Abstract
Organic–inorganic hybrid materials, prepared via chemical synthesis or physical blending of functionalized nanofillers within polymer matrix, have gained an increased attention in the recent years. Polyhedral oligomeric silsesquioxane (POSS) nanoparticles, due to their nanometer size and functionalization possibilities, are applied as effective modifiers—both chemical and physical, for polymer matrices, including polyurethanes (PU). Research efforts focused on polymers incorporating polyhedral oligomeric silsesquioxane (POSS) have intensified in recent years, revealing new synthetic routes and interesting features of these composite materials. This chapter describes polyurethane/POSS systems with different architectures—with POSS molecules as pendant groups in the polyurethane chain, incorporated in the main chain and as cross-linking agents. The methods of incorporation of POSS into polymer matrices via covalent bonds or physical blending have been presented, and the influence of preparation conditions on the structure and properties of nanocomposites was discussed. Application fields, such as gas membranes or biomedical implants, have been outlined.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Polyurethanes (PU) are an important group of polymers with a wide range of applications in industry and technology. They are obtained by polyaddition reactions of isocyanates with compounds containing reactive hydrogen atoms, such as alcohols or amines. The group of polyurethane materials includes foams, varnishes, adhesives, fibers, and elastomers [1, 2].
Elastomers are characterized by a large modulus of longitudinal elasticity, tearing module, compressibility, very high resistance to scratches and attrition, and excellent elastic properties. They are resistant to aging, weathering, typical solvents, and oils. They can be manufactured in a very wide range of hardness, but—in contrast to hydrocarbon rubber—remain elastomers even at high hardness. Considering the processing, polyurethane elastomers can be divided into three main groups—cast, rolled, and thermoplast elastomers. Cast polyurethane elastomers are obtained with a ratio of isocyanate to hydroxyl and possibly amino groups nearing to stoichiometric. Because this provides the highest molecular weight of linear polyurethane, their physical properties are best among all polyurethanes [3].
Linear polyurethanes are segmented polymers consisting of rigid and flexible segments. There is a close relationship between the properties of segment polyurethanes and their chemical structure and physical structure. Rigid segments are formed by the reaction of isocyanate groups with chain extenders and consist of isocyanate residues, extenders, urethane, and possibly urea groups. On the other hand, the flexible segments are built from polyol chains and consist of methylene and ether groups or ester groups. Rigid segments are joined together via polyol flexible segments. The polyurethanes therefore resemble block copolymers with a structure of the type (AB)n, which are constructed from alternating repetitive rigid and flexible blocks (Fig. 5.1). This type of segment construction is a key feature distinguishing polyurethane elastomers from other such materials and is the main reason for the favorable mechanical properties of these materials in a wide range of temperatures [4].
Segmental construction of polyurethanes; adopted from [5]
The polar and hard-to-melt rigid segments interact strongly with each other, in particular through hydrogen bonds between the NH groups and the oxygen atoms of the ether or ester group and usually do not mix homogeneously at temperatures below 120 °C with less polar, easily meltable flexible segments. This leads to their separation and formation of the so-called rigid domains, resulting in polyurethane becoming diphase and microheterogeneous. In polyurethanes with 10% of rigid segments, phase separation takes place before the start of the reaction. If the polymer is obtained below the miscibility temperature with the chain extender—glycol, the resulting macromolecules show a higher degree of self-association than the polyurethane obtained in a homogeneous system or solution. In rigid domains, there is physical cross-linking; thus, they fulfill a function similar to the function of the polymer reinforcing filler. At room temperature, almost all NH groups in polyurethanes are bound by hydrogen bonds, therefrom only 30–60% with carbonyl groups of urethane groups. Rigid domains are the phase of ordered rigid segments, permeating with the phase (usually continuous) of flexible segments [3]. Even in the case of very good phase separation, interfacial areas can be distinguished which at the border between soft and hard domains. These areas have intermediate properties between flexible and rigid properties, and their dimensions are a measure of the degree of phase separation [1].
Due to stronger interaction with the urethane groups of segments of rigid ester groups of oligoester chains than with ether linkages of oligoether chains, the microphase separation takes place to a greater extent in polyetherurethanes than in polyesterurethanes. Flexible segments in the non-deformed state are distributed statistically, as a result of which the soft domains are isotropic and amorphous. The flexible segments are arranged approximately perpendicular to the longitudinal axis of the hard domains, which makes these domains locally anisotropic. Because rigid domains are also statistically arranged, the polymer as a whole exhibits mechanical isotropy and optical isotropy.
Rigid polyesterurethane domains are 3–10 nm in size and polyetherurethane 5–10 nm and consist of 2–4 repeating segments. Rigid domains both of polyether and polyesterurethane have a fringed-layered structure with a thickness equal to the length of the rigid segment, the average distance between the centers 10–25 nm and the width below several dozen nanometers. Such domains act as cross-linkers bond; they limit chain relaxation and allow crystallization of the segments after application of stress, thereby increasing the tensile strength of the polymer and its thermal resistance. In many polyurethane systems, in addition to the microphase structure, the oriented domains also form a super spherulitic structure. The spherulite radius is proportional to the size of the rigid segments, and the bigger is the radius, the larger the phase separation. In the center of the spherulite, the content of rigid segments is very large and decreases in the radial direction. The spherulites in polyetherurethanes made of 4,4′-methylenediphenyl diisocyanate (MDI) have a diameter of 1–10 μm and an open fiber structure [3].
The occurrence of a two-phase domain structure causes high strength of polyurethanes. The presence of resistant to sticky flow of rigid domains in which the rigid segments strongly interact with each other, in particular the formation of hydrogen bonds, reduces the intensity of the stress line due to domains deflection, forking of their cracks and their plastic deformation. The domains of the flexible segments additionally strengthen the polymer as a result of viscoelastic dissipation of energy in the immediate vicinity of the fractured blade, and thanks to the progressive ordering and crystallization during deformation. With the increase in the content of rigid segments, the degree of phase separation increases and the mechanical properties improve. The content and structure of flexible segments also significantly affect the properties of polyurethanes. Polyestroles provide excellent mechanical properties, while polyetheroles have better microbial resistance and hydrolysis. On the other hand, increasing the molecular weight of the elastic segment causes increase in the glass transition temperature of the obtained polyurethanes. Polyethers have poorer miscibility with MDI than polyesters; the elastomers obtained from them show a higher degree of phase separation, moreover the rigid domains forming in them are larger and their structure is more complex than in the case of polyestroles. The phase separation tendency also increases as the hydrocarbon chain length increases in polyetheroles and polyestroles due to the decreasing polarity of the polyol [4, 6].
As commonly used diisocyanates—(MDI) and toluene 2,4-diisocyanate (TDI) are reported to exert harmful effects if they enter the body through inhalation, skin (open wounds) or eye contact [7], and their production process is based on the reaction of toxic phosgene with amines, alternative synthetic routes have been proposed that do not require the use of diisocyanates. Three approaches have been applied for the synthesis of non-isocyanate PU (NIPU)—by step-growth polyaddition, polycondensation, and ring-opening polymerization. From industrial perspective, synthesis of polyurethanes in the reaction of multifunctional cyclic carbonates with aliphatic primary diamines or polyamines proceeding via step-growth polyaddition is the most viable option [8]. In the polyaddition reaction, two isomers are formed, one with a secondary β-hydroxyl group and the other possessing a methylol group, which form intramolecular hydrogen bonds with the urethane group—Fig. 5.2.
Reaction of cyclic carbonate with amine; adopted from [9]
NIPU show relatively low sensitivity to moisture in the surrounding environment, and the hydroxyl groups formed at the β-carbon atom of the urethane group cause an increase in adhesion properties. The presence of intra and intermolecular hydrogen bonds [10] as well as the lack of unstable biuret and allophanate units [11] lead to an improved thermal stability and hydrolytic stability/chemical resistance of NIPU to nonpolar solvents, as compared to conventional PU.
The properties of polyurethanes can be changed by using different types of fillers that do not mix homogeneously with the polyurethane components. Classic powder or fibrous fillers increase the hardness and improve the mechanical properties of polyurethanes; however, they significantly increase the density of the material. In recent years, polyurethane/nanofiller composites have been extensively studied in which the particles of the reinforcing phase have nanometric dimensions. At this length scale, significant improvements of some polymer properties with a relatively low content of the nanofiller can occur. As nanoadditives metal nanoparticles, organophilized layered silicates, including montmorillonite, zinc oxide, aluminum oxide, titanium oxide, silica or carbon nanotubes have been applied [12,13,14,15,16].
A new and promising group of materials are hybrid polyurethane/functionalized polyhedral oligomeric silsesquioxane (POSS) materials, which, due to the possibility of chemical bonding of nanoparticles with organic matrix, show improved properties compared to classical polymer/nanofiller systems.
The dimensions of POSS molecules do not exceed a few nm; therefore, they are classified as 0-D nanofillers with a spherical structure. Many POSS derivatives containing reactive functional groups attached to corners of Si-O cage have already been synthesized. The presence of such groups makes POSS capable of reacting with traditionally used monomers. Materials created in this way can be included in the group of (nano) hybrid materials in which oligosilsesquioxane molecules are covalently bound to organic polymers. POSS derivatives containing hydroxyl, amino, or isocyanate groups can be used for chemical modification of all kinds of polyurethane materials, such as elastomeric, foamed, and coating materials [17].
The number and functionality of the substituents attached to the POSS cage determine the way of incorporation of these compounds into the PUR structure as:
-
a side group of the main chain or end group terminating the polymer chain (monofunctional POSS),
-
a main chain fragment (di-functional POSS), or
-
a network node (multifunctional POSS).
For the synthesis of hybrid polyurethanes, numerous POSS have been used, including POSS monofunctional compounds with isocyanate or amino substituents, POSS compounds with one dihydroxyalkyl and dihydroxyaryl reactive group, di-functional POSS compounds with two hydroxyl substituents, and multifunctional POSS with a partially caged structure or a fully condensed cage with isocyanate, hydroxyl, or amino substituents [17, 18].
2 Chemical Modifications
POSS molecules can be incorporated in the polymer matrix by copolymerization, grafting, or reactive blending. Numerous possibilities of chemical decoration of POSS molecules with organic substituents open up new perspectives for synthesis of organic–inorganic polyurethane hybrid materials in which silsesquioxanes can be incorporated in the macrochains as pendant groups, network nodes, or fragments of the main chain. This gives the opportunity to design novel materials with a broad spectrum of known and yet unknown properties [19]. Therefore, for several years, a significant increase in the use of POSS compounds as an inorganic nanoadditive in polymeric materials has been observed.
Polyhedral silsesquioxanes can be incorporated into the polymer chain chemically by several methods [18, 20,21,22,23]:
-
copolymerization of organic monomers with POSS compounds having at least two reactive functional groups—hybrid polymers are obtained with covalently bound POSS nanoparticles as a side group of the macrochain (Fig. 5.3a), a polymer backbone fragment (Fig. 5.3b), or a polymer network node (Fig. 5.3c),
-
chemical modification of polymers by grafting them with POSS molecules with one reactive group—systems with a covalently bond POSS molecule constituting a side group of the main chain of macromolecules or telechelic polymers terminated with POSS molecules are obtained.
