Abstract
Because there is no effective treatment to reverse or stop the progression of placental insufficiency, fetal surveillance and decision regarding when delivery should take place are the main strategies in the management of these fetuses. Although many studies have been conducted, there is a lack of consistent evidence to safely recommend a specific time of delivery in cases of fetal growth restriction (FGR). The objective of a clinical protocol for the management of FGR is to combine the existing evidence on various methods for assessing fetal vitality (cardiotocography, biophysical profile, and Doppler) to achieve better growth and lung maturity and minimize the risk of morbidity as well as fetal and neonatal mortality. A type of management described in the literature is to group patients into stages of development on the basis of the follow-up results, birth time, and similar fetal risks. In this chapter, we will describe a management protocol based on the evidence available in the literature and the characteristics of our practice and of our patient population. A uniform management, based on protocol, improves perinatal outcome, reducing stillbirths.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The biggest challenges in the management of fetal growth restriction (FGR) are the precise diagnosis of fetuses at risk of adverse perinatal outcomes, prevention of fetal death , and timing of delivery [1]. Because there is no effective treatment to reverse or stop the progression of placental insufficiency yet, fetal vitality assessment and the decision regarding timing of delivery are the main strategies in the management of these fetuses [2]. However, despite numerous studies, the literature lacks a consensus on how to monitor and when and how to delivery in FGR (expectant management, labor induction, or elective cesarean section) [1].
A clinical trial titled The Growth Restriction Intervention Trial randomly divided pregnant women with FGR between 24 and 36 weeks into two groups: immediate delivery (n = 296) and expectant management (n = 292); the patients were assigned when obstetricians were in doubt about when to recommend delivery. Of these patients, 40% had absent or reversed end-diastolic flow in the umbilical artery Doppler. The number of fetal deaths was lower in the immediate delivery group than in the expectant management group (two versus nine). There was no statistically significant difference in the combined rates of neonatal death and severe disability at the age of 2 years between the immediate delivery group and the expectant management group [19% versus 16%, odds ratio (OR) 1.1, confidence interval (CI) 95% (0.7–1.8)]; however, the percentage of pregnancies under 31 weeks was 13% in the immediate delivery group and 5% in the expectant management group [2]. The follow-up of children aged 6–13 years showed no difference between the groups in terms of cognition, language, and motor and behavioral development [3]. These data suggest that expectant management of very premature growth-restricted fetuses , when there is doubt about the timing of delivery, results in more fetal deaths, but immediate delivery resulted in a greater number of neonatal deaths and that neither of the two produced a better neurological prognosis [3, 4].
The study “TRUFFLE—Trial of Randomized Umbilical and Fetal Flow in Europe ” assessed neurological development in infants aged 2 years with early FGR born before 32 weeks of pregnancy. The patients were divided into three groups of recommended timing of delivery according to different strategies of assessing fetal vitality, such as reduction in the computerized cardiotocography short-term variation, early changes in the ductus venosus (DV) Doppler (pulsatility index above 95th percentile), and late changes in the DV Doppler (absent A-wave). Most of the infants had their deliveries recommended for reasons other than those in the protocol for each group (maternal or other fetal conditions). Only 32% of the patients had their delivery recommended based on study criteria. The groups based on DV Doppler used cardiotocography as a safety criterion, whereas the reverse did not apply, i.e., DV Doppler was not a safety criterion for the cardiotocography group. Survival without impairment at the age of 2 years in the group based on the reduction in cardiotocography short-term variation was worse (77%) than that in the two groups that used DV Doppler (83%), without any statistically significant difference. However, on analyzing the surviving infants, the groups that used DV Doppler showed half the prevalence of neurological impairment in comparison to the cardiotocography group (7% versus 15%, p = 0.049). The hypothesis was that the slightly worse prognosis in the cardiotocography group is explained by the absence of information on the DV Doppler. Therefore, they concluded that, in order to optimize the decision on the timing of delivery in early FGR, fetuses should be monitored longitudinally with the DV Doppler and computerized cardiotocography [5].
