Abstract
Ecological acoustic recorders (EARs) have been deployed at several locations in Hawaii and in other western Pacific locations to study the foraging behavior of deep-diving odontocetes. EARs have been deployed at depths greater than 400 m at five locations around the island of Kauai, one at Ni’ihau, two around the island of Okinawa and four in the Marianas (two close to Guam, one close to Saipan, and another close to Tinian). The four groups of deep-diving odontocetes were blackfish (mainly pilot whales and false killer whales), sperm whales, beaked whales (Cuvier and Bainsville beaked whales), and Risso’s dolphin. In all locations, the biosonar signals of blackfish were detected the most followed by either sperm or beaked whales depending on specific locations with Risso’s dolphin being detected the least. There was a strong tendency for these animals to forage at night in all locations. The detection results suggest a much lower population of these four groups of odontocetes around Okinawa and in the Marianas and then off Kauai in the main Hawaiian Island chain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
The use of autonomous remote passive acoustic monitoring (PAM) continues to grow as new and varied devices become commercially available. These devices are extremely useful in order to collect long-term (months) acoustic data on the presence of marine mammals in any area of interests, especially remote areas and in areas that are difficult to get to on a regular basis. The advantages and disadvantages of using PAM including the early history of the use of PAM have been discussed in Chap. 1 of this book and will not be repeated here. However, we emphasize again that PAM represent one of the few ways to obtain data in remote locations of the world and over periods of months and years.
Our knowledge of the distribution of cetacean in a large part of the Pacific has come mainly from shipboard visual line-transect cetacean surveys for over 30 years and are now conducted with combined visual and acoustics methods for over several years (Rankin et al. 2008). However, these surveys tend to occur infrequently with surveys occurring at intervals between half a year to several years. Only a handful of surveys have been performed around the Hawaiian Islands and more research needs to be conducted. In recent years large ship surveys have been complemented by shall-boat surveys close to shore (Barid et al. 2013). Very little survey efforts have been spent in other areas of the Pacific west of Hawaii including the northwest Hawaiian Islands. This chapter focuses mainly on monitoring efforts around the island of Kauai (Au et al. 2013) in the main Hawaiian Island chain with additional data from Okinawa and the Marianas.
There are approximately 18 species of odontocetes and six species of baleen whales that can be found in Hawaiian waters (Baird et al. 2009). Except for spinner dolphins (Stenella longirostris) and humpback whales (Megaptera novaeangliae) the locations and time of occurrence of these cetaceans cannot be predicted with any degree of certainty. Knowing what animals are present in a given body of water at any given time is important in order to understand the overall cetacean population dynamics. Where and when animals might be present may provide insights as to how different species utilize a given habitat. For example, spinner dolphins in Hawaii typically rest during the day in several different known locations along a coast. In the late afternoon and at night they may travel along the entire coastline at varying distances from shore foraging for food. They move with the mesopelagic boundary community throughout the night to optimize their foraging effort (Benoit-Bird and Au 2003).
The presence of deep-diving odontocetes around the island of Kauai detected by a number of autonomous remote PAM devices operating nearly simultaneously has been studied by Au et al. (2013, 2014). The studies by Au and his colleagues have been focused on Blainville’s beaked whale s, Mesoplodon densirostris, Cuvier beaked whales, Ziphius cavirostris sperm whale s, Physeter macrocephalus, short-finned pilot whales , Globicephala macrorhynchus, and Risso’s dolphin (Grampus griesus). These species are known to be present in Hawaiian waters (Baird et al. 2009, 2013) and they typically forage at depths as far down to approximately 1200 m. To complicate the study of these animals beaked whales and sperm whales do not emit whistle signals but only click signals, most of which are biosonar click signals. The same type of studies have been conducted in the Marianas and off Okinawa , in waters used by the US military.
Kauai is of special interest to the Navy since the Pacific Missile Range facility (PMRF) underwater test range exists in waters along the west and southwest coast. Our objectives were to determine the daily pattern of detection of different species, relative number of detections, and the seasonal and diurnal patterns at five locations around Kauai. The diurnal behavior of biosonar activity was previously reported by Au et al. (2013, 2014). The Marianas is also of special interest to the US Navy with bases and facilities on the island of Guam with training exercise areas in the waters of Saipan and Tinian . The US Marines are presently on Okinawa although they will eventually close their bases there in the near future.
Our knowledge of the behavior of deep-diving odontocetes has expanded manyfold with the introduction of the D-tag (digital acoustic record ing tag) developed at Woods Hole Oceanographic Institute (Johnson and Tyack 2003). DTAGs have been used to study deep-diving odontocetes such as Blainville’s and Cuvier beaked whale s (Johnson et al. 2004; Madsen et al. 2005), sperm whale s (Miller et al. 2004), and short-finned pilot whales (Aguilar de Soto 2006; Aguilar de Soto et al. 2008). These same species are also present in Hawaiian waters (Baird et al. 2009, 2013).
Beaked whales, sperm whale s, short-finned pilot whales , and Risso’s dolphin are some of the deep-diving odontocete species that forage in a depth regime between several hundred meters down to slightly over 1200 m using their biosonar to hunt for prey (Johnson et al. 2004; Aguilar de Soto 2006). Johnson et al. (2004, 2006) reported that beaked whale s can dive to depths on the order of 1200 m but do not emit biosonar signals until they descend below approximately 200 m below the surface. DTAG data collected by Miller et al. (2004) showed the steady use of regular biosonar clicks with creaks produced during the deepest part of dives by sperm whales. Aguilar de Soto et al. (2008) reported that short-finned pilot whales forage at depths between 250 and 1000 m using their biosonar to detect prey. Deep-diving odontocetes such as pilot whales, sperm whales, beaked whales, and Risso’s dolphins prey on squids and deep-dwelling demersal fish .
Another device that has contributed to our expanding knowledge of deep-diving foraging odontocete is the autonomous high-frequency acoustic record ing package (HARP ) developed at the Scripps Institute of Oceanography (Wiggins and Hildebrand 2007). Use of the HARP was also accompanied by research to identify odontocetes by their biosonar signals. The use of a HARP off the Cross Seamount (Johnston et al. 2008; McDonald et al. 2009) and another in the waters of Palmyra Atoll (Baumann-Pickering et al. 2010) have successfully confirmed the presence of foraging beaked whale s in both locations. Soldevilla et al. (2008, 2010) reported on the presence and behavior of Risso’s dolphin (Grampus griesus) and Pacific white-sided dolphin (Lagenorhynchus obliquidens) in the Southern California Bight. These studies have demonstrated that some species of echolocating odontocetes can be identified by characteristics of their biosonar signals and autonomous remote recorders can collect data to study the long-term behavior of deep-diving odontocetes in a single location. Chap. 2 will be devoted to important findings from the use of Harps.
5.2 Identifying Odontocete Species by Their Biosonar Signals
The prevalent notion for many years was that species and groups of odontocetes could not be identified by their broadband biosonar clicks. Beam pattern measurements on the bottlenose dolphins ( Tursiops truncatus ), a false killer whale (pseudorca crassidens) and a beluga whale ( Delphinapterus leucas ) summarized in Au (1993) indicated that signals measured at angles away from the beam axis were distorted when compared with the signals measured on the beam axis. Not only were the off-axis signals distorted but the waveform and spectrum varied almost randomly as a function of angle in three-dimensional space. Measurements done in the field on the white-beaked dolphin (Lagenorhynchus albirostris) by Rasmussen et al. (2002), killer whale ( Orcinus orca ) by Au et al. (2004), Atlantic spotted dolphin (Stenella frontalis) by Au and Herzing (1997), dusky dolphins (Lagenorhynchus obscurus) by Au and Würsig (2004), and spinner dolphin (Stenella longirostris) and pantropical spotted dolphin (Stenella attenuata) by Schotten et al. (2003) all indicated that off-axis signals were distorted. However, measurements of biosonar signals produced by odontocetes that forage at deep depths indicate that these animals can be identified by their biosonar signals.
