Abstract
Purpose
We aim to compare the perinatal outcomes of two consecutive management strategies for fetal growth restriction (FGR), with or without the inclusion of additional Doppler parameters.
Methods
A quasi-experimental before/after study was conducted in which we compared a composite perinatal outcome, prematurity rate, and neonatal complications between two management strategies in small fetuses. In the strategy 1 (S1), the management was based on fetal biometry and umbilical artery Doppler. The second strategy (S2) added the assessment of uterine and middle cerebral artery Doppler. We also compared outcomes between strategies according to early (≤ 32 weeks) and late (> 32 weeks) diagnosis subgroups.
Results
We included 396 patients, 163 in S1 and 233 in S2. There were no significant differences in the perinatal composite outcome (p 0.98), prematurity (p 0.19), or in the subgroup analysis. We found a significant reduction in respiratory distress syndrome (RDS) rate with S2 both globally (OR 0.50, p 0.02), and in the early diagnosis subgroup (OR 0.45, p 0.01). In addition, we observed a significant reduction in the incidence of sepsis with S2 both globally (OR 0.30, p 0.04) and in the early diagnosis subgroup (OR 0.25, p 0.02). We did not observe significant differences in necrotizing enterocolitis (p 0.41) and intraventricular hemorrhage (p 1.00).
Conclusion
The expanded strategy for the management of FGR did not show significant differences in the primary composite outcome or prematurity. However, it was associated with a lower incidence of RDS and neonatal sepsis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fetal growth restriction (FGR) due to placental insufficiency is defined by an estimated fetal weight (EFW) lower than the 10th percentile with Doppler signs of placental insufficiency [1,2,3]. It is one of the leading causes of perinatal morbidity and mortality, associated with up to 30% of intrauterine deaths in the third trimester [4]. Recently, it has also been linked to a greater predisposition to pediatric and adult non-communicable diseases such as diabetes mellitus, obesity, high blood pressure, and cardiovascular disease [5,6,7].
Although several interventions have been proposed to improve the prognosis of these fetuses, none of them have shown to be effective [8,9,10,11,12,13,14,15]. For this reason, so far, the only available tool to manage these cases is early detection and timely delivery.
Traditionally, screening and management were based exclusively on ultrasound biometry and umbilical artery Doppler [1, 16,17,18]. According to this strategy, fetuses with an EFW below the 10th percentile with normal umbilical artery Doppler were considered to be small for gestational age (SGA), while those with abnormal umbilical artery Doppler were labeled as growth-restricted [19,20,21]. However, different groups and scientific societies have recently advocated for the introduction of a more comprehensive definition for FGR that includes the evaluation of other Doppler parameters to standardize the obstetric management of this condition [4, 22, 23]. This new definition includes extremely small fetuses (EFW less than the 3rd percentile) and fetuses with EFW between the 3rd and 10th percentile and at least one of the following abnormal Doppler parameters: umbilical artery pulsatility index (UmA-PI) over the 95th percentile, middle cerebral artery pulsatility index (MCA-PI) below the 5th percentile, the ratio between these two vessels (cerebro-placental ratio, CPR) below the 5th percentile, and mean pulsatility index of uterine arteries (UtA-PI) over the 95th percentile [24].
The aim of this study was to compare the perinatal outcomes between these two strategies for the management of FGR during two consecutive periods in a single tertiary center.
Methods
Study design
This was a quasi-experimental before/after study in which we compared a perinatal composite outcome between two management strategies in patients with small fetuses followed in our Unit from June 2009 to April 2019.
Groups and definitions
We included all singleton pregnancies with an EFW less than the 10th percentile and available perinatal outcomes. We excluded all cases with fetal genetic and structural anomalies and prenatal infections.
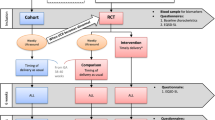
Patients were classified and managed from June 2009 to July 2014 according to Strategy 1 (S1) (Fig. 1) [18], and from July 2014 to April 2019 according to Strategy 2 (S2) (Fig. 2) [24].
