Abstract
Extracorporeal life support (ECLS) encompasses a spectrum of temporary mechanical support for patients with heart or lung dysfunction that does not respond to traditional critical care therapy. The term ECMO (extracorporeal membrane oxygenation) is often used when the primary indication for support is failure of gas exchange. The physiologic goals vary with patient needs but may include oxygenation and CO2 removal, increased oxygen delivery via improved perfusion and the provision of lung rest and cardiac unloading. ECLS is available in an increasing number of specialised centres and may be of short duration (hours) to facilitate a surgical procedure or provide support for prolonged periods (weeks to months).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Extracorporeal support is an established therapy in perioperative care of lung transplant recipients.
-
New applications of intraoperative extracorporeal life support (ECLS) are increasingly being explored in thoracic surgery.
-
In clinical scenarios which compromise oxygenation, VV-ECMO is most often used. These include procedures for patients with airway obstruction, require an open airway or have advanced lung disease.
-
Surgery which will compromise cardiac function or great vessel flow is best supported by VA-ECMO. These include procedures for life-threatening mediastinal masses and locally advanced malignancies requiring resection of the atrium or great vessels.
-
Unanticipated perioperative respiratory failure may require extracorporeal support.
-
Meticulous and multidisciplinary preoperative assessment and planning are essential. The anaesthesiologist should understand the technical capability and limitations of the ECLS device.
-
Critically ill patients may require cannulation of vessels under local anaesthesia, which may be technically challenging and uncomfortable but best done electively rather than emergently.
-
ECLS applied to thoracic surgery is not as yet supported by strong medical evidence, but can be life-saving in individual cases.
Introduction
Extracorporeal life support (ECLS) encompasses a spectrum of temporary mechanical support for patients with heart or lung dysfunction that does not respond to traditional critical care therapy. The term ECMO (extracorporeal membrane oxygenation) is often used when the primary indication for support is failure of gas exchange. The physiologic goals vary with patient needs but may include oxygenation and CO2 removal, increased oxygen delivery via improved perfusion and the provision of lung rest and cardiac unloading. ECLS is available in an increasing number of specialised centres and may be of short duration (hours) to facilitate a surgical procedure or provide support for prolonged periods (weeks to months).
The dramatic expansion of ECLS applications during the last decade is evidenced by an ever-increasing number of annual ECLS-related publications. It is a challenge for the interested clinician to keep up with the latest literature on the topic [1]. Use of ECLS for cardiac failure and cardiac procedures is beyond the scope of this chapter. The highest level of evidence for the clinical benefit of all the ECLS technologies in both respiratory and cardiac disease are randomised controlled trials of ECMO in acute respiratory distress syndrome (ARDS); all other applications are described in case series or cohort studies [2].
This chapter endeavours to provide the anaesthesiologist with a clear understanding of the nomenclature used to describe ECLS, the configurations of ECLS circuits and their physiological effects and the applications described for support of the thoracic surgical patient population both in the critical care unit and the operating room. Most applications in thoracic surgery require some degree of gas exchange support and therefore for the purposes of this chapter will be referred to as ECMO.
Brief History
ECMO first became an established practice in neonates with hyaline membrane disease and has been used in that context since the 1970s. In adults, although it had been occasionally reported as a rescue technique for the critically ill for decades, high mortality and significant neurological complication rate prevented its widespread use. However, interest was renewed in the intensive care population during the influenza outbreak of 2009 [3]. The CESAR trial demonstrated a 6-month survival benefit with ECMO use versus optimal conventional ventilator management of ARDS [4]. Additionally, streamlined ECLS technology has improved the feasibility of transporting critically ill patients from outside facilities to specialised ECLS-capable centres. In the last decade, ECMO has become a widely accepted supportive treatment for severe respiratory failure in the ICU. Improved survival has also been observed in end-stage lung disease patients supported with ECMO as a bridge to transplant and for severe primary graft dysfunction as a bridge to recovery. The growing experience of thoracic surgical teams in operating on patients on ECMO, either from the ICU for diagnostic or therapeutic procedures or for lung transplantation, has meant that this form of support is increasingly for elective surgical situations where adequate gas exchange cannot be guaranteed. Historically, full cardiopulmonary bypass (CBP) has been the most typical form of extracorporeal mechanical and gas exchange support for complex thoracic surgery. Use of CPB has the known disadvantages of the need for full heparinisation, increased transfusion requirements, pump-related inflammatory response and the potential recirculation of tumour cells in oncology cases. Technological improvements offered by ECMO include the miniaturisation of circuits requiring lower priming volumes, limited air/blood contact by closed circuits without cardiotomy suction/reservoir and improved biocompatibility of material used in circuit components [5].
Components of the ECMO Circuit
The equipment used for mechanical cardiac or respiratory support have evolved from components of cardiopulmonary bypass for cardiac surgery. The original ECMO circuits used a CPB circuit stripped down to an oxygenator, pump, heating coil and minimal volume tubing, with no reservoir. There now exists a wide spectrum of different circuit configurations and cannulation options for mechanical support, but the basic components remain similar. It is important to understand the different components of the circuit being used, as they directly affect aspects of anaesthetic management such as oxygenation, anticoagulation, monitoring, transfusion, inotrope and volume status management (see Fig. 27.1).
Typical bedside ECLS circuit (back and front views shown). (a) Display with revolutions per minute, pump flow and resistance display (lower line). (b) Pump control terminal. (c) Oxygenator heater, (d) Air and oxygen wall gas inlet, (e) Sweep gas flowmeter, (f) Centrifugal pump, (g) Oxygenator (note oxygenated blood line marked with red tape)
Oxygenator
The oxygenator in an ECMO circuit channels blood down hollow fibres, with a counter current gas flow surrounding the fibres. Gases diffuse down concentration gradients, a similar mechanism to haemodialysis. The rate of gas exchange along the fibres can be controlled by altering the gas flow (sweep) and blood flow to change the partial pressure difference of oxygen and carbon dioxide across the membrane. Oxygenation is mainly a function of blood flow, while CO2 removal can be modified by regulating the sweep. Modern membrane oxygenators are compact and have a very low resistance to flow. They are susceptible to fibrin clots making them first less efficient and then eventually fail; however, this usually occurs after weeks of support, and anticoagulation is used to delay this. In addition, their delicate structure makes them susceptible to trauma.
Pump (When Applicable)
In CPB both roller pumps and centrifugal pumps are used. However, centrifugal pumps cause less haemolysis during long-term use, so in ECMO they are used almost exclusively. Centrifugal pumps are composed of a disposable cone which sits on top of a spinning magnet and rotate through magnetic coupling. The centrifugal force generated by this rotation causes a pressure difference across the cone which drives the blood flow. Pump flow is determined by this pressure difference, as well as preload and afterload, in a non-linear way. There is limited scope for the perfusionist to increase the cardiac output by turning up the revolutions per minute, as decoupling of the magnets can take place. Compared to CPB, the amount of flow is much more limited by technical design of the pump. Crucially, centrifugal pump flows are dependent on both preload and afterload conditions. If preload drops or the drainage line occludes, the centrifugal pump generates high negative pressures, and the cone slows down causing the flow to drop. This is seen clinically as “chugging” of the drainage line requiring restoration of patient volume to maintained desired flow. If afterload suddenly increases, the pressure difference across the pump drops and flow slows. Retrograde flow can happen, which would exsanguinate the patient or aspirate air from the reinfusion cannula site. If centrifugal pump flows drop too much, the perfusionist must clamp the arterial line and come off support altogether until the problem is rectified. This means that in a haemodynamic crisis, support may be suddenly terminated which can be difficult to manage.
Reservoir, Circuit and Connectors
A major step in the evolution of ECMO from CPB pumps was the removal of components such as the venous reservoir, three-way taps, stop cocks and shortening of lines. This has the effect of reducing the priming volume required for an ECMO circuit, minimising dilution. The use of heparin-bonded circuit components and removal of the large reservoir, filled with static blood often with an air/blood interface, means that the anticoagulation requirement is much lower (activated clotting times of 160–200 s compared to >400 s for CPB [6]). Since the ECMO circuit is completely closed, the risk of accidental air entrainment is much reduced (a requirement for long-term use). Unlike a CPB circuit, there are no infusion ports meaning that all drugs, blood products and replacement volume must be delivered by direct intravenous access into the patient. Shed blood must be processed in a cell saver prior to reinfusion.
ECMO Configurations
Configurations of ECMO
Several different ECMO configurations are possible, with varying levels of gas exchange capacity and haemodynamic support (summarised in Table 27.1).
Veno-venous ECMO
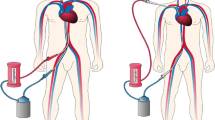
VV-ECMO is used in refractory respiratory failure and requires only peripherally placed venous catheters. Blood is drained from and reinfused into central veins. In the most common configuration with early use of VV-ECMO, deoxygenated blood drained via a femoral catheter was actively pumped though the membrane oxygenator and returned via a jugular catheter (Fig. 27.2). Oxygenated blood is reinfused into the right atrium (RA) and ejected into the pulmonary circulation by the patient’s own cardiac output, mixing with the deoxygenated venous return. VV-ECMO has no direct effect on cardiac function; however, myocardial dysfunction associated with hypoxia and respiratory acidosis will improve. Additionally, oxygenated blood entering the pulmonary circulation will reduce hypoxic pulmonary vasoconstriction, increasing the shunt fraction but decreasing right heart afterload. It is not unusual for depressed right ventricular function to improve after institution of VV-ECMO. In TEE assessment of the reinfusion cannula, the recommended position of the tip is in the RA just beyond the IVC/RA junction, a safe distance away from the intra-atrial septum and the tricuspid valve [9].