Sperm whales, being the largest of all odontocetes, should produce the biosonar signals with the lowest peak frequency (frequency of maximum energy). Madsen et al. (2002) and Mohl et al. (2003) reported peak frequency between 8 and 15 kHz for sperm whale s. The physics of sound production indicate that sperm whales are probably the only animals that can produce biosonar clicks with such low peak frequency. Therefore, detected biosonar signals with peak frequency in this frequency range can only be produced by sperm whales. Beaked whales are the only odontocetes known to consistently produce biosonar signal that is frequency modulated (Johnson et al. 2004; Madsen et al. 2005; Zimmer et al. 2005). The spectra of Risso’s dolphin biosonar clicks typically have a rippled feature between 20 and 30 kHz (Soldevilla et al. 2008). The biosonar signals of the Pacific white-sided dolphin (Lagenorhynchus obliquidens) also have a similar rippled structure between 20 and 30 kHz (Soldevilla et al. 2008); however, this species has not been seen in Hawaiian waters. According to Bauman-Pickering et al. (2011) the biosonar clicks of short-finned pilot whales and false killer whales ( Pseudorca crassidens ) have a peak frequency close to 30 kHz and it is often difficult to tell the two species apart acoustically. However, the sighting rate of false killer whales on visual surveys around the Hawaiian Islands is approximately 10 % of short-finned pilot whale sightings (Barid et al. 2013). Representative signals and spectrum from a sperm whale, a Risso’s dolphin, and a short-finned pilot whale are shown in Fig. 5.1. The waveform and Wigner-Ville distribution showing frequency versus time distribution of a beaked whale biosonar signal are also shown in Fig. 5.1. In this chapter, no distinction is made between Blainville’s and Cuvier’s beaked whales. Both species are lumped into a single category.
Examples of waveforms and frequency spectra of biosonar clicks produced by sperm whale s, short-finned pilot whales , and Risso’s dolphins . The waveform and Wigner-Ville time-frequency distribution are shown for a representative beaked whale signal. All signals were extracted from EAR recording being discussed in this manuscript
5.3 Marine Mammal Monitoring on Navy Ranges (M3R) Software
The acoustic data of Au et al. (2013, 2014) were analyzed with the class -specific support vector machine (CS-SVM) portion of the M3R software (Jarvis et al. 2008; Jarvis 2012) and custom Matlab programs. The M3R (Jarvis et al. 2008; Jarvis 2012) is the primary Navy software used to detect and identify deep-diving odontocetes at the following US Navy ranges: AUTEC (Atlantic Undersea Test and Evaluation Center), SCORE (Southern California Offshore Range), and PMRF (Pacific Missile Range Facility). It has undergone detailed testing and found to be reliable and robust . The CS-SVM portion of the M3R software uses nine-dimensional feature vectors formed by computing the time between 6 zero crossings about the peak and 3 normalized envelope amplitude peaks. M3R software contain templates of biosonar signals from the short-finned pilot whale, Risso’s dolphin , sperm whale s, Cuvier and Blainville beaked whale s, and spinner dolphins (Stenella longirostris). A preliminary performance check can be found in Jarvis et al. (2008) and a more detailed performance evaluation can be found in Jarvis (2012). Au et al. (2013, 2014) performed separate validation test of the M3R performance using a totally different method than that used by Jarvis (2012). The classification precision of the M3R on test data sets for all the species was high, 85 % or higher depending on the species (Jarvis 2012). We combined the Cuvier and Blainville beaked whales together under the beaked whale category. We also combined all dolphin biosonar signals except those of the short-finned pilot whales and Risso’s dolphins as unknown dolphins, which would include a number of inshore species that typically do not dive to deep depths but their biosonar clicks can occasionally be detected by a deep-moored PAM.
5.3.1 Independent Validation Test of M3R
Validation test of the M3R algorithm was conducted by Jarvis et al. (2008) and Jarvis (2012) using test data that contained the biosonar signals of specific species. An independent validation test was performed in the study of Au et al. (2014) using data collected by one of our EARs using a completely different technique than Jarvis (2012). The validation test consisted of examining 100 files per species for each of the four species, sperm whale s, short-finned pilot whales , Risso’s dolphins , and beaked whale s that were detected by the CS-SVM algorithm. The waveform, frequency spectrum, and the Wigner-Ville time-frequency distribution for each biosonar click were displayed on a computer monitor and a decision made by a human operator as to the species producing the signal. The frequency spectrum was used to determine the presence of sperm whales, short-finned pilot whales, and Risso’s dolphin . The Wigner-Ville distribution was used to determine the presence of beaked whales. The time waveform was also used for further confirmation of the presence of a particular species. If the visual inspection indicated that at least five signals were from the designated species then that file was accepted as a correctly identified file. The specific clicks detected by the M3R algorithm were not singled out so that the clicks used to identify the desired species were not necessarily the same clicks detected by M3R. The results of the validation tests are shown in Table 5.1.
Not surprising is that all the files in which M3R indicated the presence of sperm whale s were verified since Jarvis (2012) also had 100 % correct detection for sperm whales. Sperm whale biosonar clicks are probably the most unique of all odontocetes since it is the only species with clicks that have peak frequencies between approximately 5 and 15 kHz (Mohl et al. 2003). There is a remote possibility that highly off-axis clicks from bottlenose dolphin (Tursiops truncatus) could be confused with clicks from short-finned pilot whales . Examples of the clicks measured at aspect angles of ±90° by Au et al. (2012a, b) have a peak frequency close to 20 kHz, similar to the spectrum in Fig. 5.2 for the short-finned pilot whale. However, at such a wide off-axis angle, the signal level is 45–55 dB below the on-axis source level. With an EAR at depths below 600 m, it is highly unlikely that such extreme off-axis clicks from bottlenose dolphins at shallow depths would be regularly detected in comparison to the signals of short-finned pilot whales which consistently forage at much deeper depths. The M3R software was originally developed to analyze data from deep bottom-mounted hydrophones on Navy acoustic ranges.
Another independent validation study was conducted by Bio-wave Inc. under contract to HDR Inc. to visually examine some randomly selected EAR files collected off the island of Niihau. This analysis concentrated on beaked and sperm whale s. When the same data set used by Bio-wave Inc. was analyzed with the M3R algorithm, performance accuracy was 99.4 % correct on the 746 files used to look for Ziphius signals and 98 % correct on the 748 files used for Mesoplodon.
5.4 Deployment of Deep EARs in Western Pacific
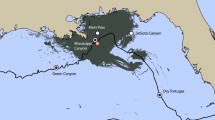
Five EARs were deployed around the island of Kauai in the approximate locations shown in Fig. 5.2 between February 2009 and January 2011. These were refurbished by swapping out the batteries and the laptop disk used to store the data at intervals of approximately 4–5 months. One was deployed off Ni’ihau in June 2010 and retrieved in December 2010. The depth of the EARs at the different locations is indicated in the figure. The original data acquisition rate for the EARs around Kauai was 64 kHz but was eventually modified to 80 kHz (Au et al. 2013). Only data collected at a sample rate of 80 kHz have been used in the M3R program. The sample rate for the EAR off Ni’ihau was 80 kHz. All of the EARs acquired data over a 30-s period every 5 min. After collecting data for 30 s, the EAR would enter a “sleep” mode to conserve battery and storage and wake up 4.5 min later.
Three EARs were deployed in waters of Okinawa , Japan, between November 2011 and May 2012. However, only two of the EARs were in deep waters (greater than 400 m). The western site was designated as Le Shima (in the East China Sea) and the eastern site (in the Philippines Sea) as Schwab South. Deep EARs were also deployed in the Marianas, in waters off Guam , Saipan , and Tinian between September 2011 and September 2012. The approximate location and depth of the EARs deployed off Okinawa (deep EARs only) and in the Marianas are shown in Fig. 5.3. The depth of each EAR is shown in the figure. In order to increase the recording time, the duty cycle for the EARs in the Marianas was changed to 30 s of recording every 10 min instead of every 5 min.
Detection of deep-diving odontocetes was reported by Au et al. (2013, 2014) in terms of the number of 30-s files that contained biosonar signals from the different species. Each file can be considered an observation period (OBSP) and if the 30-s data acquisition period occurred every 5 min, then there were 288 OBSP per day. The EARs in the Marianas had a duty cycle of 10 min so that there were 144 OBSP per day. The longer duty cycle was used in order to extend the battery life of each EAR .