2.1 POSS Molecules as Pendant Groups in the Polyurethane Chain
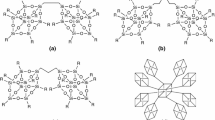
One of the first papers dealing with the subject of polyurethane hybrid materials, containing POSS compounds in their structure, were prepared by Schwab et al. [24, 25] Diol (bisphenol A—BPA)-POSS was used as a chain extender, the isocyanate component was 4,4′-diphenylmethane diisocyanate (MDI), and soft segments were composed of flexible polyoxytetramethylene diol (PTMG) with an average molecular weight of 2000. The catalyst for the reaction between isocyanate and hydroxyl groups was dibutyltin dilaurate (DBTDL). Later, these studies were continued by Fu et al. who determined the influence of BPA-POSS on the structure and properties of polyurethane elastomers—Fig. 5.4 [26, 27].
Schematic diagram of the synthesis for POSS-polyurethane: a octacyclohexyl-POSS, with R = cyclohexyl; b hydrido-POSS; c BPA-POSS; and d POSS-PU [27]
The synthesized materials contained 21 and 34 wt% of BPA-POSS. Structural investigations using the WAXD and SAXS methods revealed that BPA-POSS creates crystallites in the polymer, whose presence is manifested by clear reflections on WAXD patterns. The formation of crystallites is most likely induced by polyurethane phase separation of rigid and elastic domains, which was confirmed by SAXS studies. The value of the great period was 111 Å for hybrids with 34 wt% of POSS and 164 Å for materials containing 21 wt% of POSS. The size of rigid domains determined by the SAXS method was 34 Å for both BPA-POSS loads. When polyurethane/POSS elastomers were subjected to uniaxial deformation, enhanced tensile modulus and strength of the hybrid polymers were recorded. TEM observations on a stretched and relaxed PUR/POSS materials indicated that tensile stretching broke the large hard segment domains into disk-like smaller domains with plane normals almost parallel to the stretching direction, as shown in Fig. 5.5.
Schematic diagram of microphase changes during stretching: a no deformation or low deformation (POSS molecules form nanocrystals in the hard segment domains); b intermediate deformation (e.g., 400%) (some POSS crystals are destroyed in the hard segment domains); and c large deformation after relaxation (e.g., 700%) as in TEM [27]
Interesting research results were presented in the work of Hoflund and coworkers [28] on the resistance of PUR/POSS coatings used in space satellites for erosion caused by atomic oxygen. Polyurethane nanohybrid systems were prepared using MDI, polytetramethylene diol as an elastic component, and 1,4-butanediol and trimethylpropane substituted with a group containing heptacyclopentyl-POSS (TMP-POSS). XPS surface tests revealed that in the samples exposed to the oxygen atom stream for 63 h, the carbon content on their surface was reduced from 72.5 to 37.8%, while the concentration of oxygen and silicon on the surface increased with prolonged exposure time, from the initial value of 2.28–2.93%. The obtained data suggest that atomic oxygen destroys cyclopentyl rings in POSS-TMP, forming CO and/or CO2 and H2O that desorb from the material surface. Extended exposure time results in the formation of a silica layer on the surface of the sample, which favorably protects the inner polymer layers against the influence of atomic oxygen.
POSS nanohybrid polyurethanes for coatings, made of dihydroxy isobutyl-POSS (R = isobutyl), isophorone diisocyanate, dimethylolpropionic acid, and ethylenediamine as a chain extender, have been prepared by Turri [29, 30]. POSS particles easily integrated into the polyurethane structure, and the resulting materials formed stable aqueous dispersions if the POSS content did not exceed 10%. X-ray analysis showed the presence of crystallites in the obtained coatings even at low POSS content. The results of dynamic mechanical analysis show a reinforcing effect, mainly in the case of polytetramethylene diol with a molecular weight of 2000. In addition, polyurethanes with built-in POSS were characterized by lower surface wettability and lower value of the polar surface energy component than pure polymer, even with relatively low silsesquioxane content. This effect may be due to both a stratification of apolar components of the coating close to polymer–air interface and a topographical change of the surface due to formation of nanosized structures.
The phenomenon of wetting the surface of polyurethane composites with 1-(2,3-propanediol)propoxy-3,5,7,9,11,13,15-heptaisobutylpentacyclo-POSS (PHI-POSS)was studied by Zhang et al. [31]. It was shown that the contact angle values increase with the increase of silsesquioxane content, whereas free surface energy decreases more than twice for a sample containing 8.5 wt% of PHI-POSS in oligoestrodiol, compared to unmodified polyurethane. As AFM studies showed, the average surface roughness (Ra) increased with the increase of silsesquioxane content; however, Ra values were much lower than 100 nm, and thus the impact of roughness on the contact angle value was insignificant. As the reason for the reduction of surface energy of the materials studied, migration of the polyester segments toward the polimer surface was named. This effect intensifies after incorporation of silsesquioxane containing alkyl substituents which strengthen the migration effect of the elastic segments; it leads to a decrease in free energy and to an increase in the contact angle values. WAXD results showed that for lower PHI-POSS contents, the silsesquioxane nanoparticles are well dispersed in the polymer matrix, which limits the tendency to form crystallites. However, for hybrids containing 8.5 wt% of silsesquioxane, PHI-POSS forms crystallites, and there is a tendency for phase separation between the oligoester segments and silsesquioxane moieties. Besides, stiff POSS cages hinder crystallization of oligoestrodiol segments. The DMA analysis revealed an increase in the glass transition temperature of the elastic segments phase with an increase in silsesquioxane content, most likely due to limiting the mobility of oligoester chains by large silsesquioxane side groups. Thermal stability (decomposition onset temperature determined by TGA) under inert gas atmosphere was increasing with an increase of POSS content.
Surface roughness tests of coatings obtained from urethane cationomers synthesized from oligooxypropylene diol and modified PHI-POSS showed that at even a relatively small (1%) PHI-POSS content the roughness increases, as revealed by confocal laser microscopy results [32, 33]. Thermal and rheological properties of poly (urethane–urea) ionomers containing 3-(2-aminoethyl)amino)propylheptaisobutyl-POSS were discussed by Madbouly et al. [34, 35]. It was observed that the dependence of the imaginary component of the stiffness modulus versus the vibration frequency deviates from the straight line at 140 and 160 °C for a pristine polymer and polymer containing POSS, respectively. Such deviation indicates that above these temperatures the Williams–Landel–Ferry equation ceases to describe their viscoelastic properties due to the phase separation phenomena of rigid and elastic segments. The increase of this temperature value for nanohybrid polyurethane, as compared to the unmodified polyurethane, demonstrates that POSS has increased the miscibility between rigid and flexible segments, which leads to a material with a relatively higher homogeneity at elevated temperature. The increase in miscibility has also been confirmed by TEM microscopy—Fig. 5.6.
TEM photographs for pure PU and PU/POSS composite (10 wt% POSS). The dark particles represent the hard segments, and the bright matrix represents the polyester soft segments. The sample of 10 wt% POSS shows finer morphology than that of pure PU [34]
Thermogravimetric analysis of the obtained hybrid ionomers under inert atmosphere showed the beginning of the decomposition process at a temperature of 270 °C, regardless of the content of POSS in the polymer. The authors linked the first stage of thermal degradation of the material with the distribution of elastic segments, whereby in the second stage starting at ca. 350 °C, the rigid segments are decomposed. These results indicate that the presence of silsesquioxane in polyurethane does not significantly increase the polymer resistance to thermodegradation under inert conditions. In the oxidizing atmosphere, nanohybrid polyurethane is more stable at high temperatures compared to the unmodified polymer, and there is more solid (char) residue after decomposition.
Hybrid coatings based on polyurethanes/polyhedral oligomeric silsesquioxanes were investigated by Lai et al. [36]. Hydroxy-terminated polybutadiene (HTPB), isophorone diisocyanate (IPDI), and trans-cyclohexane diolisobutyl-POSS were used as precursors for the hybrid coatings which were spin-coated to form films. Electrochemical characteristics showed that the PU/POSS hybrids offer very good corrosion protection. In comparison with the value for the untreated aluminum alloy (AA) (2.48–8.22×10−3 Acm−2), the measured corrosion electric current (Icorr) value decreased significantly for the PU/POSS hybrids on the AA (1.24×10−6–7.61×10−8 Acm−2) and was lower than that of the PU film on AA (1.32–1.37×10−5 Acm−2). The result obtained can be explained by the formation of denser hybrid films that were less susceptible to localized pitting.
Hu et al. [37] synthesized POSS molecule with two functional amino groups—3-(2-aminoethylamino)propylheptaphenylPOSS (AA-POSS). Incorporation of AA-POSS to polyurethane matrix causes changes in water uptake and contact angle. With an increase of AA-POSS content, the lower water uptake was observed from 13.6 to 3.2 wt% for pristine PU and PU/POSS (4 wt%), respectively. It was postulated that POSS migrates to the surface, thus preventing the solvent molecules to penetrate into the interior, as well as POSS molecules, as highly hydrophobic, lower the polymer surface–water interactions. DSC data showed increase of Tg from 75 °C (for reference PU) to 99 °C for PU containing 12 wt% of silsesquioxane; chemical incorporation of rigid POSS moieties effectively hinders the macrochains motions. The enhanced thermal stability of AA-POSS-modified polyurethanes, as found by TGA, could be caused by suppressing the molecular mobility of polyurethane chains by bulky POSS substituents which provide additional heat capacity, thereby stabilizing materials during the thermal degradation process.
Nanohybrid-segmented polyurethanes were obtained through solvent-free polymerization in mass by Janowski et al. [38]. 1-(1-(2,3-dihydroxypropoxy) butyl)-3,5,7,9,11,15-isobutyl-pentacyclo [9.5.1.1.(3.9).1(5.5).1(7,13)] octasiloxane (PHI-POSS), 4,4′-diphenylmethane diisocyanate (MDI), 1,4-butanediol (chain extender), and polyoxytetramethylene diol (PTMG) with an average molecular weight of 1000, 1400, and 2000 (soft segment) were used. Schematic route of polyurethane-/POSS-segmented elastomers synthesis is shown in Fig. 5.7.