Classification and management recommended by Strategy 2 EFW estimated fetal weight, UmA-PI umbilical artery pulsatility index, MCA-PI middle cerebral artery pulsatility index, CPR cerebro-placental ratio, UtA-PI mean pulsatility index of the uterine arteries, SGA small for gestational age, FGR fetal growth restriction, > p95 greater than the 95th percentile, < p5 below the 5th percentile, UmA umbilical artery, DV Ductus venosus, DV-PI pulsatility index of the ductus venosus, EDF end-diastolic flow
Briefly, in S1, SGA was defined as an EFW below the 10th percentile with normal umbilical artery Doppler and in S2, as an EFW between the 3rd and 10th percentile with normal Doppler parameters (UmA-PI, MCA-PI, CPR, or UtA-PI). In S1, the diagnosis criterion for FGR was an EFW below the 10th percentile with an abnormal umbilical artery Doppler defined as UmA-PI over the 95th percentile. In S2, the diagnosis of FGR was based on the updated definition proposed in 2014 [24], i.e., either an EFW below the 3rd percentile or an EFW between the 3rd and 10th percentile with any of the following abnormal Doppler: MCA-PI below the 5th percentile, CPR below the 5th percentile, UmA-PI over the 95th percentile, and UtA-PI over the 95th percentile [25, 26]. Each strategy had its own recommended follow-up intervals and gestational ages for delivery according to severity (Figs. 1, 2).
In both strategies, the decision to administer steroids and magnesium sulfate was based on the gestational age (GA) at birth. Our local protocol consisted of administering two doses of 12 mg of betamethasone every 24 h to pregnant women expected to deliver between 24 + 0 and 34 + 6 weeks and magnesium sulfate to those expected to deliver between 24 + 0 and 31 + 6 weeks. There were no changes in this protocol during the 2009–2019 period.
Outcomes
The primary outcome was a composite of perinatal death or the need for mechanical ventilation (NMV) or admission to the Neonatal Intensive Care Unit (NICU) for at least 1 day.
The secondary outcomes were: operative delivery rate, Apgar score < 7 at 5 min, the incidence of prematurity and associated complications, such as respiratory distress syndrome (RDS), sepsis, intraventricular hemorrhage (IVH), and necrotizing enterocolitis (NEC). Operative delivery was defined as delivery by cesarean section, vacuum, or forceps. Prematurity was defined as GA at birth < 37 weeks. RDS diagnosis was based on the clinical picture of a neonate with respiratory failure confirmed by a chest X-ray. IVH was defined by clinical suspicion added to signs of intraventricular bleeding on transfontanellar ultrasound. Sepsis was defined as positive blood culture requiring treatment with antibiotics, and necrotizing enterocolitis as the presence of pneumatosis or perforation on X-ray or disease identified by laparotomy. Any grade of RDS, NEC, and IVH was considered.
We compared the primary and secondary outcomes between strategies according to early (≤ 32 weeks) and late (> 32 weeks) diagnosis [27].
Statistical analysis
We analyzed the primary and secondary outcomes as dichotomous variables. We calculated the odds ratios (OR) using a simple logistic regression model to analyze the association between the two strategies and the primary and secondary outcomes. We analyzed separately each component of the composite outcome using independent logistic regression models.
Numerical variables were described with mean and standard deviation or median and interquartile range according to distribution and categorical variables with percentage and absolute frequency. T test or Mann–Whitney test was used for continuous variables according to distribution and Chi2 or Fisher’s test for categorical variables.
All data analysis was done using the Stata 13.0 software package.
A p value of less than 0.05 was considered statistically significant.
The study was approved by the institutional review board (P20-035).
Results
Among 812 pregnancies with an EFW less than the 10th percentile, 172 were excluded for having normalized growth in subsequent controls, 163 for fetal pathology, and 81 for incomplete perinatal data. 396 met the inclusion criteria, 163 in S1 and 233 in S2 (Fig. 3). The baseline characteristics of the population are presented in Table 1.