5.5 Daily Pattern of Biosonar Detections
5.5.1 Off the Island of Kauai
The results for the four groups of deep-diving odontocetes listed in Table 5.1 along with a group denoted as unknown dolphins are shown in Fig. 5.4 for the SW location of Kauai during the period between January 26 and May 4, 2010. The plots all have the same vertical scale so that a quick visual inspection will portray the relative number of detection between the species. The results indicate that at least one of the species of interest was detected every day. There were many days in which multiple species were detected. The daily occurrence of at least one group is typical for all sites regardless of the time period. The data indicate that pilot whales were detected most often followed by sperm and beaked whale s. The highest detection rate occurred on April 23 with a rate of 52 %. This means that of the 288 observation periods during that day, 150 contained biosonar signals of pilot whales. However, the daily number of detection also varied considerably. Two days before the day of highest detection only 8 % or 23 of the OBSP and 2 days after only 6 % or 17 of the OBSP contained pilot whale biosonar signals. At least six dolphin species can be lumped into the unknown dolphin category, Pacific bottlenose dolphin ( Tursiops truncatus gilli), Hawaiian spinner dolphins (Stenella longirostris), pantropical spotted dolphin (Stenella attenuata), striped dolphin (Stenella coeruleoalba), rough-toothed dolphin (Steno bredanensis), and Fraser’s dolphin (Lagenodelphis hosei). These dolphins emit very similar and highly variable biosonar signals that are not species specific and at this time impossible to separate (Au 1993; Au and Hasting 2008; Schotten et al. 2003).
The graphs in Fig. 5.4 also show a pattern of detection that was similar for the five groups of odontocetes. There were periods of relatively high detection in March and after the first week in April for all groups. The pattern may be difficult to visualize because of the day-to-day variations in detecting the biosonar signals of the five groups of odontocetes. Furthermore, the two peaks in detection occurred at approximately the same days.
The spatial distribution of detected biosonar clicks for pilot whales and beaked whale s at the four EAR locations are shown in Figs. 5.5 and 5.6, respectively, for one deployment period.
Similar graphs can be drawn for the other species but short-finned pilot whales were chosen because they had the highest detection levels and beaked whale s were chosen because of the high interest in beaked whales close to PMRF in the waters of SW and NW Kauai. The results in Fig. 5.5 clearly indicate that short-finned pilot whales were detected most often at the SW and NW locations. The results in both Figs. 5.5 and 5.6 clearly indicate that more biosonar signals were detected on the west side of Kauai than on the east side. The average percent of OBSP with biosonar signals had its highest value in April and June for all species at the SW location. The April value at the NW location was higher for all the species than during any months at the NE and SE locations.
The percentage of observation periods per day during the January 26 to May 4, 2010, time period that contained biosonar clicks at the different locations and different groups of odontocetes is shown in Fig. 5.7. Biosonar signals of short-finned pilot whales were detected the most at all locations although the mean values of detection of pilot whale signals were very similar at all locations, approximately 30 % of all OBSP. Biosonar signals of sperm and beaked whale s were close to 20 % and there were no significant difference between the percent of sperm and beaked whale signals detected at all locations during this time period.
5.5.2 Off Okinawa and in the Marianas
The percent of the observation periods with biosonar detected on a daily basis at the two locations in Okinawa was similar to Fig. 5.3 for the island of Kauai with detections made every day during the time period of March 2 to May 17, 2012. Instead of presenting the daily distribution results, the averaged daily results are shown in Fig. 5.8. In this figure, the pilot whale designation was changed to “blackfish,” a group of odontocetes that include short-finned and long-finned pilot whales , false killer whales, and melon-headed whales. Unlike the water in the main Hawaiian Island, it is not known if pilot whale abundance is considerably higher than the other species in this category as is in Hawaii .
Although the biosonar signals of at least one species of deep-diving odontocetes were detected every day, the actual numbers of observation periods for all species were much smaller than off Kauai. The mean values for detection of pilot whale signals were close to 30 % or about four times more in the waters of Kauai compared to about 7 % in the waters of Okinawa . Approximately six times more files with sperm whale clicks and about five times more with beaked whale clicks were detected off Kauai than Okinawa. The detection rate at Le Shima was about the same as at Schwab South for the four groups of odontocetes being compared.
The average and standard deviation of the percent detections at the four locations in the Marianas are shown in Fig. 5.9 within the time period of Sept 11, 2011, and Jan 6, 2012. The EAR at Tinian started recording on September 11 while the other three started recording on September 12. Although the EARs were programmed to record over the same time period, the number of days of recordings varied with 106 days at Saipan , 78 days at Tinian, 118 days at NW Guam , and 108 days at SW Guam because of variations in the battery life. Nevertheless, the daily average of detection shown in Fig. 5.9 should be a good representation of the biosonar activity by the different groups of deep-diving odontocetes at the four different locations.
The duty cycle for the results shown in Fig. 5.9 was 10 min versus 5 min for all the other EARs used in Hawaii and Okinawa , so caution must be taken in comparing the results obtained in the Marianas with results obtained in the other locations. It would be expedient to discuss the possible effect of having a lower duty cycle for the Marianas data at this time so that more confidence can be placed on the meaning of the results. Assume that we have two EARs, one with a 5-min duty cycle (EAR -1) and the second with a 10-min duty cycle (EAR-2) both with the same period so that EAR-1 will have two data sample periods for each of EAR-2. Therefore, any signals detected by EAR-1 during the first sampling period will also be detected by EAR-2 while any signals detected during the second sampling period of EAR-1 would not be available to EAR-2. Assuming a random detection rate, there is a 50 % probability that signals detected by EAR-1 will be during the first sampling period. Therefore, EAR-2 will have approximately one-half the number of OBSP with detections than EAR-1 and since EAR-2 will have ½ the total sampling periods per unit time, the percent of OBSP with biosonar clicks will be the same as for EAR-1. Now if it turns out that there is a slight imbalance in detection so that 55 % of all the clicks detected by EAR-1 occurs in the second sampling period and 45 % in the first sampling period then percent of OBSP for EAR-2 will only be 3.5 % lower than for EAR-1. The reverse could also happen where first sampling period of EAR-1 detects 55 % of all the click for EAR-1 and the second period detects 45 %; then EAR-2 will report a 3.5 % higher detection rate than EAR-1, again a relatively small difference. Therefore we should have good confidence that the low percentage of detection of deep-diving odontocetes in the Marianas is real and that like Okinawa, the detection percentage in the Marianas is approximately 4–7 times lower than off Kauai. The number of detections of the four groups of animals off Tinian was much lower than for the other locations in the Marianas. The number of detections of beaked whale s off NW Guam was very low, indicating nearly an absence of beaked whales at that location. Finally, blackfish had the most detections in all four areas. The data indicate a trend in which the number of deep-diving odontocetes around Okinawa and in the Marianas is considerable lower than around the island of Kauai in the main Hawaiian Island chain. If the percent of detection per OBSP is related to the abundance of the different group of odontocetes, then the population of deep-diving odontocetes in this part of the Pacific Ocean is much lower than in Hawaii.
5.5.3 Diurnal Pattern Biosonar Detections
The diurnal behavior of foraging by deep-diving odontocetes off Kauai and Ni’ihau was examined by Au et al. (2013) by dividing the 24 h in a day into two 12-h periods. The dawn-dusk-night or twilight-night period was defined from 6:00 PM until 6:00 AM and the day period between 6:00 AM and 6:00 PM. At the latitude of the main Hawaiian Islands (19°–22° N) the time difference between sunrise on the longest day and the shortest day is only about 1 h. An example of average number of files in which signals from the various species were detected is shown in Fig. 5.10 for the time period between October 20, 2010, and January 11, 2011, at the SW Kauai location.
An example of the average number of files in which foraging clicks from the different species were detected on an hourly basis for the time period between Oct 20, 2010, and Jan 26, 2011, at the SW location of Kauai. The percentage of twilight-night detection is shown in the shaded block of each histogram
The shaded areas on each histogram plot represent the twilight-nighttime period. The twilight period is often referred to the crepuscular period where many animals display increased activity. The shaded block with a percentage value attached to each histogram is the percentage of time that files with biosonar signals were detected during the twilight-nighttime period. The percent of observation periods with biosonar clicks detected during the twilight-night period at the different EAR locations and for different deployment periods is summarized in Table 5.2 for the locations around Kauai and one at Ni’ihau.
The results in Table 5.2 are consistent with the results of Fig. 5.10 in that most of the foraging clicks were detected at night, although there was a fair amount of variability depending on location, time period, and species and without any other obvious trends. For example, the smallest percentage of foraging clicks detected during the twilight-nighttime period was 57 % for sperm whale at the SW location during the Jun 13 to Sept 19, 2010, period. Yet at the NE location for this same time period, the highest percentage of nighttime foraging clicks of 86 % for sperm whale was recorded. During the Oct 20, 2010 to Jan 26, 2011, time period, the smallest percentage of nighttime foraging clicks for short-finned pilot whale was 62 % at the SE location while during this same time period the highest percentage was 80 % occurred at the NE location. Beaked whales also had a strong tendency to forage at night with foraging mainly occurring 80 % or greater in 7 out of 12 cells in Table 5.2.