Schematic presentation of the synthesis route for PU-/POSS-segmented elastomers [38]
Thermo (oxidative) stability of novel PU/POSS nanohybrids was investigated by thermogravimetry (TG) [39]—the highest thermal stability, both of inert and oxidative atmosphere, have the PU containing 4 and 6 wt% of PHI-POSS. Temperatures of maximum rate of degradation, defined as the first maximum in DTG curve (TDTGmax), were shifted toward higher temperatures for polyurethanes containing 6 wt% of PHI-POSS (PTMG 1000) and for all nanohybrids based on PTMG 1400. In case of PTMG 2000, a slight decrease of TDTGmax was observed. The results obtained suggest that there is a restricted molecular mobility of PU macrochains in the presence of POSS and reduction of rate of volatile products’ emission. Lewicki et al. [40] investigated mechanisms by which POSS influences the molecular dynamics and phase separation behavior of both the hard- and soft-block segments. Different solid NMR techniques were used to probe the segmental dynamics of a model elastomeric system that incorporates POSS (at a range of loadings) into the elastomer hard block. NMR characterization of these novel systems has shown that the incorporation of POSS significantly alters the dynamical behavior of the soft-block interphase region and may be negatively impacting the crystalline order of the nanostructured hard-block domains. The obtained NMR data strongly suggest that at comparatively low polyol molecular weights, where overall phase separation is less favorable, there is an increase in the rigid domain fraction with increasing POSS loadings.
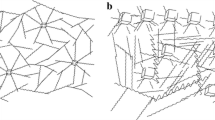
Raftopoulos et al. [41–43] investigated morphology of a series of PHI-POSS/PU hybrid materials on the basis of poly (tetramethylene glycol) as the soft component, 4,4′-diphenylmethane diisocyanate and 1,4-butanediol as chain extender. AFM measurements indicate the formation of POSS crystallites in the PU matrix, with extended structures at low POSS content and more regular structures at higher POSS content. Figure 5.8 shows lateral force AFM images for polyurethanes with POSS content 4 and 10 wt%. For the PU hybrids with smaller filler content, the POSS molecules aggregate to nanometer-size longitudinal crystallites (about 60–70 nm in length) (Fig. 5.8, top), which form spherulites of several microns average sizes (Fig. 5.8, bottom left). At higher filler content (10 wt%), POSS forms more regular crystallites of ca. 120 nm size diameter (Fig. 5.8, bottom right). PHI-POSS displays tendency to form crystallites in the PU matrix, such as extended structures for lower POSS content and more regular structures for the higher silsesquioxane load (PU10).
Lateral force atomic force microscope images for PU04 (area 1.62 × 1.62 µm, top left; 0.7 × 0.7 µm, top right; 4.5 × 4.5 µm, bottom left) and for PU10 (4 × 4 µm, bottom right) [41]
The unique POSS cage structure, vast functionalization possibilities, and ease of their chemical incorporation into polymer matrices are the key factors deciding about silsesquioxanes application in the biomedical field. The main features that allow the medical use of POSS additives are biocompatibility, biodegradability, cytological compatibility, as well as no toxicity and thermodynamic stability [44,45,46].
Recently, numerous works have been devoted to polycarbonate-based materials with POSS molecules built in as pendant groups in the main chain [47,48,49]. The presence of POSS in the polymer chain results in increased mechanical strength and thermal stability [50]. The hybrid composites are also much more resistant to enzymatic and oxidative biodegradation [51]. Moreover, they suffer from calcination to a much lesser extent than unmodified polycarbonate urethane [52]. Through the incorporation of POSS, a continuous, porous matrix is formed, with a pore size of 150–250 μm [53], which turns out to be an excellent substrate for propagation and cell differentiation [50]. Materials of this kind do not degrade in a living organism, so they can be a better substitute for silicones [54].
Kannan et al. patented a nanocomposite polymer based on poly(carbonate–urea)urethane (PCU) with trans-cyclohexanediolisobutyl-silsesquioxane as a pendant group [55]. Further developments include poly(carbonate–urea)urethane (PCU)/POSS hybrids for potential use in cardiovascular bypass grafts and as microvascular components of artificial capillary beds [56,57,58]. A POSS-PCU blood vessel implant with controlled porosity was obtained by foaming extrusion, applying NaHCO3 as the blowing agent [59]. The obtained materials were found to show better resistance to hydrolysis and oxidative degradation, as well as viscoelasticity similar to that of the biological vessels [60, 61]. The PCU-POSS hemocompatibility was also tested, and it was found that silsesquioxane molecules repel platelet and fibrin adsorption because of their variable surface tension, and on a vascular interface, they would contribute to the increased thromboresistance [62,63,64]. Moreover, POSS cages are thought to exhibit optimal cytocompatibility [65].
Improved biocompatibility and biostability against oxidation, hydrolysis, and enzymatic attack under in vitro and in vivo conditions [66, 67] led to the design of the first artificial heart valve made of PCU-POSS nanocomposite [68]. Surface thrombogenicity and mechanical failure of polymeric heart valves are primarily associated with the choice of valve material. Polymer thicknesses of 100, 150, and 200 µm were selected to investigate the mechanical properties and the suitability of these nanocomposites for valve leaflet application. The mechanical test results (Table 5.1) showed that the tensile strength of POSS-PCU increased as compared to PCU. The Young’s modulus of POSS-PCU was significantly greater in comparison with the control PCU for 100 µm thickness.
POSS-PCU showed no significant difference in tear strength compared to PCU. In addition, POSS-PCU demonstrated comparable tear strength to the commercially available PU materials (Table 5.2).
Since PCU/POSS nanocomposites possess excellent mechanical strength, good surface properties, and resistance to platelet adhesion, they were used to design prototype of an artificial heart valve—Fig. 5.9.
Synthetic heart valve (i) design and (ii) prototype made with the POSS-PCU nanocomposite polymer developed at UCL and currently under investigation [68]
In the course of other studies, Ghanbari and colleagues [69] proved that the presence of POSS compounds in the PCU/POSS nanocomposite reduces the tendency of PCU to calcification and thus extends the duration of use of these materials in vivo.
PCU/POSS hybrid nanocomposites were utilized by Chaloupka et al. [70] for fabrication of lacrimal ducts. Different manufacturing techniques were applied to develop a small diameter conduit for the lacrimal duct reconstruction. The aim was to obtain a conduit with distinct outer and inner wall surfaces, characterized by controlled porosity forfacile integration and regeneration into native surrounding tissue.
It was found that by using cast PCU/POSS for the inner wall, formation of scar tissue is prevented and drainage is secured. This wall can thereafter be covered with a coagulated layer allowing suturing of the conduit to the residual lacrimal duct and preventing implant displacement.
The research on PCU/POSS nanocomposites was also carried out by Bakhshi et al. [71] As biocompatibility of PCU containing silsesquioxane is much better than this of nickel and titanium alloy used for shape-memory stents fabrication, an attempt was made to electrohydrodynamically sputter of organic–inorganic hybrids on metallic stents. The peel strength of the deposit was studied before and after degradation of the coating. It has been shown that the surface modification increases the peel strength by 300%. It was also shown how the adhesion strength of the PCU/POSS coating changes after exposure to physiological solutions composed of hydrolytic, oxidative, peroxidative, and biological media. Interestingly, it turned out that NiTi alloys coated with the PCU/POSS layer undergo integration with the stent after long-term (70 days) exposure to biological environments. The applied polymer coating also increases the corrosion resistance. Antithrombogenic properties have also been obtained, and facilitated endothelialization has been observed [72, 73]. Coating of NiTi alloys with poly(carbonate–urea)urethane/POSS layer extends the service life of metallic stents by about 10 years [74].
2.2 POSS Incorporated in the Main Chain
The process of obtaining polyurethanes with POSS cores incorporated in the main chain was studied by Oaten and Choudhury [75]. An open cage POSS with three functional hydroxyl groups (trisilanol) was used, and the synthesis of hybrid polyurethane relied on the direct reaction between an aliphatic diisocyanate (HDI) and trisilanolisobutyl-POSS in toluene, catalyzed by dibutyl tin dilaurate (DBTL). The transparent PU-POSS thin films obtained were used as a protective barrier to cover steel. The 29Si NMR and angle-resolved (AR-XPS) investigations showed that PU-POSS forms a 3-D network which consists of polymer chains cross-linked through urethane bridges and due to condensation of free silanol groups from POSS units. Hydrogen bond interactions of the amide groups in the urethanes and the long-range hydrophobic interactions of the hexamethylene and isobutyl groups contribute to the lamellar structure formation—Fig. 5.10. Thermogravimetric studies revealed a three-stage decomposition of the polymers obtained. The first stage begins at a temperature of 200 °C and is related to the breakdown of urethane bonds and degradation of isobutyl groups of trisilanol. The second stage, starting at 415 °C, and the third stage, commencing at 470 °C, are related to the thermal degradation of the Si-O bonds of the silsesquioxane. DSC studies in combination with photoacoustic Fourier transform infrared spectroscopy (PA-FTIR) have revealed the formation of a silica layer that protects the material onto which the hybrid polyurethane was applied prior to thermal degradation.
Proposed structure of the hybrid thin film on steel [75]
Pistor et al. [76] described the effect of isobutyl trisilanol POSS on the crystalline structure of thermoplastic polyurethane. POSS nanocages introduced in the polyurethane matrix tended to form aggregates, and an increase in the rate of crystal formation was observed with the increase of POSS content in the PU matrix. At the content of 1.14 wt% of POSS, there was one crystallization stage, leading to formation of smaller crystals in the form of disks. With an increase of the POSS load, two stages of crystallization occurred. In the first stage, disks were created, while in the second stage, spherulites were formed—Fig. 5.11. The obtained results confirm that POSS nanoparticles have a strong influence on the crystallization mechanism.
Schematics for the PU and the nanocomposites nucleation and crystal growth phenomena [76]
Pan et al. [77, 78] obtained polyurethane/trisilanolisobutyl polyhedral oligomeric silsesquioxane (PU/TSI-POSS) hybrid composites using MDI and glycerol propoxylate as the PU components. The chemical structure of TSI-POSS and the synthesized hybrids are shown in Fig. 5.12.
Chemical structures of TSI-POSS monomer and PU/TSI-POSS–hybrid composites [77]
X-ray diffractograms showed that by increasing the content of TSI-POSS in polyurethane matrix (above 22 wt%), larger cluster-like crystallites are formed in rigid segments, increasing thus their volume. Glass transition temperature of hybrid composites was found to increase with TSI-POSS content. Due to the presence of isobutyl groups in the TSI-POSS, which are sensitive to oxygen, a reduction in the decomposition temperature of the PU/TSI-POSS composites was observed with an increase of POSS content in the polymeric material [79, 80].
Our research group [81] incorporated disilanol isobutyl-POSS (DSI-POSS) in a PU synthesized using PTMG, MDI, and BD. The obtained hybrid materials were studied toward molecular dynamics behavior, and it was found that incorporation of DSI-POSS does not exert any significant effect on Tg, indicating that incorporation of POSS is practically not affecting the mobility of the soft phase. MDSC results revealed three distinct regions—the first weak and broad endotherm at ca. 70 °C has been associated with the disruption of ordering of short MDI-BD sequences or glass transition of hard segmental microdomains. The next endotherm around 170 °C was due to the main phase separation effect. The last peak commencing at ca. 250 °C was attributed to the thermal decomposition process. By utilizing pyrolysis-GC/MS and DSC methods, we have also studied the effects of DSI-POSS on the mechanisms of thermal degradation of polyurethane matrix [82]. The results of analytical pyrolysis assays of the polyurethane systems demonstrated that low levels of POSS substitution (<10 wt%) lead to a significant increase in both the onset temperature of thermal depolymerization and a reduction in the yield of volatile degradation products [83].