Veno-venous (VV)-ECMO with drainage of deoxygenated blood from the inferior vena cava (IVC) via cannulation of a femoral vein and reinfusion of oxygenated blood into the superior vena cava (SVC) via cannulation of the jugular vein. Oxygenated blood is ejected into the pulmonary circulation by the patient’s right heart function. (a) Mid-oesophageal TEE view verifies the position of the outflow cannula in the SVC. (b) TTE subcostal view visualises the inflow cannula within the long axis of the IVC. (Adapted with permission from Doufle et al. [8])
The development of bi-caval dual-lumen catheters allowed for inflow and outflow from a single percutaneous site, usually the right jugular vein (Fig. 27.4). Optimal cannula positioning is essential for good oxygenation; specifically, the infusion jet should be directed towards the tricuspid valve. TEE assessment of position and flow is very helpful, although fluoroscopy may also be used to guide venous catheter placement. By avoiding femoral cannulation, patient mobilisation is improved and participation in rehabilitation is possible, which is important in patients being bridged to transplant.
In VV-ECMO there is a functional dissociation of decarboxylation and oxygenation. Oxygenation varies primarily with blood flow through the membrane oxygenator, while CO2 removal is dependent on the gas sweep past the membrane. Oxygenation also varies with the ratio of the circuit blood flow to the patient’s cardiac output. Blood flow of 3–6 L/min is typically required to maintain acceptable oxygenation in patients with severe lung injury. The higher range of ECMO blood flow may be required to oxygenate an unventilated surgical patient with a hyperdynamic circulation. If the lungs are not severely injured and can be partially ventilated with oxygen, oxygenation can be maintained with lower flows. Low-flow VV-ECMO via small-sized cannula can achieve blood flow of up to 1.0 L/min which is sufficient for CO2 removal but provides very little oxygenation [10].
Veno-arterial ECMO
VA-ECMO is used for cardiocirculatory support with or without respiratory failure. Blood is drained from the venous side and reinfused arterially; for peripheral VA-ECMO in adults, the femoral vessels are most often used for cannulation (Fig. 27.3). Blood drained from the RA is pumped through the membrane oxygenator and reinfused in the mid-aorta. Oxygenated blood mixes with the blood ejected from the heart with direct perfusion of the central organs. Physiological effects include unloading of the RV; when ECMO blood flows are high, much of the patient’s venous return bypasses the pulmonary circulation. While organ perfusion pressure is improved, left ventricular afterload is increased as blood from the ECMO circuit is reinfused at arterial pressure. The cannula chosen should be large enough to allow VA-ECMO blood flow up to 2 L/min higher than the calculated cardiac output [11]. Because of the large size of the femoral arterial cannula required, distal limb perfusion may be compromised, and a distal arterial perfusion catheter is routinely placed. Disadvantages of VA-ECMO include the risk of arterial cannulation: arterial injury, bleeding and embolism, distal limb ischemia and cardiac thrombus if low flows are maintained through the heart. TEE can be used to assessing the drainage cannula position, ideally in the lower part of the RA, and decompression of the right side of the heart, as well as overall heart function.
Veno-arterial (VA)-ECMO with drainage of deoxygenated blood from the IVC via cannulation of a femoral vein and reinfusion of oxygenated blood into the ascending aorta via cannulation of a femoral artery. (a) Four-chamber view visualised by a TTE four-chamber view to assess cardiac function. (Adapted with permission from Doufle et al. [8])
Central cannulation directly in the left atrium and ascending aorta enables placement of larger cannula and higher ECMO blood flows but requires an open chest and is generally reserved for operative cases that require haemodynamic support, including lung transplantation.
Patients on VV-ECMO for respiratory failure who subsequently develop haemodynamic instability can be supported by the addition of an arterial cannula for a hybrid configuration called veno-veno-arterial ECMO. Both venous catheters are used for drainage into a single ECMO circuit with reinfusion via a third (usually femoral) catheter.
AV-ECMO (Pumpless)
The Novalung interventional lung assist device (Novalung GmbH, Hechingen, Germany) is a low-resistance gas exchanger designed for pulsatile blood flow driven by the patient’s cardiac output. A drainage catheter is inserted into a femoral artery and reinfusion catheter into a femoral vein. The two are connected to the Novalung via a short, pumpless circuit. The blood flows generated are dependent on cardiac function and arterial blood pressure and are in the range of 1–2.5 L/min. This allows full decarboxylation, but only partial oxygenation, so this modality is particularly useful for treating hypercapnic respiratory failure [10].
Pumpless configurations have the advantage of greater portability and low priming volumes. They are practical for inter-hospital transport [12]. Once hypercarbia is controlled, many patients can be extubated, be mobilised and participate in rehabilitation [13].
PA-LA Novalung (Pumpless)
Another pumpless configuration is applicable only to patients with pulmonary hypertension and impending right heart failure. The Novalung is connected via a short tubing circuit between a drainage cannula placed directly into the main PA and reinfusion catheter inserted in the left atrium via the right upper pulmonary vein. High right-sided pressures drive the flow through the low-resistance Novalung. This oxygenated right to left shunt has the effect of unloading the RV, reduced septal shift, allowing better LV filling, coronary perfusion and leading to improved RV function. This modality involves central cannulation via a sternotomy; patients who are awake but haemodynamically unstable may need femoral cannulation for peripheral VA-ECMO to safely tolerate the induction of general anaesthesia, with planned weaning when the right heart is decompressed. Intraoperative TEE does not direct the cannula positioning; however, it is vital for assessing adequate unloading of the RV and the status of LV function. It is crucial to verify that the LV is able to tolerate increased blood flow without failing. In the event of LV decompensation, longer-term VA-ECMO would be required. At the end of the procedure, the PA and LA cannulas are advanced through the skin of the upper abdomen and the chest closed. Used as a bridge to transplant, many patients can be extubated and subsequently mobilised [7, 14].
Applications in Thoracic Surgery
Lung Transplantation
ECMO is being used at an increasing number of specialised centres to bridge deteriorating patients with end-stage lung disease to lung transplant surgery. ECMO-bridged patients are at an increased risk of perioperative morbidity and mortality but do have an excellent 1-year survival (>90%) [2]. Given that without pre-transplant ECLS most of the ECMO-bridged patients would have died, this is increasingly an accepted practice. Many patients with hypercapnic respiratory failure can be bridged with pumpless AV-ECMO or preferably low-flow VV-ECMO. Those with significant hypoxia will require full-flow VV or if haemodynamically unstable peripheral VA-ECMO. In the subgroup with severe pulmonary hypertension, VA-ECMO or PA-LA Novalung is used [14].
Lung transplant surgery is characterised by dynamic haemodynamic and respiratory compromise, the most challenging being systemic hypotension, pulmonary hypertension and hypoxemia (see Chap. 47). ECMO is supplanting CBP as the favoured form of ECLS to stabilise patients. Typically, when ECMO is initiated intraoperatively, a VA-ECMO configuration via central cannulation sites is used. In a recent case-control cohort study of patients where cardiopulmonary support was used intraoperatively, those managed with ECMO had better early outcomes including duration of mechanical ventilation, ICU and hospital length of stay than patient who went on CBP. Blood product usage was significantly less in the ECMO patients [15].
VV-ECMO is the most common mode for preoperative bridging. When these patients undergo their transplant, some patients can be adequately supported with continued VV support. Many though require higher flows or increased haemodynamic support, often with a hybrid configuration. Patients who arrive in the operating room with a jugular dual-lumen catheter and with the addition of a central aortic catheter can receive veno-venous-arterial support with drainage via the bi-caval lumen and reinfusion of oxygenated blood into both the atrial lumen and into the aorta. Alternately, the VV-ECMO can be maintained unchanged via the jugular catheter, and additional central IVC and aortic cannulas can be placed as separate circuit for simultaneous VA-ECMO (personal communication Dr. Marcelo Cypel). The VV-ECMO is maintained during the surgery to avoid any thrombotic problem in the circuit and thus can be resumed postoperatively after weaning VA-ECMO if necessary.
Upon completion of the transplant, ECMO will be weaned for assessment of graft function. While desirable to decannulate the patient, the function of the allograft dictates the withdrawal of support. Some patients are decannulated of all extracorporeal support, patients with hypoxemia may be converted to VV, and those with pulmonary hypertension or severe myocardial dysfunction need continued VA-ECMO, requiring a later return to the operating room for decannulation. Any form of postoperative ECMO has the disadvantage of increased bleeding risk from continued anticoagulation after major surgery. Lung transplant recipients requiring ECMO for PGD have decreased survival as compared to those without PGD, but similar outcomes to patients with PGD managed without ECMO. Bridge to recovery support with VA-ECMO has a much higher 30-day mortality than VV-ECMO [2].