In order to obtain a broad and general appreciation of the amount foraging during the twilight-nighttime period around Kauai and Ni’ihau, the total number of files detected for each day and for all time periods and locations was summed for each species. The corresponding number of files that pertained to the twilight-night period was summed and the percent of detection of foraging clicks during twilight-night period is summarized in Table 5.3. The results clearly show a definite preference for twilight-nighttime foraging by the different species.
The diurnal variation in foraging behavior by deep-diving odontocetes in the waters of Okinawa was examined by dividing the 24 h in a day into two 12-h periods in the same manner as for the Kauai data. Sunrise on 15 December 2011 in Okinawa occurred at approximately 07:00, so the dusk-night-dawn period was defined from 19:00 until 07:00 AM and the day period as 07:00–19:00. The average numbers of observation periods in which signals from the various species were detected during the twilight-nighttime periods are shown in Table 5.4. The percentage of observation periods with biosonar clicks detected during the twilight-night periods was considerably higher than the day-time period for each of the five groups. Foraging also occurred during the day, but not as much as during the night.
The EAR at the Schwab South location had a stronger tendency for nighttime foraging than the Le Shima location. Sperm whales had only a slight tendency to forage at night at the Le Shima location but a strong tendency for nighttime foraging at Schwab South location in the Philippine Sea.
The detection of biosonar clicks by the EARs deployed in the Marianas is summarized in Table 5.5. The tendency for nighttime foraging was strong at the four locations for all the marine mammal groups considered. Sperm whales detected at the NW Guam location had the lowest tendency for nighttime foraging but yet 61 % of sperm whale clicks detected at this location occurred at night.
The amount of nighttime clicks was extremely high at all locations for Risso’s and unknown or unidentified small dolphin species in the Marianas and were highest than all the locations in Okinawa , Kauai, and Ni’ihau.
5.6 Percentage of Biosonar Signals by the Different Species
A general insight into the relative number or the relative biosonar activity of the different groups of deep-diving odontocetes can be obtained by examining the percentage of biosonar clicks detected for the different groups at a specific site. This approach was taken by Au et al. (2014) for all the locations around Kauai. The results of Au et al. (2014) separated into four locations around Kauai are shown in Fig. 5.11. An interest feature of Fig. 5.11 is the similarity of the results showing very little differences in the four locations. The percent of biosonar clicks for the short-finned pilot whale varied between 27 and 31 %. The percentage values varied from 19 to 22 % for sperm whale s, 22–25 % for beaked whale s, and 14–17 % for Risso’s dolphins .
The number of observation periods containing beaked whale s and sperm whale s clicks was almost even with beaked whales having a slightly higher detection rate. The unknown dolphin category had the least number of click s which is not surprising because the animals in the unknown category are usually found close to shore and do not normally dive to deep depths. The rate of detection of Risso’s dolphins was only slightly higher than that of unknown dolphins. The largest variation was only 4 % and most locations had no more than 3 % variation. Since the variations in the percent of detections for each species were so small at all locations, a gross estimate of the percentage of signals emitted by the different species around Kauai was calculated based on all the deployment periods and all the locations summed together.
The percentage of observation periods containing biosonar clicks emitted from the different groups of animals at the two locations in the waters of Okinawa is shown in Fig. 5.12. Unlike the Kauai results, there are larger variations between species for this data set. The percentage of clicks attributed to blackfish is 8 % higher off Okinawa than for pilot whales off Kauai.
The amount of clicks attributed to sperm whale s and beaked whale s was higher at Schwab than at Le Shima suggesting that sperm and beaked whales made up a higher percentage of deep-diving odontocetes at Schwab South than at Le Shima. The percentage of clicks attributed to the unknown dolphin category in both locations was much lower than off Kauai.
The percentage of observation periods containing biosonar clicks emitted from the different groups of animals at the four locations in the Marianas is shown in Fig. 5.13.
The amount of biosonar signals attributed to the different groups of deep-diving odontocetes had larger variations in the Marianas than either Kauai and Okinawa . The amount of clicks attributed to sperm whale s at the NW Guam location was highest for all locations in Kauai, Okinawa, and the Marianas. Biosonar clicks attributed to blackfish had the higher rate of detection of all clicks detected in the Marianas. Sperm whales at the NW Guam location emitted approximately 30 % of the clicks detected which is at least 8 % higher than the locations around Kauai and 13 and 17 % higher than the Le Shima and Schwab South locations, respectively, off Okinawa. Conversely, the proportion of clicks attributed to beaked whale s of 1 % was the lowest of all the locations around the Marianas, Kauai, and Okinawa.
5.7 Seasonal Variations of Foraging
The time period of EAR data collected off the island of Kauai was 1 month short of a year but nevertheless the results can provide some insight into how foraging behavior varies during the course of a year. Data from Okinawa and Guam did not extend beyond several months and so these data cannot provide any seasonal insights. The monthly averages of the percent of OBSP per day with biosonar signals are shown in Fig. 5.14 for the different groups of odontocetes. The vertical scale of each plot is the same so that the relative number of occurrences over the same time period at the different locations can be easily observed. The number of days during the beginning and end of each deployment period was limited. There were 7 months in which data existed for every day of the month. Only 5 days of data existed for January 2010 and 4 days for May 2010 and so these are not shown in the figure. The other 4 months had approximately 2 weeks of data associated with them and should serve as a good representation of that particular month.
Each species at the different locations seems to follow a general pattern specific to each location suggesting some general and common conditions (the availability of prey and environmental conditions) that influence each animal group. However, each location had its own trend. At the NW location, the peak monthly average occurred in April 2010. Unfortunately, the EAR did not surface after the second deployment so data and use of that EAR were lost for the rest of the project. The monthly averages showed little variations at the NE location with very small peaks in February and July 2010 and January 2011. At the SE location, two peaks can be seen, one in August and the other in January. There were two peaks, one in April and the other in June for the SW location.
5.8 Discussion and Conclusions
5.8.1 Occurrence
The daily occurrence of at least one group and on most days several groups of deep-diving odontocetes at some locations around Kauai was unexpected and the results suggest that these animals are around a given body of water more often than previously realized. Unfortunately there are no data on visual sighting rate of these animals over periods greater than several weeks. These results strongly suggest that the use of remote acoustic record ers is a good method to obtain quantitative information of which type of animals frequent a particular location. In addition, the data provide daily occurrence pattern and daily behavior of some species of odontocetes. Barlow and Taylor (2005) found that sperm whale s were detected more often acoustically than visually. The acoustic detection ranges were also much larger than the visual detection ranges. Au et al. (2013a, 2014) have shown that most of the biosonar click detections occur during the twilight and nighttime hours when visual surveys are not possible. This pattern of foraging behavior could not possibly be uncovered without long-term acoustic monitoring spanning several months.
One of the important considerations to keep in mind is the fact that food resource is extremely important to any animal and that animals tend to congregate in area of high prey level. In the case of the odontocetes being considered here, their diet consists mainly of squid and some demersal fish that inhabit deep depths. Since these deep-diving odontocetes use their biosonar to forage, other animals in the vicinity should know that foraging is taking place and by knowing the length of foraging bouts and the level of biosonar activity these other animals can ascertain the relative abundance of prey in an area. Furthermore, since odontocetes have a directional hearing system, they can probably localize the depth and locations where different animals are foraging. This type of activity will probably attract them into the area and perhaps prompt them to begin foraging.
It is not obvious why these deep-diving odontocetes would favor certain locations around an island such as the western side of the island of Kauai. The trade winds generally come from a northeast direction so that the western side of the island may be slightly calmer than the eastern side but the difference is not enough to significantly affect the noise received by the EARs. The SW location is directly opposite from the northeast direction of the trade wind and most detections were obtained at this location. Little or no research has been done on whether dolphins and whales prefer calm or rough ocean conditions. It is also not obvious why the population of the four groups of deep-diving odontocetes investigated around Okinawa and in the Marianas is so much lower than around Kauai and why the population at Tinian is so much lower than in other locations in the Marianas. Different prey distribution and behavior will definitely affect which areas they forage in and at this time there is little information on the distribution and behavior of the prey fields that these deep-diving odontocetes feed on in Hawaiian waters or any place in the world. However, other oceanographic conditions such as water temperature, salinity, bottom conditions, and noise conditions are among some of the variables; in addition, the availability of prey should also affect the size of a population in a given body of water. Finally, the presence of predator could affect the population of odontocetes in different sites.