2.3 POSS in a Polyurethane Network Node
POSS can also be used as cross-linkers in polymeric systems. This is accomplished through the functionalization of POSS with eight identical groups that may react to form materials with a high cross-link density. Functional groups that have been employed in the preparation of cross-linked hybrid materials are epoxy, amine, methacryloyl, vinyl, alkyl halide, and hydroxyl groups. Neumann et al. [83, 84] synthesized POSS with eight –NCO groups, using hydrosilylation of octakis (dimethylsiloxy) octasilsesquioxane. As the cross-linking agent, POSS reacted with poly(ethylene glycol) to form cross-linked polyurethane. Nuclear magnetic resonance (29Si NMR) showed that the cage structure of POSS was not damaged after chemical incorporation in the PU matrix [85].
Liu and Zheng [86, 87] used octaaminophenyl polyhedral oligomeric silsesquioxane as a cross-linking agent and 4,4′-methylenebis-(2-chloroaniline) to prepare polyurethane networks containing POSS. DMA investigations showed that in the glassy state ((−75)–(−25) °C), the dynamic storage moduli of all the POSS-containing hybrids are significantly higher than that of the control PU. The TGA results indicated that the thermal stabilities of the nanocomposites were improved, as evidenced by the rate of volatile release from the materials and the enhanced char yields together with ceramic yields. Moreover, contact angle measurements showed that the organic–inorganic nanocomposites with POSS displayed a significant enhancement in surface hydrophobicity. Significant nanoscale reinforcement effect of the POSS cages on the polyurethane matrix and the formation of robust structure of the POSS-containing PU networks were postulated to be the main reasons for properties enhancements caused by silsesquioxanes moieties [88–90].
A functional N-phenylaminomethyl POSS was synthesized by Zhang and coworkers [91] and then used as a cross-linker for polyurethane elastomers—Fig. 5.13. The obtained PU-POSS elastomers showed no macrophase separation up to 52 wt% of POSS load. HRTEM images revealed a well-separated nanostructure of POSS with a typical phase size of 5–10 nm. The storage modulus (DMA), Tg, DSC, and Young modulus (tensile test) were increased with increasing POSS concentration. Thanks to good compatibility of POSS and PU, a cross-linker could be dispersed in PU matrix in nanoscale, restricting efficiently polyurethane chains mobility. As POSS concentration further increased, the cross-linking density also increased, and, when POSS concentration reached 26 wt%, a permanent network was formed.
Synthesis of PU-POSS hybrids [91]
The same authors studied the process of gelation of cross-linked PU-POSS composites at various curing temperatures, times, and POSS concentration [92]. After the completion of the curing reaction, the critical concentration of POSS beyond which the gelation of PU-POSS composites happens was found around 2.5 wt%. In the materials between 2.7 and 6 wt%, formation of self-similarity network near the critical gel occurred. This revealed that different structures were formed, which are shown in Fig. 5.14.
Schematic diagram of PU-POSS composites at different POSS concentration. Cubics represent the POSS; lines represent the strands between POSS [92]
A new class of ester–amine-functionalized silsesquioxane macromers, synthesized by Madhavan et al. [93,94,95,96], was introduced into the polyurethane matrix. DSC showed that the glass transition temperature corresponding to the hard segment increases with an increase in the POSS-amine content as POSS rigid cubes restrict the free rotation of the macromolecular chains. The contact angle measurements revealed its increase with the increase of POSS-amine load for all hybrid materials. This was due to formation of granular POSS aggregates of highly hydrophobic nature on the PU surface. Moreover, permeability coefficient decreases with an increase the POSS concentration, and the selectivities of O2/N2 and CO2/N2 gas pairs increased with an increase in the POSS concentration.
Hu et al. [97, 98] used octa(aminopropyl) silsesquioxane (OapPOSS) for functionalization of graphene sheets (GO) via amide group formation between the amine groups at the eight vertices of POSS and the oxygen-containing groups of graphene oxide. The obtained functionalized graphene (OapPOSS-GO) was used to reinforce waterborne polyurethane (WPU) to obtain OapPOSS-GO/WPU nanocomposites by in situ polymerization. Morphological studies using field emission SEM (FESEM) showed a faster OapPOSS-GO development in the polyurethane matrix compared to GO and OapPOSS. It may be explained by a synergistic effect: The OapPOSS grafted onto the GO surface can lead to a large interlayer spacing, providing effective physical interactions such as van der Waals forces and hydrogen bonds, thus resulting in a better dispersion; on the other hand, OapPOSS-GO surface can provide abundant amine functional groups for grafting PU chains, thus introducing covalent bonds between OapPOSS-GO and PU chains through in situ polymerization. In addition, a significantly better hydrophobicity was achieved for OapPOSS-GO/WPU (contact angle values of ca. 94o) compared to the GO/WPU and OapPOSS/WPU matrix.
Hybrid polyurethane elastomers with octakis [m-isopropyl-α, α’-dimethylbenzylisocyanatodimethylsiloxy]octa silsesquioxane (NCO-POSS) were synthesized by Prządka et al. [99] using IPDI and polyoxypropylene triol. The obtained polyurethane hybrids showed an increase in the M100 module (stress at 100% of the relative elongation) (Fig. 5.15) up to 2.5% mass of NCO-POSS and decrease above this concentration of silsesquioxane.
Stress of hybrid polyurethane materials at 100% elongation as a function of NCO-POSS content [99]
NCO-POSS added in a small amount caused an increase in polyurethane stiffness, while its larger load led to excessive cross-linking, which disturbed the formation of hydrogen bonds with carbonyl groups, causing lower separation of rigid and soft segments and consequently reduction in the mechanical properties of polyurethane composites [100–103].
Star-shaped polyhedral oligomeric silsesquioxane-polycaprolactone-urethane hybrid materials were obtained by Teng et al. [104, 105] who used POSS with eight organic, functional arms chemically bonding to the polymer matrix. By incorporating organic groups in the core through extension with PCL and cross-linking with PU, organic–inorganic nanocomposites with enhanced cell attachment profile and biodegradability could be synthesized [106, 107]. The PCL-PU-POSS films revealed a high porosity of >75% and intrinsic nanoscale features that enhance cell attachment and growth. They exhibited negligible weight loss during the initial 24 weeks of degradation, followed by a large increase in the weight loss of 18% in the following 28 weeks. As cell viability is still maintained at >95%, even after 48 h of incubation with the by-products of degradation, one can consider these hybrid materials to be promising candidates for scaffolds in tissue engineering.
Star-like PU hybrid films using functional cubic silsesquioxanes (CSSQ) (with up to eight reactive sites at the corner of POSS) were developed by Mya et al. [108, 109] Octakis-(dimethylsilyloxy) silsesquioxane isopropenyldimethylbenzylisocyanate (OS-PDBI), and octakis-(dimethylsilyloxy) hydroxypropyl silsesquioxane (HPS) were prepared by hydrosilylation reaction and used as nano-cross-linkers. Additionally, different types of PU hybrids were also prepared by using OS-PDBI macromonomer with hexane diol (HD) monomer. AFM images showed that no phase separation in the macroscopic level occurs for PU hybrid films and incorporation of CSSQ in PU structure provides enhanced thermal stability and increased cross-link density, as revealed by TGA and DMA results. Moreover, the presence of cage structure improved oxidation resistance and mechanical strength.
Pielichowski’s group [110] incorporated into polyurethane elastomer octa-OH-functional POSS as a relatively massive and robust three-dimensional cross-linking core—Fig. 5.16. The influence of this cross-linking moiety on the morphology and molecular dynamics of the PU system was studied. AFM images for a polyurethane matrix showed numerous globules and spherulites of rigid segment domains dispersed in a flexible continuous phase with dimension 3–6 μm. After introducing the POSS molecules embedded as nodes of the polymer network, the globules sizes have decreased, and they assumed more spherical shapes ranging from 10 to 300 nm. On the phase images of hybrid elastomers with POSS, rigid spherical structures with softer center were observed. These new structures have been interpreted as forms consisting of POSS molecules connected to MDI, which have become soft cores surrounded by rigid segments. Additionally, despite extensive chemical cross-linking, the rubbery mechanical modulus decreased on addition of POSS, confirming that hard microdomains reinforce the matrix to a greater extent than chemical cross-links [111].
Reaction route and sketch of the anticipated complex network. Some links have been omitted for clarity [110]
Blattmann and Mülhaupt [112] reported on the synthesis of novel multifunctional polyhedral oligomeric silsesquioxane (POSS) cyclic carbonates and their cure with diamines to produce POSS/NIPU nanocomposites with variable POSS content—Fig. 5.17.
SEM images of NIPU hybrid materials; SEM samples were obtained from NIPU specimens for tensile testing using the cryomicrotome technique [112]
Authors found that Young’s modulus and tensile strength were improved with an increase of POSS content as a result of higher cross-link density and silica content. Moreover, the incorporation of POSS considerably improved scratch resistance of optically transparent and colorless NIPU/POSS coatings as evidenced by the surface gloss and SEM images.
A series of NIPU coatings was prepared by Liu et al. through the ring-opening polymerization of rosin-based cyclic carbonate with amines, then the NIPUs were modified with epoxy and cyclic carbonate-functionalized POSS to form NIPU/POSS coatings [113]. Incorporation of POSS moieties into the NIPU networks caused an improvement in thermal stability, water tolerance, and pencil hardness of the hybrid coatings with an increase of the POSS content, whereby the impact strength, adhesion, and flexibility of the coatings were not observed to be affected to a significant extent. In an another development, this research group has obtained biomass-based NIPU coatings via the reactions of gallic acid-based cyclic carbonate with diamines which were then modified with epoxy-functionalized POSS to form NIPU/POSS coatings with chemically linked POSS groups [114]. The results revealed that the gallic acid-based NIPU coatings show excellent impact strength, adhesion, flexibility, pencil hardness, and thermal stability, but are suffering from low water resistance. Incorporation of POSS into the NIPU networks enhanced the pencil hardness, water resistance, and thermal stability of the NIPU/POSS coatings; however, the adhesion of the coatings was slightly decreased.
3 Physical Blending
Incorporation of POSS through physical blending offers several advantages, such as ease of processing, versatility, being fast, and cost-effective. The successful dispersion of POSS into polymeric matrices depends on the surface interactions of POSS, such as van der Waals and hydrogen bonding, with polymers [115,116,117]. However, interparticle interactions often result in the aggregation of POSS particles which may be a serious technological problem [118]. Polyurethane and POSS can be blended by using a solution or melt method [85].
Bourbigot et al. [119] reported on the preparation of POSS-containing composites based on PU by melt blending. Thermoplastic polyurethane was mixed with 10 wt% of poly(vinylsilsesquioxane) using a Brabender mixer running in nitrogen flow at 50 rpm for 10 min and at 180 °C. In the TEM image obtained at smaller magnification (17,000x), POSS particles were visible evenly dispersed in a thermoplastic polyurethane. At high magnification (30,000x), it turned out that POSS tended to create aggregates with micron size probably formed in the melt during processing. The silsesquioxane particle size was in the range of 200–400 nm, and the particles had an ellipsoidal shape.