The mean GA at diagnosis was 29.4 ± 4 weeks in S1 and 29.2 ± 4 weeks in S2. The proportion of FGR was 25% (95% CI 18–32) in S1 and 59% (95% CI 53–65) in S2 (p < 0.001). In S1, the proportion of pregnancies with abnormal Doppler was: UmA-PI over the 95th percentile 22.7% (95% CI 17–29), and ductus venosus pulsatility index (DV-PI) over the 95th percentile 1.8% (95% CI 0.6–6). In S2, the proportion of abnormal Doppler was: UtA-PI over the 95th percentile 37.9% (95% CI 32–44), UmA-PI over the 95th percentile 15% (95% CI 11–20), MCA-PI below the 5th percentile 9.9% (95% CI 7–14), CPR below the 5th percentile 16.7% (95% CI 12–22), and DV-PI over the 95th percentile 0.9% (95% CI 0.2–3). The median GA at birth for both strategies was 37 weeks (S1 37.8, IQR 35.1–39.4; S2 37.7 IQR 35.9–39).
There were no significant differences in the perinatal composite outcome between the two strategies (OR 0.99, 95% CI 0.7–1.5, p 0.98) (Table 2), or in the subgroup analysis (early vs. late diagnosis) (Table 3).
No differences were found in the rate of operative delivery (OR 0.93, 95% CI 0.6–1.4, p 0.75) nor in the proportion of newborns with Apgar score < 7 at 5 min (OR 0.69, 95% CI 0.3–1.7, p 0.41) (Tables 2, 3).
There were no differences in the global incidence of preterm birth (S1: 40%, 95% CI 33–48; S2: 33%, 95% CI 27–39; OR 0.76, 95% CI 0.5–1.2, p 0.19) (Table 2). Subgroup analysis according to GA at diagnosis showed a trend for lower rate of preterm birth in the early diagnosis subgroup for S2, not reaching statistical significance (Table 3).
In relation to complications of preterm birth, we found a significant reduction in the incidence of RDS in S2, both globally (OR 0.50, 95% CI 0.3–0.9, p 0.02), and in the early diagnosis subgroup (OR 0.45, 95% CI 0.2–0.8, p 0.01) (Tables 2, 3). In addition, we observed a significant reduction in the incidence of sepsis in S2, both globally (OR 0.30, 95% CI 0.09–0.9, p 0.04) and in the early diagnosis subgroup (OR 0.25, 95% CI 0.08–0.8, p 0.02) (Table 2, 3).
We did not observe significant differences in the other prematurity-related complications, such as NEC (p 0.41) and IVH (p 1.00) (Tables 2, 3).
Discussion
This study provides comparative data on the implementation of an expanded strategy for the prenatal management of small fetuses with respect to the traditional one, based exclusively on biometry and umbilical artery Doppler assessment. There were no differences in the perinatal composite outcome or preterm birth rate between both strategies. However, we found a reduction in RDS and sepsis in the cases managed with the expanded strategy.
Although the composite perinatal outcome rates found in this study were higher than in previously published studies, we attribute these differences to the outcome definition. The PORTO study [28] reported a composite perinatal adverse outcome of 5.2%, 7 times lower than the present study, but this included more severe conditions such as death, intraventricular hemorrhage, periventricular leukomalacia, hypoxic-ischemic encephalopathy, necrotizing enterocolitis, bronchopulmonary dysplasia, and sepsis. The inclusion of NICU admission in our study resulted in a higher incidence of this composite outcome.
The group who initially described S2 published the results of a cohort of 1197 pregnancies with low fetal weight, in which they reported a composite adverse outcome rate of 9.7% [29]. Although this composite outcome consisted of the same variables as our study except for metabolic acidosis, they only included fetuses with late FGR, which are known to have a lower risk of perinatal complications. As our study also included early FGR, the global perinatal outcomes were worse, including a two-to-three times higher rate of preterm birth.
In our study, we did not observe significant differences in the preterm birth rate between both strategies. However, S2 showed a lower incidence of two complications related to prematurity, such as RDS and sepsis. This could be related to the fact that S2 provides more information about the fetal hemodynamic status, which could in turn translate into a better optimization of the moment of birth.
Strengths and limitations
To the best of our knowledge, this is the first study that compares the perinatal outcomes of two consecutive prenatal strategies for the management of small fetuses based on an updated definition of FGR which included not only late-onset but also early diagnosed FGRs. Another strength is that this study included a considerable number of pregnancies with low fetal weight, with a low percentage of loss to follow-up.