A peculiarity of the results around Kauai is the seemly low seasonal variation in most locations. There was an indication at the SW location of Kauai of a fairly large seasonal variation that was not present at the other locations. Between the months of March and June, the detection rate was much higher at this location than then at any time in the other locations. Between the months of July 2010 and January 2011, the detection rate was similar for the SW, SE, and NE locations. The reasons for such a seasonal variation are not known.
One of the difficulties in analyzing acoustic data recorded remotely is in obtaining an accurate identification of the whale or dolphin species that emitted the sounds. This applies to both whistle and echolocation signals. To complicate matters, in the marine environment it is often difficult to obtain visual validation or confirmation of the species emitting the sounds. Finally if most of the sounds are emitted at night, the problem of assessing the accuracy of the acoustic identification becomes almost impossible. In general whistles are easier to identify than clicks since clicks are very short in duration and the waveform and the subsequent spectrum will vary according to the geometry between the marine mammal and the sensor. On the positive side, there are some species of odontocetes that emit relatively low-frequency biosonar signals that seem to be species specific and these animals can be identified by their click emissions. However, there are some serious issues involving species identification. In the study of Au et al. (2013, 2014), biosonar clicks identified as originating from Risso’s dolphins consisted of approximately 15 % of all clicks by deep-diving odontocetes around the island of Kauai. However, Risso’s dolphins are not detected very often in visual surveys conducted in Hawaiian waters (Barid et al. 2013). So we are left with a conundrum in regard to this species. The M3R has been validated by two independent methods and with different data sets and shown to accurately label Risso’s dolphins. We cannot deny that clicks that best represent Risso’s dolphins were detected about 15 % of the time and that the clicks cannot at this time be assigned to other species of dolphins. We could choose to assign these clicks to the unknown dolphin category. However, these clicks were very consistent in their characteristics and to the best of our knowledge resemble Risso’s dolphin signals. Therefore, we chose to assign the signals to Risso’s dolphins. Other investigators may choose to assign them to the unknown dolphin category and that would be their prerogative.
One should keep in mind the situation with minke whale s and boing sounds. Boing sounds have been detected in Hawaiian waters as early as the 1950s (Wenz 1962). Yet it was not until 2002 that it was confirmed by visual and acoustic record ings that boing sounds were produced by minke whales (Rankin and Barlow 2005). Not one single minke whale was sighted during more than 10 years of aerial survey effort over Hawaiian waters (Mobley et al. 2000; Mobley 2004). Additionally, despite significant shipboard survey efforts, there have been only a handful of verified sightings in Hawaiian waters. A juvenile minke whale was observed riding the bow wave of a navy ship in the 1970s (Balcomb, pers. comm.), long-line fishery observers have reported four confirmed minke whale sightings (Carretta et al. 2005), three minke whales were encountered in Hawaiian waters during a 5-month visual and acoustic survey of marine mammal abundance (Barlow et al. 2004), and one minke whale was sighted during a 1-week visual and acoustic survey focused on minke whales in waters offshore of Oahu and Kauai (Rankin et al. 2008). The minke whale experience can be summarized by the statement “just because you can’t see them doesn’t mean that they are not there, especially if you can hear them.”
Discrepancies between visual surveys and sighting and results from acoustic record ings are expected since both the methodologies have their own strengths and weaknesses (Barlow and Taylor 2005; Barlow and Rankin 2007). For example, Barid et al. (2013) reported that the sighting rate of short-finned pilot whales around Kauai was much lower than for other areas around the main Hawaiian Islands. Yet our results indicated that short-finned pilot whales were detected the most often of all the deep-diving odontocetes. For the most comprehensive understanding of the relative abundance of marine mammals including their distribution, time of occurrence, and movement patterns both visual and acoustic data should be collected and their results should be considered complementary rather than contrary. The “dual” approaches have been used for several years by NOAA, pioneered by Dr. Jay Barlow and Shannon Rankin at the Southwest Fishery Science Center in La Jolla, outside of San Diego, California.
It is important to emphasize that in order to make more progress in the field of acoustically identifying odontocetes by their biosonar signals more data in field need to be collected. In previous measurements of biosonar signals in the field, the focus was to obtain clicks that were emitted along the major axis of the animal’s beam. Those efforts should continue since there are many species from which biosonar signals have not been collected. However, field efforts should also include the collection of off-axis signals and laboratory effort should include measurements around the bodies of animals as was done by Au et al. (2012a, b) for Tursiops truncatus . A priority of laboratory measurements should be on different species including porpoises and dolphins that emit narrow-band biosonar signals.
The distribution of deep-diving odontocetes and their seasonal variations could only be obtained with remote autonomous PAM devices. Having visual survey teams at multiple locations that operated around the clock would be prohibitively expensive. Furthermore, many detections of these deep-diving odontocetes occurred at night (Au et al. 2013). It would also be very expensive to have a system in which hydrophones are connected to shore by cables as in the hydrophone arrays on the PMRF range. Finally, battery-operated radio telemeter systems (modified sonobuoys) powered by photovoltaic cells on the surface would be subject to theft or damage by boaters and their anchor cables could possibly be hazardous to the marine mammals that frequent the area.
5.8.2 Relative Abundance
The percentage of clicks from the different groups can be used to provide a first-order or ballpark estimate of the relative abundance of these deep-diving odontocetes. However, to go beyond this is not warranted. There are many criteria that need to be satisfied before a definitive statement can be made. The source levels of the different species would need to be almost the same and there are no data to support this criterion. The higher the source level the greater distance clicks can be detected and more clicks can also be detected. The beam pattern of each group of animals should be similar in order to estimate relative abundance. Once again, there is no data to support this contention. The beam pattern has been measured completely around an echolocating Tursiops truncatus (Au et al. 2012a, b) and estimates have been made of the beam pattern for Ziphius cavirostris by Zimmer et al. (2005) and by Shaffer et al. (2013) for Mesoplodon densirostris. The results of Au et al. (2012a) indicated that for large angles from the beam axis the source level can vary by as much as 50–60 dB! Another criterion that should be satisfied for an accurate abundance estimate has to do with the depth of the echolocating animal. The transmission loss may be different depending on the depth of the animal. Finally, the group size of the different animals would need to be similar since the greater the group size the more signals may be detected.
The results shown in this chapter clearly indicate that biosonar signals from short-finned pilot whales were detected the most often at all locations around Kauai and Okinawa and in the Marianas. Sperm whale and beaked whale clicks were detected almost equally around Kauai and other western Pacific locations but were detected less often than pilot whale clicks. Blackfish biosonar signals were also detected the most for EARs deployed near the Josephine Seamount off Portugal (Giorli et al. 2015). It is interesting that in very vastly separated locations that the biosonar signals of blackfish were detected the most of all the different deep-diving odontocetes.
Although PAM technology can provide valuable information about the occurrence of marine mammals and indications of the relative abundance of different species, there some serious limitations with the use of a single device which will hopefully be addressed in future generations of remote recorders. The range at which biosonar signals are being detected, the number of animals being detected at a given time, the depth at which different animals are detected, the relationship of the depth of foraging animals as a function of the time of day, and the movement pattern of foraging animals are but some of the few questions that should be addressed. To address some of these questions, array of remote recorders in which the data acquisition sample process is synchronized need to be developed. Such arrays exist in Navy ranges; however, these facilities not being available to most researchers present some serious problems and the hydrphone spacing and array configuration may not be optimal to address some questions. Therefore, the advancement in technology that is required has to do with being able to localize and track animals over a scale of several kilometers in three dimensions.
5.8.3 Diurnal Variation
There is a strong inclination of different deep-diving echolocating odontocetes to foraging at night. Johnston et al. (2008) using a HARP reported that beaked whale s at the Cross Seamount foraged mainly at night. Soldevilla et al. (2010) using recording from six HARPs moored between 300 and 1300 m found that Risso’s dolphins in the southern California Bight forage mainly at night. However, data from tagged beaked whales have shown no difference between day and night in the foraging patterns of beaked whale in the Tongue of the ocean, Bahama (Hazen et al. 2011), and off El Hierro, in the Canary Islands (Arranz et al. 2011). Baird et al. (2008) using time-depth recorders on six Blainville and two Cuvier’s beaked whale off the Hawaii Island also found that deep foraging dives occurred at the same rate during the day and night. The Seaglider experiment off the Kona coast of Hawaii Island indicated that Cuvier’s beaked whales and sperm whale did not display any difference between day and night foraging patterns (Klinck et al. 2012). It should be recognized that different types of information on odontocete foraging behavior are being gathered by PAM devices, time-depth recording, acoustic tags, and ocean gliders . Tags can obtain detailed temporal and spatial information on a few subjects for a short period of time whereas PAM devices sample a population for an extended period of several months. The differences between PAMs, tags, and ocean gliders can lead to different results and conclusions.