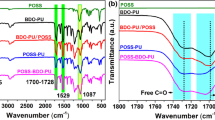
Figure 5.18 shows a large reduction of the maximum peak of heat release rate (PHRR) in thermoplastic composites from 430 to 80 kW/m2 as compared to pure TPU. The intumescent material has been found to be composed of ceramified char made of silicon network in a polyaromatic structure. This intumescent PU nanocomposite acts as a thermal barrier at the surface of the substrate limiting thus the heat and mass transfer as evidenced by lowered HRR [85, 120].
Heat release rate (HRR) as a function of time of virgin TPU compared to TPU-POSS composite (external heat flux = 35 kW/m2) [116]
Reactive blending was applied by Montecelli et al. too [121]. to prepare polyurethane/POSS hybrids As a result of controlled scission of the thermoplastic polyurethane, isocyanate functional groups are formed during melt mixing, which react with OH groups in the functionalized POSS molecules: octaisobutyl POSS or trans-cyclohexanediolisobutyl POSS. These systems were prepared for mixing at 220 °C under the inert atmosphere for 10 min. The morphology studies using TEM microscopy revealed that there is no segregation or aggregation of POSS particles in TPU/POSS-OH (5 wt%), thus indicating that POSS-OH has chemically reacted with the polymer. DSC data showed an increase in glass transition temperature with increasing POSS content in TPU matrix from −48 °C for pristine TPU to −37 °C for TPU/POSS-OH (10 wt%).
Gu et al. [122] introduced octa(3-hydroxypropyl) polyhedral oligomeric silsesquioxane (POSS-(OH)8) into ester-based thermoplastic polyurethane matrix by solution blending. The obtained composites displayed glass transition temperature around −25 °C, which can be used as trigger temperature of the shape-memory recovery. Moreover, they showed high shape fixity and shape recovery ratios with low trigger temperature.
Blended hybrid systems of PU with POSS having eight poly(ethylene glycol) (PEG) vertex groups (PEG-POSS) were prepared and characterized by Pielichowski’s group [123]. The main change induced by the PEG-POSS is a plasticization effect which is observed by lowering the glass transition temperature. AFM and SEM analyses showed that hard segments form spherulites, and their size increases with the increase of POSS content. No POSS aggregates are observed while the storage modulus (DMA) in the rubbery phase decreases in the blends. This change in morphology, combined with the softening of the material on addition of POSS, suggests decreased microphase separation in polyurethane. Based on the results obtained, microstructure morphology development has been postulated—Fig. 5.19.
Sketch of the mesostructure and organization of the microstructure of the reference polyurethane (left) and the PU/PEG-POSS blend (right) [120]
4 Application of PU-POSS Hybrid Materials
Hybrid materials by combining the features of organic and inorganic matter offer advantageous properties for different materials science applications. One of the application areas is polyurethane/POSS materials with controlled gas transport properties, as well as multilayer membranes [94, 124]. Specifically, by using amino-POSS to modify PU matrix, a reduction in permeability was achieved. The O2/N2 permeability and CO2/N2 permeability could be controlled by the POSS load and the PU structure [75]. It was postulated that bulky POSS moieties located on the polymer surface hinder transport of gas molecules. Moreover, POSS substituents influence formation of ordered crystalline structures [95, 124].
Hybrid thermally stable materials [38, 119, 125, 126], as well as materials with high dielectric constant, resistant to creeping currents [127], are considered as potential candidates for future applications. For instance, incorporation of PHI-POSS into polyurethane matrix composed of MDI and polytetramethylene glycol caused an increase in temperatures at which the degradation rate was the highest and a decrease in the intensity of emission of volatile decomposition products. This is most likely related to the reduced mobility of polymer chains in the presence of chemically linked POSS molecules [38]. Octaminophenyl-POSS (OapPOSS), added to epoxy-imine-polyurethane resins, caused an improvement in the heat resistance and in the Young’s modulus values [125].
POSS nanoadditives were tested toward flame retardancy behavior of various polymers, and the best synergistic effects were found for a combination of conventional flame retardants with silsesquioxanes [126]. POSS mode of action includes preventing the fire by creation of ceramic, fire-resistant char layer on the surface of the burning material [116].
Special attention is focused on protective polymeric materials with POSS—due to good adhesion and improved durability, an effective protection against corrosion [128] and weathering effects has been reported [129]. Incorporation of POSS moieties into the polymer chain resulted in increased compressive strength [130], stretching [26], and improved thermoplasticity [128]. By using octaphenyl and glycidoxypropyl-POSS in the polyurethane matrix, glues with improved adhesion and shear strength could be obtained [131, 132]. Moreover, POSS nanofillers have been successfully used to obtain novel aerogels [130, 133] and shape-memory composites [134].
POSS modified polyurethane materials can also be used as high-strength coatings. The electrochemical and salt spray test evaluation of the PU films demonstrated the barrier and corrosion resistance properties improved by the POSS. The low current density over a wide potential indicated that the PU/POSS film provided an effective barrier to water. Additionally, the POSS components retain their partial cage structure, which give the product a high glass transition and enhanced thermal stability as compared to conventional polyurethanes, which is essential in their application as weatherable coatings [135, 136]. PU/POSS show also potential as flame retardants in textile coatings applications [137]. The unique POSS cage structure, non-toxicity, and ease of properties control decided on the use of silsesquioxanes in the biomedical field. The main features that determine the medical applicability of POSS-containing materials are biocompatibility, biodegradability, cytological compatibility, and dimensional stability, as well as the already-mentioned no toxicity. Vast attention was paid to polycarbonate urethane-based (PCU) materials with POSS embedded in the main chain [138,139,140]. An increased mechanical strength and thermal stability [138], as well as better resistance to enzymatic and oxidative biodegradation, compared to pristine polymer, were reported [142]. Importantly, it was also found that PCU/POSS composites undergo calcination to a much lesser extent than non-modified polycarbonate urethane [69]. Through the incorporation of POSS, a continuous, porous matrix is formed, with a pore size of 150–250 μm [143], which turns out to be an excellent substrate for propagation and cell differentiation [141]. Materials of this type do not degrade inside mammalian organisms; therefore, they can be considered as a replacement for silicones [54, 144].
In general, research works on PCU/POSS hybrids are focused on three groups of biomaterials—blood vessel implants, lacrimal canal implants, and scaffolds that help regenerate tissues in the nervous system [142, 145]. As the fabrication route foaming extrusion proved to be a useful technique, enabling control of the porous structure formed [58].
Promising path is covering of shape-memory stents, made of nickel and titanium alloy (Nitinol), by electrohydrodynamic sputtering of PCU/POSS hybrids [70]. The layer formed shows antithrombogenic properties and facilitates endothelialization process [57, 71]. Noteworthy, Nitinol coating with POSS-containing polymer layer extends the usefulness of stents by about 10 years [74].
Another use of PCU/POSS materials in medicine is as scaffolds. These are mostly cell scaffolds capable of trapping cells and facilitating the creation of new tissues, such as RGD peptide (arginine-glycine-aspartic acid)-grafted PCU [146, 147]. The obtained materials are currently in the phase of clinical trials, and it is possible that they will initiate a new generation of scaffolds capable of regenerating nerves to a larger than 30 mm barrier. Using cell scaffolds, it is also possible to treat liver [148] and brain damages [149].
Materials containing POSS and nanotubes that can be used in the treatment of serious diseases are also of great interest. In this case, the nanotubes are coated with the PCL/POSS composite to ensure a biocompatible surface. Such materials exhibit strong near infrared absorption, emitting large amounts of thermal energy, and can be used for destruction of cancer cells [150].
5 Conclusions
In this work, diverse and significant research on polyurethane/POSS–hybrid materials has been presented. Two major routes of silsesquioxanes incorporation into polyurethane matrix—via chemical or physical path, leading to various organic–inorganic architectures, have been described. The crucial role of POSS functionalization in the synthesis and during processing of polymers was highlighted. POSS moieties in nanohybrid polyurethanes influence the crystallization process of polymer matrix, leading to formation of stable morphological forms. The aggregation or crystallization behavior of POSS in nanocomposites plays an important role in manipulating the physical properties of materials, such as viscosity and melt elasticity. It is also important to limit the segment vibrations by large POSS substituents, which increases the glass transition temperature. There is also the possibility of occurrence favorable second-order interactions between macrochains containing POSS in their structure. The described polyurethane-POSS systems, due to their special properties, can find numerous applications, such as membranes, high-strength coatings and adhesives, aerogels, flame retardant materials, and shape-memory materials. The unique POSS cage structure, its non-toxicity, and broad functionalization possibilities make them useful modifiers for polyurethanes devoted to biomedical applications.