Airway Surgery
ECMO may be used to support patients with critical airway obstruction. Lesions above the larynx may be managed with awake fibre-optic intubation or tracheostomy under local anaesthesia. When severe obstruction is encountered below the larynx, while intubating the vocal cords may not be difficult, ventilation may be impossible. While some lesions may be managed by rigid bronchoscopy and debridement with intermittent ventilation (see Chaps. 11 and 13), extensive tracheal obstruction, need for prolonged debridement and significant bleeding into the airway present formidable problems. Historically, full CPB support was instituted via femoral venous and arterial catheters placed under local anaesthesia [16]. More recently, VV-ECMO support has been reported for endotracheal tumour resection [17, 18], dislodged stents [17, 19], foreign body removal [20], control of haemoptysis [21], debridement of papillomatosis [22] and relief of external compression [17, 23, 24] in adults. A variety of cannulation strategies have been reported: femoral and jugular single-lumen catheters [17, 20, 22] and bi-caval dual-lumen catheter in the right jugular vein [18, 24] have been described. Death as a result of post-resection haemoptysis has been reported likely exacerbated by anticoagulation for ECMO [17]. Relief of airway obstruction in small children has been performed with both VV- and VA-ECMO [25].
ECMO management has been reported after resuscitation from cardiopulmonary arrest due to airway obstruction; both VA [19] and VV [18] have been used. While VV support was successful in a hypoxic patient where haemodynamic stability had been restored, if there is postarrest myocardial dysfunction, VA-ECMO is a better choice. Both reports describe ECMO support during restoration of airway patency, conversion to conventional mechanical ventilation and a short period of observation for neurological sequelae, followed by successful extubation.
An additional challenge presented by tracheal resection and reconstruction is continued oxygenation and ventilation during the period with an open airway. Distal airway management often involves cross-field ventilation with an endotracheal tube or jet ventilation with a tracheal catheter, both of which may obstruct the surgical field (see Chap. 13). Complex reconstruction may be facilitated with ECMO support, as can open airway procedures in patients with limited respiratory reserve [26, 27]. Advanced tumours requiring extensive en bloc resection have been managed with VA-ECMO due to the need for vigorous retraction of the heart and great vessels. Both central and peripheral cannulation has been described [26]. VV-ECMO support is more often reported and is suitable for tracheal and carinal resection including carinal pneumonectomy [28,29,30]. An unusual case of repair of a complex tracheoesophageal fistula was reported using pumpless AV-ECMO, with a low-resistance circuit placed between femoral arterial and venous catheters for CO2 removal. Apnoeic oxygenation was provided via a small endotracheal tube at the carina, allowing 12 h of surgery under without ventilation of the lungs [31]. Tracheal resection has also been described with AV-ECMO [32]. When VV-ECMO provides insufficient oxygenation during tracheal surgery, supplemental catheter oxygen insufflation had also been used [29].
Impossible One-Lung Ventilation
Patients under consideration for thoracic surgery may have limited pulmonary reserve, making oxygenation with one-lung ventilation impossible. Successful prior management of lung cancer may result in patients who have undergone pneumonectomy presenting with contralateral disease. Post-pneumonectomy patients have undergone contralateral wedge resection and segmentectomy for second malignancies [30, 33], VATS bullectomy [34] and esophagectomy [35] with VV-ECMO support. A low-flow VV-ECMO was noted to suffice in a subgroup of 3 of these patients undergoing segmentectomy. A dual-lumen catheter inserted in a femoral artery and an average of 1.6 L/min blood flow and sweep gas flow of 5 L/min through the oxygenator maintained stable blood gases for over 45 min. Pumpless AV-ECMO has also been used to facilitate wedge resection and decortication in post-pneumonectomy patients [32].
Patients who have advanced pulmonary parenchymal lung disease may present for vital thoracic surgery procedures; however, may not tolerate OLV. There are no guidelines to determine which patients will need extracorporeal support and who will tolerate the procedure without. Arterial PaO2 on two-lung ventilation, FEV1/FVC ratio and side of operation are usually good predictors of hypoxia on OLV [36]; however, this information may be limited on critically ill patients and may not reflect new pulmonary co-morbidities such as empyema or an air leak. Patients for empyema surgery frequently have necrotising pneumonia or bronchiectasis, and VV-ECMO has been instituted intraoperatively to allow aggressive decortication of empyema [37]. The bilateral decortication of critically ill, VV-ECMO-dependent patients was proposed to be a crucial step in their weaning from ECMO, followed by successful extubation [38].
Patients with emphysema are prone to ruptured bullae and prolonged air leaks, and VATS resection of bullae has been with both VV-ECMO support [30] and AV-ECMO [32]. In the late 1990s, elective intraoperative initiation of ECMO was used to support three COPD patients with severe hypercapnia undergoing LVRS, who therefore did not meet standard selection criteria for this procedure [39]. VA-ECMO was chosen due to the anticipated haemodynamic instability often seen with positive pressure of patients with pulmonary hyperinflation. This must be described as a controversial treatment plan as severe hypercapnia is a risk factor for perioperative mortality. This practice has not been reported by other groups. VV-ECMO has been used to facilitate LVRS/major bullectomy to liberate COPD patients from mechanical ventilation [40, 41].
Additionally, VV-ECMO has been used to enable patients with significant loss of lung parenchyma to tolerate resections: metastasectomy after prior extensive lung resection [30], bullectomy [42] and resection of aspergilloma [43] after scarring from tuberculosis have been reported.
Finally, patients with alveolar proteinosis with progressive hypoxia require whole lung lavage of both lungs with large volumes of saline to remove lipoprotein deposits. Classically, this has been done sequentially via a double-lumen tube, but severely affected patients may not tolerate this, and there are many case reports describing VV-ECMO support to enable lavage in this patient group [44,45,46,47,48,49,50,51,52,53,54,55].
Mediastinal Masses
Large masses occupying the anterior mediastinum have the potential to cause compression of great vessels and right atrium, causing reduction in preload and cardiac output. In the awake patient, the negative pressure generated by inspiration reduces this effect so that compression is minimal until surgical disease is advanced. However, this is abolished by muscle relaxants, and positive pressure ventilation can precipitate collapse of cardiac output and arrest. This is a particular risk for children with large anterior lymphomas. Historically, this has been treated with CPB support [56,57,58,59]. More recently, preinduction femoral VA-ECMO while maintaining spontaneous ventilation has been described in children [60] and adults [61]. The VA configuration is warranted by the potential for cardiovascular collapse. This is a well-known problem in thoracic anaesthesia, and the risk assessment for extracorporeal support is described in more detail in Chap. 14.
Advanced Surgical Resections
Late presentation of intrathoracic cancer with local invasion historically was deemed unresectable. However, tertiary referral centres are increasingly undertaking resection of advanced lung and oesophageal malignancies as part of multimodal therapy and reporting reasonable survival [62,63,64,65,66,67,68,69,70,71,72]. The most common extrapleural site of invasion for lung cancer is the left atrium. Resection requires clamping the affected area of the atrial wall, which causes haemodynamic instability which can be prolonged as a patch repair or primary closure of the atrium is required before the clamp can be released. This can be achieved in some cases without extracorporeal support [63, 73]. Resection of lung malignancies with reconstruction of the superior or inferior vena cava, left atrium, distal aorta and carina has been described with VA-ECMO support [71] as have oesophageal malignancies invading carina [5]. Cannulation sites are chosen according to the planned resection and may be central or peripheral [71]. Resection of complex thoracic malignancies with extension into the pulmonary trunk, the aortic arch or the heart requiring opening a cardiac chamber is only amenable to traditional CPB [71, 74,75,76].
Thoracic Emergencies
ECMO has been described as a life-saving emergency treatment of massive haemoptysis [21, 77, 78]. Intraoperative iatrogenic large vessel injury with massive bleeding is most often managed with CPB, which provides the advantages of rapid infusion, autotransfusion and delivery of cardioplegia for cardiac arrest or deep hypothermic circulatory arrest if needed [5]. In the event of pulmonary artery injury, the reduction of blood flow through the pulmonary trunk enables surgical repair [5, 79], whereas ECMO has been shown to be of benefit in a wide variety of tracheal injuries to facilitate tracheal repair and healing. These include iatrogenic injury, postoperative fistulae and trauma [27, 80].
ECMO for primary graft dysfunction is frequently required after lung transplantation, to support the lungs, while reperfusion injury abates but has also been instituted emergently for other perioperative aetiologies of lung failure. These include haemodynamic stabilisation in patients with acute severe pulmonary embolism [81] to allow pulmonary embolectomy [82, 83], reperfusion injury after pulmonary thromboendarterectomy [84,85,86] for chronic thromboembolic pulmonary hypertension and the indications and methodology of which have recently been reviewed [7]. Support for postoperative transfusion-associated lung injury (TRALI) is also well-described [87, 88]. The importance of advanced planning for emergencies and effective multidisciplinary team-working has recently been emphasised in maintaining good patient outcomes [5]. In addition, ECMO has been used outside the thoracic operating rooms as part of resuscitation and surgical repair for thoracic trauma [37, 89,90,91,92].
The choice of VV- versus VA-ECMO in emergency circumstances is dependent on the need for primarily oxygenation versus the need for concomitant haemodynamic support. When urgent ECMO is instituted intraoperatively, available venous and arterial cannulation sites may be dictated by patient position and available vessels [5].
Preoperative Planning of ECMO
For any patient who requires extracorporeal support, the surgical checklist should include the planned ECMO configuration, cannulation sites, timing of cannulation (before or after induction of general anaesthesia) and anticipated timing of weaning and decannulation. Patient position and planned incision will influence cannulation sites [5]. If patients are transferred to the operating room already on ECMO, any required modification of support or additional cannulation should be discussed. This allows the anaesthesiologist to optimise intravenous and arterial access for monitoring and volume infusion.