The results obtained with EARs around Kauai and in other western Pacific locations strongly indicate that deep-diving odontocetes forage mainly at night. The nighttime foraging behavior applies to four groups of deep-diving odontocetes, blackfish, sperm whale s, beaked whale s, and Risso’s dolphin . Sperm whales exhibited the lowest tendency to forage at night but the results are highly variable. Overall, the results for sperm whale indicated that approximately 70 % of their foraging activities occur at night but there were at one location (Le Shima off Okinawa ) in which only 54 % of the foraging was done at night.
The foraging pattern of any animal is dependent on the dynamic behavior of the prey and in order to obtain an appreciation of the foraging process, an understanding of the prey field is required. Research in the Hawaiian Islands has shown that the dynamic behavior of the mesopelagic boundary community (MBC) consisting of myctophid, shrimp , and small squid has an overriding influence on the natural history of spinner dolphins (Benoit-Bird et al. 2001; Benoit-Bird and Au 2003).
The prey field essentially dictates where on the coast spinner dolphins rest, where they forage, how they forage, and when they forage. A similar type of relationship would not be surprising between deep-diving foraging odontocetes and the prey they depend on for their survival. Unfortunately there is a poor understanding of the dynamic behavior of the prey fields of the deep-diving odontocetes and the rationale for nighttime foraging is much more difficult to understand. Short-finned pilot whales , sperm whale s, Risso’s dolphins, and beaked whale s all feed mainly on squids and occasionally on some unspecified species of fish . Seagars and Henderson (1985) reported that short-finned pilot whales in the Pacific west coast feed primarily on neritic squid Loligo sp. Mintzer et al. (2008) found that oceanic squid Brachioteuthis riisei was the main prey of short-finned pilot whales in the Atlantic; however Taonius pavo and Histioteuthis reversa were also a part of their diet. Sperm whales feed mainly on mesopelagic and benthic habitats on squids of different species and occasionally fish. Giant squid (Architeuthis sp.) and jumbo squid (Dosidicus sp.) and Antarctic colossal squid (Mesonychoteuthis hamiltoni) (Clarke et al. 1993; Whitehead 2003) are some prey species of sperm whales. Risso’s dolphins feed mainly on squid and other cephalopods (Clarke and Pascoe 1985). Off the California coast the jumbo squid (Dosidicus gigas) and the California market squid (Loligo opalescens) are common prey (Orr 1966; Kruse 1989). Beaked whales tend to prefer deepwater squid but there exist sufficient data to suggest that the prey specimens include a variety of demersal and mesopelagic fishes (Mead 2002; Pitman 2002; Ohizumi et al. 2003).
Although the habitat of the deep-diving odontocetes consists of the mesopelagic and upper bathypelagic zones of the ocean, it would not be surprising if some sort of habitat partitioning occurs as a function of depth . We can assume that sperm whale s must forage for larger prey than the smaller odontocetes. Short-finned pilot whales and beaked whale s are of similar size and it is conceivable that the prey species may be similar. Risso’s dolphins weigh approximately 1/2 that of short-finned pilot whales and it would be reasonable that they would forage for smaller prey. It would not be surprising that there are niches mediated by bottom depth for the different species of squids. Yet the various prey species behave in such a manner as to make it advantageous for the different species of deep-diving odontocetes to forage at night.
At the current level of understanding we can only speculate on the advantages of nighttime foraging . There has not been much research done on the foraging ecology of deep-diving odontocetes. It is well known that the deep scattering layer (DSL) and other mesopelagic layer of organisms migrate vertically towards the surface. However, scientific echosounder result indicates that the biomass structure in the offshore mesopelagic region can be very complicated.
Echosounding data obtained off the Kona coast of Hawaii Island shown in Fig. 5.14 indicate that there are usually two strong mesopelagic layers: one at deep depth on the order to 400–600 m and a shallower layer between 0 and 250 m.
The data also indicate that the layer structure is often complex with several “weaker” layers between the two strong layers. The surface layer became more dense at night with the vertical migration beginning at dusk as early as 17:10–20:00 HST. The organisms migrated from the surface to a deeper forging layer at dawn starting at 4:00–5:50 HST. A portion of the deep layer does not migrate vertically very much, remaining within a small range of depth . The depth of this deep layer appears to be relatively independent of the bottom depth. As depth increases beyond 600 m, the spacing between the deep layer and the bottom increases. The sloping bottom dropping off the chart can be seen in the echogram of Fig. 5.15 with the deep layer remaining relatively constant in depth. Echosounding inshore (approximately 1000 m bottom depth) and offshore (3000 m plus bottom depth) indicated that the biomass tends to be denser closer to shore. The peak densities of the top and bottom layer are approximately the same although the deep layer is considerably wider in depth.
The question is how does the migration of mesopelagic organisms affect the squid species and consequently the top marine mammal predators. If the squid prey also migrate from deep waters to forage on the organisms of the DSL, the squids would rise into depth strata that would be more beneficial for deep-diving odontocetes to forage on them. Furthermore, not all species of squid will behave in the same manner. Smaller species may migrate vertically higher in the layer than layer species of squid, which would create a partitioning of the prey field for deep-diving odontocetes. Arranz et al. (2011) have found that Blainville’s beaked whale s spend most of their foraging time in the lower part of the DSL or near the bottom in the Canary Islands. From a biosonar perspective, the DSL represents a volume reverberation environment and finding prey within such a layer of scatterers would represent a difficult sonar task. To detect and localize targets below the DSL or even off the bottom may be a simpler task than attempting to do so in the DSL. Andrews et al. (2011) using satellite time depth recorders found that short-finned pilot whales off the island of Hawaii do most of the foraging at night and that the night dives are slightly but not statistically significantly shallower than the daytime dives. The mean depth of dives for eight subjects varied between 293 and 502 m. However, more data from more species are needed in order to draw stronger conclusions about the diving and foraging behavior of not only pilot whales but the other deep-diving odontocetes. Other species of deep-diving odontocetes may not vary their foraging depth between day and night hours. Until data can be obtained from tag animals in different locations around the world, our understanding of the foraging ecology of deep-diving odontocetes will be severely limited. The one solid piece of knowledge that we have is the fact that there is a strong bias by these animals to foraging at night and dive to deep depths beyond 200 or so meters.
5.9 Closing Remarks
The data collected by a stationary PAM device such as the EAR make it possible to study the diurnal foraging behavior of deep-diving odontocetes over a long time period. Other instruments such as acoustic and time-depth recording tags and acoustic gliders have not uncovered the twilight-night foraging behavior of deep-diving odontocetes. The Seagilder experiment found a twilight-nigh sound emission for delphinids but these were probably from spinner dolphins which are known to forage mainly at night in swallow waters. The Seaglider experiment was also performed off the Kona coast of Hawaii Island instead of Kauai and geographic differences may have been a factor in not detecting a strong twilight-nighttime foraging tendencies in sperm and beaked whale s. Nevertheless, the results of this study indicate a strong tendency for twilight-nighttime foraging by deep-diving odontocetes around Kauai and Ni’ihau. The reasons for this foraging behavior are not known and will continue to be an area of interesting research.
The results collected by deep-moored EARs in the western Pacific are new and were obtained with a relatively new measurement technique and a signal processing technique (CS-SNM portion of the M3R algorithm) to identify species by the clicks they emit. In this type of situation, a considerable amount of consternation can arise among those not familiar with passive acoustic methods leading to much skepticisms. That is not a bad thing in science. However, in this case, it is a relatively simple process to detect the presence of biosonar clicks. It is also very easy to identify clicks from sperm and beaked whale s. Sperm whales are the only species that emit click with peaked frequency between 5 and 15 kHz. Beaked whales are the only odontocetes that emit clicks with fm modulation. As best as we know, short-finned pilot whale and Risso’s dolphins emit clicks with characteristics that are unique to them. Needless to say, more research in this area is warranted.