References
Florjańczyk Z, Penczek S (1998) Chemia polimerów, v.II, Oficyna Wydawnicza Politechniki Warszawskiej, Warszawa
Wirpsza Z (1991) Poliuretany: chemia, technologia, zastosowanie. Wydawnictwo Naukowo-Techniczne, Warszawa
Randall D, Lee S (2002) The polyurethane book. Wiley Ltd., New York
Szycher M (2003) Szycher’s handbook of polyurethane. CRC Press, Taylor &Francis Group, Boca Raton
Prisacariu C (2011) Polyurethane Elastomers – from morphology to mechanical aspects. Springer Wien, New York
Prociak A, Rokicki G (2014) Materiały poliuretanowe. Polskie Wydawnictwo Naukowe, Warszawa
Allport DC, Gilbert DS, Outterside SM (2003) MDI and TDI: a safety Health and the environment. Wiley, Chichester
Rokicki G, Parzuchowski PG, Mazurek M (2015) Non-isocyanate polyurethanes: synthesis, properties, and applications. Polym Adv Technol 26:707–761
Kathalewar MS, Joshi PB, Sabnis AS et al (2013) Non-isocyanate polyurethanes: from chemistry to applications. RSC Adv 3:4110
Yaocheng H, Liyun L, Xu R et al (2011) Nonisocyanate polyurethanes and their applications. Prog Chem 23(6):1181–1188
Beniah G, Fortman DJ, Heath WH et al (2017) Non-Isocyanate polyurethane thermoplastic elastomer: amide-based chain extender yields enhanced nanophase separation and properties in polyhydroxyurethane. Macromolecules 50(11):4425–4434
Guo Z, Kim TY, Lei K, Pereira T et al (2008) Strengthening and thermal stabilization of polyurethane nanocomposites with silicon carbide nanoparticles by a surface-initiated-polymerization approach. Compos Sci Technol 62(1):164–170
Kuan HC, Ma CC, Chang WP et al (2005) Synthesis, thermal, mechanical and rheological properties of multiwall carbon nanotube/waterborne polyurethane nanocomposite. Compos Sci Technol 65(11):1703–1710
Koerner H, Liu W, Alexander M et al (2005) Deformation morphology correlations in electrically conductive carbon nanotube thermoplastic polyurethane nanocomposites. Polymer 46:4405–4420
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1):293–346. https://doi.org/10.1021/cr030698+
Kuo SW, Chang FC (2011) POSS related polymer nanocomposites. Prog Polym Sci 36:1649–1696. https://doi.org/10.1016/j.progpolymsci.2011.05.002
Kickelbick G (2007) Hybrid materials. synthesis, characterization and application. Wiley-VCH, Weinheim
Markovic E, Constantopolous K, Janis G (2011) Polyhedral oligomeric silsesquioxanes: from early and strategic development through to materials application. In: Matisons Hartmann-Thompson C (ed) Applications of polyhedral oligomeric silsesquioxanes. Springer, New York, pp 1–46
Harrison PG (1997) Silicate cages: precursors to new materials. J Organomet Chem 542(2):141–183. https://doi.org/10.1016/S0022-328X(96)06821-0
Pielichowski K, Njuguna J, Janowski B, Pielichowski J (2006) Polyhedral oligomeric silsesquioxanes (POSS)-containing nanohybrid polymers. In: supramolecular polymers polymeric betains oligomers, Springer, Berlin, Heidelberg, pp 225–296. https://doi.org/10.1007/11614784
Pan G (2007) Polyhedral oligomeric silsesquioxane (POSS). In: Mark JE (ed) Physical properties of polymers handbook, Springer, New York, pp 577–584. https://doi.org/10.1007/978-0-387-69002-5
Janowski B, Pielichowski K (2008) Polimery nanohybrydowe zawierające poliedryczne oligosilseskwioksany. Polimery 53:87–98
Cordes DB, Lickiss PD, Rataboul F (2010) Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev 110:2081–2173
Schwab JJ, Lichtenhan JD, Mather PT, Romouribe A et al, (1996) 21th meeting of the American Chemical Society, New Orleans, LA
Feher F, Schwab J, Tellers D, Burstein A (1998) A general strategy for synthesizing cubeoctameric silsesquioxanes containing polymerizable functional groups. Main Group Chem 2(3):169–181
Fu BX, Hsiao BS, White H, Rafailovich M et al (2000) Nanoscale reinforcement of polyhedral oligomeric silsesquioxane (POSS) in polyurethane elastomer. Polym Int 49:437–440
Fu BX, Hsiao BS, Pagola S, Stephens P et al (2001) Structural development during deformation of polyurethane containing polyhedral oligomeric silsesquioxanes (POSS) molecules. Polymer 42:599–611
Hoflund GB, Gonzalez RI, Philips SHJ (2001) In situ oxygen atom erosion study of a polyhedral oligomeric silsesquioxane-polyurethane copolymer. Adhesion Sci Technol 15:1199–1211. https://doi.org/10.1163/156856101317048707
Turri S, Levi M (2005) Preparation and characterization of polyurethane hybrids from reactive polyhedral oligomeric silsesquioxanes. Macromolecules 38:5569–5574
Turri S, Levi M (2005) Wettability of polyhedral oligomeric silsesquioxane nanostructured polymer surfaces. Macromol Rapid Commun 26:1233–1236. https://doi.org/10.1002/marc.200500274
Zhang S, Zou Q, Wu L (2006) Preparation and characterization of polyurethane hybrids from reactive polyhedral oligomeric silsesquioxanes. Macromol Mater Eng 291:895–901. https://doi.org/10.1002/mame.200600144
Król B, Król P (2010) Materiały powłokowe otrzymywane z kationomerów poliuretanowych modyfikowanych funkcjonalizowanym silseskwioksanem. Cz. I. Budowa chemiczna kationomerów. Polimery 55(6):440–451
Król B, Król P (2010) Materiały powłokowe otrzymywane z kationomerów poliuretanowych modyfikowanych funkcjonalizowanym silseskwioksanem. Cz. II. Właściwości użytkowe. Polimery 55(11–12):855–862
Madbouly SA, Otaigbe JU, Nanda AK et al (2007) Rheological behavior of POSS/polyurethane-urea nanocomposite films prepared by homogeneous solution polymerization in aqueous dispersions. Macromolecules 40:4982–4991
Madbouly SA, Otaigbe JU (2009) Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and film. Prog Polym Sci 34:1283–1332. https://doi.org/10.1016/j.progpolymsci.2009.08.002
Lai YS, Tsai CW, Yang HW, Wang GP et al (2009) Structural and electrochemical properties of polyurethanes/polyhedral oligomeric silsesquioxanes (PU/POSS) hybrid coatings on aluminum alloys. Mater Chem Phys 117:91–98
Hu J, Li L, Zhang S, Gong L et al (2013) Novel phenyl-POSS-polyurethane aqueous dispersions and their hybrid coatings. J Appl Polym Sci 130:1611–1620. https://doi.org/10.1002/APP.39303
Janowski B, Pielichowski K (2008) Thermo(oxidative) stability of novel polyurethane/POSS nanohybrid elastomers. Thermochim Acta 478:51–53
Lewicki JP, Pielichowski K, TremblotDeLaCroix P, Janowski B et al (2010) Thermal degradation studies of polyurethane/POSS nanohybrid elastomers. Polym Degrad Stab 95(6):1099–1105. https://doi.org/10.1016/j.polymdegradstab.2010.02.021
Lewicki JP, Mayer BP, Pielichowski K, Janowski B et al (2010) Synthesis and characterization via solid state NMR of novel POSS-polyurethane nanohybrid elastomers. Polymer Preprints 51(2):343–344
Raftopoulos KN, Pandis Ch, Apekis L, Pissisa P et al (2010) Polyurethane–POSS hybrids: molecular dynamics studies. Polymer 51:709–718
Raftopoulos KN, Jancia M, Aravopoulou D, Hebda E et al (2013) POSS along the hard segments of polyurethane. Phase separation and molecular dynamics. Macromolecules 46:7378–7386. https://doi.org/10.1021/ma401417t
Raftopoulos KN, Janowski B, Apekis L, Pissis P et al (2013) Direct and indirect effects of POSS on the molecular mobility of polyurethanes with varying segment Mw. Polymer 54:2745–2754. https://doi.org/10.1016/j.polymer.2013.03.036
Cui D, Tian F, Ozkan CS, Wang M et al (2005) Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett 155(1):73–85. https://doi.org/10.1016/j.toxlet.2004.08.015
Kim SK, Heo SJ, Koak JY, Lee JH et al (2007) A biocompatibility study of a reinforced acrylic-based hybrid denture composite resin with polyhedraloligosilsesquioxane. J Oral Rehabil 34(5):389–395. https://doi.org/10.1111/j.1365-2842.2006.01671.x
Punshon G, Vara DS, Sales KM, Kidane AG et al (2005) Interactions between endothelial cells and a poly(carbonate-silsesquioxane-bridge-urea)urethane. Biomaterials 26(32):6271–6279. https://doi.org/10.1016/j.biomaterials.2005.03.034
Lakhani HA, Mel A, Seifalian AM (2015) The effect of TGF-β1 and BMP-4 on bone marrow-derived stem cell morphology on a novel bioabsorbable nanocomposite material. Artif Cells Nanomedicine Biotechnol 43(4):230–234. https://doi.org/10.3109/21691401.2013.856015
Maqsood A, Hamilton G, Seifalian AM (2010) Viscoelastic behaviour of a small calibre vascular graft made from a POSS-nanocomposite. In: 32nd annual international conference of the IEEE engineering in medicine and biology 251–254. https://doi.org/10.1109/iembs.2010.5627472
Kannan RY, Salacinski HJ, Sales KM, Butler PE et al (2006) The endothelialization of polyhedral oligomeric silsesquioxane nanocomposites: an in vitro study. Cell Biochem Biophys 45(2):129–136
Guo YL, Wang W, Otaigbe JU (2010) Biocompatibility of synthetic poly(ester urethane)/polyhedral oligomeric silsesquioxane matrices with embryonic stem cell proliferation and differentiation. J Tissue Eng Regen Med 4(7):553–564
Wu J, Gu X, Mather PT (2010) Biostable multiblock thermoplastic polyurethanes incorporating poly(ε-caprolactone) and polyhedral oligomeric silsesquioxane (POSS). Trans Annu Meet Soc Biomater 1(84)
Ghanbari H, Cousins BG, Seifalian AM (2011) A nanocage for nanomedicine: polyhedral oligomeric silsesquioxane (POSS). Macromol Rapid Commun 32(14):1032–1046. https://doi.org/10.1002/marc.201100126
Gupta A, Vara DS, Punshon G, Sales KM et al (2009) In vitro small intestinal epithelial cell growth on a nanocomposite polycaprolactone scaffold. Biotechnol Appl Biochem 54(4):221–229
Kannan RY, Salacinski HJ, Odlyha M, Butler PE et al (2006) The degradative resistance of polyhedral oligomeric silsesquioxane nanocore integrated polyurethanes: an in vitro study. Biomaterials 27(9):1971–1979
Salacinski HJ, Handcock S, Seifalian AM (2005) Polymer for use in conduits and medical devices. Patent Number: WO2005070998, 4 Aug 2005
Kannan RY, Salacinski HJ, Edirisinghe MJ, Hamilton G et al (2006) Polyhedral oligomeric silsequioxane-polyurethane nanocomposite microvessels for an artificial capillary bed. Biomaterials 27:4618–4626
Kannan RY, Salacinski HJ, Groot JD, Clatworthy I et al (2006) The antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocomposite. Biomacromolecules 7:215–223