However, this study also has several limitations. Since the Delphi consensus [3] was published after the implementation of the S2, the decrease of more than 50 percentile points of EFW was not taken into account for the diagnosis of FGR in either of the two strategies unless it met some criteria described in each protocol. Due to the study design, the results could have been influenced by a temporal bias related to potential changes in the neonatal protocols. Since it was an observational study, there may be unmeasured confounding influencing the results. Additionally, this study did not evaluate the possible effects of these interventions in the medium and long term, such as cardio-metabolic changes and neurocognitive development.
Conclusion
The implementation of an expanded strategy for the management of small fetuses based on the inclusion of additional Doppler parameters did not show significant differences in the primary composite outcome, operative delivery rate, Apgar < 7 at 5 min, or the proportion of preterm birth when compared with a strategy based exclusively on fetal biometry and umbilical artery Doppler. However, the expanded strategy was associated with a lower incidence of RDS and neonatal sepsis, possibly related to an optimization in gestational age at birth according to risk.
The use of additional Doppler parameters such as UtA-PI, MCA-PI, and CPR included in an updated definition of FGR, together with a standardized management algorithm, may improve the detection of small fetuses with a higher risk of complications and consequently improve their perinatal outcomes.
Additional studies are warranted to validate these results in other populations.
References
American college of obstetricians and gynecologists (2013) Practice bulletin No. 134: fetal growth restriction. Obstet Gynecol 1122–1133. https://doi.org/10.1097/01.aog.0000429658.85846.f9
McCowan LM, Figueras F, Anderson NH (2018) Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2017.12.004
Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN et al (2016) Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 48:333–339. https://doi.org/10.1002/uog.15884
Lees CC, Stampalija T, Baschat A, da Silva CF, Ferrazzi E, Figueras F et al (2020) ISUOG practice guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol 56:298–312. https://doi.org/10.1002/uog.22134
Salam RA, Das JK, Bhutta ZA (2014) Impact of intrauterine growth restriction on long-term health. Curr Opin Clin Nutr Metab Care 17:249–254. https://doi.org/10.1097/MCO.0000000000000051
Kopec G, Shekhawat PS, Mhanna MJ (2017) Prevalence of diabetes and obesity in association with prematurity and growth restriction. Diabetes Metab Syndr Obes 10:285–295. https://doi.org/10.2147/DMSO.S115890
Ross MG, Beall MH (2008) Adult Sequelae of Intrauterine Growth Restriction. Semin Perinatol. https://doi.org/10.1053/j.semperi.2007.11.005
Hawkes N (2018) Trial of Viagra for fetal growth restriction is halted after baby deaths. BMJ. https://doi.org/10.1136/bmj.k3247
Villanueva-García D, Mota-Rojas D, Hernández-González R, Sánchez-Aparicio P, Alonso-Spilsbury M, Trujillo-Ortega ME et al (2007) A systematic review of experimental and clinical studies of sildenafil citrate for intrauterine growth restriction and pre-term labour. J Obstet Gynaecol. https://doi.org/10.1080/01443610701194978
Sharp A, Cornforth C, Jackson R, Harrold J, Turner MA, Kenny LC et al (2018) Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child Adolesc Health 2:93–102. https://doi.org/10.1016/S2352-4642(17)30173-6
Spencer R, Ambler G, Brodszki J, Diemert A, Figueras F, EVERREST Consortium et al (2017) EVERREST prospective study: a 6-year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction. BMC Pregnancy Childbirth. https://doi.org/10.1186/s12884-017-1226-7
Say L, Metin Gülmezoglu A, Justus HG (2003) Maternal oxygen administration for suspected impaired fetal growth. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd000137
Laurin J, Persson P-H (1987) The effect of bedrest in hospital on fetal outcome in pregnancies complicated by intrauterine growth retardation. Acta Obstet Gynecol Scand. https://doi.org/10.3109/00016348709022043