This chapter has illustrated how PAMs can be valuable instruments to determine the presence of sound-producing marine mammals. In this chapter, the diurnal and seasonal patterns of deep-diving odontocetes were examined. Yet the use of a single hydrophone can be a serious limitation. On one hand, an EAR can provide valuable information but on the other hand the limitation of the information provided can trigger deeper questions that are important to understand the behavior of marine mammals in a given body of water. Questions like how many animals are present, how far away are they, and how deep are they diving to cannot be addressed by present single hydrophone PAMs.
The results of our use of EARs in the western Pacific Ocean suggest that future studies of deep-diving odontocetes should have a strong ecological emphasis. The composition and dynamics of the prey field need to be examined more deeply. How the prey field of squid interacts with the mesopelagic layers is one area of study that is important and basis in order to understand the foraging behavior of deep-diving odontocetes. The role of the bottom topography is also a factor that should be considered in future studies. The bottom off the Hawaiian Islands rises steeply from the deep into the air as can be seen in Fig. 5.1. This type of topography is rather different than a seamount which basically represents submerged isolated bathymetric feature on the abyssal plane. Canyons, like the one at the AUTEC range, have steep walls that rise to an underwater plateau on both sides. The oceanographic conditions for these bottom types can be expected to be very different and these differences will affect the dynamic behavior of squid and fish prey. There are also many other factors that affect prey behavior that we can only speculate on without any detailed measurements. Furthermore, the geographic locations and atmospheric and oceanic patterns will all be contributing factors in a complex interactive web of variables that affect squid and fish prey behavior. In the end, the general prey field behavior around Pacific islands like Kauai, Ni’ihau, Okinawa , Guam , Tinian , and Saipan is such that deep-diving odontocetes must have a distinct advantage foraging at night rather than during the day. In summary, better and more sophisticated PAMs are needed and complementary ecological studies should be conducted with PAMs being but one of the instruments involved. PAMs definitely have a role in studies to understand the foraging behavior of deep-diving odontocetes.
References
N.A. Aguilar deSoto, M.P. Johnson, P.T. Madsen, F. Díaz, I. Domínguez, A. Brito, P. Tyack, Cheetahs of the deep sea: deep foraging sprints in short-finned pilot whales off Tenerife (Canary Islands). J. Anim. Ecol. 77, 936–947 (2008)
P. Arranz, N. Aqiliar de Soto, P.T. Madsen, A. Brito, F. Bordes, M.P. Johnson, Following a foraging fish-finder: Diel habitat use of Blainville’s beaked whales revealed by echolocation. PLoS One 6, e28353 (2011)
R.D. Andrews, G.S. Schorr, R.W. Baird, D.L. Webster, D.J. McSweeney, M.B. Hanson, New satellite-linked depth-recording LIMPET tags permit monitoring for weeks to months and reveal consistent deep nighttime feeding behavior of short-finned pilot whales in Hawai‘. Fourth international science symposium on bio-logging, Hobart, Tasmania, 2011
W.W.L. Au, The sonar of dolphins (Springer-Verlag, New York, 1993)
W.W.L. Au, D.L. Herzing, Measurement of the echolocation signals of the Atlantic Spotted Dolphin Stenella frontalis in the waters off the Grand Bahamas. J. Acoust. Soc. Am. 101, 3137–3138 (1997)
W.W.L. Au, B. Würsig, Echolocation signals of dusky dolphins (Lagenorhyn chus obscurus) in Kaikoura, New Zealand. J. Acoust. Soc. Am. 115, 2307–2313 (2004)
W.W.L. Au, J.K.B. Ford, J.K. Horne, K.A. Newman-Allman, Echolocation signals of free-ranging killer whales (Orcinus orca) and modeling of foraging for chinook salmon (Oncorhynchus tshawytscha). J. Acoust. Soc. Am. 116, 901–909 (2004)
W.W.L. Au, M.C. Hastings, The principles of marine bioacoustics (Springer-Verlag, New York, 2008)
W.W.L. Au, B. Branstetter, P.W. Moore, J.J. Finneran, The biosonar field around an Atlantic bottlenose dolphin (Tursiops truncatus). J. Acoust. Soc. Am. 131, 569–576 (2012a)
W.W.L. Au, B. Branstetter, P.W. Moore, J.J. Finneran, Dolphin biosonar signals measured at extreme off-axis angles: insights to sound propagation in the head. J. Acoust. Soc. Am. 132, 1199–1206 (2012b)
W.W.L. Au, G. Giorli, J. Chen, A. Copeland, M.O. Lammers, M. Richlen, S. Jarvis, R. Morrissey, D. Moretti, H. Klinck, Nighttime Foraging by deep diving echolocating odontocetes off the Hawaiian Islands of Kauai and Ni’iahu as determined by passive acoustic monitors. J. Acoust. Soc. Am. 133, 3119–3127 (2013)
W.W.L. Au, G. Giorli, J. Chen, A. Copeland, M.O. Lammers, M. Richlen, S. Jarvis, R. Morrissey, D. Moretti, Presence and seasonal variation of deep diving foraging odontocetes around Kauai, Hawaii using remote autonomous acoustic recorders. J. Acoust. Soc. Am. 135, 521–530 (2014)
R.W. Baird, D.L. Webster, G.S. Schorr, D.J. McSweeney, J. Barlow, Diel variation in beaked whale diving behavior. Mar. Mamm. Sci. 24, 630–642 (2008)
R.W. Baird, A.M. Borgone, D.J. McSweeney, A.D. Ligon, M.H. Deakos, D.L. Webster, G.S. Schorr, K.K. Martien, Population structure of island-associated dolphins: evidence from photo-identification of common bottlenose dolphins (Tursiops truncatus) in the main Hawaiian Islands. Mar. Mamm. Sci. 25, 251–274 (2009)
R.W. Barid, D.L. Webster, J.M. Aschettino, G.S. Schorr, D.J. McSweeney, Odontocete cetacean around the main Hawaiian islands: Habitat use and relative abundance from small-boat sighting surveys. Aquat. Mamm. 39, 253–269 (2013)
J. Barlow, S. Rankin, E. Zele, J. Appler, Marine mammal data collected during the Hawaiian island cetacean ecosystem assessment (HICEA) conducted aboard the NOAA ships McArthur and David Starr Jordan, July–December, 2002, NOAA/NMFS Technical Memorandum NOAA-TM-NMFS-SWFSC-362, 2004
J. Barlow, B.L. Taylor, Estimates of sperm whale abundance in the northeastern temperate Pacific from a combined acoustic and visual survey. Mar. Mamm. Sci. 21, 429–445 (2005)
J. Barlow, S. Rankin, False killer whale abundance and density: preliminary estimate for the PICEAS study area south of Hawaii and new estimates for the US EEZ around Hawaii, Administrative Report LJ-07-02 (Southwest Fisheries Science Center, La Jolla, CA, 2007)
S. Baumann-Pickering, M.A. Roch, H.-U. Schnitzler, J.A. Hildebrand, Echolocation signals of a beaked whale at Palmyra Atoll. J. Acoust. Soc. Am. 127, 3790–3799 (2010)
S. Baumann-Pickering, A.W. Simonis, E.M. Oleson, S. Rankin, R.W. Baird, M.A. Roch, S.M. Wiggins, J.A. Hildebrand, False killer whale and short-finned pilot whale acoustic occurrences around the Hawaiian Islands. Nineteenth Biennial Conference on the Biology of Marine Mammals, 27 November–2 December 2011
K.J. Benoit-Bird, W.W.L. Au, Prey dynamics affect foraging by a pelagic predator (Stenella longirostris) over a range of spatial and temporal scales. Behav. Ecol. Sociobiol. 53, 364–373 (2003)
N. Aguilar de Soto, Acoustic and diving behaviour of the short finned pilot whale (Globicephala macrorhynchus) and Blainville’s beaked whale (Mesoplodon densirostris) in the Canary Islands. Implications on the effects of man-made noise and boat collisions. PhD dissertation, La Laguna University, Tenerife, Canary Islands, 2006