A 2010 clinical trial with women with suspected FGR between 36 and 41 weeks showed no increased neonatal morbidity or incidence of cesarean section or operative vaginal delivery , when comparing groups of labor induction and expectant management. The authors concluded that expectant management could be conducted with strict control of fetal vitality, but it would be wise to induce labor at term in order to prevent neonatal morbidity and fetal death [6]. A 2017 study by Pilliod et al. concluded that at 38 weeks and later, the risk of fetal death in expectant management for another week exceeded the risk of immediate delivery, regardless of whether the estimated fetal weight was below the 10th, 5th, or 3rd percentile. However, the lower the percentile, the higher the risk [7]. A retrospective study published in 2018 assessed 2232 patients with FGR (characterized in the study as estimated fetal weight below the 10th percentile) and compared labor induction with expectant management between 34 and 38 + 6 weeks. The authors concluded that labor induction at 37 weeks decreases the prevalence of fetal death and, additionally, in late preterm, it is associated with lower rates of neonatal death and non-reassuring cardiotocography pattern [8].
Although many studies have been conducted, there is a lack of consistent evidence to safely recommend the timing of delivery in FGR. The aim of a FGR clinical management protocol is to combine the existing evidence on the various methods of evaluation of fetal vitality (cardiotocography, fetal biophysical profile, and Doppler), in order to achieve the best growth and lung maturity and thus minimize the risks of fetal and neonatal morbidity and mortality. This decision is often based on gestational age, etiology of growth restriction, and degree of fetal vitality impairment, in addition to the experience and technological resources available to assess the fetus and treat the neonate, who preferably should be delivered in a tertiary hospital. One type of management considered ideal by many authors and used in our service is longitudinal monitoring of fetal vitality , starting between 24 and 26 weeks (depending on the viability gestational age used by the service), with ultrasound, biophysical, and Doppler velocimetry methods . Combining multiple tests in the evaluation of fetal vitality improves the prediction of acidemia and fetal death in comparison to isolated tests [9]. The intervals for this evaluation depend on gestational age and signs of placental insufficiency.
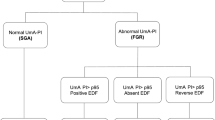
In the management of these fetuses, the first important step is trying to distinguish actual FGR, associated with placental insufficiency and worse perinatal prognosis, from fetuses of small constitution, with practically normal perinatal prognosis [10]. Early and late FGRs are distinguishable when considered in groups. Early FGR usually starts with an abnormal umbilical artery Doppler, progressing to brain-sparing, abnormal venous Doppler, abnormal computerized cardiotocography, and finally abnormal fetal biophysical profile [9]. The primary change in late FGR is observed in the middle cerebral artery Doppler or the umbilical artery Doppler, without significant changes in the venous Doppler. Changes in the cerebroplacental ratio (CPR) might be the only existing sign of hypoxemia. Furthermore, fetal death is faster and more unexpected in late FGR; thus, fetal vitality control must be intensified from 34 weeks onward [9].
Despite pathophysiological differences in placental insufficiency , when dealing with individual fetuses, clinical features can overlap, especially at borderline gestational age. Therefore, the same management protocol can be used to monitor and decide the timing of delivery in both groups [10]. Grouping patients according to the stage of evolution, with similar monitoring, timing of delivery, and fetal risks is a type of management described in the literature [10]. Based on evidence available in the literature and the features of our service and of the population of patients and obstetricians in the Department of Obstetrics, Paulista School of Medicine – Federal University of São Paulo (EPM-UNIFESP), Brazil, we follow a management protocol based on the stages of evolution of FGR [11]. The protocol is summarized in Table 12.1.
Small for Gestational Age Fetuses
In fetuses with estimated weight between the 3rd and 10th percentiles, without changes in the Doppler, fetal vitality (Doppler and fetal biophysical profile) and fetal growth can be assessed every 2 weeks [10]. If the patient does not go into labor spontaneously, it can be induced at 40 weeks. Prostaglandins can be carefully used for labor induction, with strict control of intrapartum vitality, owing to the risk of hyperstimulation in fetuses that could present some degree of placental injury [11].