Are the Proposed Vessels Big Enough?
The sizes of the cannulas currently available for ECMO are summarised in Table 27.2.
The size of the cannula required will depend on the patient’s body surface area (aiming for 2.5 L/min/m2), the achievable flow rate through the cannula without excessively high pressures (a technical property of the cannula available from the manufacturer). A large adult will need femoral vessels in the range of 8–10 mm in diameter, without atherosclerosis, stricture or tortuosity to be successful. This can be checked preoperatively with ultrasound or ultimately with angiography. Central cannulation typically allows placement of larger cannulas and consequently higher flows than peripheral ECMO.
Will the Patient Tolerate the Procedure?
An intrathoracic mass large enough to cause obstruction on induction may also cause positional symptoms, and such patients can often not lie flat for any length of time. Airway tumours also often cause positional dyspnoea. It is very difficult for the surgeon to insert these large cannulas into the groin with the patient in a semi-sitting position, and this must be done carefully to avoid iatrogenic vessel injury. In addition, even with meticulous local anaesthetic, the procedure can be painful for the patient as the cannulas are introduced and as the innervation of the vessels is visceral rather than somatic. Judicious sedation and analgesia may be helpful while maintaining respiratory drive and muscle strength. In the authors’ experience, a vasovagal response to awake femoral cannulation can provoke cardiopulmonary arrest, so cannulation should only be done in a fully monitored setting with resuscitative drugs and equipment immediately available.
Are the Cannulas Correctly Positioned?
Malpositioned cannulas have the potential to become obstructed or create recirculation (see “Troubleshooting ECMO”), causing the patient to become hypoxic. The position can be checked with TEE or fluoroscopically. The chosen imaging modality should be in place prior to induction.
What Are the Risks of Anticoagulation, and How Should It Be Managed?
Once the cannulas are in place, the patient must be sufficiently anticoagulated to prevent thrombosis, either at the tips of the cannulas or in the circuit. This potentially has implications for neuraxial analgesia and surgical blood loss, which can be significant and rapid, so appropriate intravenous access should be gained prior to insertion.
Historically, the predominant causes of adverse outcome from ECMO on ICU patients with respiratory failure have been major bleeding requiring surgical intervention, and intracerebral haemorrhage, particularly in neonates. ICU patients on long-term ECMO have low platelet function and numbers, fibrinogen deficiency, loss of vitamin K-dependent clotting factors and acquired Von Willebrand’s disease resulting in complex coagulopathy, which has been recently reviewed [94]. International guidelines from the Extracorporeal Life Support Organization (ELSO) [95] are summarised in Table 27.3. For CPB, anticoagulation practices are well-established [96], and a typical institutional protocol is given in Table 27.3 for comparison.
It should be emphasised that the risk/benefit gained from anticoagulation in the intraoperative setting is potentially different, and these guidelines were not designed for short-term operative use. For example, the use of a heparin-bonded circuit can reduce the heparin requirement substantially for the first 6 h of its use [95]. This doesn’t reduce the heparin requirement in the long term, but could substantially reduce the thrombosis risk in the intraoperative situation. Similarly, patients undergoing procedures with major blood loss will also be deficient in platelets, fibrinogen and other clotting factors, which will potentially prolong coagulation in the absence of heparin. There are multiple reports of surgical interventions with high bleeding risk using ECMO without heparin [21, 32, 33, 37, 38]. In our institution, we routinely target an ACT of 160–180 s for these reasons, checking at least every 30 min and giving small boluses of heparin as needed rather than running an infusion. We also routinely use ROTEM™ and platelet function assay, as an adjunct to ACT, to detect other potential causes of bleeding such as factor deficiency, platelet dysfunction or fibrinolysis.
Vascular Access
Two-cannula peripheral ECMO may use femoral vessels (often on both sides) or jugular and femoral vessels. A single-stage VV-ECMO cannula is usually placed in the right internal jugular, but axillary [97, 98] and supraclavicular cannulation sites have also been described [99, 100]. Placement of central lines should be planned in discussion with the surgeon, leaving the sites with the highest calibre for ECMO cannulas. If ECMO is already in place and central venous line placement is required, the potential for air entrainment is increased by the negative pressure generated by the drainage cannula. In VA-ECMO there is a risk of paradoxical arterial air embolism from venous bubbles, via the arterial return cannula (the ECMO circuit contains no bubble traps or filters).
Ventilation Strategies for ECMO
All configurations of ECMO are extremely efficient at clearing carbon dioxide, which means that in the ECMO patient the main determinant of paCO2 is sweep gas flow and carbon dioxide tension, not the patient’s minute volume. Alveolar pO2 is still important to preserve when possible, as it contributes to oxygenating the remaining pulmonary blood flow. Tidal volumes and respiratory rate can be reduced far beyond what is normally possible. Given the established link between mechanical ventilation and lung injury, particularly in thoracic surgery and one-lung ventilation [101, 102], it would seem prudent to adopt a lung-protective ventilation strategy even in patients without prior injury, with tidal volumes 6–8 ml/kg (ideal body weight) preventing volutrauma. Prevention of atelectasis with 5-10 mmHg of PEEP will reduce atelectotrauma. Reducing the ventilator rate to 6–10 breaths a minute will allow for reduction in ventilator mean pressures, and longer rise times, with consequent reduction in strain forces which may be associated with acute lung injury. High alveolar oxygen tensions can exacerbate perioperative lung injury, so titrating FiO2 as low as saturations allow is advisable [103].
The efficacy of ECMO means that the patient can receive little to no ventilation and remain stable – so “ultraprotective” lung ventilation has been advocated for the ARDS patient group [104]. This comprises tidal volumes of less than 4 ml/kg, with PEEP >10 mmHg and a respiratory rate of 6 breaths/minute. The degree of protection is not mandatory for the perioperative patient, but is easy to achieve with ECMO support.
Monitoring Patients on ECMO
Monitoring considerations for patients on ECMO in the ICU has recently been authoritatively reviewed, with particular emphasis on the importance of TEE for cannula positioning [9]. Arterial lines are best placed in the right arm to measure pressure and PaO2 downstream from the brachiocephalic artery, which correlates most closely with conditions in the right carotid artery and coronary arteries. Central venous line location most likely will be dictated by the surgical incision, but care should be taken not to advance the tip of the catheter too far into the central circulation, so that it is not aspirated into the lumen of the ECMO cannula. Once the ECMO is started, central venous pressure monitoring may not be reliable. Pulmonary artery (PA) catheters may be very difficult to insert if upper body ECMO cannulas are already in place, both in terms of passing the catheter through the occupied SVC and in floating into the right heart with ongoing drainage into the ECMO circuit. It is possible for the PA catheter balloon to be aspirated into a drainage cannula and occlude it completely. Seeing a pulsatile PA tracing is a reassuring sign that volume replacement is adequate.
Peripheral saturation probes should be placed on the right hand, to guard against diffusion hypoxia. Consideration should be given to cerebral saturation monitoring with near-infrared spectroscopy, as an indicator of cerebral hypoxia. In a short retrospective case series using this monitor, Wong et al. found a 100% rate of cerebral desaturation on initiation of ECMO, 80% of which were reversible with changes in haemodynamic management [105].
Troubleshooting ECMO
Hypoxia
Flows Are Insufficient
VV-ECMO is dependent on sufficient venous blood flow through the oxygenator before returning it to the right atrium. If flow through the oxygenator is much less than the total cardiac output, the difference will pass through the unventilated pulmonary circulation, and the arterial saturation will be reduced by this mixing. In severe respiratory failure, VV-ECMO flows of 60% of cardiac output are required to maintain an SpO2 over 90% [106]. The solution is to oxygenate via the lungs if possible, optimise venous drainage, increase the ECMO flows if possible, increase the sweep gas to ensure full oxygenation of the return blood and if necessary reduce the cardiac output. When this problem occurs on the ICU, beta-blockers are occasionally used, but their use should only be considered carefully in the unstable patient in the operating room.
Recirculation
In VV-ECMO, recirculation of blood between the reinfusion and drainage catheters can occur. The intent is for the oxygenated return blood to pass through the tricuspid valve; therefore, the reinfusion ports should be in the right atrium. Oxygenated reinfused blood may flow into a single-lumen drainage catheter if the catheters are too close together. Recirculation can also occur with bi-caval dual-lumen catheters particularly if malpositioned and when higher flow rates are used but is less common than with two cannula configurations [107]. Echocardiography using colour Doppler can be useful for assessing ECMO cannula positions and direction of flows. The IVC cannula should be below the hepatic vein, SVC cannula should be at the SVC-RA border, and double-lumen cannulas should have the return port pointed at the tricuspid valve.
Differential Hypoxia/Watershed Phenomenon
In peripheral VA-ECMO, oxygenated arterial flow is retrograde from the femoral arterial reinfusion catheter back to the aortic arch. Reinfused blood competes with anterograde (deoxygenated) cardiac output ejected from the left ventricle. This means that while the lower body is perfused with highly oxygenated blood, there may be ongoing upper body hypoxia (see Fig. 27.4). Importantly, this may result in deoxygenated blood flow to the brain and coronary arteries [11]. This is a particular risk in the patient with poor lung function in the presence of preserved myocardial function. The solution is to increase ECMO blood flow: the effect of this is twofold, and it will reduce pulmonary blood flow while increasing the ECMO flow itself. The replacement of inotropes by purely vasopressor medications may also be helpful (Fig. 27.5).
