymphoma. Am J Gastroenterol. 2002;97(7):1802–6.
Gisbert JP, et al. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125(6):1723–32.
Hermine O, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347(2):89–94.
Silvestri F, et al. Hepatitis C virus infection among cryoglobulinemic and non-cryoglobulinemic B-cell non-Hodgkin’s lymphomas. Haematologica. 1997;82(3):314–7.
Kawamura Y, et al. Viral elimination reduces incidence of malignant lymphoma in patients with hepatitis C. Am J Med. 2007;120(12):1034–41.
Maciocia N, O’Brien A, Ardeshna K. Remission of follicular lymphoma after treatment for hepatitis C virus infection. N Engl J Med. 2016;375(17):1699–701.
Ennishi D, et al. Hepatic toxicity and prognosis in hepatitis C virus–infected patients with diffuse large B-cell lymphoma treated with rituximab-containing chemotherapy regimens: a Japanese multicenter analysis. Blood. 2010;116(24):5119–25.
Deybach JC, et al. European Porphyria Initiative (EPI): a platform to develop a common approach to the management of porphyrias and to promote research in the field. Physiol Res. 2006;55(Suppl 2):S67–73.
Alla V, Bonkovsky HL. Iron in nonhemochromatotic liver disorders. Semin Liver Dis. 2005;25(4):461–72.
Elder GH. Update on enzyme and molecular defects in porphyria. Photodermatol Photoimmunol Photomed. 1998;14(2):66–9.
Liu LU, Phillips J, Bonkovsky H. Familial porphyria cutanea tarda. In: Adam MP, et al., editors. GeneReviews®. Seattle: University of Washington; 1993.
Frank J, Poblete-Gutierrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24(5):735–45.
Besur S, et al. Clinically important features of porphyrin and heme metabolism and the porphyrias. Meta. 2014;4(4):977–1006.
Bonkovsky HL, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27(6):1661–9.
Garcovich S, et al. Cutaneous manifestations of hepatitis C in the era of new antiviral agents. World J Hepatol. 2015;7(27):2740–8.
Nishina S, et al. Hepatitis C virus–induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134(1):226–38.
Gisbert JP, et al. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39(4):620–7.
Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26(3):225–32.
Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375(9718):924–37.
Schulenburg-Brand D, et al. The cutaneous porphyrias. Dermatol Clin. 2014;32(3):369–84, ix.
Linet MS, et al. Primary liver cancer, other malignancies, and mortality risks following porphyria: a cohort study in Denmark and Sweden. Am J Epidemiol. 1999;149(11):1010–5.
Sayiner M, et al. Dermatologic manifestations of chronic hepatitis C infection. Clin Liver Dis. 2017;21(3):555–64.
Szlendak U, Bykowska K, Lipniacka A. Clinical, biochemical and molecular characteristics of the main types of porphyria. Adv Clin Exp Med. 2016;25(2):361–8.
Balwani M, Desnick RJ. The porphyrias: advances in diagnosis and treatment. Hematol Am Soc Hematol Educ Program. 2012;2012:19–27.
Fernandez I, et al. Porphyria cutanea tarda as a predictor of poor response to interferon alfa therapy in chronic hepatitis C. Scand J Gastroenterol. 2003;38(3):314–9.
Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913–5.
Desai TK, et al. Phlebotomy improves therapeutic response to interferon in patients with chronic hepatitis C: a meta-analysis of six prospective randomized controlled trials. Dig Dis Sci. 2008;53(3):815–22.
Aguilera P, Laguno M, To-Figueras J. Treatment of chronic hepatitis with boceprevir leads to remission of porphyria cutanea tarda. Br J Dermatol. 2014;171(6):1595–6.
Le Cleach L, Chosidow O. Clinical practice: lichen planus. N Engl J Med. 2012;366(8):723–32.
Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995;123(8):615–20.
Shengyuan L, et al. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol. 2009;145(9):1040–7.
Nagao Y, et al. Genome-wide association study identifies risk variants for lichen planus in patients with hepatitis C virus infection. Clin Gastroenterol Hepatol. 2017;15(6):937–44. e5
Abdallah MA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53(2):247–51.
Khanna VJ, et al. Necrolytic acral erythema associated with hepatitis C: effective treatment with interferon alfa and zinc. Arch Dermatol. 2000;136(6):755–7.
Hivnor CM, et al. Necrolytic acral erythema: response to combination therapy with interferon and ribavirin. J Am Acad Dermatol. 2004;50(5 Suppl):S121–4.
Tsai TF, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63(1):40–6.