Stage 1: Fetal Growth Restriction with Normal Doppler (Mild Placental Insufficiency)
Stage 1 is characterized by an estimated fetal weight below the 3rd percentile, without changes in the Doppler. Fetal growth and vitality (Doppler and fetal biophysical profile) can be assessed every 2 weeks up to 34 weeks and weekly after that [11]. Delivery can be carefully induced at 38 weeks, avoiding, however, the use of prostaglandins [1]. If the estimated weight is below the 1st percentile, delivery is considered at 37 weeks [10, 11] (Fig. 12.1).
Stage 2: Fetal Growth Restriction with Moderate Placental Insufficiency (with Changes in the Doppler)
Stage 2 is characterized by the following changes in the Doppler: umbilical artery pulsatility index (PI) >95th percentile, middle cerebral artery PI ˂5th percentile, or CPR ˂5th percentile. Weekly assessment of fetal vitality (Doppler and fetal biophysical profile) is acceptable [10, 12]. In our service, we monitor fetal vitality twice a week and consider hospitalization of the patient after 34 weeks to optimize clinical control and check vitality daily [11]. Evidence suggests a low risk of fetal deterioration before term, but it also shows no benefits in maintaining pregnancy after reaching term. Delivery induction at 37 weeks is acceptable, avoiding, however, the use of prostaglandins. There is a higher risk of intrapartum fetal distress [12]. Resolution by elective cesarean section is acceptable for patients with an unfavorable cervix and changes in the CPR [in our service, we consider it altered when it is less than 1] [13].
There are FGR management protocols in the literature that include mean uterine artery PI >95th percentile in the category above and recommend the resolution of delivery at 37 weeks [10]. A few studies showed that small fetuses with changes in the uterine artery Doppler have twice the risk of developing changes in the middle cerebral artery Doppler before delivery, which could be useful in planning fetal surveillance [14, 15]. However, longitudinal studies with serial assessment of uterine arteries failed to show any worsening of Doppler from diagnosis to delivery, so its use as a method to evaluate fetal vitality is questionable [15]. At our service, we use uterine artery Doppler as one of the FGR diagnostic criteria [according to the Delphi consensus [16]] but not as a management criterion (Fig. 12.2).
Stage 3: Fetal Growth Restriction with Severe Placental Insufficiency (Umbilical Artery Doppler with Absent End-Diastolic Flow)
Stage 3 is defined by the absence of end-diastolic flow in the umbilical artery Doppler or reversed end-diastolic flow in the aortic isthmus Doppler. Fetal monitoring every 2 days is acceptable [5]. To optimize the control of fetal vitality, in our service, patients are hospitalized after the limit of viability and evaluated on a daily basis (Doppler, fetal biophysical profile, and computerized cardiotocography) [11]. Delivery is recommended at 34 weeks by elective cesarean section, because the risk of fetal distress in labor induction exceeds 50% [10, 11] (Fig. 12.3).
Stage 4: Fetal Growth Restriction with Advanced Fetal Deterioration (Umbilical Artery Doppler with Reversed End-Diastolic Flow or Ductus Venosus with Pulsatility Index ˃95th Percentile)
Stage 4 is defined by reversed end-diastolic flow in the umbilical artery Doppler or a DV Doppler with PI ˃95th percentile. There is a high risk of fetal death and impairment of neurological development, and the following protocol should be followed: hospitalization and daily monitoring of fetal vitality with Doppler, fetal biophysical profile, and computerized cardiotocography . Some protocols in the literature recommend delivery from 30 weeks onward [10]; however, in our service, we have adopted delivery by elective cesarean section, after the neonatal intensive unit care (NIUC) limit of viability (26 weeks and estimated fetal weight ≥500 g or 28 weeks regardless of estimated fetal weight) [11]. In this stage, before 30 weeks, we can use the fetal biophysical profile to evaluate the possibility of expectant management at least for corticosteroid therapy and transfer to a tertiary service [9]. A fetal biophysical profile score of less than 6/10 is a recommendation for birth at the limit of viability due to its high association with acidemia [1]; nonetheless, we must emphasize that the fetal biophysical profile before 28 weeks changes on an average of 1 week after the changes in the venous Doppler [17], a period that could increase neonatal survival by 14% [1] (Fig. 12.4).