Veno-venous (VV)-ECMO via a bi-caval dual-lumen catheter inserted via the jugular vein. Deoxygenated blood is aspirated via holes located in the drainage lumen of the catheter: proximally in the SVC and distally in the IVC. Oxygenated blood is reinfused via the atrial lumen with infusion port oriented towards the tricuspid valve (inset). (a) TEE mid-oesophageal bi-caval view of the cannula within the right atrium. (b) TTE subcostal view of the injection port facing the tricuspid valve and the tip of the cannula within the IVC. (Adapted with permission from Doufle et al. [8])
Differential hypoxia or the watershed phenomenon. In peripheral VA-ECMO, oxygenated blood reinfused into the descending aorta mixes with blood ejected from the left ventricle. The mixing point (or watershed) is typically located at the base of the aortic root but will vary depending on the ECMO flows and the patient’s cardiac output. In situations of severe lung injury with preserved myocardial function, deoxygenated blood ejected from the heart can force the watershed distally, resulting in hypoxemia of the heart and brain. The SaO2 in the right arm will be lower than the other limbs. This phenomenon is alternately called the “harlequin syndrome”
Hypotension
VA-ECMO Flows Are Insufficient
Check the drainage cannula: if it is chugging, flow may be obstructed (ask surgeon to check), the line may be kinked (perfusionist can check), the patient’s great veins are compressed (e.g. by extrinsic pressure or retraction), or the patient may be hypovolemic. Intravascular volume status is the responsibility of the anaesthesiologist, and volume must be delivered intravenously as the perfusionist has no reservoir. Check the reinfusion cannula; low flows can occur if the line is kinked or cannula is misplaced.
The Patient’s Afterload Is Too Low
Support of the patient’s SVR on ECMO is the responsibility of the anaesthesiologist, requiring infusion of vasopressors via a central venous line separate from the ECLS device. In addition, the ECMO flows vary with the level of support required and surgical manipulation. Inotropy is frequently required.
Weaning ECMO
VA-ECMO
At the end of the surgical procedure, if ECMO support is not planned into the postoperative period, VA-ECMO can be weaned by sequentially dropping the flows on the pump over a period of 10–20 min, with the lungs well recruited, suctioned and ventilated at protective tidal volumes. In preparation for this, the anaesthesiologist must ensure adequate filling, afterload and inotropy and be prepared to react quickly to deterioration. The right heart in particular is at risk of failure, as no cardioprotection is used during ECMO, and coronary perfusion may be threatened by the watershed phenomenon as described above. Pulmonary vascular resistance may be altered by the surgical procedure, and clots can form in the relatively empty heart on ECMO. A thorough TEE evaluation prior to and during weaning ECMO, monitoring right ventricular size and function in real time as flows are reduced, should be considered. Once flows are down to around 1 L/min, the ECMO pump is stopped altogether and the arterial line clamped. This process often reveals hypovolemia, which the anaesthesiologist should be prepared to treat. If the patient remains stable and the arterial blood gas is adequate after 5 min, the patient can be decannulated.
VV-ECMO
Weaning VV-ECMO takes place with full flows running and the sweep gas speed and FiO2 slowly being reduced. This gradually reduces the diffusion gradient of oxygen and carbon dioxide across the oxygenator membrane to zero, allowing the lungs to take over the process of gas exchange. Since flows are maintained, this process can be done slowly if necessary, with time for serial arterial blood gases, suctioning, bronchoscopy, recruitment manoeuvres and trials of high PEEP to optimise gas exchange. If blood gases remain good on low sweep flows, the pump can be stopped, and decannulation can take place.
Complications of ECLS
Most of the studies describing ECMO complications (see Table 27.4) have been in ICU populations, where ECMO use lasts for weeks rather than hours. The extent to which these risks can be extrapolated to short-term intraoperative use is unknown. Acute kidney injury, haemolysis and HIT are all complications of long-term ECMO use and are unlikely relevant intraoperative considerations. Incidence of infection is also correlated to the duration of ECMO [116]. In intraoperative ECMO, cannulation and surgical site haemorrhage, thrombotic events and vascular complications are probably the most relevant issues.
Neurological complications in ECMO are insidious and therefore potentially more common than Table 27.4 would suggest. For example, in a retrospective case series of adults who received ECMO for an average of 91 h, 50% suffered a variety of complications including subarachnoid haemorrhage, watershed ischemia and hypoxic-ischemic encephalopathy [117]. Overt stroke was rarely clinically diagnosed, in keeping with a rate of 2–4% as above, but nine out of ten patients who died had cerebral infarcts on autopsy. Similarly, in an autopsy study of patients who received emergency ECMO post cardiotomy, Rastan et al. [118] found an unrecognised cerebral infarct rate of 9%, unrecognised venous thromboembolism in 32% and unrecognised systemic embolism in 31%. In total 69–75% of long-term ECMO recipients were having subclinical thrombosis on autopsy [118, 119]. In addition, the rate of gaseous microembolism during ECMO support rivals that of CPB, with intravenous injections being the most common culprit, with potential for postoperative neurological morbidity [120]. For the perioperative context, simple maintenance of peripheral saturation and blood pressure are not necessarily sufficient to ensure adequate oxygen delivery to the brain – some form of active cerebral monitoring, such as near-infrared spectroscopy, should be considered [9, 105].
Fatal air embolism has been reported associated with performance of tracheostomy while on high-flow VV-ECMO with drainage via a bi-caval dual-lumen jugular catheter [121]. Inadvertent breach of the neck veins enabled the negative pressure in the SVC to aspirate air into the ECMO circuit. Suggested measures for avoiding this complication include temporary reduction of ECMO flows, performance of tracheotomy with the patient in a head down position and coverage of the puncture sites with wet compresses. The risk of air embolism by a similar mechanism could occur during placement of upper body central venous lines.
Abbreviations
- ACT:
-
Activated clotting time
- APTT:
-
Activated partial thromboplastin time
- CPB:
-
Cardiopulmonary bypass
- ECLS:
-
Extracorporeal life support
- ECMO:
-
Extracorporeal membrane oxygenation
- FEV1:
-
Forced expiratory volume in 1 second
- FVC:
-
Forced vital capacity
- HIT:
-
Heparin-induced thrombocytopenia
- ICU:
-
Intensive care unit
- INR:
-
International normalised ratio
- IVC:
-
Inferior vena cava
- OLV:
-
One-lung ventilation
- RA:
-
Right atrium
- SVC:
-
Superior vena cava
- SVR:
-
Systemic vascular resistance
- TEE:
-
Transoesophageal echocardiography
- TTE:
-
Transthoracic echocardiography
References
Squiers JJ, Lima B, DiMaio JM. A call for standardized end point definitions regarding outcomes of extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2017;153(1):147–8.
Squiers JJ, Lima B, DiMaio JM. Contemporary extracorporeal membrane oxygenation therapy in adults: fundamental principles and systematic review of the evidence. J Thorac Cardiovasc Surg. 2016;152(1):20–32.
Dalton HJ. Extracorporeal life support: moving at the speed of light. Respir Care. 2011;56(9):1445–53.
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet (London, England). 2009;374(9698):1351–63.
Machuca TN, Cypel M, Keshavjee S. Cardiopulmonary bypass and extracorporeal life support for emergent intraoperative thoracic situations. Thorac Surg Clin. 2015;25(3):325–34.
Esper SA, Levy JH, Waters JH, Welsby IJ. Extracorporeal membrane oxygenation in the adult: a review of anticoagulation monitoring and transfusion. Anesth Analg. 2014;118(4):731–43.
Machuca TN, de Perrot M. Mechanical support for the failing right ventricle in patients with precapillary pulmonary hypertension. Circulation. 2015;132(6):526–36.
Doufle G, Roscoe A, Billia F, Fan E. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care. 2015;19:326.
Doufle G, Ferguson ND. Monitoring during extracorporeal membrane oxygenation. Curr Opin Crit Care. 2016;22(3):230–8.
Gattinoni L, Carlesso E, Langer T. Clinical review: extracorporeal membrane oxygenation. Crit Care. 2011;15(6):243.
Reeb J, Olland A, Renaud S, Lejay A, Santelmo N, Massard G, et al. Vascular access for extracorporeal life support: tips and tricks. J Thorac Dis. 2016;8(Suppl 4):S353–63.
Zimmermann M, Bein T, Philipp A, Ittner K, Foltan M, Drescher J, et al. Interhospital transportation of patients with severe lung failure on pumpless extracorporeal lung assist. Br J Anaesth. 2006;96(1):63–6.
Schellongowski P, Riss K, Staudinger T, Ullrich R, Krenn CG, Sitzwohl C, et al. Extracorporeal CO2 removal as bridge to lung transplantation in life-threatening hypercapnia. Transpl Int. 2015;28(3):297–304.
de Perrot M, Granton JT, McRae K, Cypel M, Pierre A, Waddell TK, et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. 2011;30(9):997–1002.
Machuca TN, Collaud S, Mercier O, Cheung M, Cunningham V, Kim SJ, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg. 2015;149(4):1152–7.
Jensen V, Milne B, Salerno T. Femoral-femoral cardiopulmonary bypass prior to induction of anaesthesia in the management of upper airway obstruction. Can Anaesth Soc J. 1983;30(3 Pt 1):270–2.