Imafuku S, Naito R, Nakayama J. Possible association of hepatitis C virus infection with late-onset psoriasis: a hospital-based observational study. J Dermatol. 2013;40(10):813–8.
Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. Br J Dermatol. 2011;165(5):1037–43.
Cohen AD, et al. Psoriasis associated with hepatitis C but not with hepatitis B. Dermatology. 2010;220(3):218–22.
Snast I, et al. Risk for hepatitis B and C virus reactivation in patients with psoriasis on biologic therapies: a retrospective cohort study and systematic review of the literature. J Am Acad Dermatol. 2017;77(1):88–97.e5.
Cacoub P, et al. Extrahepatic manifestations associated with hepatitis C virus infection: a prospective multicenter study of 321 patients. The GERMIVIC. Groupe d’Etude et de Recherche en Medecine Interne et Maladies Infectieuses sur le Virus de l’Hepatite C. Medicine (Baltimore). 2000;79(1):47–56.
Himoto T, Masaki T. Extrahepatic manifestations and autoantibodies in patients with hepatitis C virus infection. Clin Dev Immunol. 2012;2012:871401.
Hsieh MY, et al. Antinuclear antibody is associated with a more advanced fibrosis and lower RNA levels of hepatitis C virus in patients with chronic hepatitis C. J Clin Pathol. 2008;61(3):333–7.
Younossi Z, et al. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599–608.
Antonelli A, et al. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117(1):10–3.
Prummel MF, Laurberg P. Interferon-alpha and autoimmune thyroid disease. Thyroid. 2003;13(6):547–51.
Mohammed RH, et al. Prevalence of rheumatologic manifestations of chronic hepatitis C virus infection among Egyptians. Clin Rheumatol. 2010;29(12):1373–80.
White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49(5):831–44.
Petit JM, et al. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J Hepatol. 2001;35(2):279–83.
Bigam DL, et al. Hepatitis C–related cirrhosis: a predictor of diabetes after liver transplantation. Hepatology. 2000;32(1):87–90.
Moucari R, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134(2):416–23.
Milner KL, et al. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology. 2010;138(3):932–41.e1–3.
Romero-Gomez M, et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48(5):721–7.
Arase Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49(3):739–44.
Conjeevaram HS, et al. Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology. 2011;140(2):469–77.
Johnson RJ, et al. Hepatitis C virus–associated glomerulonephritis: effect of alpha-interferon therapy. Kidney Int. 1994;46(6):1700–4.
McGuire BM, et al. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med. 2006;144(10):735–41.
Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;109:S1–99.
Morsica G, et al. Detection of hepatitis C virus genomic sequences in the cerebrospinal fluid of HIV-infected patients. J Med Virol. 1997;53(3):252–4.
Cozzi A, et al. Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J Viral Hepat. 2006;13(6):402–8.
Byrnes V, et al. Effects of anti-viral therapy and HCV clearance on cerebral metabolism and cognition. J Hepatol. 2012;56(3):549–56.
Thein HH, et al. Improved cognitive function as a consequence of hepatitis C virus treatment. HIV Med. 2007;8(8):520–8.
Kallman J, et al. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci. 2007;52(10):2531–9.
Heeren M, et al. Active at night, sleepy all day—sleep disturbances in patients with hepatitis C virus infection. J Hepatol. 2014;60(4):732–40.
Bonkovsky HL, et al. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46(3):420–31.
Marcellin P, et al. Adherence to treatment and quality of life during hepatitis C therapy: a prospective, real-life, observational study. Liver Int. 2011;31(4):516–24.
Younossi ZM, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH-C). J Hepatol. 2014;60(4):741–7.
Fukui M, et al. Hepatitis C virus and atherosclerosis in patients with type 2 diabetes. JAMA. 2003;289(10):1245–6.
Domont F, Cacoub P. Chronic hepatitis C virus infection, a new cardiovascular risk factor? Liver Int. 2016;36(5):621–7.
Gill K, et al. Hepatitis C virus as a systemic disease: reaching beyond the liver. Hepatol Int. 2016;10(3):415–23.
Hsu YC, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64(3):495–503.
Wong RJ, et al. Hepatitis C virus infection and coronary artery disease risk: a systematic review of the literature. Dig Dis Sci. 2014;59(7):1586–93.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Karaca, Ç. (2019). Extrahepatic Manifestations of Hepatitis C Virus Infection. In: Ozaras, R., Salmon-Ceron, D. (eds) Viral Hepatitis: Chronic Hepatitis C. Springer, Cham. https://doi.org/10.1007/978-3-030-03757-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-03757-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03756-7
Online ISBN: 978-3-030-03757-4
eBook Packages: MedicineMedicine (R0)