Ahmed M, Ghanbari H, Cousins BG, Hamilton G et al (2011) Small calibre polyhedral oligomeric silsesquioxane nanocomposite cardiovascular grafts: influence of porosity on the structure, haemocompatibility and mechanical properties. Acta Biomater 7(11):3857–3867
Ahmed M, Hamilton G, Seifalian AM (2014) The performance of a small-calibre graft for vascular reconstructions in a senescent sheep model. Biomaterials 35(33):9033–9040. https://doi.org/10.1016/j.biomaterials.2014.07.008
Tai NR, Salacinski HJ, Edwards A, Hamilton G et al (2000) Compliance properties of conduits used in vascular reconstruction. Br J Surg 87(11):1516–1524. https://doi.org/10.1046/j.1365-2168.2000.01566.x
Salacinski HJ, Tai NR, Carson RJ, Edwards A et al (2002) In vitro stability of a novel compliant poly(carbonate-urea)urethane to oxidative and hydrolytic stress. J Biomed Mater Res 59(2):207–218
Silver JH, Lin JC, Lim F, Tegoulia VA et al (1999) Surface properties and hemocompatibility of alkyl-siloxane monolayers supported on silicone rubber: effect of alkyl chain length and ionic functionality. Biomaterials 20(17):1533–1543
Park JH, Bae YH (2002) Hydrogels based on poly(ethylene oxide) and poly(tetramethylene oxide) or poly(dimethyl siloxane): synthesis, characterization, in vitro protein adsorption and platelet adhesion. Biomaterials 23(8):1797–1808. https://doi.org/10.1016/S0142-9612(01)00306-4
Kannan RY, Salacinski HJ, Butler PE, Seifalian AM (2005) Polyhedral oligomeric silsesquioxane nanocomposites: the next generation material for biomedical applications. Acc Chem Res 38(11):879–884. https://doi.org/10.1021/ar050055b
Kannan RY, Salacinski HJ, Sales K, Butler P et al (2005) The roles of tissue engineering and vascularisation in the development of micro-vascular net-works: a review. Biomaterials 26:1857–1875
Salacinski HJ, Tai NR, Punshon G, Giudiceandrea A et al (2000) Optimal endothelialisation of a new compliant poly(carbonate-urea)urethane vascular graft with effect of physiological shear stress. Eur J Vasc Endovasc Surg 20(4):342–352
Chawla R, Tan A, Ahmed M, Crowley C et al (2014) A polyhedral oligomeric silsesquioxane–based bilayered dermal scaffold seeded with adipose tissue–derived stem cells: in vitro assessment of biomechanical properties. J Surg Res 188(2):361–372. https://doi.org/10.1016/j.jss.2014.01.006
Kidane AG, Burriesci G, Edirisinghe M, Ghanbari H et al (2009) A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater 5(7):2409–2417. https://doi.org/10.1016/j.actbio.2009.02.025
Ghanbari H, Kidane AG, Burriesci G, Ramesh B et al (2010) The anti-calcification potential of a silsesquioxane nanocomposite polimer under in vitro conditions: potential material for synthetic leaflet heart valve. Acta Biomater 6(11):4249–4260. https://doi.org/10.1016/j.actbio.2010.06.015
Chaloupka K, Motwani M, Seifalian AM (2011) Development of a new lacrimal drainage conduit using POSS nanocomposite. Biotechnol Appl Biochem 58(5):363–370. https://doi.org/10.1002/bab.53
Bakhshi R, Darbyshire A, Evans JE, You Z et al (2011) Polymeric coating of surface modified nitinol stent with POSS-nanocomposite polimer. Colloids Surf B 86(1):93–105. https://doi.org/10.1016/j.colsurfb.2011.03.024
Kannan RY, Salacinski HJ, De Groot J, Clatworthy I et al (2006) The antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocomposite. Biomacromol 7(1):215–223. https://doi.org/10.1021/bm050590z
Farhatnia Y, Pang JH, Darbyshire A, Dee R et al. (2016) Next generation covered stents made from nanocomposite materials: a complete assessment of uniformity, integrity and biomechanical properties. Nanomed Nanotechnol Biol Med 12(1):1–12. https://doi.org/10.1016/j.nano.2015.07.002
Desai M, Bakhshi R, Zhou X, Odlyha M et al (2012) A sutureless aortic stent-graft based on a nitinol scaffold bonded to a compliant nanocomposite polymer is durable for 10 years in a simulated in vitro model. J Endovasc Ther 19(3):415–427. https://doi.org/10.1583/11-3740MR.1
Oaten M, Choudhury NR (2005) Silsesquioxane-urethane hybrid for thin film applications. Macromolecules 38(15):6392–6401. https://doi.org/10.1021/ma0476543
Pistor V, Conto D, Ornaghi FG, Zattera AJ (2012) Microstructure and crystallization kinetics of polyurethane thermoplastics containing trisilanol isobutyl POSS. J Nanomaterials 2012, Article ID 283031, 8 pages. https://doi.org/10.1155/2012/283031
Pan R, Shanks R, Kong I, Wang L (2014) Trisilanolisobutyl POSS/polyurethane hybrid composites: preparation, WAXS and thermal properties. Polym Bull 71:2453–2464. https://doi.org/10.1007/s00289-014-1201-7
Pan R, Shanks R, Wang L (2015) Crystallite cluster structure formation resulting from semi-enclosed cage interaction in TSI-POSS/PU hybrid composites. Adv Mater Res 1091:19–23
Pan R, Shanks R, Liu Y (2015) The effect of humping semi-enclosed cage structure on polymer chains characteristics of TSI-POSS/PU hybrid composites. Appl Mech Mater 751:30–34
Pan R, Wang LL, Shanks R, Liu Y (2016) The influence of trisilanolisobutyl POSS on domain microstructure of a polyurethane hybrid composite: A molecular simulation approach. Silicon. https://doi.org/10.1007/s12633-016-9463-3
Raftopoulos KN, Jancia M, Aravopoulou D, Hebda E et al (2013) POSS along the hard segments of polyurethane. Phase Sep Molecular Dyn Macromol 46(18):7378–7386. https://doi.org/10.1021/ma401417t
Lewicki JP, Pielichowski K, Jancia M, Hebda E et al (2014) Degradative and morphological characterization of POSS modified nanohybrid polyurethane elastomers. Polym Degrad Stab 104:50–56
Neumann D, Fisher M, Tran L, Matisons JG (2002) Synthesis and characterization of an isocyanate functionalized polyhedral oligosilsesquioxane and the subsequent formation of an organic-inorganic hybrid polyurethane. J Am Chem Soc 124(47):13998–13999. https://doi.org/10.1021/ja0275921
Markovic E, Nguyen K, Clarke S, Constantopoulos K et al (2013) Synthesis of POSS–polyurethane hybrids using octakis (m-isoprenyl-α, α′-dimethylbenzylisocyanato dimethylsiloxy) octasilsesquioxane (Q8M8TMI) as a crosslinking agent. J Polym Sci Polym Chem 51(23):5038–5045. https://doi.org/10.1002/pola.26934
Diao S, Mao L, Zhang L, Key YW (2015) POSS/Polyurethane hybrids and nanocomposites: a review on preparation, structure and performance elastomers and composites. 50(1):35–48
Liu H, Zheng S (2005) Polyurethane networks nanoreinforced by polyhedral oligomeric silsesquioxane. Macromol Rapid Commun 26:196–200
Liu H, Zheng S (2006) Polyurethane networks modified with octa(propylglycidyl ether) polyhedral oligomeric silsesquioxane. Macromol Chem Phys 207:1842–1851
Zhang Q, He H, Xi K, Huang X et al (2011) Synthesis of N-phenylaminomethyl POSS and its utilization in polyurethane. Macromolecules 44(3):550–557. https://doi.org/10.1021/ma101825j
Zhang Q, Huang X, Wang X, Jia X et al (2014) Rheological study of the gelation of cross-linking polyhedral oligomeric silsesquioxanes (POSS)/PU composites. Polymer 55:1282–1291
Zhang Q, Huang X, Meng Z, Jia X et al (2014) N-phenylaminomethyl hybrid silica, a better alternative to achieve reinforced PU nanocomposites. RSC Adv 4(35):18146–18156. https://doi.org/10.1039/C4RA01419G
Zhang Q, He H, Xi K, Huang X et al (2011) Synthesis of N-phenylaminomethyl POSS and its utilization in polyurethane. Macromolecules 44:550–557. https://doi.org/10.1021/ma101825j
Zhang Q, Huang X, Wang X, Jia X et al (2014) Rheological study of the gelation of cross-linking polyhedral oligomeric silsesquioxanes (POSS)/PU composites. Polymer 55:1282–1291. https://doi.org/10.1016/j.polymer.2014.01.040
Madhavan K, Reddy BSR (2009) Synthesis and characterization of polyurethane hybrids: influence of the polydimethylsiloxane linear chain and silsesquioxane cubic structure on the thermal and mechanical properties of polyurethane hybrids. J Appl Polym Sci 113:4052–4065
Madhavan K, Reddy BSR (2009) Structure–gas transport property relationships of poly(dimethylsiloxane–urethane) nanocomposite membranes. J Membr Sci 342(1–2):291–299
Madhavan K, Reddy BSR (2006) Poly(dimethylsiloxane-urethane) membranes: effect of hard segment in urethane on gas transport properties. J Membr Sci 283:357
Madhavan K, Gnanasekaran D, Reddy BSR (2011) Poly(dimethylsiloxane-urethane) membranes: effect of linear siloxane chain and caged silsesquioxane on gas transport properties. J Polym Res 18(6):1851–1861. https://doi.org/10.1007/s10965-011-9592-8
Hu L, Jiang P, Bian G, Huang M et al. (2017) Effect of octa(aminopropyl) polyhedral oligomeric silsesquioxane (OapPOSS) functionalized graphene oxide on the mechanical, thermal, and hydrophobic properties of waterborne polyurethane composites. J Appl Polym Sci 44440–44450. https://doi.org/10.1002/app.44440
Xue Y, Liu Y, Lu F, Qu J et al (2012) Functionalization of graphene oxide with polyhedral oligomeric silsesquioxane (POSS) for multifunctional applications. J Phys Chem Lett 3:1607–1612. https://doi.org/10.1021/jz3005877
Prządka D, Jęczalik J, Andrzejewska E, Marciniec B et al (2013) Novel hybrid polyurethane/POSS materials via bulk polymerization. React Funct Polym 73:114–121. https://doi.org/10.1016/j.reactfunctpolym.2012.09.006
Ti Y, Chen D (2008) Temperature dependence of hydrogen bond in Fe-OCAP/polyurethane blends. J Appl Polym Sci 130(4):2265–2271
Zhang J, Hu CP (2008) Synthesis, characterization and mechanical properties of polyester-based aliphatic polyurethane elastomers containing hyperbranched polyester segments. J Am Chem Soc 44:3708
Prządka D, Jęczalik J, Andrzejewska E, Dutkiewicz M (2013) Synthesis and properties of hybrid urethane polymers containing polyhedral oligomeric silsesquioxane crosslinker. J Appl Polym Scihttps://doi.org/10.1002/app.39385
Kim EH, Myoung SW, Jung YG, Paik U (2009) Polyhedral oligomeric silsesquioxane-reinforced polyurethane acrylate. Prog Org Coat 64(2–3):205–209. https://doi.org/10.1016/j.porgcoat.2008.07.026
Teng CP, Mya KY, Win KY, Yeo CC et al (2014) Star-shaped polyhedral oligomeric silsesquioxane-polycaprolactone-polyurethane as biomaterials for tissue engineering application. NPG Asia Mater 6:e142. https://doi.org/10.1038/am.2014.102
Liu Y, Yang X, Zhang W, Zheng S (2006) Star-shaped poly(ε-caprolactone) with polyhedral oligomeric silsesquioxane core. Polymer 47(19):6814–6825. https://doi.org/10.1016/j.polymer.2006.07.050