Hong Y, Jo KW, Lyu J, Huh JW, Hong SB, Jung SH, et al. Use of venovenous extracorporeal membrane oxygenation in central airway obstruction to facilitate interventions leading to definitive airway security. J Crit Care. 2013;28(5):669–74.
Ko M, dos Santos PR, Machuca TN, Marseu K, Waddell TK, Keshavjee S, et al. Use of single-cannula venous-venous extracorporeal life support in the management of life-threatening airway obstruction. Ann Thorac Surg. 2015;99(3):e63–5.
Willms DC, Mendez R, Norman V, Chammas JH. Emergency bedside extracorporeal membrane oxygenation for rescue of acute tracheal obstruction. Respir Care. 2012;57(4):646–9.
Higashi K, Takeshita J, Terasaki H, Tanoue T, Esaki K, Sakamoto M, et al. A case of acute airway obstruction with sharp sawdust particles, successfully treated by extracorporeal lung assist. Kokyu To Junkan. 1989;37(3):329–33.
Park JM, Kim CW, Cho HM, Son BS, Kim DH. Induced airway obstruction under extracorporeal membrane oxygenation during treatment of life-threatening massive hemoptysis due to severe blunt chest trauma. J Thorac Dis. 2014;6(12):E255–8.
Smith IJ, Sidebotham DA, McGeorge AD, Dorman EB, Wilsher ML, Kolbe J. Use of extracorporeal membrane oxygenation during resection of tracheal papillomatosis. Anesthesiology. 2009;110(2):427–9.
Fung R, Stellios J, Bannon PG, Ananda A, Forrest P. Elective use of veno-venous extracorporeal membrane oxygenation and high-flow nasal oxygen for resection of subtotal malignant distal airway obstruction. Anaesth Intensive Care. 2017;45(1):88–91.
Natt B, Knepler J Jr, Kazui T, Mosier JM. The use of extracorporeal membrane oxygenation in the bronchoscopic management of critical upper airway obstruction. J Bronchology Interv Pulmonol. 2017;24(1):e12–e4.
Park AH, Tunkel DE, Park E, Barnhart D, Liu E, Lee J, et al. Management of complicated airway foreign body aspiration using extracorporeal membrane oxygenation (ECMO). Int J Pediatr Otorhinolaryngol. 2014;78(12):2319–21.
Lang G, Ghanim B, Hotzenecker K, Klikovits T, Matilla JR, Aigner C, et al. Extracorporeal membrane oxygenation support for complex tracheo-bronchial procedures. Eur J Cardiothorac Surg. 2015;47(2):250–5. discussion 6
Johnson AP, Cavarocchi NC, Hirose H. Ventilator strategies for VV ECMO management with concomitant tracheal injury and H1N1 influenza. Heart Lung Vessel. 2015;7(1):74–80.
Kim CW, Kim DH, Son BS, Cho JS, Kim YD, I H, et al. The feasibility of extracorporeal membrane oxygenation in the variant airway problems. Ann Thorac Cardiovasc Surg. 2015;21(6):517–22.
Keeyapaj W, Alfirevic A. Carinal resection using an airway exchange catheter-assisted venovenous ECMO technique. Can J Anaesth. 2012;59(11):1075–6.
Redwan B, Ziegeler S, Freermann S, Nique L, Semik M, Lavae-Mokhtari M, et al. Intraoperative veno-venous extracorporeal lung support in thoracic surgery: a single-centre experience. Interact Cardiovasc Thorac Surg. 2015;21(6):766–72.
Walles T, Steger V, Wurst H, Schmidt KD, Friedel G. Pumpless extracorporeal gas exchange aiding central airway surgery. J Thorac Cardiovasc Surg. 2008;136(5):1372–4.
Wiebe K, Poeling J, Arlt M, Philipp A, Camboni D, Hofmann S, et al. Thoracic surgical procedures supported by a pumpless interventional lung assist. Ann Thorac Surg. 2010;89(6):1782–7. discussion 8
Gillon SA, Toufektzian L, Harrison-Phipps K, Puchakayala M, Daly K, Ioannou N, et al. Perioperative extracorporeal membrane oxygenation to facilitate lung resection after contralateral pneumonectomy. Ann Thorac Surg. 2016;101(3):e71–3.
Oey IF, Peek GJ, Firmin RK, Waller DA. Post-pneumonectomy video-assisted thoracoscopic bullectomy using extra-corporeal membrane oxygenation. Eur J Cardiothorac Surg. 2001;20(4):874–6.
Schiff JH, Koninger J, Teschner J, Henn-Beilharz A, Rost M, Dubb R, et al. Veno-venous extracorporeal membrane oxygenation (ECMO) support during anaesthesia for oesophagectomy. Anaesthesia. 2013;68(5):527–30.
Slinger P, Suissa S, Adam J, Triolet W. Predicting arterial oxygenation during one-lung ventilation with continuous positive airway pressure to the nonventilated lung. J Cardiothorac Anesth. 1990;4(4):436–40.
Brenner M, O'Connor JV, Scalea TM. Use of ECMO for resection of post-traumatic ruptured lung abscess with empyema. Ann Thorac Surg. 2010;90(6):2039–41.
Bressman M, Raad W, Levsky JM, Weinstein S. Surgical therapy for complications of pneumonia on extracorporeal membrane oxygenation can improve the ability to wean patients from support. Heart Lung Vessel. 2015;7(4):330–1.
Tsunezuka Y, Sato H, Tsubota M, Seki M. Significance of percutaneous cardiopulmonary bypass support for volume reduction surgery with severe hypercapnia. Artif Organs. 2000;24(1):70–3.
Li X, He H, Sun B. Veno-venous extracorporeal membrane oxygenation support during lung volume reduction surgery for a severe respiratory failure patient with emphysema. J Thorac Dis. 2016;8(3):E240–3.
Redwan B, Ziegeler S, Semik M, Fichter J, Dickgreber N, Vieth V, et al. Single-site cannulation venovenous extracorporeal CO2 removal as bridge to lung volume reduction surgery in end-stage lung emphysema. ASAIO J. 2016;62(6):743–6.
Kim JD, Ko ES, Kim JY, Kim SH. Anesthesia under cardiopulmonary bypass for video assisted thoracoscopic wedge resection in patient with spontaneous pneumothorax and contralateral post-tuberculosis destroyed lung. Korean J Anesthesiol. 2013;65(2):174–6.
Julien F, Mosolo A, Hubsch JP, Souilhamas R, Safran D, Cholley B. Respiratory support using veno-venous ECMO during lung resection for aspergilloma. Ann Fr Anesth Reanim. 2010;29(9):645–7.
Sivitanidis E, Tosson R, Wiebalck A, Laczkovics A. Combination of extracorporeal membrane oxygenation (ECMO) and pulmonary lavage in a patient with pulmonary alveolar proteinosis. Eur J Cardiothorac Surg. 1999;15(3):370–2.
Sihoe AD, Ng VM, Liu RW, Cheng LC. Pulmonary alveolar proteinosis in extremis: the case for aggressive whole lung lavage with extracorporeal membrane oxygenation support. Heart Lung Circ. 2008;17(1):69–72.
Krecmerova M, Mosna F, Bicek V, Petrik F, Grandcourtova A, Lekes M, et al. Extracorporeal membrane oxygenation to support repeated whole-lung lavage in a patient with pulmonary alveolar proteinosis in life threatening dyspnoe – a case report. BMC Anesthesiol. 2015;15:173.
Kim KH, Kim JH, Kim YW. Use of extracorporeal membrane oxygenation (ECMO) during whole lung lavage in pulmonary alveolar proteinosis associated with lung cancer. Eur J Cardiothorac Surg. 2004;26(5):1050–1.
Kaya FN, Bayram AS, Kirac F, Basagan-Mogol E, Goren S. Abstract OR001: extracorporeal membrane oxygenation to support whole lung lavage in a patient with pulmonary alveolar proteinosis. Anesth Analg. 2016;123(3 Suppl 2):1.
Hurrion EM, Pearson GA, Firmin RK. Childhood pulmonary alveolar proteinosis. Extracorporeal membrane oxygenation with total cardiopulmonary support during bronchopulmonary lavage. Chest. 1994;106(2):638–40.
Hasan N, Bagga S, Monteagudo J, Hirose H, Cavarocchi NC, Hehn BT, et al. Extracorporeal membrane oxygenation to support whole-lung lavage in pulmonary alveolar proteinosis: salvage of the drowned lungs. J Bronchology Interv Pulmonol. 2013;20(1):41–4.
Guo WL, Chen Y, Zhong NS, Su ZQ, Zhong CH, Li SY. Alveolar proteinosis in extremis: a critical case treated with whole lung lavage without extracorporeal membrane oxygenation. Int J Clin Exp Med. 2015;8(10):19556–60.
Cohen ES, Elpern E, Silver MR. Pulmonary alveolar proteinosis causing severe hypoxemic respiratory failure treated with sequential whole-lung lavage utilizing venovenous extracorporeal membrane oxygenation: a case report and review. Chest. 2001;120(3):1024–6.
Chauhan S, Sharma KP, Bisoi AK, Pangeni R, Madan K, Chauhan YS. Management of pulmonary alveolar proteinosis with whole lung lavage using extracorporeal membrane oxygenation support in a postrenal transplant patient with graft failure. Ann Card Anaesth. 2016;19(2):379–82.