J.V. Carretta, K.A. Forney, K.A. Muto, J. Barlow, J. Baker, B. Hanson, M.S. Lowry, U.S. Pacific marine mammal stock assessments 2004. NOAA Tech. Mem. NOAA-TM-NMFS-SWFSC-375, Southwest Fisheries Science Center, La Jolla, CA, 2005
M.R. Clarke, H.R. Martins, P. Pascoe, The diet of sperm whales (Physeter macrocephalus Linnaeus 1758) off the Azores. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 339, 67–82 (1993)
M.R. Clarke, P.L. Pascoe, The stomach contents of a Risso’s dolphin (Grampus griseus) stranded at Thurlestone, South Devon. J. Mar. Biol. 65, 663–665 (1985)
G. Giorli, W.W.L. Au, H. Ou, S. Jarvis, R. Morrissey, D. Moretti, Passive acoustics monitoring of Odontocetes foraging activity in the Josephine seamount Marine Protected Area. Submitted to J. Acoust. Soc. Am. (2015)
E.L. Hazen, D.P. Nowacek, L.S. Laurent, P.N. Halpin, D.J. Moretti, The relationship among oceanography, prey fields, and beaked whale foraging habitat in the Tongue of the Ocean. PLoS One 1–10, e19269 (2011)
S. Jarvis, A novel method for multiclass classification using support vector machines. PhD dissertation, University of Massachusetts Dartmouth, Dartmouth, MA, 2012
S. Jarvis, N. DiMarzio, R. Morrissey, D. Moretti, A novel multi-class support vector machine classifier for automated classification of beakd whales and other small odonotocetes. Can. Acoust. 36, 34–40 (2008)
M. Johnson, P.L. Tyack, A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Ocean. Eng. 28, 3–12 (2003)
M. Johnson, P.T. Madsen, M.X. Zimmer, N. Aquilar de Soto, P.L. Tyack, Breaked whales echolocate on prey. Proc. R. Soc. Lond. B. 271, S383–S386 (2004)
D.W. Johnston, M. McDonald, J. Polovina, R. Domokos, S. Wiggins, J. Hildebrand, Temporal patterns in the acoustic signals of beaked whales at Cross Seamount. Biol. Lett. 4, 208–211 (2008)
H. Klinck, D.K. Mellinger, K. Klinck, N.M. Bogue, J.C. Luby, W.A. Jump, G.B. Shilling, T. Litchendorf, A.S. Wood, G.S. Schorr, R.W. Baird, Near-real-time acoustic monitoring of beaked whales and other cetaceans using a Seaglider M. PLoS One 7, e36128 (2012)
S.L. Kruse, Aspects of the biology, ecology, and behavior of Risso’s dolphins (Grampus griseus) off the California Coast, Master’s thesis, University of California, Santa Cruz, CA, 1989
P.T. Madsen, R. Payne, N.U. Kristiansen, M. Wahlberg, I. Kerr, B. Mohl, Sperm whale sound production studied with ultrasound time/depth-recording tag. J. Exp. Biol. 205, 1899–1906 (2002)
P.T. Madsen, M. Johnson, N. Aquilar de Soto, M.X. Zimmer, P.L. Tyack, Biosonar performance of foraging beaked whales (Mesoplodon densirostris). J. Exp. Biol. 208, 181–194 (2005)
M.A. McDonald, J.A. Hildebrand, S.M. Wiggins, D.W. Johnston, J.J. Polovina, An acoustic survey of beaked whales at Cross Seamount near Hawaii. J. Acoust. Soc. Am. 125, 624–627 (2009)
J.G. Mead, Beaked whales, overview Ziphiidae, in Encyclopedia of marine mammals, ed. by W.F. Perrin, B. Würsig, J.G.M. Thewissen (Academic Press, San Diego, 2002), pp. 81–84
P.J.O. Miller, M.P. Johnson, P.L. Tyack, Sperm whale behaviour indicates the use of echolocation click buzzes ‘creaks’ in prey capture. Proc. R. Soc. Lond. B 271, 2239–2247 (2004)
V.J. Mintzer, D.P. Gannon, N.B. Barros, A.J. Read, Stomach contents of mass-stranded short-finned pilot whales (Globicephala macrorhynchus) from North Carolina. Mar. Mamm. Sci. 24, 290–302 (2008)
J.R. Mobley Jr., S.S. Spitz, K.A. Forney, R.A. Grotefendt, P.H. Forestell, Distribution and abundance of odontocete species in Hawaiian waters: preliminary results from 1993-98 aerial surveys. Admin. Rep. LJ-00-14C, Southwest Fisheries Science Center, La Jolla, CA, 2000
J.R. Mobley Jr, Results of marine mammal surveys on U.S. Navy underwater ranges in Hawaii and Bahamas, Final Report (Office of Naval Research, Arlington, VA, 2004), http://socrates.uhwo.hawaii.edu/SocialSci/jmobley/ONRfinal.pdf
B. Mohl, M. Wahlberg, P.T. Madsen, The monopulsed nature of sperm whale clicks. J. Acoust. Soc. Am. 114, 1143–1154 (2003)
H. Ohizumi, T. Isoda, T. Kishiro, H. Kato, Feeding habits of Baird’s beaked whales Berardius bairdii, in the western North Pacific and Sea of Okhotsk off Japan. Fish. Sci. 69, 11–20 (2003)
R.T. Orr, Risso’s dolphin on the Pacific coast of North America. J. Mammal. 47, 341–343 (1966)
R.L. Pitman, Mesoplodont whales, in Encyclopedia of marine mammals, ed. by W.F. Perrin, B. Würsig, J.G.M. Thewissen (Academic Press, San Diego, 2002), pp. 738–741
S. Rankin, J. Barlow, Source of the North Pacific ‘boing’ sound attributed to minke whales. J. Acoust. Soc. Am. 118, 3346–3351 (2005)
S. Rankin, J. Barlow, J. Oswald, L. Ballance, Acoustic studies of marine mammals during seven years of combined visual and acoustic line-transect surveys for cetaceans in the Eastern and Central Pacific Ocean. NOAA Technical Memorandum NOAA-TM-NMFS-SWFSC-429, 2008, 59 pp
M.H. Rasmussen, L.A. Miller, W.W.L. Au, Source levels of clicks from free-ranging white-beaked dolphins (Lagenorhynchus albirostris Gray 1846) recorded in Icelandic waters. J. Acoust. Soc. Am. 111, 1122–1126 (2002)
J.W. Shaffer, D. Moretti, S. Jarvis, P. Tyack, M. Johnson, Effective beam pattern of the Blainville’s beaked whale (Mesoplodon densirostris) and implications for passive acoustic monitoring. J. Acoust. Soc. Am. 133, 1770–1784 (2013)
M. Schotten, W.W.L. Au, M.O. Lammers, R. Aubauer, Echolocation recordings and localizations of wild spinner dolphins (Stenella longirostris) and pantropical spotted dolphins (Stenella attenuata) using a four hydrophone array, in Echolocation in Bats and Dolphins, ed. by J. Thomas, C. Moss, M. Vater (University of Chicago Press, Chicago, IL, 2003), pp. 393–409
D.J. Seagars, J.R. Henderson, Cephalopod remains from the stomach of a short-finned pilot whale collected near Santa Catalina Island, California. J. Mamm. 66, 777–779 (1985)
M.S. Soldevilla, E.E. Henderson, G.S. Campbell, S.M. Wiggins, J.A. Hildebrand, M.A. Roch, Classification of Risso’s and Pacific white-sided dolphins using spectral properties of echolocation clicks. J. Acoust. Soc. Am. 124, 609–624 (2008)
M.S. Soldevilla, S.M. Wiggins, J.A. Hildebrand, Spatial and temporal patterns of Risso’s dolphin echolocation in the Southern California Bight. J. Acoust. Soc. Am. 127, 124–132 (2010)
G.M. Wenz, Acoustic ambient noise in the ocean: spectra and sources. J. Acoust. Soc. Am. 34, 1936–1956 (1962)
H. Whitehead, Sperm whales: social evolution in the ocean (University of Chicago Press, Chicago, 2003)
S.M. Wiggins, J.A. Hildebrand, High-frequency Acoustic Recording Package (HARP) for broad-band, long-term marine mammal monitoring. International symposium on underwater technology, Institute of Electrical and Electronics Engineers, Japan, 2007, pp. 551–557
W.M.X. Zimmer, M.P. Johnson, P.T. Madsen, P.L. Tyack, Echolocation clicks of free-ranging Cuvier’s beaked whales ( Ziphius cavirostris ). J. Acoust. Soc. Am. 117, 3919–3927 (2005)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Au, W.W.L., Giorli, G. (2016). Studying the Biosonar Activities of Deep Diving Odontocetes in Hawaii and Other Western Pacific Locations. In: Au, W., Lammers, M. (eds) Listening in the Ocean. Modern Acoustics and Signal Processing. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3176-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3176-7_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3175-0
Online ISBN: 978-1-4939-3176-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)