She MS, Lo TY, Hsueh HY, Ho RM (2013) Nanostructured thin films of degradable block copolymers and their applications. NPG Asia Mater 5:e42. https://doi.org/10.1038/am.2013.5
Wu J, Mather PT (2009) POSS polymers: physical properties and biomaterials applications. J Macromol Sci Polym Rev 49(1):25–63. https://doi.org/10.1080/15583720802656237
Mya KY, Wang Y, Shen L, Xu J et al (2009) Star-like polyurethane hybrids with functional cubic silsesquioxanes: preparation, morphology, and thermomechanical properties. J Polym Sci Part A Polym Chem 47:4602–4616. https://doi.org/10.1002/pola.23512
Mya KY, He CB, Huang J, Xiao Y et al (2004) Preparation and thermomechanical properties of epoxy resins modified by octafunctional cubic silsesquioxane epoxides. J Polym Sci Part A Polym Chem 42(14):3490–3503. https://doi.org/10.1002/pola.20168
Raftopoulos KN, Koutsoumpis S, Jancia M, Lewicki JP et al (2015) Reduced phase sparation and slowing of dynamics in polyurethanes with three-dimensional POSS-based cross-linking moieties. Macromolecules 48(5):1429–1441. https://doi.org/10.1021/ma5023132
Raftopoulos KN, Pielichowski K (2015) Segmental dynamics in hybrid polymer/POSS nanomaterials. Prog Polym Sci 52:136–187. https://doi.org/10.1016/j.progpolymsci.2015.01.003
Blattmann H (2016) Mülhaupt R (2016) Multifunctional POSS cyclic carbonates and non-isocyanate polyhydroxyurethane hybrid materials. Macromolecules 49(3):742–751. https://doi.org/10.1021/acs.macromol.5b02560
Liu G, Wu G, Chen J et al (2016) Synthesis, modification and properties of rosin-based non-isocyanate polyurethanes coatings. Prog Org Coat 101:461–467. https://doi.org/10.1016/j.porgcoat.2016.09.019
Liu G, Wu G, Chen J et al (2015) Synthesis and Properties of POSS-containing Gallic acid-based non-isocyanate polyurethanes coatings. Polym Degrad Stab 121:247–252. https://doi.org/10.1016/j.polymdegradstab.2015.09.013
Wu J, Mather PT (2009) POSS polymers: physical properties and biomaterials applications. Polym Rev 49(1):25–63 https://doi.org/10.1080/15583720802656237
Striolo A, McCabe C, Cummings PT (2005) Thermodynamic and transport properties of polyhedral oligomeric sislesquioxanes in poly(dimethylsiloxane). J. Phys. Chem. B 109(30):14300–14307. https://doi.org/10.1021/jp045388p
Striolo A, McCabe C, Cummings PT (2006) Organic-inorganic telechelic molecules: solution properties from simulations. J Chem Phys 125(10):104904. https://doi.org/10.1063/1.2348641
Ayandele E, Sarkar B, Alexandridis P (2012) Polyhedral oligomeric silsesquioxane (POSS)-containing polymer nanocomposites. Nanomaterials 2(4):445–475. https://doi.org/10.3390/nano2040445
Bourbigot S, Turf T, Bellayer S, Duquesne S (2009) Polyhedral oligomeric silsesquioxane as flame retardant for thermoplastic polyurethane. Polym Degrad Stab 94:1230–1237. https://doi.org/10.1016/j.polymdegradstab.2009.04.016
Majka TM, Raftopoulos KN, Pielichowski K (2018) The influence of POSS nanoparticles on selected thermal properties of polyurethane-based hybrids. J Therm Anal Calorim 133(1):289–301. https://doi.org/10.1007/s10973-017-6942-8
Monticelli O, Fina A, Cavallo D, Gioffredi E et al (2013) On a novel method to synthesize POSS-based hybrids: an example of the preparation of TPU based system. Express Polym Lett 7(12):966–973. https://doi.org/10.3144/expresspolymlett.2013.95
Gu SY, Jin SP, Liu LL (2015) Polyurethane/polyhedral oligomeric silsesquioxane shape memory nanocomposites with low trigger temperatur and quick response. J Polym Res 22:142. https://doi.org/10.1007/s10965-015-0779-2
Koutsoumpis S, Raftopoulos KN, Jancia M, Pagacz J et al (2016) POSS moieties with PEG vertex groups as diluent in polyurethane elastomers: morphology and phase separation. Macromolecules 49(17):6507–6517. https://doi.org/10.1021/acs.macromol.6b01394
Gnanasekaran D, Walter PA, Parveen AA, Reddy BSR (2013) Polyhedral oligomeric silsesquioxane-based fluoroimide-containing poly(urethane-imide) hybrid membranes: synthesis, characterization and gas-transport properties. Sep Purif Technol 111:108–118. https://doi.org/10.1016/j.seppur.2013.03.035
Song J, Chen G, Wu G, Cai C et al (2001) Thermal and dynamic mechanical properties of epoxy resin/poly(urethane-imide)/polyhedral oligomeric silsesquioxane nanocomposites. Polym Adv Technol 22(12):2069–2074
Bourbigot S., Duquesne S., Fontaine G., Bellayer S et al. (2008) Characterization and reaction to fire of polymer nanocomposites with and without conventional flame retardants. Mol Cryst Liq Cryst 486(1): 325/1367–339/1381
Fomenko AA, Gomza YP, Klepko VV, Gumenna MA et al (2009) Dielectric properties, conductivity and structure of urethane composites based on polyethylene glycol and polyhedral silsesquioxane. Polym J 31(2):137–143
Oaten M, Choudhury NR (2005) Synthesis and characterization of a POSS-urethane hybrid coating for use in the corrosion protection of metal. J Metastable Nanocrystalline Mater 23:231–234
Imai G, Inada Y, Matsuura Y, Nagai A (2013) Radiation-curable compositions with good curability under oxygen, and their coated scratch-resistant articles. JP Patent 2013018848
Hebda E, Ozimek J, Raftopoulos KN, Michałowski S et al (2015) Synthesis and morphology of rigid polyurethane foams with POSS as pendant groups or chemical crosslinks. Polym Adv Technol 26(8):932–940. https://doi.org/10.1002/pat.3504
Schwab J, Lichtenhan J, Carr M, Chaffee K et al (1997) Hybrid nanoreinforced polyurethanes based on polyhedral oligomeric silsesquioxanes. Polym Mater Sci Eng 77:549–550
Efrat T, Dodiuk H, Kenig S, Mccarthy S (2006) Nanotailoring of polyurethane adhesive by polyhedral oligomeric silsesquioxane (POSS). J Adhes Sci Technol 20(12):1413–1430
Jana SC, Duan Y, Wang X, Shinko A (2013) Chemical and engineering issues of functional polymer aerogels. In: Abstracts of papers of the American Chemical Society Spring Meeting, New Orleans, 7–11 April 2013
Xu J, Song J (2007) Biodegradable shape memory poly (ester-urethane) nanocomposites strengthened by polyhedral silsesquioxane (POSS) core. In: Abstracts of the 23th ACS National Meeting, Boston, 2007
Lai YS, Tsai CW, Yanga HW, Wang GP et al (2009) Structural and electrochemical properties of polyurethanes/polyhedral oligomeric silsesquioxanes (PU/POSS) hybrid coatings on aluminum alloys. Mater Chem Phys 117(1):91–98. https://doi.org/10.1016/j.matchemphys.2009.05.006
Wang X, Hu Y, Song L, Xing W et al (2011) UV-curable waterborne polyurethane acrylate modified with octavinyl POSS for weatherable coating applications. J Polym Res 18:721–729. https://doi.org/10.1007/s10965-010-9468-3
Devaux E, Rochery M, Bourbigot S (2002) Polyurethane/clay and polyurethane/POSS nanocomposites as flame retarded coating for polyester and cotton fabrics. Fire Mater 26(4–5):149–154. https://doi.org/10.1002/fam.792
Lakhani HA, Mel A, Seifalian AM (2015) The effect of TGF-β1 and BMP-4 on bone marrow-derived stem cell morphology on a novel bioabsorbable nanocomposite material. Artif Cells Nanomed Biotechnol 43(4):230–234
Maqsood A, Hamilton G, Seifalian AM (2010) Viscoelastic behaviour of a small calibre vascular graft made from a POSS-nanocomposite. In Abstracts 2010 annual international conference of the IEEE engineering in medicine and biology
Kannan R, Salacinski HJ, Sales KM, Butler PE et al (2006) The endothelialization of polyhedral oligomeric silsesquioxane nanocomposites: an in vitro study. Cell Biochem Biophys 45(2):129–136
Guo YL, Wang W, Otaigbe JU (2010) Biocompatibility of synthetic poly(ester urethane)/polyhedral oligomeric silsesquioxane matrices with embryonic stem cell proliferation and differentiation. J Tissue Eng Regen Med 4(7):553–564
Wu J, Gu X, Mather PT (2010) Biostable multiblock thermoplastic polyurethanes incorporating poly(ε-caprolactone) and polyhedral oligomeric silsesquioxane (POSS). Trans Annu Meet Soc Biomater 1(84)
Gupta A, Vara DS, Punshon G, Sales KM et al (2009) In vitro small intestinal epithelial cell growth on a nanocomposite polycaprolactone scaffold. Biotechnol Appl Biochem 54(4):221–229
Kannan RY, Salacinski HJ, Ghanavi JE, Narula A (2007) Silsesquioxane nanocomposites as tissue implants. Plast Reconstr Surg 119(6):1653–1662. https://doi.org/10.1097/01.prs.0000246404.53831.4c
Mel A, Chaloupka K, Malam Y, Darbyshire A et al. (2012) A silver nanocomposite biomaterial for blood-contacting implants. J Biomed Mater Res Part A 100(9)
Sedaghati T, Jell G, Seifalian A (2014) Investigation of Schwann cell behaviour on RGD-functionalised bioabsorbable nanocomposite for peripheral nerve regeneration. N Biotechnol 31(3):203–213
Mel A, Punshon G, Ramesh B, Sarkar S et al. (2009) In situ endothelialisation potential of a biofunctionalised nanocomposite biomaterial-based small diameter bypass graft. Biomed Mater Eng 19(4–5):317–331. https://doi.org/10.3233/bme-2009-0597
Adwan H, Fuller B, Seldon C, Davidson B et al (2013) Modifying three-dimensional scaffolds from novel nanocomposite materials using dissolvable porogen particles for use in liver tissue engineering. J Biomater Appl 28(2):250–261
Antoniadou EV, Ahmad RK, Jackman RB, Seifalian AM (2011) Next generation brain implant coatings and nerve regeneration via novel conductive nanocomposite development. In: 2011 annual international conference of the IEEE engineering in medicine and biology society, 2011
Tan A, Madani S, Rajadas J, Pastorin G et al (2012) Synergistic photothermal ablative effects of functionalizing carbon nanotubes with a POSS-PCU nanocomposite polimer. J Nanobiotechnology 10(1):34–42
Acknowledgements
Authors are grateful to the Polish National Science Center for support under Contract No. 2017/27/B/ST8/01584.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hebda, E., Pielichowski, K. (2018). Polyurethane/POSS Hybrid Materials. In: Kalia, S., Pielichowski, K. (eds) Polymer/POSS Nanocomposites and Hybrid Materials. Springer Series on Polymer and Composite Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-02327-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-02327-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-02326-3
Online ISBN: 978-3-030-02327-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)