Cai HR, Cui SY, Jin L, Huang YZ, Wang ZY, Cao B, et al. Pulmonary alveolar proteinosis treated with whole-lung lavage utilizing extracorporeal membrane oxygenation: a case report and review of literatures. Chin Med J (Engl). 2004;117(11):1746–9.
Cai HR, Cui SY, Jin L, Huang YZ, Cao B, Wang ZY, et al. Pulmonary alveolar proteinosis treated with whole-lung lavage utilizing extracorporeal membrane oxygenation: a case report and review. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28(4):242–4.
Asai T. Emergency cardiopulmonary bypass in a patient with a mediastinal mass. Anaesthesia. 2007;62(8):859–60.
Said SM, Telesz BJ, Makdisi G, Quevedo FJ, Suri RM, Allen MS, et al. Awake cardiopulmonary bypass to prevent hemodynamic collapse and loss of airway in a severely symptomatic patient with a mediastinal mass. Ann Thorac Surg. 2014;98(4):e87–90.
Sendasgupta C, Sengupta G, Ghosh K, Munshi A, Goswami A. Femoro-femoral cardiopulmonary bypass for the resection of an anterior mediastinal mass. Indian J Anaesth. 2010;54(6):565–8.
Tempe DK, Arya R, Dubey S, Khanna S, Tomar AS, Grover V, et al. Mediastinal mass resection: Femorofemoral cardiopulmonary bypass before induction of anesthesia in the management of airway obstruction. J Cardiothorac Vasc Anesth. 2001;15(2):233–6.
Wickiser JE, Thompson M, Leavey PJ, Quinn CT, Garcia NM, Aquino VM. Extracorporeal membrane oxygenation (ECMO) initiation without intubation in two children with mediastinal malignancy. Pediatr Blood Cancer. 2007;49(5):751–4.
Felten ML, Michel-Cherqui M, Puyo P, Fischler M. Extracorporeal membrane oxygenation use for mediastinal tumor resection. Ann Thorac Surg. 2010;89(3):1012.
de Perrot M, Fadel E, Mussot S, de Palma A, Chapelier A, Dartevelle P. Resection of locally advanced (T4) non-small cell lung cancer with cardiopulmonary bypass. Ann Thorac Surg. 2005;79(5):1691–6. discussion 7
Galvaing G, Tardy MM, Cassagnes L, Da Costa V, Chadeyras JB, Naamee A, et al. Left atrial resection for T4 lung cancer without cardiopulmonary bypass: technical aspects and outcomes. Ann Thorac Surg. 2014;97(5):1708–13.
Langer NB, Mercier O, Fabre D, Lawton J, Mussot S, Dartevelle P, et al. Outcomes after resection of T4 non-small cell lung cancer using cardiopulmonary bypass. Ann Thorac Surg. 2016;102(3):902–10.
Belov YV, Komarov RN, Parshin VD, Yavorovsky AG, Chernyavsky SV, Mnatsakanyan GV. Right-sided pneumonectomy with left atrium resection under cardiopulmonary bypass in the patient with lung cancer (the first case in Russia). Khirurgiia (Mosk). 2017;(1):78–81.
Ferguson ER Jr, Reardon MJ. Atrial resection in advanced lung carcinoma under total cardiopulmonary bypass. Tex Heart Inst J. 2000;27(2):110–2.
Hasegawa S, Bando T, Isowa N, Otake Y, Yanagihara K, Tanaka F, et al. The use of cardiopulmonary bypass during extended resection of non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2003;2(4):676–9.
Kugai T, Kinjyo M, Hosokawa Y. The combined resection of left atrium for advanced lung cancer on cardiopulmonary bypass: a case report. Kyobu Geka. 1996;49(9):738–41.
Marseu K, Minkovich L, Zubrinic M, Keshavjee S. Anesthetic considerations for pneumonectomy with left atrial resection on cardiopulmonary bypass in a patient with lung cancer: a case report. A A Case Rep. 2017;8(3):61–3.
Shimizu J, Ikeda C, Arano Y, Adachi I, Morishita M, Yamaguchi S, et al. Advanced lung cancer invading the left atrium, treated with pneumonectomy combined with left atrium resection under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. 2010;16(4):286–90.
Lang G, Taghavi S, Aigner C, Charchian R, Matilla JR, Sano A, et al. Extracorporeal membrane oxygenation support for resection of locally advanced thoracic tumors. Ann Thorac Surg. 2011;92(1):264–70.
Byrnes J, Leacche M, Adnithotri A, Paul S, Bueno R, Mathisen D, et al. The use of cardiopulmonary bypass during resection of locally advanced thoracic malignancies. Chest. 2004;125(4):1581–6.
Shirakusa T, Kimura M. Partial atrial resection in advanced lung carcinoma with and without cardiopulmonary bypass. Thorax. 1991;46(7):484–7.
Kobayashi S, Sawabata N, Araki O, Karube Y, Seki N, Tamura M, et al. Dissection of a mediastinal mature teratoma requires replacement of the ascending aorta during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2007;134(5):1371–2.
Agathos EA, Lachanas E, Karagkiouzis G, Spartalis E, Tomos P. Cardiopulmonary bypass assisted resection of mediastinal masses. J Card Surg. 2012;27(3):338–41.
Mei J, Pu Q, Zhu Y, Ma L, Ren F, Che G, et al. Reconstruction of the pulmonary trunk via cardiopulmonary bypass in extended resection of locally advanced lung malignancies. J Surg Oncol. 2012;106(3):311–5.
Abrams D, Agerstrand CL, Biscotti M, Burkart KM, Bacchetta M, Brodie D. Extracorporeal membrane oxygenation in the management of diffuse alveolar hemorrhage. ASAIO J. 2015;61(2):216–8.
Patel JJ, Lipchik RJ. Systemic lupus-induced diffuse alveolar hemorrhage treated with extracorporeal membrane oxygenation: a case report and review of the literature. J Intensive Care Med. 2014;29(2):104–9.
Abbas AE. Traumatic injury of the pulmonary artery: transection, rupture, pseudoaneurysm, or dissection? Sometimes semantics do matter. J Thorac Cardiovasc Surg. 2016;152(5):1437–8.
Jeng EI, Piovesana G, Taylor J, Machuca TN. Extracorporeal membrane oxygenation to facilitate tracheal healing after oesophagogastric catastrophe. Eur J Cardiothorac Surg. 2018;53(1):288–9.
Weinberg A, Tapson VF, Ramzy D. Massive pulmonary embolism: extracorporeal membrane oxygenation and surgical pulmonary embolectomy. Semin Respir Crit Care Med. 2017;38(1):66–72.
Lebreton G, Bouabdallaoui N, Gauduchon L, Mnif MA, Roques F. Successful use of ECMO as a bridge to surgical embolectomy in life-threatening pulmonary embolism. Am J Emerg Med. 2015;33(9):1332 e3–4.
Kawahito K, Murata S, Ino T, Fuse K. Angioscopic pulmonary embolectomy and ECMO. Ann Thorac Surg. 1998;66(3):982–3.
Berman M, Tsui S, Vuylsteke A, Snell A, Colah S, Latimer R, et al. Successful extracorporeal membrane oxygenation support after pulmonary thromboendarterectomy. Ann Thorac Surg. 2008;86:1261–7.
Caridi-Scheible ME, Blum JM. Use of Perfluorodecalin for Bronchoalveolar lavage in case of severe pulmonary hemorrhage and extracorporeal membrane oxygenation: a case report and review of the literature. A A Case Rep. 2016;7(10):215–8.
Chacon-Alves S, Perez-Vela JL, Grau-Carmona T, Dominguez-Aguado H, Marin-Mateos H, Renes-Carreno E. Veno-arterial ECMO for rescue of severe airway hemorrhage with rigid bronchoscopy after pulmonary artery thromboendarterectomy. Int J Artif Organs. 2016;39(5):242–4.
Kuroda H, Masuda Y, Imaizumi H, Kozuka Y, Asai Y, Namiki A. Successful extracorporeal membranous oxygenation for a patient with life-threatening transfusion-related acute lung injury. J Anesth. 2009;23(3):424–6.
Lee AJ, Koyyalamudi PL, Martinez-Ruiz R. Severe transfusion-related acute lung injury managed with extracorporeal membrane oxygenation (ECMO) in an obstetric patient. J Clin Anesth. 2008;20(7):549–52.
Incagnoli P, Blaise H, Mathey C, Vinclair M, Albaladejo P. Pulmonary resection and ECMO: a salvage therapy for penetrating lung trauma. Ann Fr Anesth Reanim. 2012;31(7–8):641–3.
Swol J, Cannon JW, Napolitano LM. ECMO in trauma: what are the outcomes? J Trauma Acute Care Surg. 2017;82(4):819–20.
Zhou R, Liu B, Lin K, Wang R, Qin Z, Liao R, et al. ECMO support for right main bronchial disruption in multiple trauma patient with brain injury – a case report and literature review. Perfusion. 2015;30(5):403–6.
Jacobs JV, Hooft NM, Robinson BR, Todd E, Bremner RM, Petersen SR, et al. The use of extracorporeal membrane oxygenation in blunt thoracic trauma: a study of the extracorporeal life support organization database. J Trauma Acute Care Surg. 2015;79(6):1049–53. discussion 53-4
Pavlushkov E, Berman M, Valchanov K. Cannulation techniques for extracorporeal life support. Ann Transl Med. 2017;5(4):70.