Stage 5: Fetal Growth Restriction with a High Probability of Fetal Acidosis and a High Risk of Fetal Death (Ductus Venosus Doppler with Reversed A-Wave, Computerized Cardiotocography ˂3 ms, or Fetal Heart Rate Decelerations)
Stage 5 is defined by ductus venosus Doppler with reversed A-wave, computerized cardiotocography short-term variation ˂3 ms, or fetal heart rate decelerations. Delivery is recommended by elective cesarean section at the moment of diagnosis, depending on the NIUC limit of viability [10, 11] (Fig. 12.5). In earlier gestational ages, parents should be advised according to the available data of viability without impairment, and their opinion should be taken into account in the decision on delivery [10]. We must emphasize that the survival rate described in the literature for newborns between 24 and 26 weeks with FGR is less than 50%, and the risk of severe morbidity is more than 80% [18]. Survival rates surpass 50% when fetuses reach 500 g or 26 weeks [1].
In any of these stages, whenever any change can indicate accelerated progression of the disease (e.g., the co-occurrence of preeclampsia) or any signs of fetal deterioration arise, the frequency of fetal vitality assessment must be increased until the gestational age for delivery is reached [1].
Amniotic Fluid Assessment
A systematic review conducted in 2008 with low- and high-risk pregnancies compared the amniotic fluid index with the deepest vertical pocket measurement, considered normal when greater or equal than 20 mm, as a method of amniotic fluid assessment. The authors concluded that the deepest vertical pocket measurement is more beneficial because assessing the amniotic fluid index increases oligohydramnios and labor induction rates without improving perinatal prognosis [19]. A meta-analysis including 18 clinical trials showed that an amniotic fluid index less than 50 mm is associated with a lower 5-min Apgar score and increased intrapartum fetal distress; however, it showed no association with fetal acidosis or perinatal death [20]. To date, the inclusion of oligohydramnios in FGR management protocols has found no consensus in the literature, and more studies are needed to validate its use [14].
Corticosteroids and Magnesium Sulfate
Antenatal corticosteroids should be used between 24 and 34 weeks and preferably in the week prior to the scheduled delivery (maximum 2 cycles) to accelerate fetal lung maturity and reduce the risk of intracranial bleeding [21]. A systematic review of the literature with a meta-analysis published in 2016 showed the benefits of using corticosteroids between 34 and 36 6/7 weeks in patients with immediate risk of late preterm birth [22]. It also concluded that, in cesarean sections planned between 37 and 38 + 6 weeks, parents can be advised about the benefits of a single dose of corticosteroids, such as decreased respiratory distress syndrome [22]. Nevertheless, soon after the use of corticosteroids, Doppler indices may show only a transitory improvement. For births before 32 weeks, the use of magnesium sulfate is recommended for neuroprotection [20]. Further studies on the use of corticosteroids and magnesium sulfate in specific groups of patients, such as those of restricted fetal growth, must be conducted [23].
Conclusion
As there is no effective treatment of FGR, the optimal clinical management is the main goal in these fetuses. There is no consensus in the literature on how to monitor and when and how to delivery in FGR. Besides that, a uniform management, based on protocol, improves perinatal outcome. A stage-based management protocol, as described in this chapter, can help the clinicians minimizing practice variations.
References
Seravalli V, Baschat AA. A uniform management approach to optimize outcome in fetal growth restriction. Obstet Gynecol Clin North Am. 2015;42:275–88.
Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol. 2011;204:288–300.
Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M. GRIT study group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet. 2004;364:513–20.
Walker DM, Marlow N, Upstone L, Gross H, Hornbuckle J, Vail A, et al. The Growth Restriction Intervention Trial: long-term outcomes in a randomized trial of timing of delivery in fetal growth restriction. Am J Obstet Gynecol. 2011;204:34.e1–9.