Say L, Metin Gülmezoglu A, Justus HG (2003) Maternal nutrient supplementation for suspected impaired fetal growth. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd000148
Say L, Metin Gülmezoglu A, Justus HG (2001) Betamimetics for suspected impaired fetal growth. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd000036
Kingdom JCP, Burrell SJ, Kaufmann P (1997) Pathology and clinical implications of abnormal umbilical artery Doppler waveforms. Ultrasound Obstet Gynecol. https://doi.org/10.1046/j.1469-0705.1997.09040271.x
Karsdorp VHM, van Vugt JMG, van Geijn HP, Kostense PJ, Arduim D, Montenegro N et al (1994) Clinical significance of absent or reversed end diastolic velocity waveforms in umbilical artery. Lancet. https://doi.org/10.1016/s0140-6736(94)90457-x
Society for Maternal-Fetal Medicine Publications Committee, Berkley E, Chauhan SP, Abuhamad A (2012) Doppler assessment of the fetus with intrauterine growth restriction. Am J Obstet Gynecol 206:300–308. https://doi.org/10.1016/j.ajog.2012.01.022
Ott WJ (2000) Intrauterine growth restriction and Doppler ultrasonography. J Ultrasound Med 19(10):661–665. https://doi.org/10.7863/jum.2000.19.10.661
Bamfo JE, Odibo AO (2011) Diagnosis and management of fetal growth restriction. J pregnancy. https://doi.org/10.1155/2011/640715
Figueras F, Gardosi J (2011) Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 204(4):288–300. https://doi.org/10.1016/j.ajog.2010.08.055
Salomon LJ, Alfirevic Z, Da Silva CF, Deter RL, Figueras F, Ghi T et al (2019) ISUOG practice guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound Obstet Gynecol 53:715–723. https://doi.org/10.1002/uog.20272
Melamed N, Baschat A, Yinon Y, Athanasiadis A, Mecacci F, Figueras F et al (2021) FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet 152(Suppl 1):3–57. https://doi.org/10.1002/ijgo.13522
Figueras F, Gratacos E (2014) Stage-based approach to the management of fetal growth restriction. Prenat Diagn 34:655–659. https://doi.org/10.1002/pd.4412
Gómez O, Figueras F, Fernández S, Bennasar M, Martínez JM, Puerto B, Gratacós E (2008) Reference ranges for uterine artery mean pulsatility index at 11–41 weeks of gestation. Ultrasound Obstet Gynecol: Off J Int Soc Ultrasound Obstet Gynecol 32(2):128–132. https://doi.org/10.1002/uog.5315
Ciobanu A, Wright A, Syngelaki A, Wright D, Akolekar R, Nicolaides KH (2019) Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet Gynecol 53(4):465–472. https://doi.org/10.1002/uog.20157
Savchev S, Figueras F, Sanz-Cortes M, Cruz-Lemini M, Triunfo S, Botet F et al (2014) Evaluation of an optimal gestational age cut-off for the definition of early- and late-onset fetal growth restriction. Fetal Diagn Ther 36:99–105. https://doi.org/10.1159/000355525
Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O’Donoghue K et al (2013) Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol 208(290):e1-6. https://doi.org/10.1016/j.ajog.2013.02.007
Meler E, Mazarico E, Eixarch E, Gonzalez A, Peguero A, Martinez J et al (2020) A 10-year experience of protocol-based management of fetal growth restriction: perinatal outcomes in late pregnancy cases diagnosed after 32 weeks. Ultrasound Obstet Gynecol. https://doi.org/10.1002/uog.23537
Acknowledgements
The authors would like to thank Diego Hernan Giunta, Cristina Maria Elizondo, Lucas Otaño, Pablo Hernan Brener, Mariana Leda Bucich, and Maria Lourdes Posadas-Martinez for their valuable contribution to this study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: SPD, AE, and CHM; methodology: SPD and AE; acquisition of data: SPD, GD, and MCB. Formal analysis and investigation: SPD, AE, and CHM; writing—original draft preparation: SPD; writing—review and editing: AE and CM.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study protocol was approved by our institutional review board.
Consent for publication
All authors agree on the final manuscript and the order of authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demirdjian, S.P., Meller, C.H., Berruet, M.C. et al. Perinatal outcomes of two consecutive strategies for the management of fetal growth restriction: a before–after study. Arch Gynecol Obstet 307, 319–326 (2023). https://doi.org/10.1007/s00404-022-06641-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06641-x