Raiten JM, Wong ZZ, Spelde A, Littlejohn JE, Augoustides JGT, Gutsche JT. Anticoagulation and transfusion therapy in patients requiring extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2017;31(3):1051–9.
Organization ELS. ELSO anticoagulation guidelines. Ann Arbor, Michigan: Extracorporeal Life Support Organization; 2014.
O'Carroll-Kuehn BU, Meeran H. Management of coagulation during cardiopulmonary bypass. Contin Educ Anaesth Crit Care Pain. 2007;7(6):195–8.
Joffre J, Preda G, Arrive L, Maury E. Fatal aortic dissection during ECMO axillary cannulation confirmed by postmortem CT angiography. Am J Respir Crit Care Med. 2017;195:953.
Roussel A, Al-Attar N, Khaliel F, Alkhoder S, Raffoul R, Alfayyadh F, et al. Arterial vascular complications in peripheral extracorporeal membrane oxygenation support: a review of techniques and outcomes. Future Cardiol. 2013;9(4):489–95.
Bojic A, Steiner I, Gamper J, Schellongowski P, Lamm W, Hermann A, et al. The supraclavicular approach to the subclavian vein as an alternative venous access site for ECMO cannulae? A retrospective comparison. ASAIO J. 2017;63:679.
Banfi C, Pozzi M, Brunner ME, Rigamonti F, Murith N, Mugnai D, et al. Veno-arterial extracorporeal membrane oxygenation: an overview of different cannulation techniques. J Thorac Dis. 2016;8(9):E875–e85.
Slinger P, Kilpatrick B. Perioperative lung protection strategies in cardiothoracic anesthesia: are they useful? Anesthesiol Clin. 2012;30(4):607–28.
Adeniji K, Steel AC. The pathophysiology of perioperative lung injury. Anesthesiol Clin. 2012;30(4):573–90.
Kilpatrick B, Slinger P. Lung protective strategies in anaesthesia. Br J Anaesth. 2010;105(Suppl 1):i108–16.
Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care. 2014;18(1):203.
Wong JK, Smith TN, Pitcher HT, Hirose H, Cavarocchi NC. Cerebral and lower limb near-infrared spectroscopy in adults on extracorporeal membrane oxygenation. Artif Organs. 2012;36(8):659–67.
Formica F, Avalli L, Colagrande L, Ferro O, Greco G, Maggioni E, et al. Extracorporeal membrane oxygenation to support adult patients with cardiac failure: predictive factors of 30-day mortality. Interact Cardiovasc Thorac Surg. 2010;10(5):721–6.
Xie A, Yan TD, Forrest P. Recirculation in venovenous extracorporeal membrane oxygenation. J Crit Care. 2016;36:107–10.
Kilburn DJ, Shekar K, Fraser JF. The complex relationship of extracorporeal membrane oxygenation and acute kidney injury: causation or association? Biomed Res Int. 2016;2016:1094296.
Makdisi G, Wang IW. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166–76.
Ventetuolo CE, Muratore CS. Extracorporeal life support in critically ill adults. Am J Respir Crit Care Med. 2014;190(5):497–508.
Turner DA, Cheifetz IM. Extracorporeal membrane oxygenation for adult respiratory failure. Respir Care. 2013;58(6):1038–52.
Pollak U, Yacobobich J, Tamary H, Dagan O, Manor-Shulman O. Heparin-induced thrombocytopenia and extracorporeal membrane oxygenation: a case report and review of the literature. J Extra Corpor Technol. 2011;43(1):5–12.
Sy E, Sklar MC, Lequier L, Fan E, Kanji HD. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation (VA-ECMO): a systematic review and meta-analysis. J Crit Care. 2017;39:87–96.
Menaker J, Tabatabai A, Rector R, Dolly K, Kufera J, Lee E, et al. Incidence of cannula associated deep vein thrombosis after veno-venous ECMO. ASAIO J. 2017;63:588.
Petricevic M, Milicic D, Boban M, Mihaljevic MZ, Baricevic Z, Kolic K, et al. Bleeding and thrombotic events in patients undergoing mechanical circulatory support: a review of literature. Thorac Cardiovasc Surg. 2015;63(8):636–46.
Biffi S, Di Bella S, Scaravilli V, Peri AM, Grasselli G, Alagna L, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017;50(1):9–16.
Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. 2011;68(12):1543–9.
Rastan AJ, Lachmann N, Walther T, Doll N, Gradistanac T, Gommert JF, et al. Autopsy findings in patients on postcardiotomy extracorporeal membrane oxygenation (ECMO). Int J Artif Organs. 2006;29(12):1121–31.
Reed RC, Rutledge JC. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. 2010;13(5):385–92.
Jiao Y, Gipson KE, Bonde P, Mangi A, Hagberg R, Rosinski DJ, et al. Quantification of postmembrane gaseous microembolization during venoarterial extracorporeal membrane oxygenation. ASAIO J. 2018;64(1):31–7.
Lother A, Wengenmayer T, Benk C, Bode C, Staudacher DL. Fatal air embolism as complication of percutaneous dilatational tracheostomy on venovenous extracorporeal membrane oxygenation, two case reports. J Cardiothorac Surg. 2016;11(1):102.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Clinical Case Discussion
A 54-year-old man with relapsing polychondritis presented for elective rigid bronchoscopy and tracheal stent repositioning. His original silicone stent was placed 3 years ago for tracheomalacia. He had stridor on forceful inhalation from suspected granulation tissue distal to the stent, but no stridor at rest. He is able to lie supine with no dyspnoea. He had a history of well-controlled hypertension and stable ankylosing spondylitis treated with prednisone and adalimumab. He had normal cardiac function and no valvular lesions. His airway examination revealed Mallampati I view with unrestricted cervical spine motion. He underwent a titrated intravenous induction and was maintained on a propofol and remifentanil infusion with muscle relaxation and intermittent jet ventilation. Rigid bronchoscopy and debridement of granulation tissue were performed. The stent was unable to be removed after multiple attempts and was ultimately pushed further distally over the area of granulation. At the end of the case, a mucosal tear of the posterior mid-trachea was noted.
On emergence, the patient had acute stridor and hypoxemia, requiring urgent reinduction and positive pressure ventilation. Repeat rigid bronchoscopy was performed and a Y-stent was inserted at the carina. The patient developed subcutaneous emphysema of the upper chest and neck. There was suspected perforation of one arm of the Y-stent through the trachea into the mediastinum. The stent was removed, and a tear could be seen from the mid-trachea extending into the left mainstem bronchus just distal to the carina.
The thoracic surgeons proposed to repair the tracheal tear via right thoracotomy.
What is your anesthetic plan for converting to thoracotomy?
During positioning and exposure of the left thorax, the goals are to provide gas exchange while avoiding high airway pressures and expansion of the mediastinal emphysema. Our patient was intubated orotracheally with an endobronchial tube with a narrow cuff placed just below the vocal cords and above the area of airway disruption to avoid pressure on the area. We maintained the patient asleep on total intravenous anaesthesia as volatile agents could not be reliably delivered during the complex lung isolation. A radial arterial line and right internal jugular central venous catheter were placed.
What are the options for oxygenating and ventilating this patient during repair of the trachea and left mainstem bronchus?
Due to the location of the tear, oxygenation via a right- or left-sided double-lumen tube or endobronchial tube would be impossible. Likewise, cross-field ventilation from an endobronchial tube placed directly into the right mainstem bronchus would be interrupted almost continuously to allow for repair of the tear. Therefore, veno-arterial extracorporeal membrane oxygenation (ECMO) was initiated via femoral cannulation to provide continuous gas exchange, allowing the surgeons full access to open and repair the trachea and bronchus. After cannulation and heparinisation, the patient’s oxygenation improved on flows of 2–4 L/min. He was placed in the left lateral decubitus position, the right chest was opened and the trachea, and bronchi were repaired with both lungs collapsed. A Y-stent is inserted at the carina. The lungs were re-expanded with positive pressure through the endotracheal tube and new stent, and water sealing test with Valsalva did not reveal any leaks. Chest tubes were placed in the right hemithorax and the chest was closed.
How will you wean ECMO support, and what are the postoperative considerations?
The ECMO flows were weaned to 1.5 L/min and the patient was decannulated after good oxygenation and blood chemistry were confirmed. The patient was transferred to the ICU intubated. He was neurologically intact, but failed extubation twice due to hospital-acquired pneumonia and difficulty clearing secretions. He eventually required tracheostomy. He left the ICU on postoperative day 18 and was discharged home on day 45 with a speaking tracheostomy.
Summary
As the technical frontiers of thoracic procedures are pushed ever forwards by our surgical colleagues, demand for intraoperative ECLS may be predicted to increase. It is unlikely that preinduction provision of mechanical support will ever become routine, and any procedure major enough to require it should not be taken lightly; however, with the help of the principles outlined in this chapter, they can at least feel within our envelope of comfort and experience.
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sellers, D., Lam, K., McRae, K. (2019). Intraoperative Extracorporeal Life Support for Thoracic and Airway Surgery. In: Slinger, P. (eds) Principles and Practice of Anesthesia for Thoracic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-00859-8_27
Download citation
DOI: https://doi.org/10.1007/978-3-030-00859-8_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-00858-1
Online ISBN: 978-3-030-00859-8
eBook Packages: MedicineMedicine (R0)