Visser GHA, Bilardo CM, Derks JB, Ferrazzi E, Fratelli N, Frusca T, et al. Fetal monitoring indications for delivery and 2-year outcome in 310 infants with fetal growth restriction delivered before 32 weeks’ gestation in the TRUFFLE study. Ultrasound Obstet Gynecol. 2017;50:347–52.
Boers KE, Vijgen SM, Bijilenga D, van der Post JA, Bekedam DJ, Kwee A, et al. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT). BMJ. 2010;341:c7087.
Pilliod RA, Page JM, Sparks TN, Caughey AB. The growth-restricted fetus: risk of mortality by each additional week of expectant management. J Matern Fetal Neonatal Med. 2017;3:1–6. https://doi.org/10.1080/14767058.2017.1381904.
Rabinovich A, Tsemach T, Novack L, Mazor M, Rafaeli-Yehudai T, Staretz-Chacham O, et al. Late preterm and early term: when to induce a growth restricted fetus? A population-based study. J Matern Fetal Neonatal Med. 2018;31:926–32.
Baschat AA. Integrated fetal testing in growth restriction: combining multivessel Doppler and biophysical parameters. Ultrasound Obstet Gynecol. 2003;21:1–8.
Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36:86–98.
Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marçal VM, et al. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295:1061–77.
Figueras F, Gratacos E. Stage-based approach to the management of fetal growth restriction. Prenat Diagn. 2014;34:655–9.
Arias F. Accuracy of the middle-cerebral-to-umbilical-artery resistance index ratio in the prediction of neonatal outcome in patients at high risk for fetal and neonatal complications. Am J Obstet Gynecol. 1994;171:1541–5.
Cruz-Martinez R, Savchev S, Cruz-Lemini M, Mendez A, Gratacos E, Figueras F. Clinical utility of third-trimester uterine artery Doppler in the prediction of brain hemodynamic deterioration and adverse perinatal outcome in small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 2014;45:273–8.
Figueras F, Caradeux J, Crispi F, Eixarch E, Peguero A, Gratacos E. Diagnosis and surveillance of late-onset fetal growth restriction. Am J Obstet Gynecol. 2018;218:S790–S802.e1.
Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48:333–9.
Cosmi E, Ambrosini G, D’Antona D, Saccardi C, Mari G. Doppler, cardiotocography, and biophysical profile changes in growth-restricted fetuses. Obstet Gynecol. 2005;106:1240–5.
Baschat AA, Cosmi E, Bilardo CM, Wolf H, Berg C, Rigano S, et al. Predictors of neonatal outcome in early-onset placental dysfunction. Obstet Gynecol. 2007;109:253–61.
Nabhan AF, Abdelmoula YA. Amniotic fluid index versus single deepest vertical pocket as a screening test for preventing adverse pregnancy outcome. Cochrane Database Syst Rev. 2008;3:CD006593.
Chauhan SP, Sanderson M, Hendrix NW, Magann EF, Devoe LD. Perinatal outcome and amniotic fluid index in the antepartum and intrapartum periods: a meta-analysis. Am J Obstet Gynecol. 1999;181:1473–8.
Committee on Obstetric Practice. Antenatal corticosteroid therapy for fetal maturation. Committee opinion no. 713. Obstet Gynecol. 2017;130:e102–9.
Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
Ting JY, Kingdom JC, Shah PS. Antenatal glucocorticoids, magnesium sulfate, and mode of birth in preterm fetal small for gestational age. Am J Obstet Gynecol. 2018;218:S818–28.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Caetano, A.C.R., Nardozza, L.M.M. (2019). Obstetric Management. In: Nardozza, L., Araujo Júnior, E., Rizzo, G., Deter, R. (eds) Fetal Growth Restriction. Springer, Cham. https://doi.org/10.1007/978-3-030-00051-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-00051-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-00050-9
Online ISBN: 978-3-030-00051-6
eBook Packages: MedicineMedicine (R0)